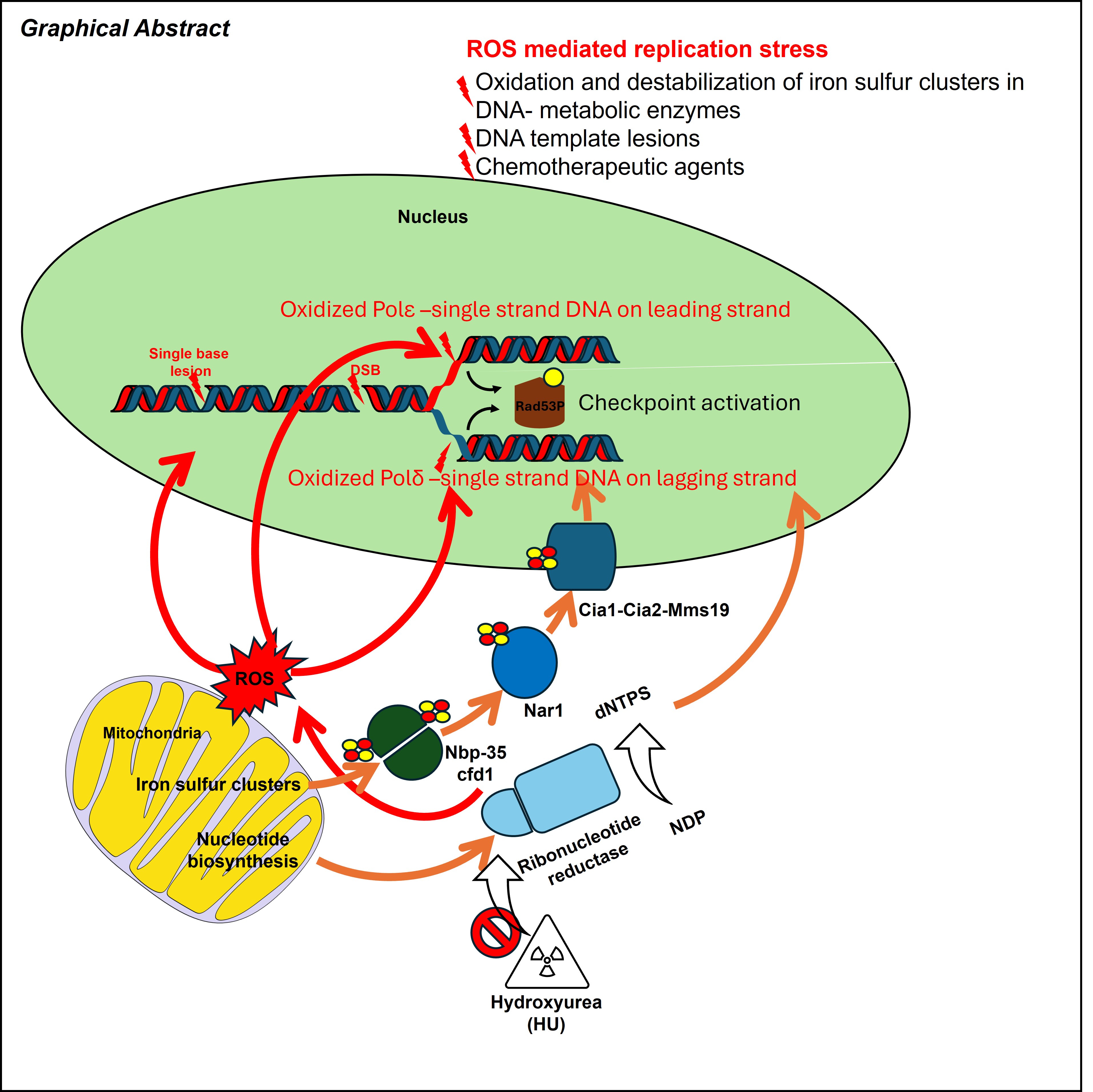
Faithful DNA replication is essential for genome stability but is constantly challenged by metabolic and oxidative stresses. Hydroxyurea (HU), a widely used antiproliferative drug, is traditionally known to inhibit ribonucleotide reductase and deplete dNTP pools. Recent studies, especially in Saccharomyces cerevisiae, reveal that HU-induced replication stress also arises from reactive oxygen species (ROS), which oxidize DNA, impair iron–sulfur–dependent replication enzymes, and disrupt replisome function. These combined effects promote helicase–polymerase uncoupling, accumulation of RPA-coated ssDNA, and activation of the Mec1–Rad53 (ATR–CHK1) checkpoint, leading to strand-specific changes such as PCNA unloading and reduced lagging-strand synthesis. When protective pathways are overwhelmed, HU-treated forks collapse, generating chromosome breaks and genome instability. This review summarizes current understanding of how HU remodels replication forks through both ROS-dependent and ROS-independent pathways and highlights emerging insights into how these mechanisms influence genome stability and may be exploited for therapeutic benefit.