Submitted:
31 January 2025
Posted:
03 February 2025
You are already at the latest version
Abstract
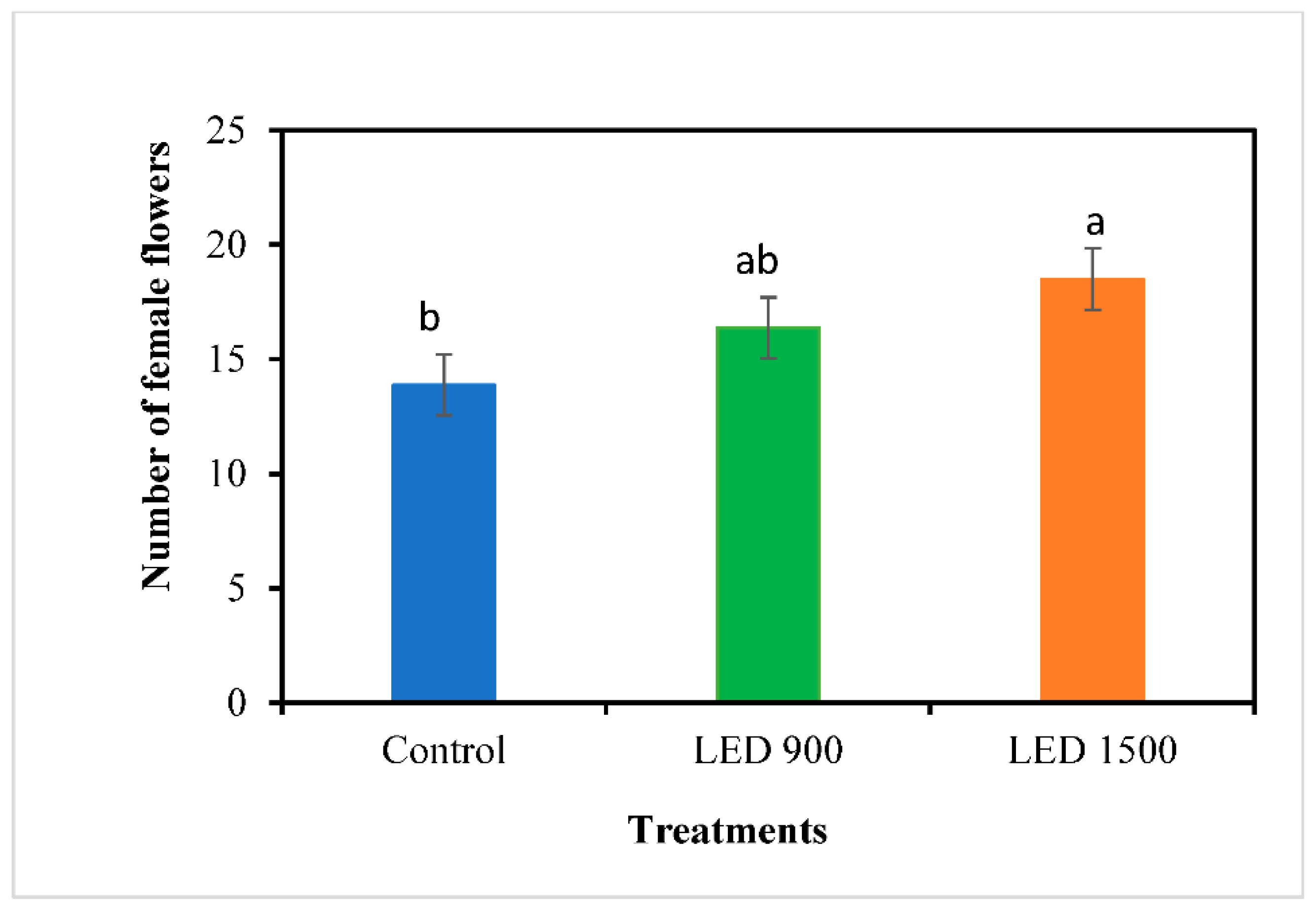
The effect of LED supplementary lighting at various intensities on winter-grown watermelon plants was evaluated to identify the optimal level for enhancing growth, yield, and quality. The plants were exposed to three lighting conditions: natural daylight (control) and LED supplementary lighting at 900 and 1500 µmol/m²/s, from 17:00 to 21:00. Supplemented LED lighting enhanced chlorophyll content, Ca2+ and Mg2+ in the leaves of fruit set region, leading to an increase in photosynthesis rate throughout the growing period and supporting consistent plant growth. The result showed that LED 900 µmol/m²/s significantly boosted the number of female flowers, fruit weight, size, and flesh thickness. Ultimately, the yield per plant increased by 31% under the LED at 900 µmol/m²/s and by 14% under the LED at 1500 µmol/m²/s compared to the control. Furthermore, high sugar and low acid contents were detected in the LED-treated fruits. These results indicate that fruits bore under LED lighting ripened faster than those in the control. In conclusion, supplemental LED lighting markedly contributes to watermelon production during winter, with a 900-µmol/m²/s LED light intensity outperforming 1500 µmol/m²/s in promoting plant growth and boosting the yield and quality.

Keywords:
1. Introduction
2. Materials and Methods
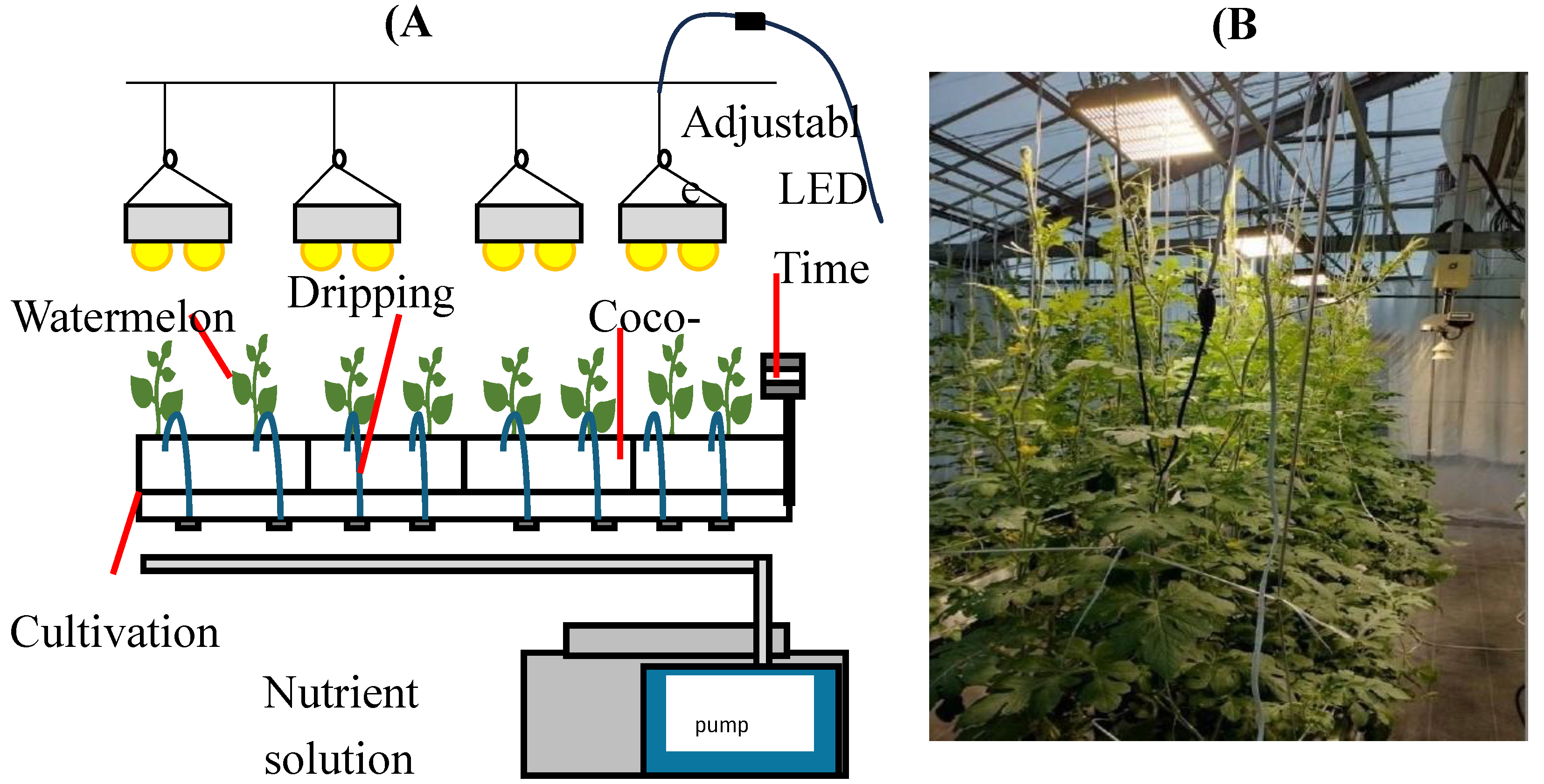
2.1. Plant Materials and Cultivation Conditions
2.2. Experimental Design and Supplementary LED Lighting Treatment
2.3. Methods of Sampling and Measurement
2.4. Statistical Analysis
3. Results
3.1. Determining Light Intensity and Environmental Conditions in Greenhouses
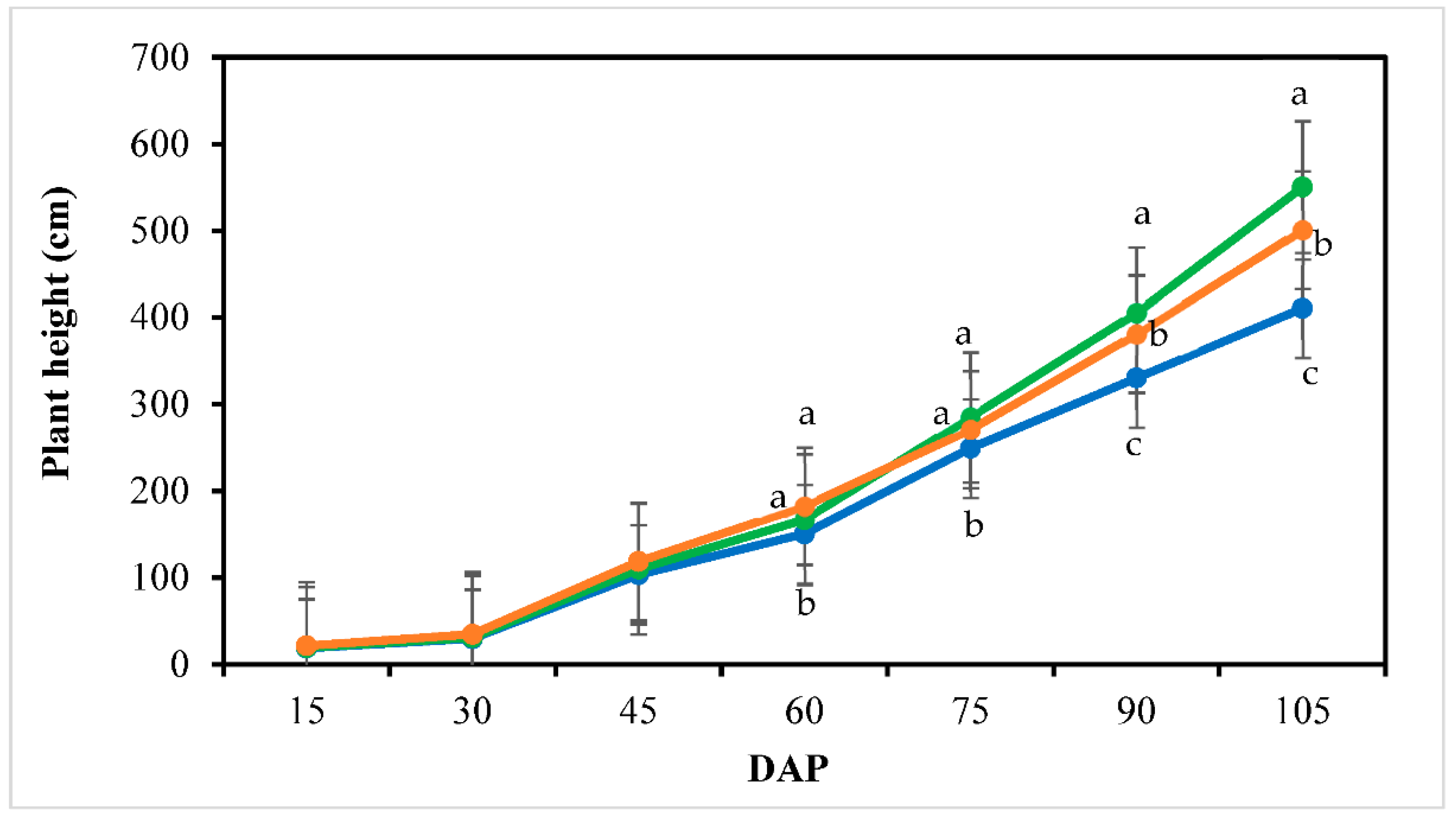
3.2. Plant Growth and Morphology
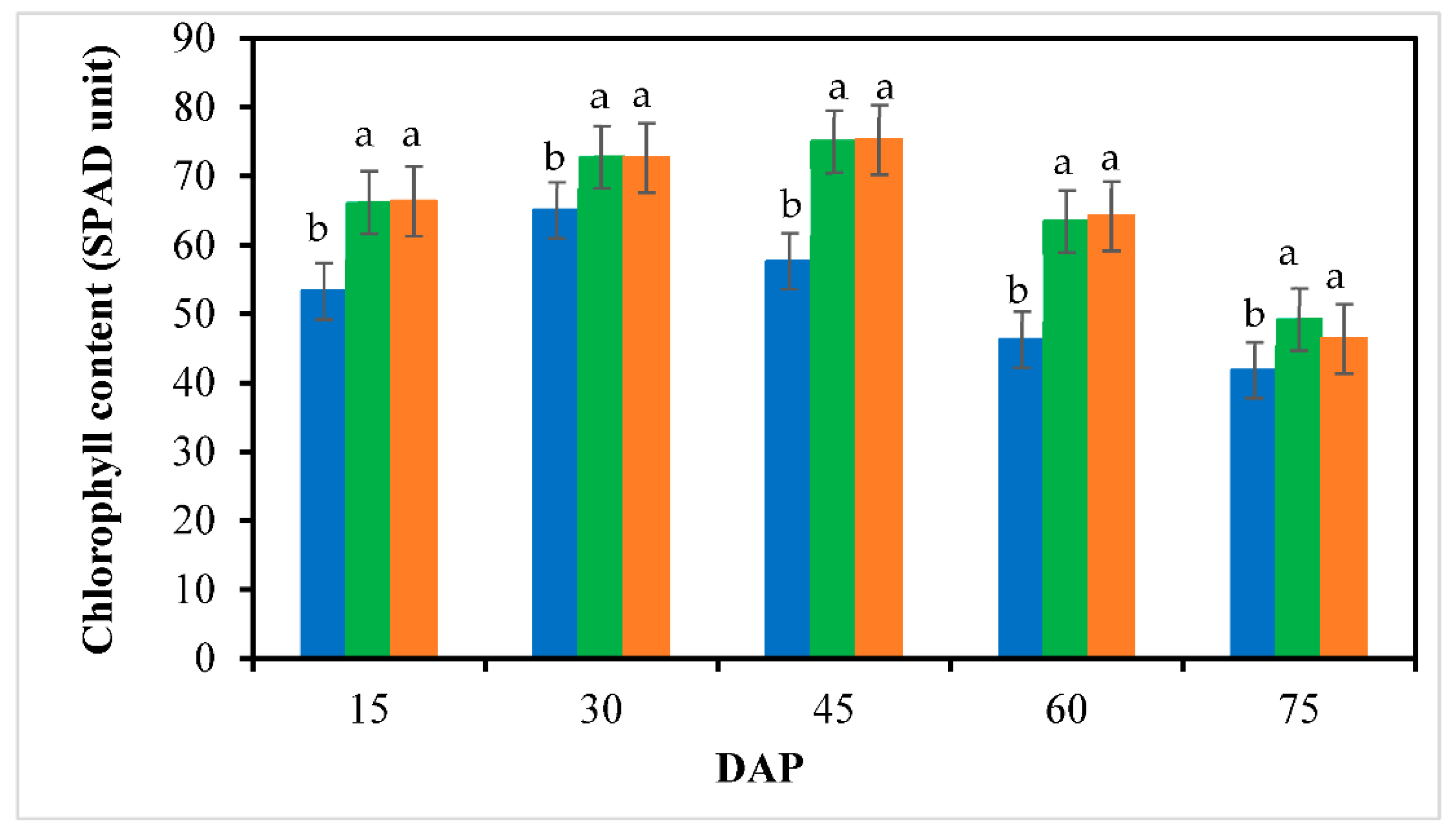
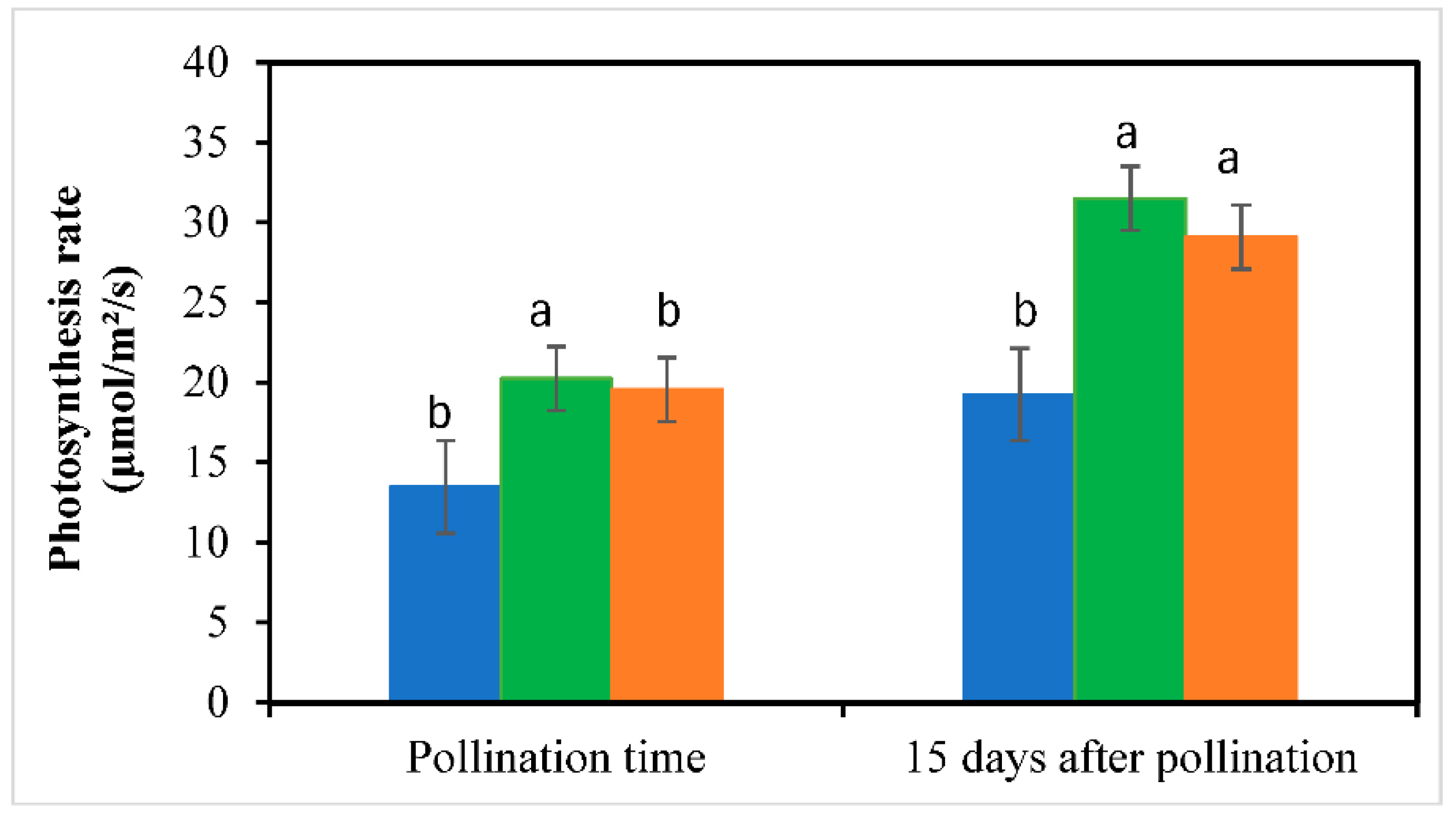
3.3. Leaf Chlorophyll Content and Photosynthesis
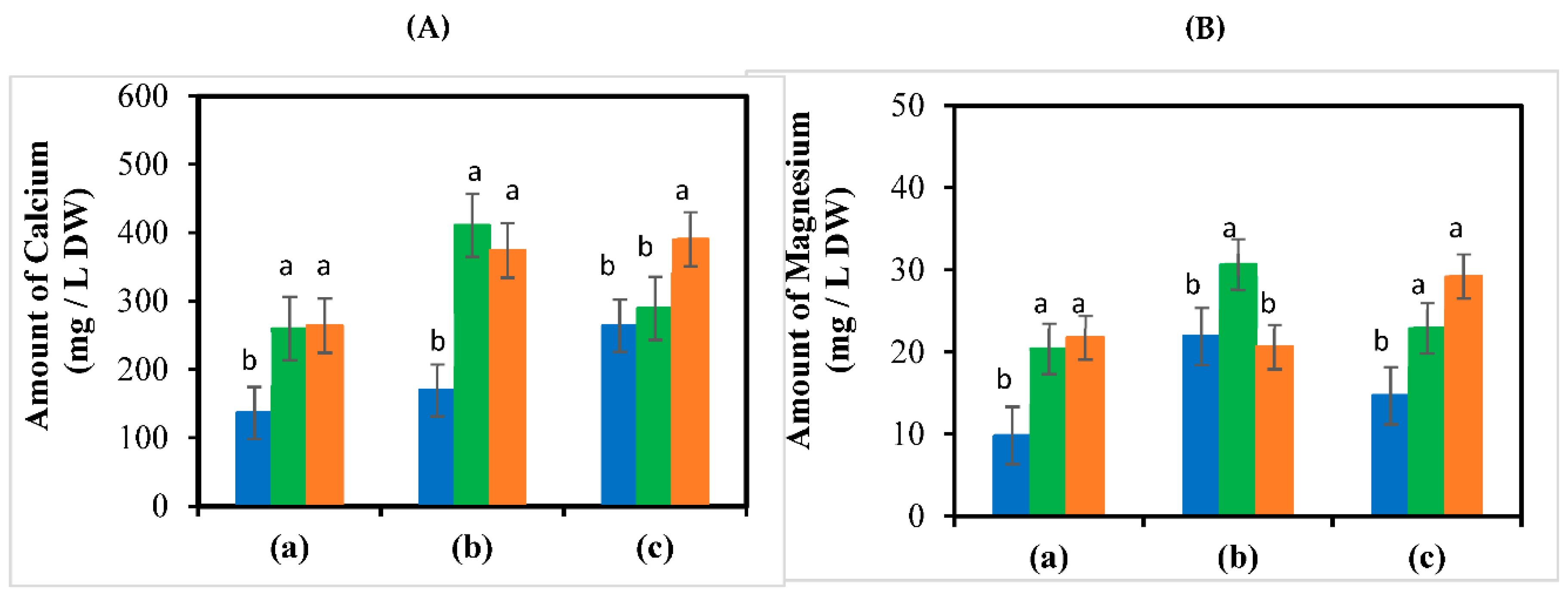
3.4. Ca2+ and Mg2+ Content in the Leaves of the Fruit Set Region
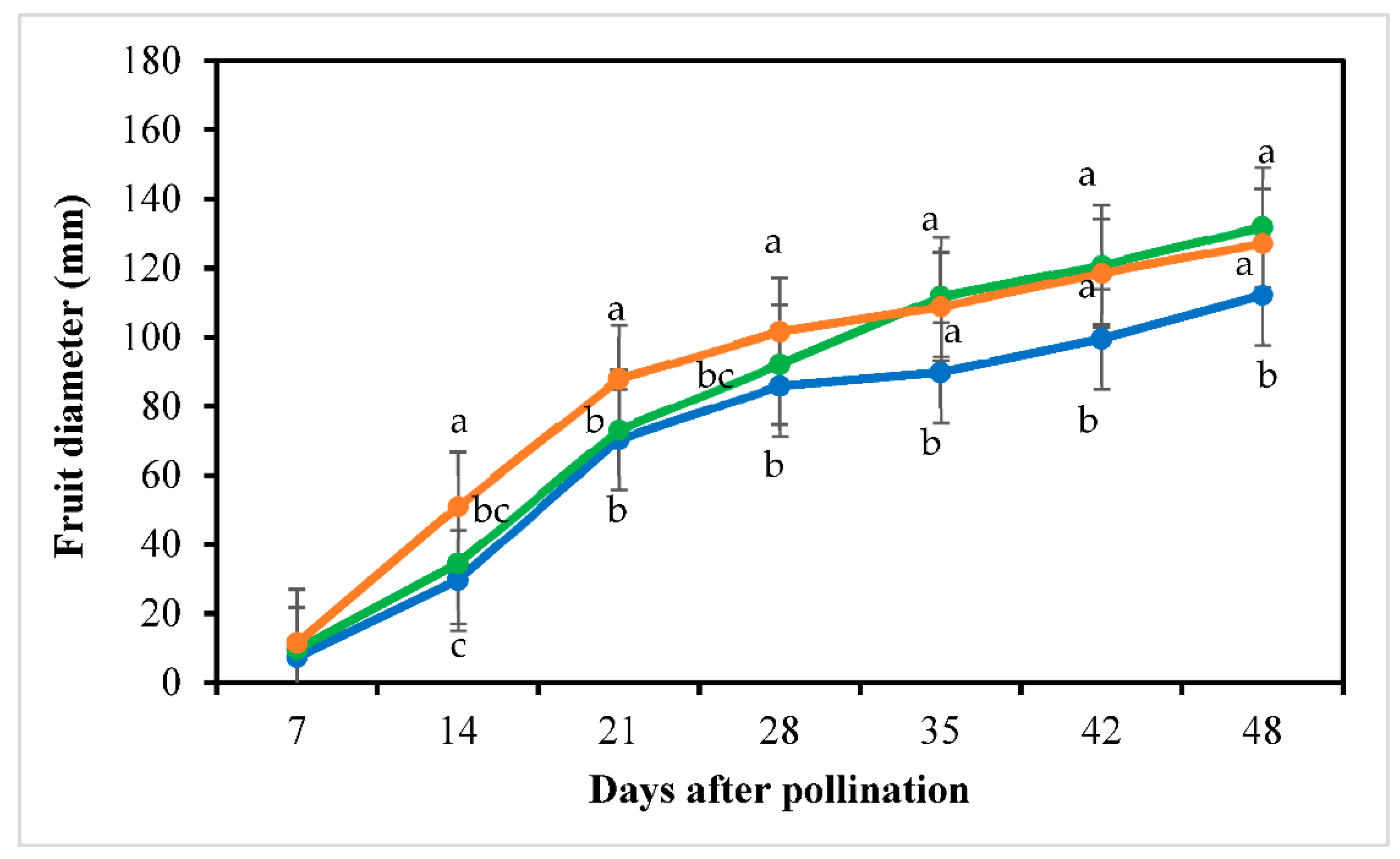
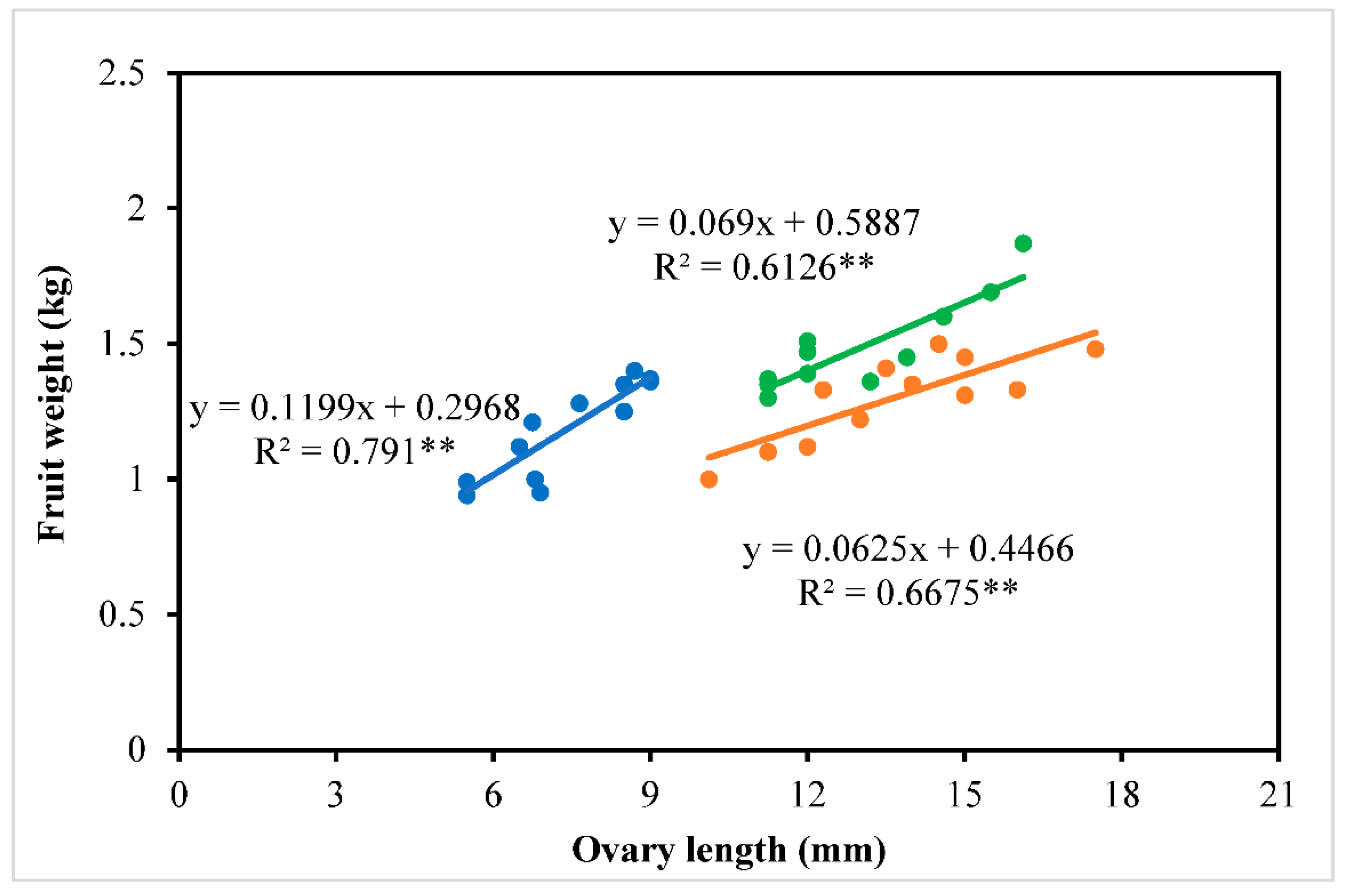
3.5. Fruit Yield and Quality
4. Discussion
4.1. LEDs Accelerate Early Growth and Have a Positive Effect Later on
4.2. In Winter, Strong Light Like That in Summer Is not Necessarily Required
4.3. Fruit Characteristics of Winter-Grown Watermelons
5. Conclusions
Supplementary Materials
Author Contributions
Data Availability Statement
Acknowledgment
Conflict of Interest
Abbreviations
| DAP | Days after pollination |
| Ca2+ | Calcium ion |
| Mg2+ | Magnesium ion |
References
- Reddy, U.K., Abburi, L., Abburi, V.L., Saminathan, T., Cantrell, R., Vajja, V.G., Reddy, R., Tomason, Y.R., Levi, A., Wehner, T.C., & Nimmakayala, P. 2015. A genome-wide scan of selective sweeps and association mapping of fruit traits using microsatellite markers in watermelon. J. Hered. 106(2):166–176. [CrossRef]
- Hashizume, T. 2019. Breeding and utilization of watermelon. J. Jpn. Soc. Food Sci. 66(8):314–318. [CrossRef]
- Levi, A., Jarret, R., Kousik, S., Patrick Wechter, W.P., Nimmakayala, P., & Reddy, U.K. 2017. Genetic resources of watermelon. In Genetics and Genomics of Cucurbitaceae, Plant Genetics and Genomics: Crops and Models (Grumet, R., Katzir, N., Garcia-Mas, J., eds.). Springer: Berlin, pp. 87–110. [CrossRef]
- Stamford, J.D., Stevens, J., Mullineaux, P.M., & Lawson, T. 2023. LED lighting: A Grower’s guide to light spectra. Hortscience 58(2):180–196. [CrossRef]
- Folta, K.M., & Carvalho, S.D. 2015. Photoreceptors and control of horticultural plant traits. Hortic. Sci. 50(9):1274–1280. [CrossRef]
- Fukuda, N., Fujita, M., Ohta, Y., Sase, S., Nishimura, S., & Ezura, H. 2008. Directional blue light irradiation triggers epidermal cell elongation of abaxial side resulting in inhibition of leaf epinasty in geranium under red light condition. Sci. Hortic. 115(2):176–182. [CrossRef]
- Olarewaju, O. O., Fajinmi, O. O., Arthur, G. D., Coopoosamy, R. M., & Naidoo, K. (2023). Effect of climate change on the production of Cucurbitaceae species in North African countries. Journal of Agriculture and Food Research, 14, 100742. [CrossRef]
- Varaldo, A., Alchera, F., Giuggioli, N. R., & Giacalone, G. (2024). LED UVB postharvest treatments modify nutraceutical quality and physical properties of ‘Cargo’ blueberries. Journal of Agriculture and Food Research, 15, 101040. [CrossRef]
- Ouzounis, T., Rosenqvist, E., & Ottosen, C.O. 2015. Spectral effects of artificial light on plant physiology and secondary metabolism: A review. Hortic. Sci. 50(8):1128–1135. [CrossRef]
- Soufi, H.R., Roosta, H.R., Fatehi, F., & Ghorbanpour, M. 2023. Spectral composition of LED light differentially affects biomass, photosynthesis, nutrient profile, and foliar nitrate accumulation of lettuce grown under various replacement methods of nutrient solution. Food Sci. Nutr. 11(12):8143–8162. [CrossRef]
- Hao, X., Little, C., Zheng, J.M., & Cao, R. 2016. Far-red LEDs improve fruit production in greenhouse tomato grown under high-pressure sodium lighting. Acta Hortic. 1134(1134):95–102.
- Park, Y.H., Seo, B.S., Seo, J.B., Shin, G.H., & Yun, B.G. 2016. Effects of supplemental light on quality, yield, and growth rate of tomatoes. Acta Hortic. 1129(1129):105–110.
- Choi, H.G., Moon, B.Y., & Kang, N.J. 2015. Effects of LED light on the production of strawberry during cultivation in a plastic greenhouse and in a growth chamber. Sci. Hortic. 189:22–31. [CrossRef]
- Pérez-Romero, L.F., Stirling, P.J., & Hancock, R.D. 2024. Light-emitting diodes improve yield, quality and inhibitory effects on digestive enzymes of strawberry. Sci. Hortic. 332:113192. [CrossRef]
- Cui, X.H., Guo, X.O., Sun, T.Y., & Qi, H.Y. 2017. Effects of LED supplementary lighting on seedling growth and fruit quality of oriental melon. Plant Physiol. J. 53(4):657–667. [CrossRef]
- Demers, D.A., Gosselin, A., & Wien, H.C. 1998. Effects of supplemental light duration on greenhouse sweet pepper plants and fruit yields. J. Am. Soc. Hortic. Sci. 123(2):202–207. [CrossRef]
- Zhou, C., Li, Z., Liu, W., Bian, Z., Lu, W., Zhou, B., Wang, S., Li, Q., & Yang, Q. (2023). High-Proportion Blue Light Irradiation at the End-of-Production Stage Promotes the Biosynthesis and Recycling of Ascorbate in Lettuce. International Journal of Molecular Sciences, 24(22), 16524. [CrossRef]
- Nadalini, S., Zucchi, P., & Andreotti, C. 2017. Effects of blue and red LED lights on soilless cultivated strawberry growth performances and fruit quality. Eur. J. Hortic. Sci. 82(1):12–20. doi:10.17660/eJHS.2017/82.1.2.
- Noh, J., Kim, J.M., Sheikh, S., Lee, S.G., Lim, J.H., Seong, M.H., & Jung, G.T. 2013. Effect of heat treatment around the fruit set region on growth and yield of watermelon [Citrullus lanatus (Thunb.) Matsum. and Nakai]. Physiol. Mol. Biol. Plants 19(4):509–514. [CrossRef]
- Lu, W., Edelson, J.V., Duthie, J.A., & Roberts, B.W. 2003. A comparison of yield between high- and low-intensity management for three watermelon cultivars. HortSci. 38(3):351–356.
- McNellis, T.W., & Deng, X.W. 1995. Light control of seedling morphogenetic pattern. Plant Cell 7(11):1749–1761. [CrossRef]
- Gómez, C., & Mitchell, C.A. 2016. Physiological and productivity responses of high-wire tomato as affected by supplemental light source and distribution within the canopy. J. Am. Soc. Hortic. Sci. 141(2):196–208.
- Tewolde, F.T., Shiina, K., Maruo, T., Takagaki, M., Kozai, T., & Yamori, W. 2018. Supplemental LED inter-lighting compensates for a shortage of light for plant growth and yield under the lack of sunshine. PLOS ONE 13(11):e0206592.
- Paponov, M., Kechasov, D., Lacek, J., Verheul, M.J., & Paponov, I.A. 2019. Supplemental light-emitting diode inter-lighting increases tomato fruit growth through enhanced photosynthetic light use efficiency and modulated root activity. Front. Plant Sci. 10:1656.
- Hanyu, H., & Shoji, K. 2002. Acceleration of growth in spinach by short-ter exposure to red, blue light at the beginning and the end of the daily dark period. Acta Hortic. 580:145–150.
- Kalathil, N., Thirunavookarasu, N., Lakshmipathy, K., Chidanand, D. V., Radhakrishnan, M., & Baskaran, N. (2023). Application of light-based, non-thermal techniques to determine physico-chemical characteristics, pungency and aflatoxin levels of dried red chilli pods (Capsicum annuum). Journal of Agriculture and Food Research, 13, 100648. [CrossRef]
- Hori, H. 1966. Gravel culture of vegetable and ornamental crops. (Japanese text) Agric. Hortic. p p 210.
- Li, Q., & Kubota, C. 2009. Effects of supplemental light quality on growth and phytochemicals of baby leaf lettuce. Environ. Exp. Bot. 67(1):59–64. [CrossRef]
- Baker, N.R. 2008. Chlorophyll fluorescence: A probe of photosynthesis in vivo. Annu. Rev. Plant Biol. 59:89–113. [CrossRef]
- Ahmed, N., Zhang, B., Bozdar, B., Chachar, S., Rai, M., Li, J., Li, Y., Hayat, F., Chachar, Z., & Tu, P. 2023. The power of magnesium: Unlocking the potential for increased yield, quality, and stress tolerance of horticultural crops. Front. Plant Sci. 14:1285512. [CrossRef]
- Wei, H., Wang, M., & Jeong, B.R. 2020. Effect of supplementary lighting duration on growth and activity of antioxidant enzymes in grafted watermelon seedlings. Agronomy 10(3):337. [CrossRef]
- Li, Y., Zhou, B., Teng, Z., Zhang, M., Yu, L., Luo, Y., Chen, P., & Sun, J. (2023). Improved metabolomic approach for evaluation of phytochemicals in mustard, kale, and broccoli microgreens under different controlled environment agriculture conditions. Journal of Agriculture and Food Research, 14, 100719. [CrossRef]
- Dong, C., Fu, Y., Liu, G., & Liu, H. 2014. Growth, photosynthetic characteristics, antioxidant capacity and biomass yield and quality of wheat (Triticum aestivum L.) exposed to LED light sources with different spectra combinations. J. Agron. Crop Sci. 200(3):219–230. [CrossRef]
- Sobczak, A., Kowalczyk, K., Gajc-Wolska, J., Kowalczyk, W., & Niedzińska, M. 2020. Growth, yield and quality of sweet pepper fruits fertilized with polyphosphates in hydroponic cultivation with LED lighting. Agronomy 10(10):1560. [CrossRef]
- Piovene, C., Orsini, F., Bosi, S., Sanoubar, R., Bregola, V., Dinelli, G., & Gianquinto, G. 2015. Optimal red:blue ratio in led lighting for nutraceutical indoor horticulture. Sci. Hortic. 193:202–208. [CrossRef]
- Choi, H.G., Moon, B.Y., & Kang, N.J. 2016. Correlation between strawberry (Fragaria ananassa Duch.) productivity and photosynthesis-related parameters under various growth conditions. Front. Plant Sci. 7:1607. [CrossRef]
- Anderson, C.M., Mattoon, E.M., Zhang, N. et al 2021. High light and temperature reduce photosynthetic efficiency through different mechanisms in the C4 model Setaria viridis. Commun. Biol. 4(1):1092. [CrossRef]
- Wimalasekera, R. 2019. Effect of light intensity on photosynthesis. In Photosynthesis, Productivity and Environmental Stress (Ahmad, P., Ahanger, M.S., Alyemeni, M.N., Alam, P., eds.), John Wiley & Sons, New Jersey, pp.65–73. [CrossRef]
- Kramchote, S., & Glahan, S. 2020. Effects of LED supplementary lighting and NPK fertilization on fruit quality of melon (Cucumis melo L.) grown in plastic house. J. Hortic. Res. 28(1):111–122. [CrossRef]
- Chamchum, W., Glahan, S., Kramchote, S., Maniwara, P., & Suwor, P. 2023. Growth and Yield of Watermelon (Citrullus lanatus) in Plastic House in Response to White LED Supplementary Lighting. Agrivita J. Agr. Sci. 45(2):271–277. [CrossRef]
- Sweetman, C., Deluc, L. G., Cramer, G. R., Ford, C. M., & Soole, K. L. (2009). Regulation of malate metabolism in grape berry and other developing fruits. Phytochemistry, 70(11–12), 1329–1344. [CrossRef]

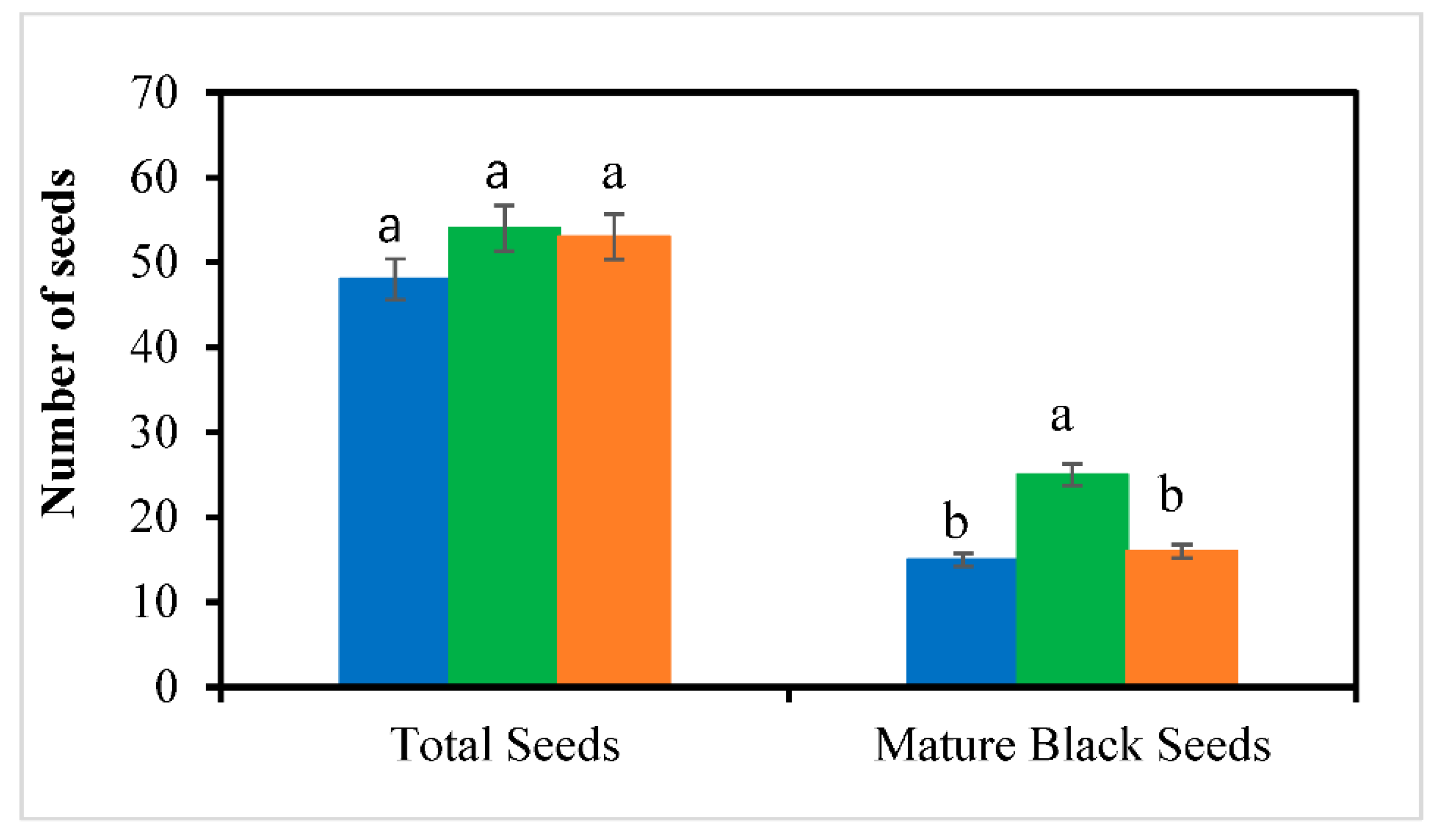
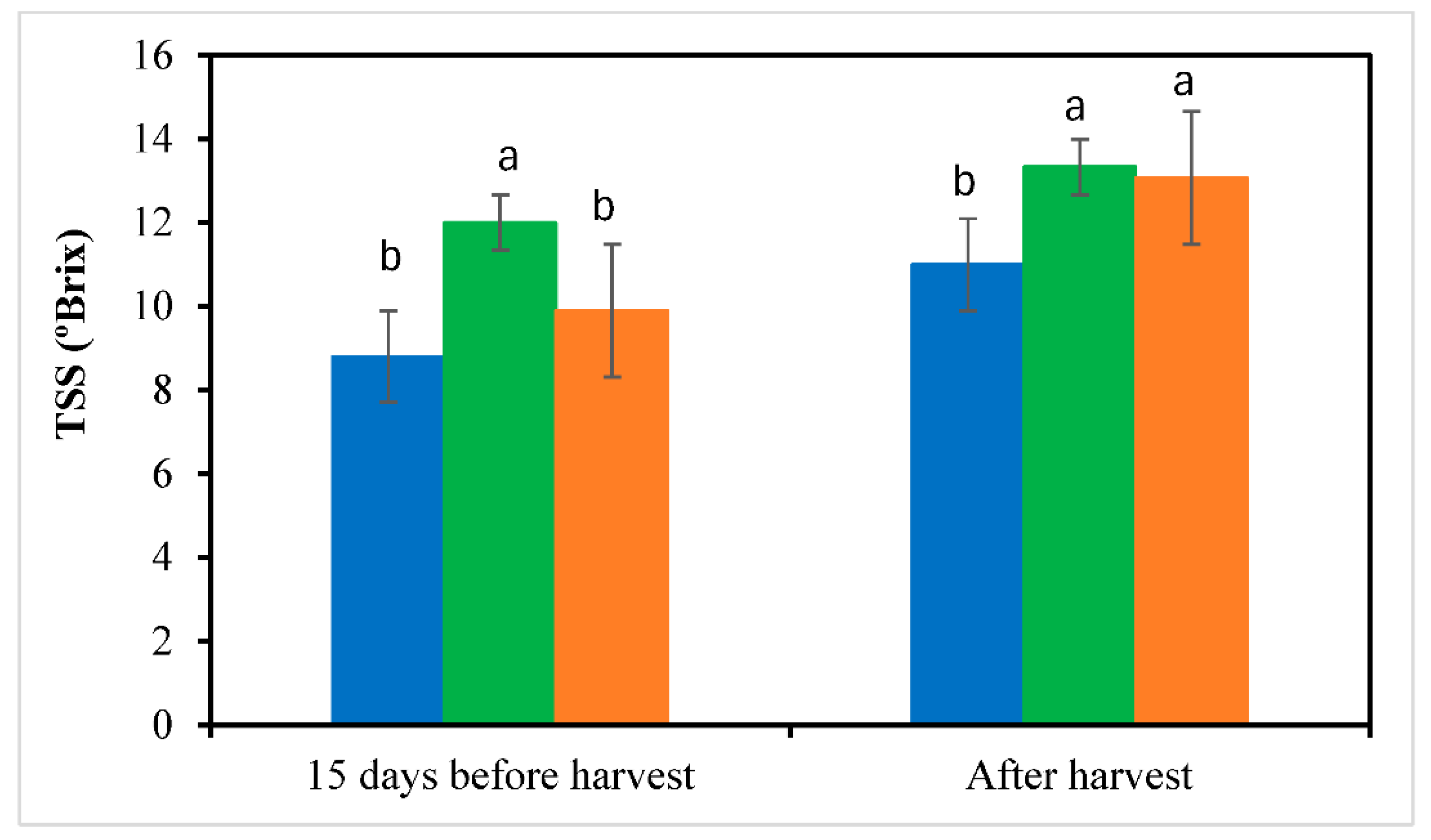
| Month | T (ºC) | RH (%) | DLI (mol m⁻²d⁻¹) | T (ºC) | RH (%) | DLI (mol m⁻²d⁻¹) | ||
|---|---|---|---|---|---|---|---|---|
| Control | LED 900 | LED 1500 | ||||||
| January | 19.5 | 52 | 6.25 | 9.55 | 11.25 | 7.1 | 54 | 10.2 |
| February | 20.5 | 65 | 6.50 | 10.25 | 13.5 | 8.0 | 63 | 11.1 |
| March | 22.0 | 60 | 9.50 | 15.0 | 17.2 | 9.6 | 59 | 15.3 |
| April | 22.5 | 69 | 10.5 | 16.5 | 19.5 | 17.1 | 72 | 15.3 |
| May | 25.5 | 69 | 12.0 | 17.1 | 21.2 | 20.0 | 72 | 18.2 |
| Supplemen tary LED Lighting | Plant Height (cm) | Shoot dry Weight (g) | Root dry Weigh t (g) | Root Length (cm) | Number of Nodes | No. of Leaves | Stem Diam eter (mm) | Leaf Area (cm2) |
|---|---|---|---|---|---|---|---|---|
| Control | 20.0 b | 26.8 b | 3.89 c | 11.0 b | 5.0 b | 8.1 b | 4.1 b | 131.2 b |
| LED 600 | 22.6 b | 31.2 ab | 5.15 b | 13.6 a | 5.7 ab | 9.8 ab | 4.2 b | 135.1 b |
| LED 900 | 29.5 a | 35.65 a | 6.97 a | 13.6 a | 7.0 a | 11.6 a | 5.3 a | 157.3 a |
| LED 1200 | 26.0 ab | 32.12 a | 5.56 b | 12.1 b | 6.3 a | 11.1 a | 5.0 a | 143 ab |
| Supplementary LED Lighting | No of Leaves | No. of Nodes | Stem Diameter (mm) |
Leaf Length (cm) |
Leaf Area (cm2) | Fresh Weight of Plant (g) | Dry Weight of Plant (g) |
|---|---|---|---|---|---|---|---|
| 56 DAP | |||||||
| Control | 87 c | 75 c | 5.19 b | 21.32 b | 187.65 c | ________ | ________ |
| LED 900 | 140 a | 103 a | 6.84 a | 24.76 a | 236.32 a | ________ | ________ |
| LED 1500 | 110 b | 93 b | 6.09 ab | 24.75 a | 226.19 b | ________ | ________ |
| 86 DAP | |||||||
| Control | 180 c | 168 c | 8.32 b | 16.07 b | 128.63 c | 754.2 c | 80.34 b |
| LED 900 | 260 a | 248 a | 10.41 a | 21.56 a | 190.37 a | 1039.8 a | 116.90 a |
| LED 1500 | 228 b | 216 b | 9.90 b | 18.09 b | 151.35 b | 866.4 b | 113.51 a |
| Supplementary LED Lighting | Fruit Length (cm) | Fruit Weight (kg) | Juice pH | Acid Content (%) | Thickness of Peel (mm) |
|---|---|---|---|---|---|
| 15 days before harvest | |||||
| Control | 11.018 b | 0.73 c | 5.34 a | 0.55 b | 4.56 a |
| LED 900 | 13.950 a | 1.30 a | 5.50 a | 0.53 a | 4.45 a |
| LED 1500 | 12.314 ab | 0.94 b | 5.48 a | 0.54 ab | 4.84 a |
| Harvest stage | |||||
| Control | 13.12 b | 1.14 c | 5.76 a | 0.47 b | 3.66 a |
| LED 900 | 14.25 a | 1.50 a | 5.80 a | 0.40 a | 3.55 a |
| LED 1500 | 13.97 a | 1.30 b | 5.82 a | 0.40 a | 3.95 a |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





