Submitted:
23 June 2025
Posted:
25 June 2025
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Results
2.1. Baseline Clinical Characteristics
2.2. Spearman’s Correlations Between the Levels of Circulating Biomarkers and Other Parameters in CKD G1-2 Patients with Asymptomatic Coronary Artery Calcification
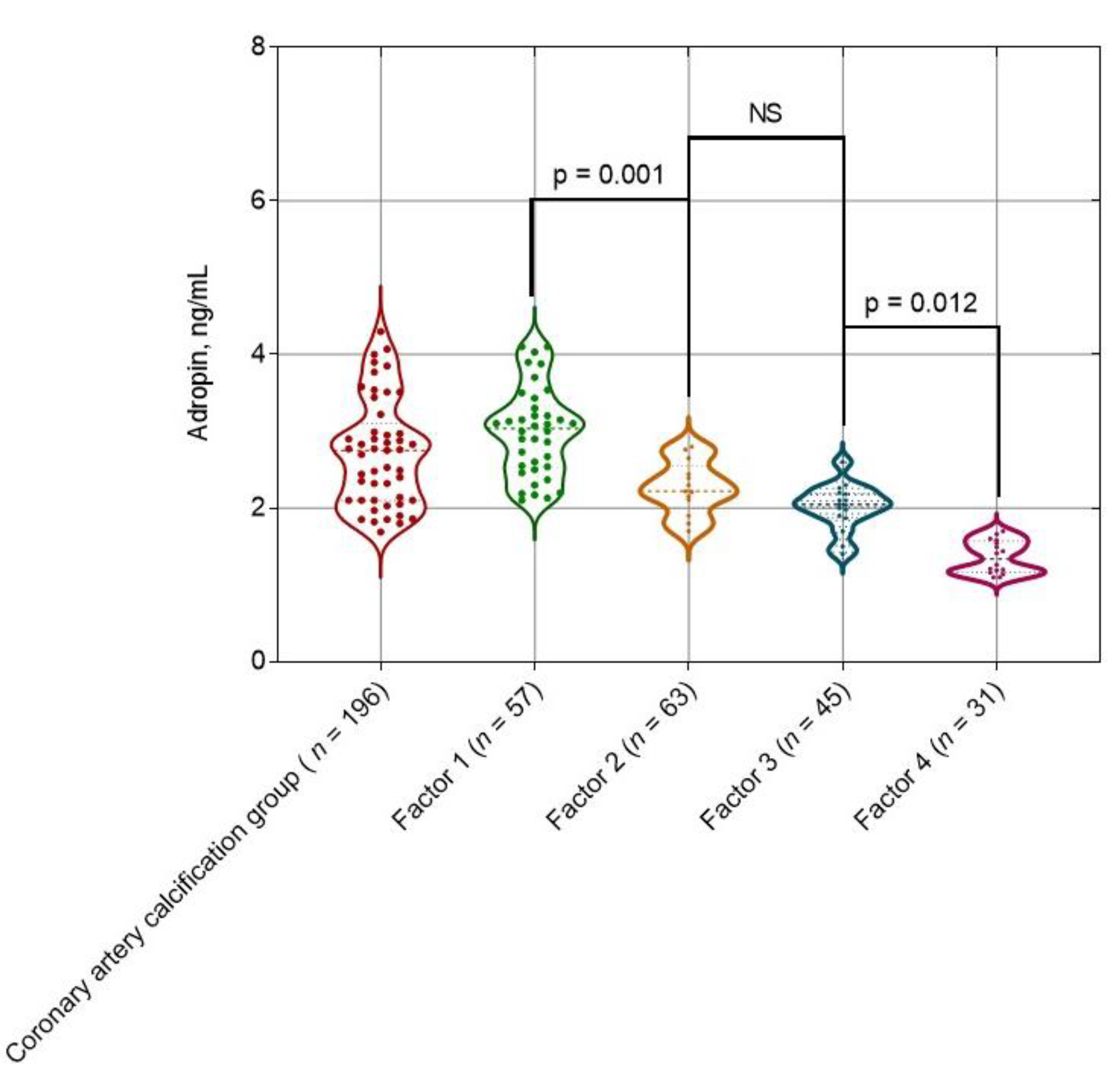
2.3. The Levels of Adropin Depending on the Weighted Sum of Coronary Artery Lesions with a Density
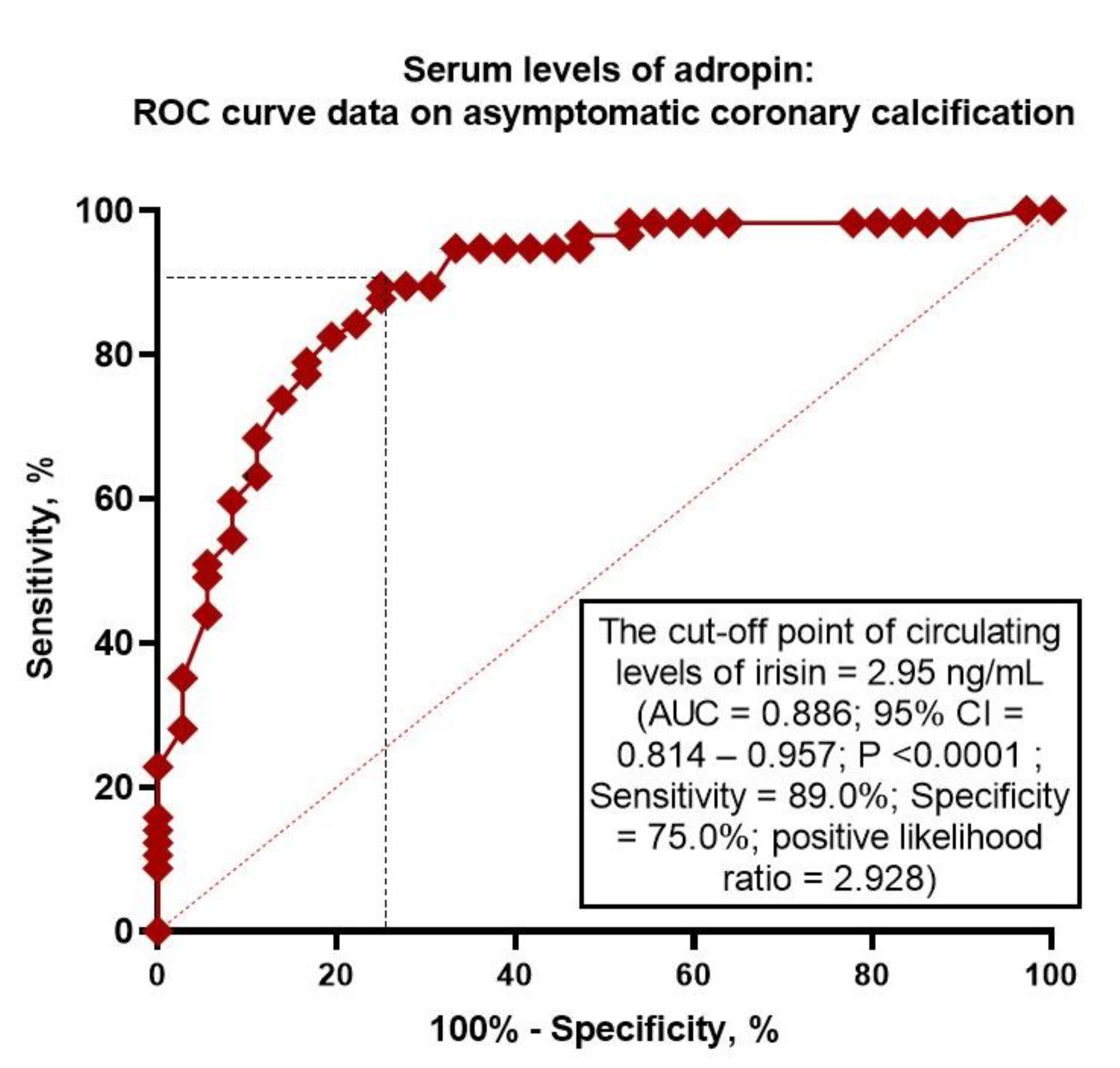
2.4. Receiver Operating Characteristic Curve Analysis for Adropin
2.5. Predictors of Asymptomatic Coronary Calcification: Univariate and Multivariate Logistic Regression Analyses
2.6. Comparison of the Predictive Models
3. Discussion
4. Materials and Methods
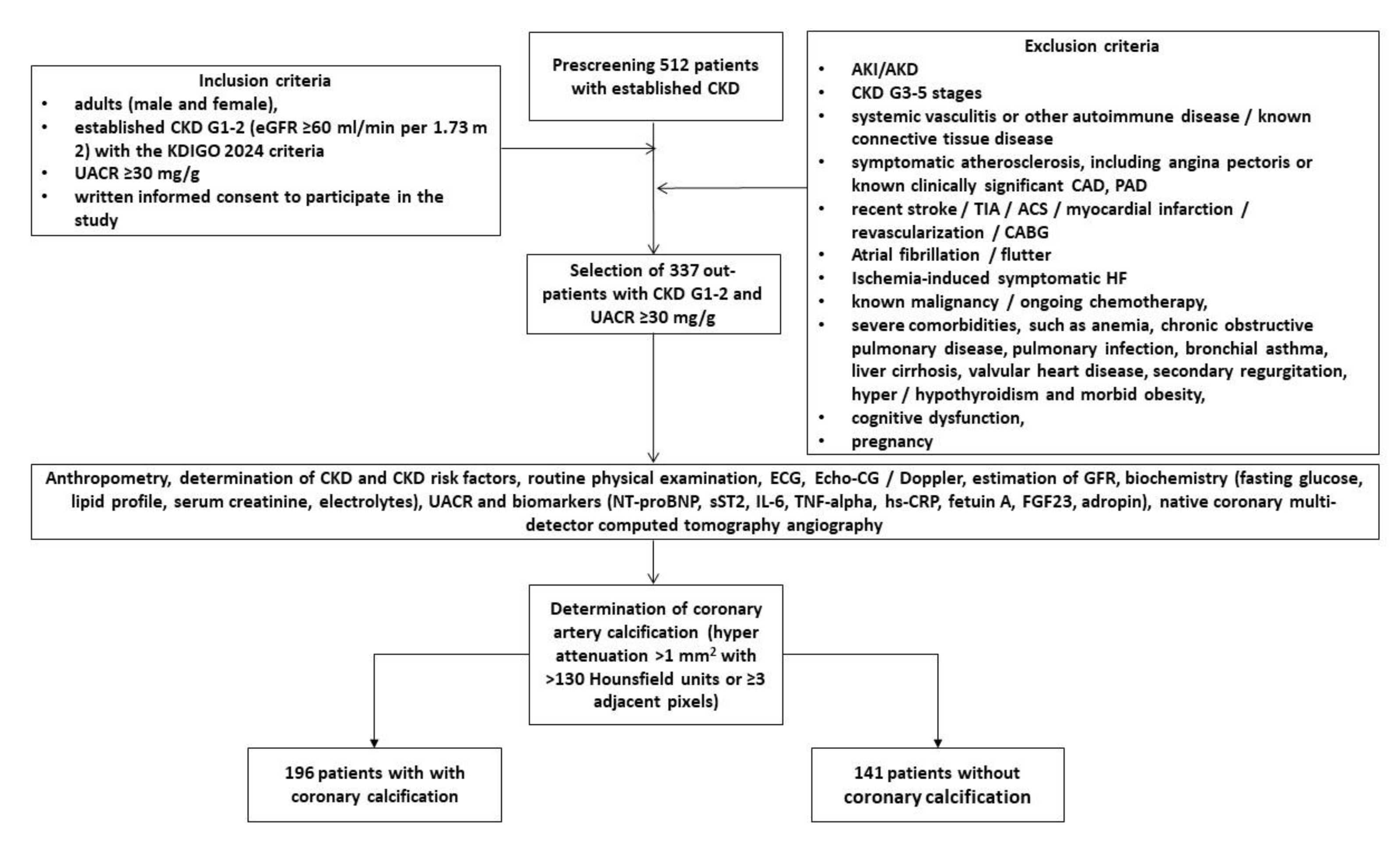
4.1. Study Population
4.2. Determination of Early Stages of CKD
4.3. Native Coronary Multi-Detector Computed Tomography Angiography
4.4. Determination of Coronary Artery Calcification
4.5. Echocardiography Examination
4.6. Clinical Data
4.7. Blood Sampling and Biomarker Assessment
4.8. Statistics
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| AUC | area under curve |
| BMI | body mass index |
| BP | blood pressure |
| CAD | coronary artery disease |
| CKD | chronic kidney disease |
| CV | cardiovascular |
| DPP-4 | dipeptidyl peptidase-4 |
| eGFR | estimated glomerular filtration rate |
| FGF-23 | fibroblast growth factor 23 |
| GLP-1 | glucagon-like peptide-1 |
| GLS | global longitudinal strain |
| HDL-C | high-density lipoprotein cholesterol |
| HFpEF | heart failure with preserved ejection fraction |
| hs-CRP | high-sensitivity C-reactive protein |
| HU | Hounsfield units |
| IL | interleukin |
| LAVI | left atrial volume index |
| LDL-C | low-density lipoprotein cholesterol |
| LVEDV | left ventricular end-diastolic volume |
| LVEF | left ventricular ejection fraction |
| LVESV | left ventricular end-systolic volume |
| LVH | left ventricular hypertrophy |
| LVMMI | left ventricle myocardial mass index |
| MRA | mineralocorticoid receptor antagonists |
| NT-proBNP | N-terminal natriuretic pro-peptide |
| PI3K | phosphatidylinositol 3-kinase |
| ROC | Receiver Operating Curve |
| SGLT2 | sodium–glucose cotransporter-2 |
| SUA | serum uric acid |
| T2DM | type 2 diabetes mellitus |
| TNF-alpha | tumor necrosis factor-alpha |
| UACR | urinary albumin/creatinine ratio |
| WHR | waist-to-hip ratio |
References
- Brück K, Stel VS, Gambaro G, Hallan S, Völzke H, Ärnlöv J, Kastarinen M, Guessous I, Vinhas J, Stengel B, Brenner H, Chudek J, Romundstad S, Tomson C, Gonzalez AO, Bello AK, Ferrieres J, Palmieri L, Browne G, Capuano V, Van Biesen W, Zoccali C, Gansevoort R, Navis G, Rothenbacher D, Ferraro PM, Nitsch D, Wanner C, Jager KJ; European CKD Burden Consortium. CKD Prevalence Varies across the European General Population. J Am Soc Nephrol, 2: 27(7), 2135. [CrossRef]
- Lv JC, Zhang LX. Prevalence and Disease Burden of Chronic Kidney Disease. Adv Exp Med Biol, 3: 1165, 1165. [CrossRef]
- Minutolo R, Gabbai FB, Chiodini P, Provenzano M, Borrelli S, Garofalo C, Bellizzi V, Russo D, Conte G, De Nicola L; Collaborative Study Group on the Conservative Treatment of CKD of the Italian Society of Nephrology. Sex Differences in the Progression of CKD Among Older Patients: Pooled Analysis of 4 Cohort Studies. Am J Kidney Dis. [CrossRef]
- Nair N, Kalra R, Chandra Bhatt G, Narang A, Kumar G, Raina R. The Effect and Prevalence of Comorbidities in Adolescents With CKD and Obesity. Adv Chronic Kidney Dis. 2022; 29(3):251-262. [CrossRef]
- Adler J, Taneva E, Ansorge T, Mertens PR. CKD prevalence based on real-world data: continuous age-dependent lower reference limits of eGFR with CKD-EPI, FAS and EKFC algorithms. Int Urol Nephrol, 2: 54(11), 2929. [CrossRef]
- Jankowski J, Floege J, Fliser D, Böhm M, Marx N. Cardiovascular Disease in Chronic Kidney Disease: Pathophysiological Insights and Therapeutic Options. Circulation, 1: 143(11), 1157. [CrossRef]
- Ding N, Lv Y, Su H, Wang Z, Kong X, Zhen J, Lv Z, Wang R. Vascular calcification in CKD: New insights into its mechanisms. J Cell Physiol, 1160. [CrossRef]
- Zoccali C, Mallamaci F, Adamczak M, de Oliveira RB, Massy ZA, Sarafidis P, Agarwal R, Mark PB, Kotanko P, Ferro CJ, Wanner C, Burnier M, Vanholder R, Wiecek A. Cardiovascular complications in chronic kidney disease: a review from the European Renal and Cardiovascular Medicine Working Group of the European Renal Association. Cardiovasc Res, 2: 119(11), 2017. [CrossRef]
- Charytan DM, Skali H, Shah NR, Veeranna V, Cheezum MK, Taqueti VR, Kato T, Bibbo CR, Hainer J, Dorbala S, Blankstein R, Di Carli MF. Coronary flow reserve is predictive of the risk of cardiovascular death regardless of chronic kidney disease stage. Kidney Int. [CrossRef]
- Murthy VL, Naya M, Foster CR, Hainer J, Gaber M, Dorbala S, Charytan DM, Blankstein R, Di Carli MF. Coronary vascular dysfunction and prognosis in patients with chronic kidney disease. JACC Cardiovasc Imaging, 1025. [CrossRef]
- Agatston AS, Janowitz WR, Hildner FJ, Zusmer NR, Viamonte M Jr, Detrano R. Quantification of coronary artery calcium using ultrafast computed tomography. J Am Coll Cardiol, 8: 15(4). [CrossRef]
- Neves PO, Andrade J, Monção H. Coronary artery calcium score: current status. Radiol Bras. [CrossRef]
- Nasir K, Clouse M. Role of nonenhanced multidetector CT coronary artery calcium testing in asymptomatic and symptomatic individuals. Radiology. 2012;264(3):637-49. [CrossRef]
- Pluquet M, Kamel S, Choukroun G, Liabeuf S, Laville SM. Serum Calcification Propensity Represents a Good Biomarker of Vascular Calcification: A Systematic Review. Toxins (Basel). 2022;14(9):637. [CrossRef]
- Liabeuf S, Okazaki H, Desjardins L, Fliser D, Goldsmith D, Covic A, Wiecek A, Ortiz A, Martinez-Castelao A, Lindholm B, Suleymanlar G, Mallamaci F, Zoccali C, London G, Massy ZA. Vascular calcification in chronic kidney disease: are biomarkers useful for probing the pathobiology and the health risks of this process in the clinical scenario? Nephrol Dial Transplant, 1: 29(7), 1275. [CrossRef]
- Bozic M, Méndez-Barbero N, Gutiérrez-Muñoz C, Betriu A, Egido J, Fernández E, Martín-Ventura JL, Valdivielso JM, Blanco-Colio LM; investigators from the NEFRONA study. Combination of biomarkers of vascular calcification and sTWEAK to predict cardiovascular events in chronic kidney disease. Atherosclerosis. [CrossRef]
- Kaur R, Krishan P, Kumari P, Singh T, Singh V, Singh R, Ahmad SF. Clinical Significance of Adropin and Afamin in Evaluating Renal Function and Cardiovascular Health in the Presence of CKD-MBD Biomarkers in Chronic Kidney Disease. Diagnostics, 3: 2023;13(19), 2023. [CrossRef]
- Berezin AE, Berezina TA, Hoppe UC, Lichtenauer M, Berezin AA. An overview of circulating and urinary biomarkers capable of predicting the transition of acute kidney injury to chronic kidney disease. Expert Rev Mol Diagn. [CrossRef]
- Chen IW, Lin CW, Lin CN, Chen ST. Serum adropin levels as a potential biomarker for predicting diabetic kidney disease progression. Front Endocrinol, 1: 2025;16, 2025. [CrossRef]
- Ali II, D'Souza C, Singh J, Adeghate E. Adropin's Role in Energy Homeostasis and Metabolic Disorders. Int J Mol Sci, 8: 23(15), 8318. [CrossRef]
- Rooban S, Arul Senghor KA, Vinodhini VM, Kumar JS. Adropin: A crucial regulator of cardiovascular health and metabolic balance. Metabol Open, 1: 23, 1002. [CrossRef]
- Wei W, Liu H, Qiu X, Zhang J, Huang J, Chen H, Qiu S, Lin R, Li S, Tu M. The association between serum adropin and carotid atherosclerosis in patients with type 2 diabetes mellitus: a cross-sectional study. Diabetol Metab Syndr. [CrossRef]
- Zhao LP, You T, Chan SP, Chen JC, Xu WT. Adropin is associated with hyperhomocysteine and coronary atherosclerosis. Exp Ther Med, 1065. [CrossRef]
- Wu L, Fang J, Chen L, Zhao Z, Luo Y, Lin C, Fan L. Low serum adropin is associated with coronary atherosclerosis in type 2 diabetic and non-diabetic patients. Clin Chem Lab Med, 7: 52(5). [CrossRef]
- El Moneem Elfedawy MA, El Sadek Elsebai SA, Tawfik HM, Youness ER, Zaki M. Adropin a candidate diagnostic biomarker for cardiovascular disease in patients with chronic kidney disease. J Genet Eng Biotechnol, 1: 22(4), 1004. [CrossRef]
- Morena-Carrere M, Jaussent I, Chenine L, Dupuy AM, Bargnoux AS, Leray-Moragues H, Klouche K, Vernhet H, Canaud B, Cristol JP. Severe Coronary Artery Calcifications in Chronic Kidney Disease Patients, Coupled with Inflammation and Bone Mineral Disease Derangement, Promote Major Adverse Cardiovascular Events through Vascular Remodeling. Kidney Blood Press Res. [CrossRef]
- Wang XR, Yuan L, Shi R, Li H, Wang DG, Wu YG. Predictors of coronary artery calcification and its association with cardiovascular events in patients with chronic kidney disease. Ren Fail, 1172. [CrossRef]
- Jin H, Ji JJ, Zhu Y, Wang XD, Li YP, Shi QY, Chen YF. Brain-Derived Neurotrophic Factor, a New Predictor of Coronary Artery Calcification. Clin Appl Thromb Hemost, 1: 27, 1076. [CrossRef]
- Berlot AA, Fu X, Shea MK, Tracy R, Budoff M, Kim RS, Naveed M, Booth SL, Kizer JR, Bortnick AE. Matrix Gla protein and the long-term incidence and progression of coronary artery and aortic calcification in the Multi-Ethnic Study of Atherosclerosis. Atherosclerosis, 1175. [CrossRef]
- Golüke NMS, Schoffelmeer MA, De Jonghe A, Emmelot-Vonk MH, De Jong PA, Koek HL. Serum biomarkers for arterial calcification in humans: A systematic review. Bone Rep, 1015. [CrossRef]
- Wong ND, Budoff MJ, Ferdinand K, Graham IM, Michos ED, Reddy T, Shapiro MD, Toth PP. Atherosclerotic cardiovascular disease risk assessment: An American Society for Preventive Cardiology clinical practice statement. Am J Prev Cardiol, 1: 10, 1003. [CrossRef]
- Berezina TA, Obradovic Z, Boxhammer E, Berezin AA, Lichtenauer M, Berezin AE. Adropin Predicts Chronic Kidney Disease in Type 2 Diabetes Mellitus Patients with Chronic Heart Failure. J Clin Med, 2231. [CrossRef]
- Butler AA, Havel PJ. Adropin: A cardio-metabolic hormone in the periphery, a neurohormone in the brain? Peptides, 1: 187, 1713. [CrossRef]
- Rooban S, Arul Senghor KA, Vinodhini VM, Kumar JS. Adropin: A crucial regulator of cardiovascular health and metabolic balance. Metabol Open, 1: 23, 1002. [CrossRef]
- Bozic J, Kumric M, Ticinovic Kurir T, Males I, Borovac JA, Martinovic D, Vilovic M. Role of Adropin in Cardiometabolic Disorders: From Pathophysiological Mechanisms to Therapeutic Target. Biomedicines, 1: 9(10), 1407. [CrossRef]
- Boric-Skaro D, Mizdrak M, Luketin M, Martinovic D, Tokic D, Vilovic M, Supe-Domic D, Kurir TT, Bozic J. Serum Adropin Levels in Patients on Hemodialysis. Life, 3: 2021;11(4), 2021. [CrossRef]
- Kiliç AF, Erkuş E, Duysak L. Measurement of serum adropin levels in chronic renal failure patients receiving routine hemodialysis treatment. Medicine, e: 2025;104(12), 2025. [CrossRef]
- Chen IW, Lin CW, Lin CN, Chen ST. Serum adropin levels as a potential biomarker for predicting diabetic kidney disease progression. Front Endocrinol, 1: 2025;16, 2025. [CrossRef]
- El Moneem Elfedawy MA, El Sadek Elsebai SA, Tawfik HM, Youness ER, Zaki M. Adropin a candidate diagnostic biomarker for cardiovascular disease in patients with chronic kidney disease. J Genet Eng Biotechnol, 1: 22(4), 1004. [CrossRef]
- Berezina TA, Fushtey IM, Berezin AA, Pavlov SV, Berezin AE. Predictors of Kidney Function Outcomes and Their Relation to SGLT2 Inhibitor Dapagliflozin in Patients with Type 2 Diabetes Mellitus Who Had Chronic Heart Failure. Adv Ther, 2: 41(1). [CrossRef]
- Kidney Disease: Improving Global Outcomes (KDIGO) CKD Work Group. KDIGO 2024 Clinical Practice Guideline for the Evaluation and Management of Chronic Kidney Disease. Kidney Int, S: 105(4S). [CrossRef]
- Levey AS, Stevens LA, Schmid CH, Zhang YL, Castro AF, 3rd; Feldman HI, et al. A New Equation to Estimate Glomerular Filtration Rate. Ann. Intern. Med. 2009, 150, 604–612. [Google Scholar] [CrossRef] [PubMed]
- Abbara S, Blanke P, Maroules CD, Cheezum M, Choi AD, Han BK, Marwan M, Naoum C, Norgaard BL, Rubinshtein R, Schoenhagen P, Villines T, Leipsic J. SCCT guidelines for the performance and acquisition of coronary computed tomographic angiography: A report of the society of Cardiovascular Computed Tomography Guidelines Committee: Endorsed by the North American Society for Cardiovascular Imaging (NASCI). J Cardiovasc Comput Tomogr. [CrossRef]
- Budoff MJ, Achenbach S, Blumenthal RS, Carr JJ, Goldin JG, Greenland P, Guerci AD, Lima JA, Rader DJ, Rubin GD, Shaw LJ, Wiegers SE; American Heart Association Committee on Cardiovascular Imaging and Intervention; American Heart Association Council on Cardiovascular Radiology and Intervention; American Heart Association Committee on Cardiac Imaging, Council on Clinical Cardiology. Assessment of coronary artery disease by cardiac computed tomography: a scientific statement from the American Heart Association Committee on Cardiovascular Imaging and Intervention, Council on Cardiovascular Radiology and Intervention, and Committee on Cardiac Imaging, Council on Clinical Cardiology. Circulation. 2006. [CrossRef]
- Mitchell C, Rahko PS, Blauwet LA, Canaday B, Finstuen JA, Foster MC, Horton K, Ogunyankin KO, Palma RA, Velazquez EJ. Guidelines for Performing a Comprehensive Transthoracic Echocardiographic Examination in Adults: Recommendations from the American Society of Echocardiography. J Am Soc Echocardiogr, 1: 32(1). [CrossRef]
- McEvoy JW, McCarthy CP, Bruno RM, Brouwers S, Canavan MD, Ceconi C, Christodorescu RM, Daskalopoulou SS, Ferro CJ, Gerdts E, Hanssen H, Harris J, Lauder L, McManus RJ, Molloy GJ, Rahimi K, Regitz-Zagrosek V, Rossi GP, Sandset EC, Scheenaerts B, Staessen JA, Uchmanowicz I, Volterrani M, Touyz RM; ESC Scientific Document Group. 2024 ESC Guidelines for the management of elevated blood pressure and hypertension. Eur Heart J, 3912. [CrossRef]
- American Diabetes Association Professional Practice Committee. 2. Diagnosis and Classification of Diabetes: Standards of Care in Diabetes-2024. Diabetes Care, S: 1). [CrossRef]
- Mach F, Baigent C, Catapano AL, Koskinas KC, Casula M, Badimon L, Chapman MJ, De Backer GG, Delgado V, Ference BA, Graham IM, Halliday A, Landmesser U, Mihaylova B, Pedersen TR, Riccardi G, Richter DJ, Sabatine MS, Taskinen MR, Tokgozoglu L, Wiklund O; ESC Scientific Document Group. 2019 ESC/EAS Guidelines for the management of dyslipidaemias: lipid modification to reduce cardiovascular risk. Eur Heart J. [CrossRef]
- McDonagh TA, Metra M, Adamo M, Gardner RS, Baumbach A, Böhm M, Burri H, Butler J, Čelutkienė J, Chioncel O, Cleland JGF, Coats AJS, Crespo-Leiro MG, Farmakis D, Gilard M, Heymans S, Hoes AW, Jaarsma T, Jankowska EA, Lainscak M, Lam CSP, Lyon AR, McMurray JJV, Mebazaa A, Mindham R, Muneretto C, Francesco Piepoli M, Price S, Rosano GMC, Ruschitzka F, Kathrine Skibelund A; ESC Scientific Document Group. 2021 ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure: Developed by the Task Force for the diagnosis and treatment of acute and chronic heart failure of the European Society of Cardiology (ESC). With the special contribution of the Heart Failure Association (HFA) of the ESC. Eur J Heart Fail. [CrossRef]
| Variables | Entire Group Patients with early (G1-2) CKD (n = 337) | Patients with coronary calcification (n = 196) | Patients without coronary calcification (n = 141) | p Value |
|---|---|---|---|---|
| Age (years) | 65 (54–77) | 68 (55–79) | 63 (52–74) | 0.044 |
| Male (n (%)) | 216 (64.1) | 125 (63.8) | 91 (64.5) | 0.822 |
| BMI (kg/m2) | 28.4 ± 6.7 | 29.6 ± 5.9 | 27.1 ± 4.6 | 0.710 |
| Waist circumference (cm) | 98 ± 5 | 98 ± 4 | 97 ± 6 | 0.810 |
| WHR (units) | 0.90 ± 0.2 | 0.91 ± 0.1 | 0.88 ± 0.1 | 0.750 |
| Smoking (n (%)) | 115 (34.1) | 71 (36.2) | 44 (31.2) | 0.870 |
| Dyslipidemia (n (%)) | 283 (84.0) | 172 (87.8) | 111 (78.7) | 0.061 |
| Hypertension (n (%)) | 269 (79.8) | 170 (86.7) | 99 (70.2) | 0.046 |
| Abdominal obesity (n (%)) | 92 (27.3) | 55 (28.1) | 37 (26.2) | 0.687 |
| T2DM, (n (%)) | 128 (38.0) | 82 (41.8) | 46 (32.6) | 0.044 |
| LVH (n (%)) | 273 (81.0) | 158 (80.6) | 115 (81.6) | 0.812 |
| HFpEF, (n (%)) | 138 (40.9) | 79 (40.3) | 59 (41.8) | 0.790 |
| Systolic BP (mm Hg) | 142 ± 10 | 143± 9 | 138 ± 7 | 0.660 |
| Diastolic BP (mm Hg) | 84 ± 8 | 86 ± 6 | 83 ± 5 | 0.830 |
| LVEDV (mL) | 149 (140–161) | 150 (140–163) | 149 (138–160) | 0.810 |
| LVESV (mL) | 68 (61–77) | 70 (62–79) | 67 (60–78) | 0.322 |
| LVEF (%) | 55 (51–59) | 53 (50–57) | 55 (51–59) | 0.384 |
| LVMMI (g/m2) | 142 ± 19 | 142 ± 16 | 140 ± 15 | 0.622 |
| LAVI (mL/m2) | 34 (31–38) | 35 (30–39) | 33 (30–37) | 0.646 |
| E/e` (units) | 13 ± 6 | 13 ± 4 | 12 ± 5 | 0.716 |
| GLS (%) | −14.5 (−11.6; −17.0) | −14.7 (−11.2; −17.2) | −14.3 (−12.1; −16.7) | 0.884 |
| eGFR (mL/min/1.73 m2) | 78 ± 15 | 75 ± 13 | 80 ± 14 | 0.776 |
| UACR, (mg/g) | 49 (33–217) | 52 (37–226) | 46 (32–211) | 0.644 |
| Fasting glucose (mmol/L) | 4.81 ± 1.24 | 5.22 ± 1.25 | 4.67 ± 1.30 | 0.292 |
| Creatinine (µmol/L) | 166 ± 39.1 | 173 ± 27 | 159 ± 24 | 0.655 |
| SUA (mcmol/L) | 365 ± 126 | 370 ± 115 | 356 ± 119 | 0.362 |
| Phosphorus (mmol/L) | 1.15 ± 0.28 | 1.15 ± 0.22 | 1.13 ± 0.20 | 0.773 |
| Calcium (mmol/L) | 2.24 (2.06–2.53) | 2.24 (2.10–2.62) | 2.22 (2.02–2.50) | 0.633 |
| Total cholesterol (mmol/L) | 5.70 ± 1.50 | 5.72 ± 1.42 | 5.66 ± 1.38 | 0.551 |
| HDL-C (mmol/L) | 0.99 ± 0.17 | 0.97 ± 0.15 | 0.99 ± 0.17 | 0.446 |
| LDL-C (mmol/L) | 3.82± 0.21 | 3.88 ± 0.20 | 3.79± 0.19 | 0.515 |
| Triglycerides (mmol/L) | 2.21 ± 0.17 | 2.27 ± 0.16 | 2.20 ± 0.15 | 0.524 |
| sST2, ng/mL | 9.8 (1.25 – 16.2) | 10.6 (0.77 – 17.1) | 8.5 (1.25 – 14.6) | 0.228 |
| hs-CRP (mg/L) | 5.15 (2.23–7.16) | 5.21 (2.30–7.30) | 5.03 (2.02–6.43) | 0.048 |
| TNF-alpha (pg/mL) | 2.61 (1.60–3.70) | 2.84 (1.92–4.15) | 2.32 (1.40–3.53) | 0.046 |
| IL-6, pg/mL | 1.67 (0.54–3.92) | 1.74 (0.62–4.15) | 1.58 (0.51–3.77) | 0.128 |
| NT-proBNP (pmol/mL) | 138 (55–219) | 142 (53–233) | 135 (47–215) | 0.563 |
| Adropin (ng/mL) | 3.50 (1.90–5.40) | 2.85 (1.85–4.07) | 3.94 (2.92–5.67) | 0.012 |
| Fetuin-A (μg/mL) | 54.2 (31.2 – 72.4) | 55.9 (33.6 – 75.1) | 53.8 (30.2 – 72.5) | 0.592 |
| FGF-23, pg/mL | 93.8 ± 15.2 | 105.5 ± 13.6 | 88.2 ± 17.8 | 0.055 |
| ACEIs (n (%)) | 217 (64.4) | 116 (59.2) | 101 (71.6) | 0.046 |
| Angiotensin-II receptor blockers (n (%)) | 48 (14.2) | 37 (18.9) | 11 (7.80) | 0.026 |
| Beta-blockers (n (%)) | 276 (81.9) | 157 (80.1) | 119 (84.4) | 0.659 |
| Ivabradine (n (%)) | 27 (8.0) | 17(8.7) | 10 (7.1) | 0.769 |
| Calcium channel blockers (n (%)) | 75 (22.3) | 37 (18.9) | 38 (27.0) | 0.040 |
| Loop or thiazide-like diuretics (n (%)) | 161 (47.8) | 95 (48.5) | 66 (46.8) | 0.725 |
| MRA (n (%)) | 95 (28.2) | 57 (29.1) | 38 (27.0) | 0.488 |
| Antiplatelet agents (n (%)) | 87 (25.8) | 51 (26.0) | 36 (25.5) | 0.873 |
| Metformin (n (%)) | 92 (27.3) | 58 (30.0) | 34 (24.1) | 0.554 |
| DPP4 inhibitors (n (%)) | 18 (5.3) | 9 (4.6) | 9 (6.4) | 0.120 |
| GLP-1 receptor agonists (n (%)) | 11 (3.2) | 5 (2.6) | 6 (4.2) | 0.066 |
| SGLT2 inhibitors (n (%)) | 65 (19.3) | 39 (19.9) | 26 (18.4) | 0.854 |
| Statins (n (%)) | 283 (84.0) | 172 (87.8) | 111 (78.7) | 0.061 |
| Variables | Adropin | hs-CRP | TNF-alpha | |||
| r | p | r | p | r | p | |
| Age (years) | -0.21 | 0.024 | 0.16 | 0.14 | 0.12 | 0.18 |
| BMI (kg/m2) | -0.23 | 0.001 | 0.19 | 0.04 | 0.18 | 0.05 |
| Systolic BP (mm Hg) | -0.25 | 0.001 | 0.08 | 0.43 | 0.12 | 0.22 |
| Diastolic BP (mm Hg) | -0.24 | 0.001 | 0.10 | 0.42 | 0.12 | 0.21 |
| LVEF (%) | 0.26 | 0.001 | -0.14 | 0.31 | -0.19 | 0.05 |
| LVMMI (g/m2) | -0.31 | 0.001 | -0.21 | 0.026 | -0.13 | 0.11 |
| LAVI (mL/m2) | -0.26 | 0.016 | 0.18 | 0.17 | 0.14 | 0.45 |
| GLS (%) | 0.32 | 0.001 | -0.17 | 0.46 | -0.13 | 0.60 |
| Agatston density range | 0.42 | 0.001 | -0.20 | 0.07 | -0.21 | 0.05 |
| eGFR (mL/min/1.73 m2) | 0.11 | 0.26 | -0.09 | 0.62 | -0.11 | 0.47 |
| UACR, (mg/g) | -0.21 | 0.012 | 0.13 | 0.47 | 0.19 | 0.12 |
| Fasting glucose (mmol/L) | -0.19 | 0.05 | 0.07 | 0.54 | 0.08 | 0.49 |
| Total cholesterol (mmol/L) | -0.25 | 0.03 | -0.08 | 0.57 | -0.10 | 0.55 |
| LDL-C (mmol/L) | -0.22 | 0.04 | 0.11 | 0.29 | 0.13 | 0.43 |
| Variables | Dependent Variable: asymptomatic coronary calcification | |||||||
| Univariate logistic regression | Multivariate logistic regression | |||||||
| OR | 95% CI | p-Value | C-Index | OR | 95% CI | p-Value | C-Index | |
| Low adropin vs. elevated adropin | 1.26 | 1.08–1.52 | 0.001 | 0.66 | 1.27 | 1.13–1.40 | 0.001 | 0.01 |
| UACR ≥49 mg/g vs. UACR <49 mg/g | 1.02 | 0.97-1.08 | 0.438 | 0.09 | - | |||
| hs-CRP ≥5.15 mg/L vs. hs-CRP <5.15 mg/L | 1.06 | 1.01-1.18 | 0.052 | 0.12 | 1.03 | 1.00-1.10 | 0.182 | 0.14 |
| TNF-α ≥2.61 pg/mL vs. TNF-α <2.61 pg/mL | 1.09 | 1.02-1.23 | 0.048 | 0.19 | 1.05 | 1.00-1.18 | 0.068 | 0.13 |
| Hypertension vs. non-hypertension | 1.09 | 1.03–1.22 | 0.044 | 0.32 | 1.09 | 1.07–1.23 | 0.042 | 0.36 |
| T2DM vs. non-T2DM | 1.07 | 1.02–1.15 | 0.042 | 0.31 | 1.05 | 1.01–1.10 | 0.044 | 0.31 |
| LVH vs. non-LVH | 1.08 | 0.96–1.25 | 0.672 | 0.11 | - | |||
| HFpEF vs. non-HFpEF | 1.11 | 1.02–1.24 | 0.046 | 0.37 | 1.14 | 1.00–1.28 | 0.422 | 0.13 |
| Administration of CCB | 0.89 | 0.71–0.99 | 0.042 | 0.39 | 0.90 | 0.70–1.02 | 0.068 | 0.22 |
| Administration of SGLT2i | 0.90 | 0.82–0.98 | 0.040 | 0.42 | 0.91 | 0.78–1.00 | 0.062 | 0.28 |
| Predictive models | Dependent Variable: asymptomatic coronary calcification | ||||||||
| AUC | NRI | IDI | |||||||
| M | 95% CI | p Value | M | 95% CI | p Value | M | 95% CI | p Value | |
| Model 1 | 0.886 | 0.814 – 0.957 | - | Reference | Reference | ||||
| Model 2 | 0.724 | 0.699 – 0.751 | 0.001 | 0.09 | 0.05 – 0.15 | 0.688 | 0.11 | 0.08 – 0.16 | 0.426 |
| Model 3 (T2DM) | 0.706 | 0.625 – 0.784 | 0.001 | 0.07 | 0.03 – 0.09 | 0.772 | 0.10 | 0.06 – 0.17 | 0.455 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





