1. Introduction
Colour is one of the most salient dimensions of visual experience, shaping not only object recognition and depth perception but also emotional appraisal and behaviour. Psychological research consistently demonstrates systematic associations between hue and affective states. Warm hues such as red and yellow are typically linked with heightened arousal and dominance, whereas cooler hues such as blue and green tend to foster calmness, pleasure, and reduced stimulation [
1,
2,
3,
4,
5]. These associations extend into cognitive and behavioural outcomes: red can increase attentional capture and avoidance motivation, while blue often facilitates creative thinking and approach behaviour [
1,
3]
Theoretical models of affect help situate these findings. Dimensional frameworks such as the Pleasure–Arousal–Dominance (PAD) model [
1] describe emotional states across three continua, and the Self-Assessment Manikin [
2] operationalises these dimensions through a pictorial, non-verbal rating tool. Such tools have become standard in colour–emotion research because they bypass linguistic biases while maintaining sensitivity to affective shifts. Importantly, colour also modulates autonomic physiology. Laboratory studies show that red environments can increase heart rate and skin conductance, whereas blue or green tones may reduce these indices [
1]. Pupil diameter has emerged as an especially robust marker of arousal, reliably reflecting both luminance changes and emotional engagement [
2]. Taken together, these strands of evidence indicate that colour exerts its influence through intertwined perceptual, affective, and physiological pathways, establishing it as a core variable in affective computing and human–computer interaction.
1.1. Colour Fidelity in Immersive VR
The importance of colour is magnified in immersive virtual reality (VR). Unlike desktop displays, head-mounted displays (HMDs) occupy over 100° of the visual field, providing full-field stimulation that recruits extensive occipital networks [
6], and magnifies behavioural impact [
6]. Under these conditions, even minor chromatic deviations become highly noticeable and can disrupt presence, realism, and comfort [
7] with colour shown to modulate affect and presence across immersive and desktop contexts [
1,
8,
9]. Performance and perception are tightly coupled in immersive interaction, where attentional and engagement demands can modulate perceptual experience [
10]. Empirical work confirms that subtle hue shifts can diminish plausibility, increase visual discomfort, and induce eyestrain [
11]. Because every pixel contributes to the sense of “being there,” colour errors that would be negligible on a flat screen can have significant psychological consequences in VR [
12].
In applied settings, reliable colour reproduction is critical. Clinical VR platforms use colour palettes to regulate anxiety during exposure therapy, with calming hues acting as “safe zones” and warmer hues supporting the graded introduction of stressors [
13]. In education, colour is used to highlight key information, direct attention, and enhance memory consolidation [
8]. Safety training relies on colour-coded hazards and warnings, where fidelity is essential to support rapid decision-making [
14]. If colours drift from their intended perceptual qualities—for example, if a cyan used to lower arousal desaturates toward grey-green—the intended psychological effect is undermined. Achieving colour fidelity in VR is technically complex. Human trichromatic vision relies on cones with peak sensitivities around 420, 534, and 564 nm [
15], but the RGB primaries of HMDs only approximate these curves [
16]. Classic accounts of human colour vision show that cone outputs are combined through opponent-process pathways [
16,
17,
18,
19], which introduce further constraints on perceptual coding. Additional distortions arise from the optical properties of the devices: the absence of ambient white points, reduced luminance ranges, chromatic aberrations between lenses, and inter-ocular hue shifts [
20]. Even calibrated HMDs exhibit gamut deformation and spatial colour non-uniformities—especially in the periphery—highlighting the necessity of device-specific calibration [
12]. Moreover, lens-induced transverse chromatic aberration introduces field-dependent hue shifts beyond ~5–6° eccentricity [
20]. Recent audits of commercial HMDs have documented gamut compression and field-of-view–dependent hue shifts that exceed perceptual threshold [
12,
21]. In colour science, a ΔE00 value below 2 is widely considered the threshold for perceptual indistinguishability [
22,
23]. Given gamut deformation and field-dependent hue shifts reported in HMDs, colour management must target perceptual tolerances (e.g., ΔE00 < 2) with device-specific LUTs and imaging colorimetry [
22,
24,
25].
Without calibration to this level, VR-based experiments risk systematic error, compromising both scientific validity and applied outcomes [
22,
23,
24,
25]. Imaging colourimetry and spectral correction pipelines are therefore indispensable for VR research on colour perception.
1.2. Existing Evidence on Colour in VR
Despite the importance of colour fidelity and the established role of hue in emotion, empirical evidence linking colour and affect in immersive VR remains limited. Systematic reviews confirm [
8] that fewer than ten immersive studies have addressed this link, typically testing only a handful of hues and relying primarily on self-report. More recent work [
11] has shown that colour effects on physiological arousal have been documented only for a few colour–lightness combinations (e.g., red vs blue), underscoring both the scarcity and specificity of such findings.
Most have tested only two to six hues, often focusing on primary colours such as red and blue, and have relied primarily on self-report measures. For example, real-world and VR exposures across several colours have been compared, showing changes in cognitive performance and self-reported arousal but without collecting physiological data [
26]. Another study contrasted red and blue VR rooms and, for the first time, measured heart rate and skin conductance, finding higher autonomic arousal in red environments [
11]. While these results are informative, they provide only limited resolution.
Related work has also shown that colour appearance interacts with spatial perception in VR, with systematic differences between real and virtual rooms [
25]. Additionally, in immersive environments, colour cues significantly modulate presence and emotional engagement [
8].
Beyond these examples, a handful of studies have explored colour-emotion associations in semi-immersive or mixed-reality contexts, but they share similar constraints: small colour sets, inconsistent calibration, and reliance on verbal self-report [
4,
9,
17]. None has systematically mapped a wide set of hues within an HMD, nor paired subjective ratings with concurrent autonomic measures such as pupil diameter and galvanic skin response. The absence of such work leaves a gap in both theory and practice. Theoretically, we lack a comprehensive hue-by-hue “emotional fingerprint” of immersive colour perception. Practically, VR designers and clinicians have little evidence to guide colour choices beyond general intuitions drawn from desktop studies. At the neural level, colour processing is supported by a distributed cortical network spanning V1, V2 and V4, which links perceptual experience to underlying mechanisms of visual coding, as comprehensively reviewed in classic accounts of cone-opponent and cortical pathways [
18]. This provides a foundation for mechanistic interpretations of colour–emotion associations under VR constraints [
19].
This study addresses this gap by providing the first systematic hue-by-hue mapping of affective and physiological responses in immersive VR. Our approach extends prior research by testing fifteen calibrated Munsell colours, ensuring perceptual fidelity (ΔE < 2), and combining self-report ratings with autonomic measures (pupil size and skin conductance). In this way, we integrate colour science with immersive VR methods to produce a more comprehensive account of colour-emotion associations. The paper is organised as follows:
Section 2 describes the materials and methods;
Section 3 presents the results;
Section 4 discusses theoretical, comparative, mechanistic, and applied implications; and
Section 5 concludes with the main findings and directions for future research.
1.3. Aims and Hypotheses
The present study was designed to map emotional and physiological responses to a broad set of calibrated hues in immersive VR. Using the Munsell system, fifteen distinct colours—ten chromatic and five achromatic—were presented within a head-tracked VR environment rendered through a high-fidelity HMD. Participants provided affective ratings along the PAD dimensions using the SAM, consistent with established desktop findings where warm hues elevate arousal and cool hues promote pleasure [
1,
27,
28], while pupil diameter and skin conductance were recorded continuously to index autonomic arousal. To examine whether stable traits modulated colour reactivity, measures of cognitive style and personality were also collected.
We formulated two hypotheses:
H1 predicted that self-reported PAD ratings would vary systematically across hues, replicating established desktop findings but with potentially stronger effect sizes under immersive conditions.
H2 predicted that physiological indices, particularly pupil dilation, would covary with hue in ways that mirror subjective arousal, while skin conductance might show weaker effects due to the brevity of exposures.
By addressing these aims, this study establishes an empirical baseline for colour-aware VR design and provides a methodological bridge between calibrated perception, multidimensional emotion, and physiological responses, complementing cross-cultural evidence for universal but contextually shaped colour-emotion associations [
9].
2. Materials and Methods
2.1. Participants
Thirty-six adults (16 women, 20 men; M = 26.0 years, SD = 5.5, range = 18–45) took part in the study. All reported normal or corrected-to-normal vision and no colour blindness. Exclusion criteria included any neurological or psychiatric disorders, as well as the use of medications or medical conditions that could influence physiological or emotional responses. Eligibility was assessed through self-report during recruitment. Written informed consent was obtained digitally, and the study was approved by the Institutional Review Board of the American College of Greece (protocol #202503487, 18 March 2025).
2.2. Hardware and Software
The experiment was conducted using a Varjo Aero XR head-mounted display (2880 × 2720 pixels per eye, 115° field of view, 90 Hz refresh rate) paired with an HTC Vive Controller 2.0 for participant input. Eye movements were recorded with the headset’s integrated binocular infrared eye tracker, operating at 200 Hz with automatic five-point calibration. These apparatus choices are consistent with recommended standards for immersive VR neuropsychological testing, emphasizing precision in tracking, reproducibility, and device selection [
29]. Galvanic skin response (GSR) was collected using a Biosignals Plux 8-Channel Hub (PLUX Biosignals, Lisbon, Portugal) at 128 Hz, with electrodes placed on the volar surfaces of the index and middle fingertips. Experimental software was developed in Unity 2022.3-LTS (Windows 11, 64-bit) and run via Varjo Base 4.1 with SteamVR 2.4.
All HMD firmware and software were updated to the latest stable releases as of April 2025. The virtual environment consisted of a plain cubic room with diffuse-only wall shaders. Colour patches were rendered on the walls and calibrated once at the beginning of the study against printed Munsell references, achieving a colour accuracy of ΔE < 2 [
22,
24]. This ΔE < 2 threshold is consistent with colour science conventions [
23] and with VR research linking perceptual fidelity in colour and scene realism [
22,
24].
2.3. Design and Stimuli
The study employed a within-subjects design in which each participant was exposed to all experimental conditions. A total of fifteen colour stimuli were presented: ten chromatic hues, three grey levels, black, and white. Each stimulus was shown once per participant in random order. Stimuli were defined in HSV colour space. To isolate the effects of hue, all chromatic stimuli were standardised to a saturation of 75% and a value (brightness) of 60%. This uniformity controlled for potential confounds of saturation and luminance on affective and physiological responses [
4,
11], in line with parametric models showing that lightness and saturation systematically modulate colour emotions and preference [
27,
28]. The achromatic stimuli (black, dark grey, medium grey, light grey, white) were defined with saturation set to 0% and value adjusted to produce the desired lightness levels.
During presentation, the entire virtual room (all six surfaces: four walls, floor, and ceiling) was rendered in the target colour using diffuse-only shaders. This created the perceptual impression of being fully surrounded by the hue, with no other visual objects or textures present. The descriptive labels, Munsell notations, and HSV coordinates of all 15 stimuli are presented in
Table 1.
2.4. Procedure
After providing consent and completing the baseline questionnaires (Cognitive Style Index, BFI-2-S), participants were seated in the experimental room and fitted with the Varjo Aero XR headset and galvanic skin response electrodes, which were placed on the volar surfaces of the index and middle fingers of the non-dominant hand (see
Figure 1). The headset was adjusted for comfort, and a five-point calibration routine in Varjo Base ensured eye-tracking accuracy within one degree of visual angle. Participants also wore closed-back headphones to minimise external noise and held the HTC Vive Controller 2.0 in their dominant hand, which was used only to provide ratings. They then completed a brief practice session (~3 minutes) in a neutral B&W VR room, which familiarised them with the headset, controller, and rating procedure. This grey environment also served as the physiological baseline.
During the experiment, each trial began with a 30 s white adaptation screen, followed by a 30 s presentation of one of the 15 colour stimuli. The entire cubic room (walls, floor, ceiling) was rendered in the target hue, producing the perceptual impression of being fully surrounded by colour. While the hue remained visible, participants rated their current state of Pleasure, Arousal, and Dominance on a –4 to +4 Self-Assessment Manikin scale using the controller. Ratings were self-paced, consistent with established colour–emotion protocols [
3,
4]. Pupil size and skin conductance were recorded continuously throughout each trial. The VR environment contained no other visual elements to minimise distraction. A full session, including the baseline and all 15 stimuli, lasted approximately 30 ± 2 minutes, and no breaks were requested.
Figure 2 illustrates the experimental setup: participants were seated in front of a desk, wearing the Varjo Aero XR headset and holding the HTC Vive Controller 2.0, while electrodermal activity was recorded using fingertip electrodes connected to the biosignals hub.
2.5 Measures
We collected subjective emotional ratings, ocular and electrodermal responses, and trait-level individual differences. A full overview of variables, instruments, and timing is provided in
Table 2.
2.5.1. Subjective Emotion
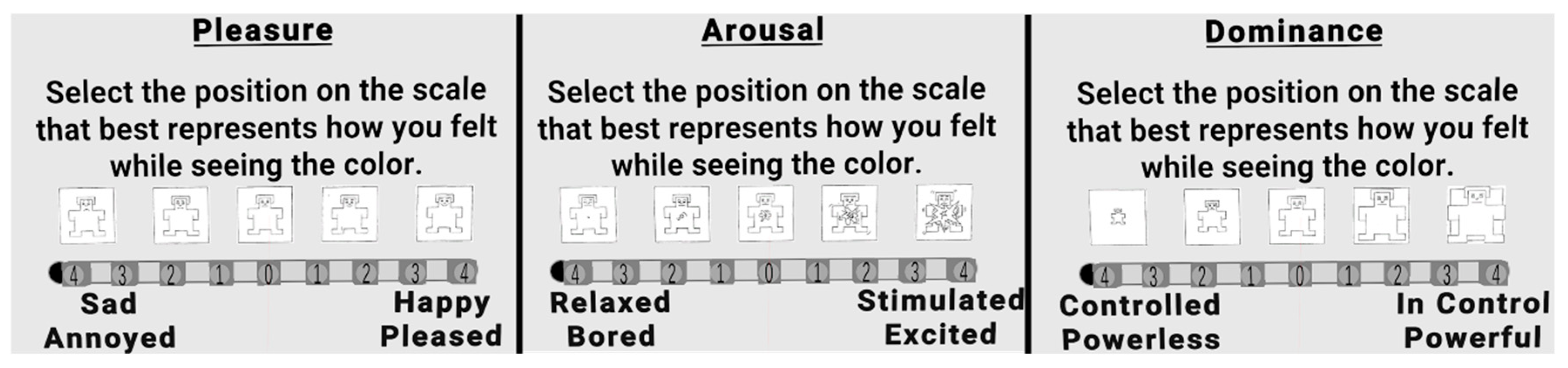
Emotional responses were assessed using the Self-Assessment Manikin (SAM) [
2] in combination with the Pleasure–Arousal–Dominance (PAD) framework [
1]. The SAM is a non-verbal, pictorial instrument that presents stylised figures depicting varying levels of each affective dimension, allowing participants to indicate their current state quickly and intuitively. For each colour exposure, participants rated their levels of Pleasure (from unpleasant to pleasant), Arousal (from calm to excited), and Dominance (from submissive to in-control) on a 9-point bipolar scale. Arousal ratings obtained with this format have been consistently shown to covary with autonomic responses such as pupil dilation and skin conductance [
32]. This format has been widely validated in affective computing and colour–emotion studies. The SAM and PAD were adapted in a 3D version to be presented in VR, where responses were provided by touching the desired response (see
Figure 2).
2.5.2. Pupil Dilation
Pupil diameter was recorded continuously through the Varjo Aero XR headset’s integrated eye tracker. For analysis, the mean pupil size (in millimetres) was calculated over the final 20 seconds of each 30-second colour presentation. This approach minimised adaptation artefacts and provided a stable index of autonomic arousal, as pupil dilation reliably tracks both luminance and emotional state [
33].
2.5.3. Electrodermal Activity
Galvanic skin response (GSR) was measured via electrodes attached to the volar surfaces of the index and middle fingers of the non-dominant hand. Mean and peak skin conductance (µS) during each colour trial were extracted, with values baseline-corrected against the preceding white adaptation screen. GSR is a sensitive measure of sympathetic activation and has been widely used to index arousal in psychophysiological studies [
34].
2.5.4. Individual Differences
To examine whether stable traits modulated colour reactivity, two questionnaires were administered prior to the VR session. The Cognitive Style Index (CSI) assesses individuals’ preference for analytic versus intuitive modes of information processing, yielding both a total score and categorical style classification [
30]. The Big Five Inventory-2 Short Form (BFI-2-S) provides brief but reliable measures of personality traits (Extraversion, Agreeableness, Conscientiousness, Negative Emotionality, and Open-Mindedness), as well as selected facets (e.g., Sociability, Trust, Energy Level) [
31]. Both instruments are validated and widely used in cognitive and personality research.
2.5.5. Cybersickness
Cybersickness was monitored using the Cybersickness in Virtual Reality Questionnaire (CSQ-VR), a brief six-item instrument validated for immersive VR (Cronbach’s α ≈ .86) [
35]. The questionnaire assesses three domains—nausea, vestibular disturbance, and oculomotor strain—each rated on a seven-point intensity scale. Subscale scores range from 2 to 14, and the total score ranges from 6 to 42, with higher values indicating more severe symptoms.
2.6. Data Pre-Processing
Ocular data were first inspected for blink artefacts, defined as frames with an eye-openness index below 50%. Such frames were excluded, and the remaining pupil traces were smoothed using a five-sample median filter to reduce high-frequency noise. Electrodermal signals were down-sampled to 10 Hz to align their temporal resolution with the ocular data and improve processing efficiency. Skin conductance values were then log-transformed using the formula log(µS + 1) to reduce positive skew and approximate normality. For each dependent variable, trial-level values were aggregated within participants: mean values were calculated for continuous measures such as pupil size, and peak amplitudes were extracted for skin conductance. This yielded one observation per condition for each participant, enabling all subsequent analyses.
2.7. Statistical Analysis
All analyses were conducted in R (version 4.4.0) [
36] using RStudio [
37]. Descriptive statistics and Bonferroni-adjusted correlation matrices were computed with the psych package. Repeated-measures ANOVAs were fitted using afex [
38], with Greenhouse–Geisser corrections (ε) applied when sphericity was violated. Partial eta squared (η²ₚ) is reported as the effect size. Estimated marginal means and pairwise contrasts were obtained with emmeans [
39], with p-values adjusted using Holm’s method for planned comparisons (warm vs. cool hues).
Exploratory analyses included Pearson correlations between physiological measures and PAD ratings, as well as regression models in which CSI scores and BFI-2-S traits predicted mean Pleasure. Regression modelling were implemented with lme4 [
40]. When variables deviated from normality, transformations were applied using bestNormalize [
41], and data were z-standardised to meet assumptions of parametric testing.
To assess robustness, non-parametric sensitivity analyses were conducted using the aligned-rank transform with ARTool [
42], and post-hoc contrasts were again performed with emmeans. All tests were two-tailed with α = .05.
3. Results
3.1. Descriptive Statistics
Descriptive statistics for all measures are summarised in
Table 2. Participants were evenly split by sex (16 women, 16 men) and ranged in age from early adulthood to midlife. Cybersickness was minimal. On the CSQ-VR, all participants reported scores of 1 (“absent”) or 2 (“very mild”) on each symptom, with the majority of responses being 1. On the CSI, they displayed a balanced distribution of cognitive styles (18 Analytic, 18 Intuitive), offering an opportunity to examine colour responses across different processing preferences. On average, self-reported emotions were close to the midpoint of the Self-Assessment-Manikin scales, with moderate pleasure, arousal, and dominance ratings. Physiologically, participants’ mean pupil diameter was 3.64 mm and their electro-dermal activity averaged 5.16 µS across trials. Notably, neither sex nor age altered the overall pattern of colour effects, although age was modestly associated with smaller pupil size and lower EDA. A slight positive skew in skin conductance data was addressed via log transformation, and non-parametric aligned-rank-transform tests confirmed that significant hue effects were robust against residual assumption violations.
For completeness,
Table 3 reports the mean and standard deviation of each measure for every colour stimulus, including pupil diameter and skin conductance (GSR).
3.2. Correlational Highlights
Bonferroni-adjusted Pearson correlation matrices (
Table 4) revealed several theoretically relevant associations. First, regarding cross-modal alignment, subjective Pleasure was strongly correlated with Dominance (
r = .62,
p < .001) and moderately with Arousal (
r = .42,
p < .001), confirming the internal coherence of the PAD model in immersive VR contexts. Second, in relation to personality variables, baseline-corrected pupil size was positively associated with Extraversion (
r = .18,
p = .010) and Energy Level (
r = .18,
p < .001), and negatively with Trust (
r = −.20,
p < .001). Participants with higher scores on Openness (BFI-2 Open-Mindedness) also tended to report greater Pleasure for blue-green and purple hues (simple-slope
rs ≈ .30–.41,
ps ≈ .02–.04), although these hue-specific effects did not survive correction for multiple comparisons.
Finally, cognitive style showed a modest effect: the total CSI score was positively associated with Arousal (r = .17, p = .010), but not with any physiological measures. This finding is consistent with prior evidence that analytic–holistic tendencies are more likely to influence self-reported affect than reflexive bodily responses.
3.3. Hue Effects: Mixed-Model ANOVA
Repeated-measures ANOVAs with factors Hue (15 levels, within) and CSI style (between) were conducted to test the central hypotheses (
Table 4). Greenhouse–Geisser corrections (ε) were applied where sphericity was violated.
For Pleasure, there was a robust main effect of Hue, F(7.64, 259.79) = 12.79, p < .001, η²ₚ = .23, indicating that colour hue explained nearly a quarter of the variance in affective ratings. A significant main effect of CSI was also observed, F(1, 34) = 7.69, p = .009, suggesting that participants with more holistic cognitive styles tended to report higher overall Pleasure. The Hue × CSI interaction was significant, F(7.64, 259.79) = 2.63, p = .010, η²ₚ ≈ .07, showing that the influence of hue on Pleasure differed according to individual differences in cognitive style.
For Arousal, Hue again exerted a strong effect, F(7.65, 260.17) = 10.54, p < .001, η²ₚ = .20, accounting for one-fifth of the variance. CSI also predicted Arousal ratings, F(1, 34) = 9.10, p = .005, η²ₚ ≈ .21, with more analytic styles associated with lower Arousal. However, the Hue × CSI interaction did not reach significance.
For Dominance, a significant main effect of Hue was found, F(7.77, 264.22) = 5.97, p < .001, η²ₚ = .12, demonstrating that hue systematically modulated participants’ sense of control within the VR environment. No main effect of CSI was observed, but the Hue × CSI interaction was significant, F(7.77, 264.22) = 2.91, p = .004, η²ₚ ≈ .08, indicating that cognitive style moderated hue-driven differences in Dominance.
For pupil size, there was a very strong effect of Hue, F(5.49, 186.58) = 95.31, p < .001, η²ₚ = .49, showing that nearly half of the variance in baseline-corrected pupil diameter was attributable to hue differences. Neither CSI nor the interaction term was significant. Finally, skin conductance did not show any reliable effects of Hue, CSI, or their interaction (all ps > .10).
As a robustness check against residual assumption violations, we also fitted aligned rank transform (ART) factorial models for each dependent variable.
Table 5 reports the Type III omnibus tests (main effects and interactions); the ART results converged with the parametric ANOVAs and thus justify the planned pairwise contrasts reported below.
Post-hoc analyses were conducted for each dependent variable to identify which hues differed significantly. Estimated marginal means were extracted from the mixed-model ANOVAs, and pairwise contrasts were tested using Holm-adjusted
p-values. For each measure, hues were ranked according to participant-level means, and significant differences are detailed in
Table 6,
Table 7 and
Table 8. As a summary, the brightest and most saturated hues (particularly yellow and cyan) tended to elicit the highest levels of Pleasure and Arousal, whereas darker hues (such as brown and dark gray) were consistently ranked lowest. For pupil size, short-wavelength hues (blue and purple) were associated with the largest dilations, whereas long-wavelength hues (red and orange) produced smaller responses, consistent with known spectral sensitivity patterns.
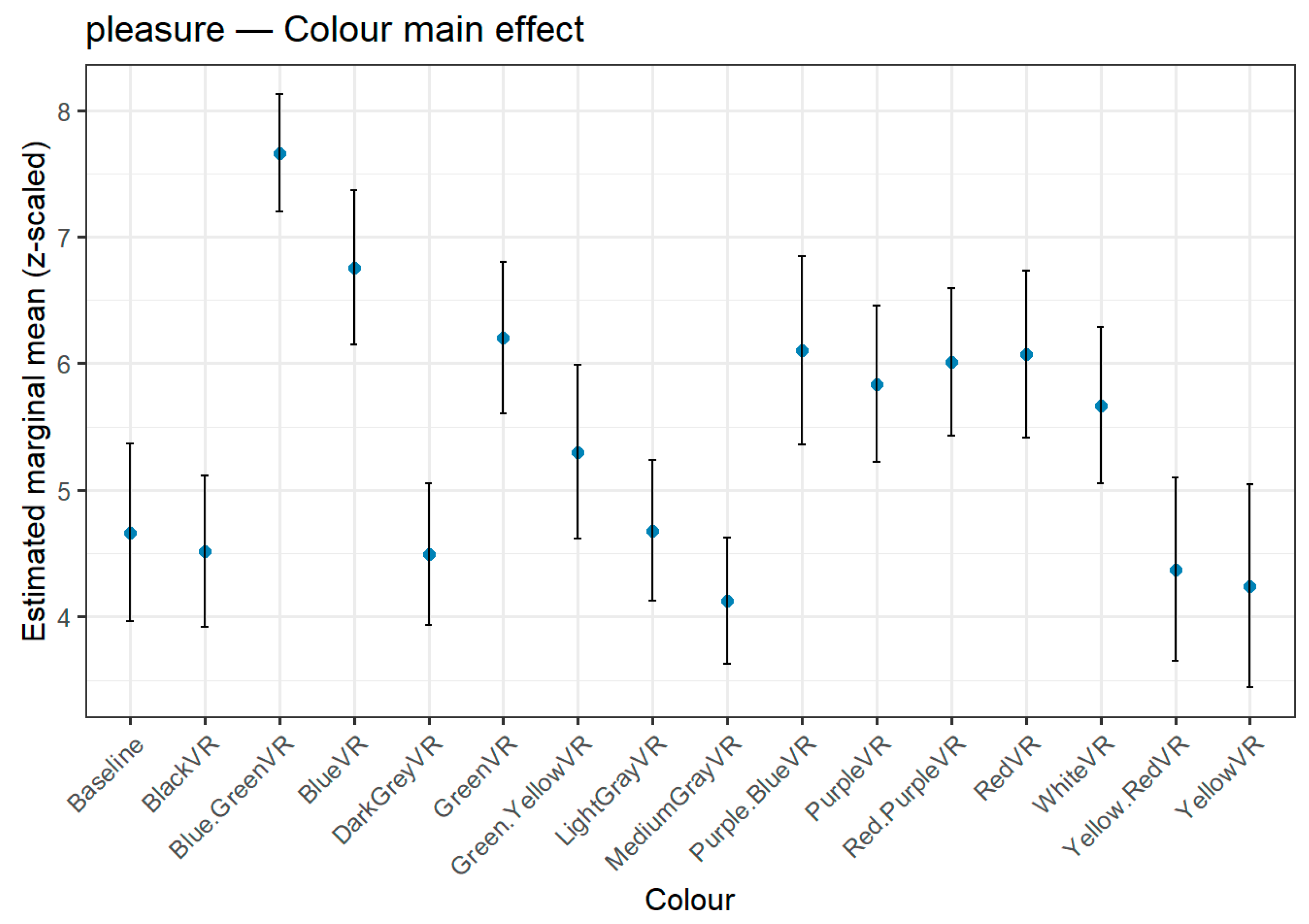
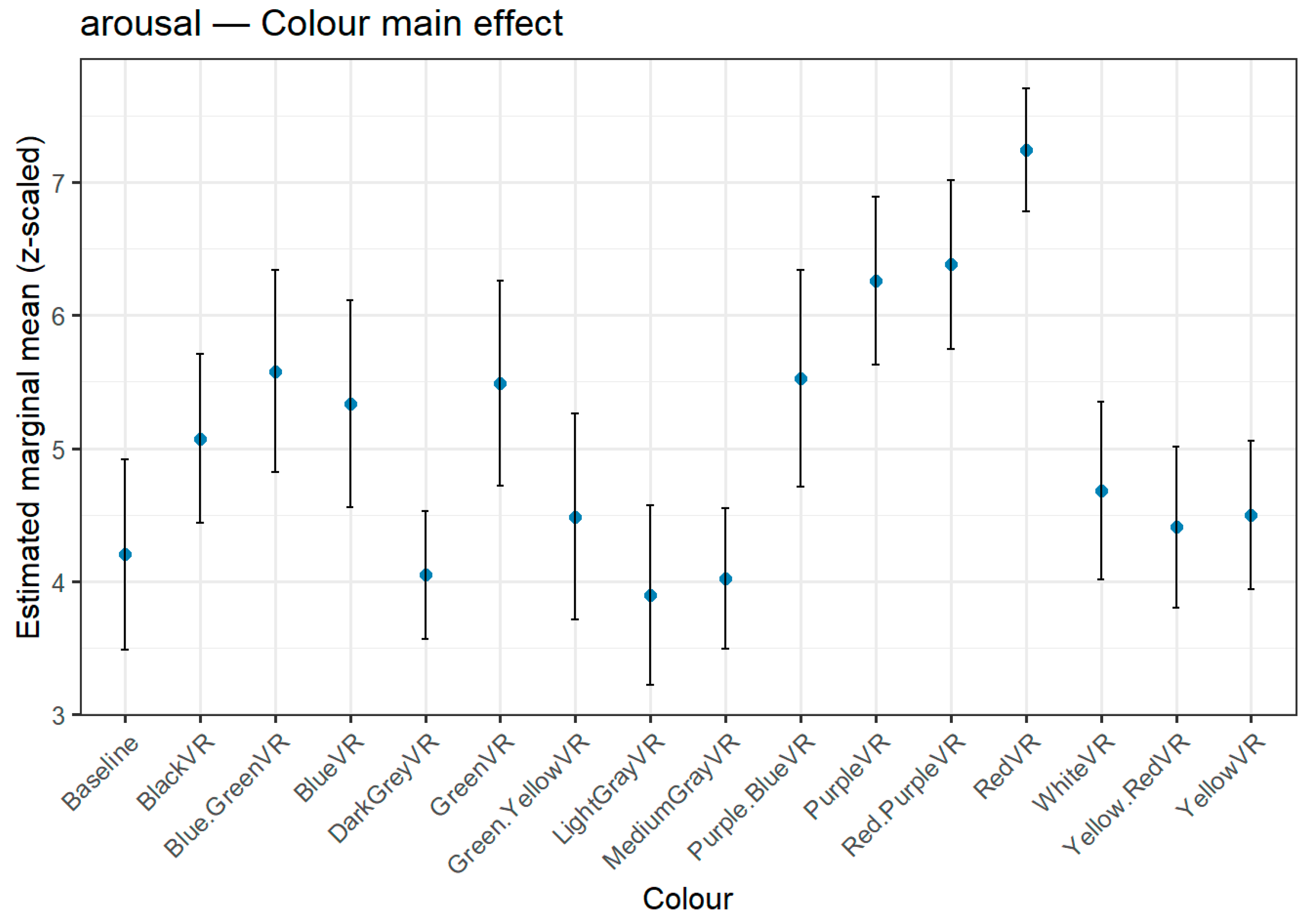
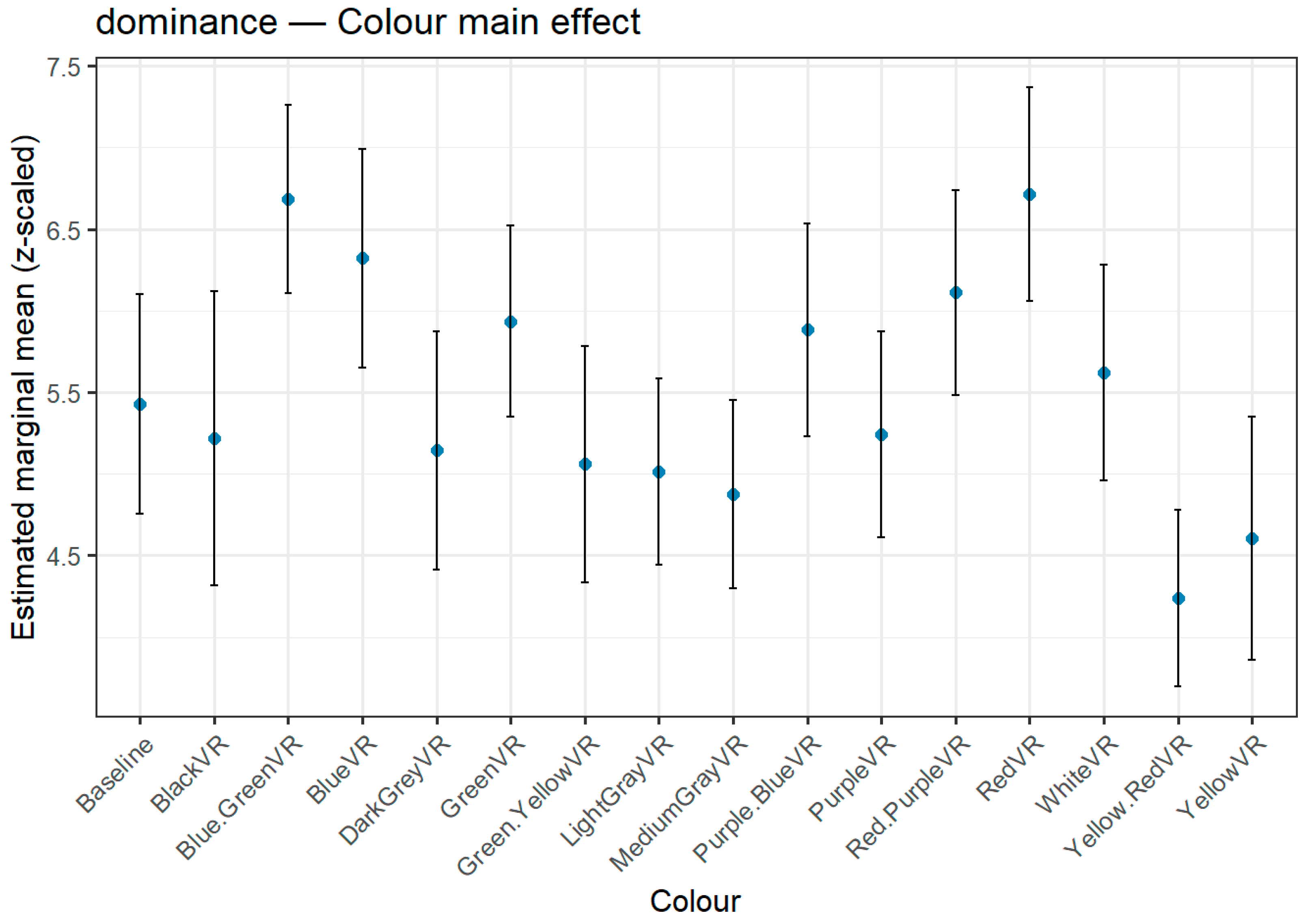
Figure 3,
Figure 4 and
Figure 5 depict the full distribution of estimated marginal means (z-scaled) across all 15 hue stimuli and the baseline condition, offering a complete visualization of hue effects. For transparency and reproducibility, extended tables with the full set of pairwise contrasts, exact p-values, and test statistics are provided in the Supplementary Material, allowing readers to inspect the complete statistical output beyond the summary rankings presented here.
3.3.1. Pleasure
The Pleasure dimension showed significant variations across hues (
Table 6,
Figure 3). Blue-Green emerged as the most pleasurable colour, with significantly higher ratings than 13 other hues, including the Baseline and all grey tones. Blue also scored highly, differing significantly from six other hues. At the lower end of the scale, Yellow was significantly lower than five other hues, while Medium Gray consistently occupied the lowest rank, differing significantly from eight other hues. These results indicate that brighter, more saturated hues—particularly those in the blue-green range—tend to evoke greater pleasure compared to less saturated or neutral tones.
3.3.2. Arousal
The Arousal dimension exhibited clear and robust differences between hues (
Table 7,
Figure 4), with warm and saturated tones occupying the upper end of the ranking. Red achieved the highest mean arousal score, showing significant pairwise differences with almost all other colours, including both neutral greys and cooler hues. Red-Purple and Purple also displayed high arousal values, significantly exceeding many of the lower-ranked colours. At the opposite end of the scale, Light Grey and Medium Grey elicited the lowest arousal levels, both being significantly lower than several high-arousal colours. This overall pattern suggests that hues in the red-to-purple range tend to evoke higher arousal, while desaturated greys are perceived as less stimulating.
3.3.3. Dominance
The Dominance ratings positioned red and Blue-Green at the top of the ranking (
Table 8,
Figure 5), with mean scores that were not statistically distinguishable from each other (p = 1.000). Both colours exhibited significantly higher dominance than several lower-ranked hues, particularly Yellow-Red, Yellow, and the grey tones. Blue followed in third position, showing significant differences from two of the lowest-ranking hues. At the lower end of the scale, Yellow and Yellow-Red consistently elicited the weakest dominance perceptions, being significantly lower than multiple high-ranking colours. These findings indicate that saturated primary hues, especially in the Red and Blue-Green range, are perceived as more dominant, whereas muted or composite hues, along with certain greys, tend to convey less dominance.
3.3.4. Pupil Size
The analysis of pupil size revealed robust differences across hues (
Table 9,
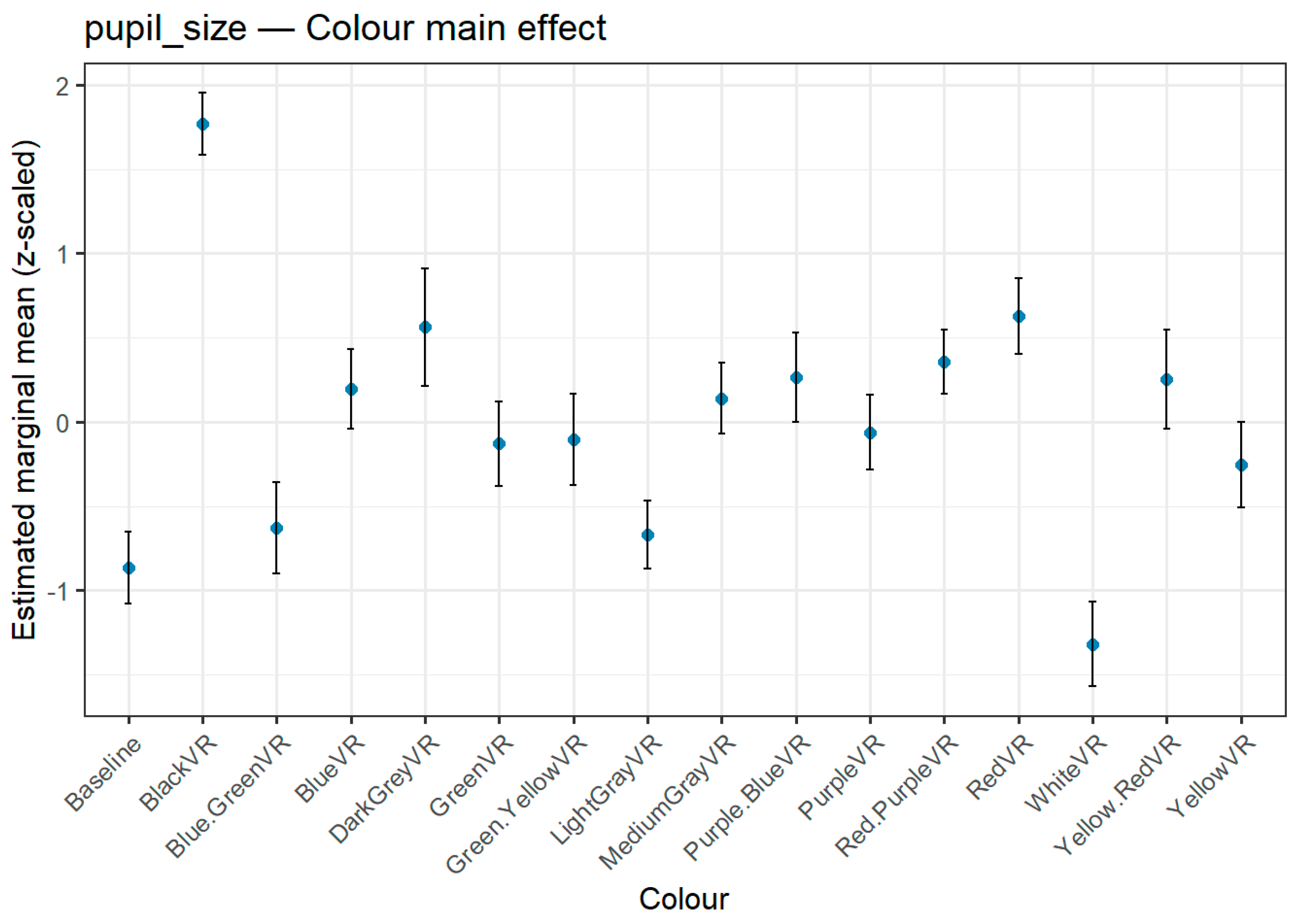
Figure 6), reflecting both luminance-driven reflexes and, for certain colours, possible affective modulation. Black elicited the greatest pupil dilation, significantly exceeding almost all other hues, followed by Red, which also produced substantial dilation and matched the top-ranking Arousal colour. At the opposite end, White produced the smallest dilation, significantly lower than nearly all other colours, with the Baseline also ranking among the lowest values. This pattern suggests that darker, high-saturation hues—particularly Black and Red—trigger stronger pupillary responses, whereas lighter and neutral tones such as White and Light Grey evoke the weakest.
3.4. Summary of Findings
The first hypothesis (H1) was confirmed: self-reported Pleasure, Arousal, and Dominance all varied significantly as a function of hue, replicating earlier desktop findings within a head-mounted display. The specific patterns were differentiated by dimension. Pleasure was highest for Blue-Green (followed by Blue) and lowest for Yellow and Medium Grey. Arousal peaked for Red, with Red-Purple and Purple also high, while Light Grey and Medium Grey consistently ranked lowest. Dominance was strongest for Red and Blue-Green, which were statistically equivalent, and weakest for Yellow and Yellow-Red.
The second hypothesis (H2) was only partially supported. Pupil size showed robust differences across hues, with Black eliciting the largest dilations, Red also producing relatively large responses, and White the smallest. These results broadly aligned with the arousal ranking of warm hues while reflecting expected luminance-driven reflexes. By contrast, electrodermal activity (EDA) showed no reliable hue effects after baseline correction, suggesting that short (30 s) passive exposures may be insufficient to engage sympathetic sweat responses.
Moderator analyses indicated that individual differences shaped the magnitude of responses but not the ordering of hues. Analytic participants reported slightly lower Pleasure and Arousal overall, and older participants showed smaller pupil sizes, yet hue-specific contrasts remained intact across sex, age, CSI style, and personality traits.
Taken together, these findings demonstrate that in an ecologically valid, head-tracked VR context, hue reliably influences both subjective experience and—via pupil diameter—bodily responses. The close coupling between arousal ratings and pupillary responses highlights the pupil as a practical and unobtrusive proxy of emotional engagement in immersive media, while the absence of hue effects on GSR underscores that not all autonomic channels are equally sensitive to chromatic stimulation.
4. Discussion
This study provides the first systematic hue-by-hue mapping of affective and physiological responses in immersive VR. Using fifteen calibrated Munsell hues, we found robust medium-to-large hue effects on subjective ratings of pleasure, arousal, and dominance. Pleasure was highest for Blue-Green and Blue, Arousal was strongest for Red and Red-Purple, and Dominance was greatest for Red and Blue-Green. Achromatic hues, particularly Medium Grey and Light Grey, were consistently rated lowest across affective dimensions.
Physiologically, pupil dilation showed large hue effects that broadly paralleled arousal ratings: Black elicited the largest dilations, Red also produced substantial dilation, and White consistently produced the smallest responses. By contrast, electrodermal activity (EDA) did not differentiate among hues once baseline was removed. Individual differences in cognitive style and personality modulated overall response magnitudes but did not alter the rank ordering of hues. Together, these findings demonstrate that colour influences both subjective states and pupillary responses in immersive VR, while GSR remains insensitive under short passive exposures.
The novelty of our study lies in three aspects: (i) the use of a high-end calibrated HMD to ensure colour fidelity, (ii) the inclusion of a broad and evenly spaced set of hues rather than a handful of “basic” colours, and (iii) the integration of self-report and physiological measures. These features allowed us to capture a fine-grained emotional “fingerprint” of colour in VR and to document stronger chromatic effects under immersive viewing than previously reported on desktop screens.
4.1. Theoretical Implications for Colour–Emotion Research
Our findings provide new support for dimensional theories of affect, especially the PAD model [
1], by showing that hue systematically modulates pleasure, arousal, and dominance in immersive contexts. The use of the Self-Assessment Manikin [
2] enabled participants to make intuitive ratings without relying on verbal labels, reducing the risk of cultural or linguistic bias.
The pattern of results replicates and extends classic findings from flat-screen studies [
1,
4]. Warm hues increased arousal and dominance, while cool hues were associated with higher pleasure. The extension to immersive VR is theoretically important because full-field visual stimulation amplifies chromatic input, making colour a more salient contextual cue. The stronger effect sizes we observed suggest that colour–emotion links are not fixed constants but scale with the ecological validity of the viewing context.
Our physiological results also contribute to the psychophysiology of colour. Pupil dilation, often studied in relation to luminance and cognitive load [
32,
43], here emerged as a sensitive index of chromatic arousal in VR. The moderate correlation between pupil change and self-reported arousal supports the idea that colour–emotion associations are embodied rather than purely symbolic [
9]. In contrast, GSR showed no hue differences, underscoring that autonomic channels vary in sensitivity and timescale. Sweat gland activity may require longer or more emotionally intense exposures to differentiate among hues, with evidence that only saturated and bright colours elicit significant GSR changes during short (≈30 s) presentations [
4].
4.2. Comparison with VR and Desktop Studies
To date, few studies have directly examined colour–emotion links in VR. One investigation compared colour exposures in real and virtual environments, finding broadly similar effects on mood and performance but without measuring physiology [
26]. Another contrasted red and blue VR rooms and reported higher heart rate and skin conductance in red environments [
11]. However, most prior VR research has been limited to a small number of hues and relied primarily on self-report [
8,
11]. Our study extends this work by testing a much larger colour set, using calibrated stimuli, and integrating ocular physiology.
Unlike prior HMD studies that sampled only a handful of hues and relied primarily on self-report, we provide a hue-by-hue map across fifteen Munsell colours coupled with concurrent pupillometry—addressing the limitations highlighted in recent immersive work [
11,
26].
Compared with desktop research [
1,
27,
28,
44], our VR results showed amplified differences. For example, the Arousal gap between Red and Dark Grey was roughly double what has been reported on monitors. This amplification likely reflects the immersive properties of VR: a head-tracked, wide-field display fills the visual surround, eliminating external reference points and magnifying chromatic contrast [
7]. Notably, physiological measures in VR also appear sensitive to environmental colour: darker rooms elicited increased heart rate and decreased heart rate variability, while red environments produced higher skin conductance than blue ones [
11]; this highlights the nuanced role of hue and lightness in shaping affective responses.
Such amplification is not trivial, it suggests that theories of colour–emotion associations derived from desktop studies may underestimate the magnitude of effects in ecologically valid environments, while cross-cultural research highlights both universal regularities and contextual variations [
9]. This aligns with evidence from immersive applications demonstrating that colour design significantly influences both the sense of presence and affective response in users [
8].
By sampling fifteen evenly spaced hues, we were also able to detect fine-grained distinctions absent in previous work. For instance, Red-Purple elicited high arousal levels comparable to Red, while Blue-Green consistently produced the highest pleasure ratings. These subtleties illustrate the value of broad, calibrated hue sets for affective science.
4.3. Mechanistic Interpretations
Several mechanisms may account for the observed hue effects. One likely explanation involves cone-opponent pathways. Long-wavelength hues asymmetrically stimulate L- and M-cones, which increases perceived brightness and may activate the retino-hypothalamic pathway linked to sympathetic arousal. This account fits well with the heightened arousal ratings and pupil dilation observed for red and red–purple stimuli, consistent with psychophysiological evidence that arousal ratings covary with autonomic responses [
32] and with cortical colour coding in extra-striate areas [
19,
45].
A second explanation concerns luminance-driven reflexes. The achromatic conditions clearly illustrate the role of lightness: black elicited the largest dilations and white the smallest, consistent with the pupil’s primary role in regulating retinal illumination [
32,
33]. Because all chromatic hues in the present study were standardised to the same lightness level (V = 60%), differences in their pupil responses can be attributed mainly to hue rather than to luminance, although prior modelling has shown that lightness and saturation can strongly influence colour emotions and preference [
27,
28].
Another possibility relates to attentional salience. In a uniform VR scene, any deviation from a neutral baseline, acts as an oddball stimulus, and longer-wavelength hues such as reds and yellows are especially likely to attract attention [
46]. This heightened attentional capture may promote increased sympathetic drive and, consequently, greater pupil dilation. Such attentional-arousal pathways are consistent with evidence that performance and perception are tightly coupled in immersive contexts [
10] and with findings that haptic feedback enhances vigilance and arousal during VR interaction [
47].
Finally, device-specific factors may have played a role. The Varjo Aero’s backlight exhibits a pronounced spectral peak near 456 nm and a secondary shoulder at 620 nm. This profile could exaggerate the perceived luminance of reds and cyans relative to greens, thereby amplifying hue differences in both subjective ratings and pupil responses. Such hardware artefacts highlight the importance of rigorous calibration in VR colour research [
12,
22,
24].
Taken together, the results are best explained by an interaction of bottom-up sensory pathways, attentional salience, and device-level optics, each contributing to the observed hue-specific differences in affect and physiology. These findings are consistent with evidence from cortical colour networks in V1, V2, and V4, which provide the neural substrate for perceptual coding [
19]. The parallel between arousal-ratings and pupil dilation aligns with dimensional accounts of affect–autonomic coupling [
32] and with immersive VR evidence showing that interaction conditions modulate perception and vigilance [
10,
45].
4.4. Practical Implications
The present findings also carry several practical implications for the design of virtual environments. In educational and training contexts, warm hues such as red and yellow can be employed sparingly to highlight feedback or mark critical elements, thereby boosting engagement, while cooler hues such as blue and blue–green can be used to sustain comfort and concentration during extended sessions [
8].
In clinical VR, particularly for anxiety management or exposure therapy, blue–green palettes—rated here as least arousing—may serve as effective default “safe zones.” Gradual transitions to warmer hues could then be used to support the graded introduction of therapeutic stressors, enabling clinicians to calibrate challenge and safety dynamically [
13].
For interface and user experience (UX) design, our results highlight pupil dilation as a real-time, unobtrusive index of arousal [
32,
43]. Designers may leverage this signal to implement adaptive systems in which brightness or chroma levels are automatically adjusted to maintain engagement while reducing the risk of visual fatigue.
Finally, the results have implications for presence and comfort in immersive media. Careful colour calibration enhances perceptual realism, which in turn supports a stronger sense of presence and greater trust in VR systems [
14,
22]. By providing empirically grounded design principles, this study bridges basic colour science with applied VR practice, illustrating how calibrated palettes can be strategically used to shape emotional tone, manage cognitive load, and enhance user experience.
4.5. Limitations & Future Directions
Several limitations of the present study should be acknowledged. First, the sample consisted of thirty-six young adults who experienced isolated colour rooms in a highly controlled laboratory setting. It remains unclear whether similar effects would be observed in older populations [
7], in clinical groups, or in more content-rich and interactive VR environments that more closely resemble everyday applications.
Second, skin conductance proved insensitive under the present paradigm. The thirty-second passive exposures to static colour walls may have been too brief to reliably elicit electrodermal responses, which typically require stronger or longer-lasting stimulation. The absence of significant GSR differences should therefore not be interpreted as definitive evidence that hue has no effect on sympathetic activity; rather, it highlights the need for paradigms better suited to capturing slower autonomic dynamics.
Third, the findings were obtained using a single high-end headset. While the Varjo Aero provided excellent fidelity and rigorous colour calibration, outcomes may vary with other display technologies, particularly devices with lower pixel density or alternative illumination methods such as OLED. Generalisability across hardware platforms thus remains to be established. Establishing such generalizability requires adherence to software design principles and technological competency frameworks for immersive VR methods [
29]. This consideration aligns with evidence that perceptual fidelity in colour strongly shapes scene realism in VR [
22,
24], underscoring the importance of calibration standards that can be generalised across display technologies.
Future studies should address these constraints in several ways. One priority is to implement dynamic colour gradients or temporal transitions to test whether changes in hue elicit stronger affective responses than static presentations. Another promising direction is the integration of VR with neuroimaging methods such as EEG or fMRI, which would allow mapping of cortical colour–emotion networks and clarify the neural substrates underlying these effects. Finally, applied research should explore adaptive colour-rendering systems capable of adjusting hues in real time to nudge users toward their preferred arousal state. Such systems could enhance engagement and emotional regulation while preserving comfort in long-duration VR sessions.
5. Conclusions
Immersive VR colours are more than decorative elements: they exert measurable influences on how users feel and how their bodies respond. In this study, reds were associated with the highest arousal and produced substantial pupil dilation, whereas blue–green tones elicited the greatest pleasure. Red and blue–green hues also conveyed a stronger sense of dominance. Black generated the largest pupil dilation overall, likely reflecting both heightened arousal and adaptation to low luminance, while white consistently produced the smallest dilation.
These findings suggest that VR creators can move beyond aesthetic considerations to employ evidence-based colour palettes that strategically shape emotional tone, manage cognitive load, and enhance the sense of presence. Warm, saturated hues may be used selectively to increase engagement, while cooler blue–green shades can help maintain comfort and reduce fatigue during prolonged exposure. By providing a systematic, hue-by-hue emotional and physiological map, this study establishes a foundation for colour-aware design principles in immersive virtual environments. By integrating calibrated hue maps with physiological metrics, our study offers a foundation for adaptive VR systems that adjust colour dynamically to optimise user comfort and engagement.
Supplementary Materials
The following supporting information can be downloaded at the website of this paper posted on Preprints.org, Supplementary reports all Holm-adjusted pairwise contrasts for Pleasure, Arousal, Dominance, Pupil Size, and Micro Siemens. The table includes the estimated contrast, standard error (SE), degrees of freedom (df), t-ratio, and adjusted p-value for every hue comparison.
Author Contributions
Conceptualization, P.K. and F.F.; methodology, P.K. and F.F.; software, P.K.; validation, P.K., F.F., S.C., and C.L.; formal analysis, P.K.; investigation, F.F. and C.L.; resources, P.K. and S.C.; data curation, F.F. and C.L.; writing—original draft preparation, F.F. and P.K.; writing—review and editing, P.K., F.F., S.C., and C.L.; visualization, P.K.; supervision, P.K.; project administration, P.K.. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
“The study was conducted in accordance with the Declaration of Helsinki, and approved by the Institutional Review Board of the American College of Greece #202503487, 18 March 2025.
Informed Consent Statement
Informed consent was obtained from all subjects involved in the study.
Data Availability Statement
The data presented in this study are available on request from the corresponding author. The data are not publicly available due to ethical approval requirements.
Conflicts of Interest
The authors declare no conflicts of interest.
Abbreviations
The following abbreviations are used in this manuscript:
| PAD |
Pleasure, Arousal, Dominance |
| SAM |
Self-Assessment Manikin |
| VR |
Virtual Reality |
| CSI |
Cognitive Style Index |
| BFQ-S |
Big Five Inventory-2 Short Traits |
| GSR |
Galvanic Skin Response |
| HMDs |
Head-Mounted Displays |
References
- Valdez, P.; Mehrabian, A. Effects of Color on Emotions. J. Exp. Psychol. Gen. 1994, 123, 394–409. [Google Scholar] [CrossRef] [PubMed]
- Bradley, M.M.; Lang, P.J. Measuring Emotion: The Self-Assessment Manikin and the Semantic Differential. J. Behav. Ther. Exp. Psychiatry 1994, 25, 49–59. [Google Scholar] [CrossRef] [PubMed]
- Elliot, A.J.; Maier, M.A. Color and Psychological Functioning. Curr. Dir. Psychol. Sci. 2007, 16, 250–254. [Google Scholar] [CrossRef]
- Wilms, L.; Oberfeld, D. Color and Emotion: Effects of Hue, Saturation, and Brightness. Psychol. Res. 2018, 82, 896–914. [Google Scholar] [CrossRef]
- Jonauskaite, D.; Epicoco, D.; Al-rasheed, A.S.; Aruta, J.J.B.R.; Bogushevskaya, V.; Brederoo, S.G.; Corona, V.; Fomins, S.; Gizdic, A.; Griber, Y.A.; et al. A Comparative Analysis of Colour–Emotion Associations in 16–88-Year-Old Adults from 31 Countries. Br. J. Psychol. 2024, 115, 275–305. [Google Scholar] [CrossRef] [PubMed]
- Malach, R.; Reppas, J.B.; Benson, R.R.; Kwong, K.K.; Jiang, H.; Kennedy, W.A.; Ledden, P.J.; Brady, T.J.; Rosen, B.R.; Tootell, R.B. Object-Related Activity Revealed by Functional Magnetic Resonance Imaging in Human Occipital Cortex. Proc. Natl. Acad. Sci. 1995, 92, 8135–8139. [Google Scholar] [CrossRef]
- Slater, M. Place Illusion and Plausibility Can Lead to Realistic Behaviour in Immersive Virtual Environments. Philos. Trans. R. Soc. B Biol. Sci. 2009, 364, 3549–3557. [Google Scholar] [CrossRef]
- Radianti, J.; Majchrzak, T.A.; Fromm, J.; Wohlgenannt, I. A Systematic Review of Immersive Virtual Reality Applications for Higher Education: Design Elements, Lessons Learned, and Research Agenda. Comput. Educ. 2020, 147, 103778. [Google Scholar] [CrossRef]
- Jonauskaite, D.; Abu-Akel, A.; Dael, N.; Oberfeld, D.; Abdel-Khalek, A.M.; Al-Rasheed, A.S.; Antonietti, J.-P.; Bogushevskaya, V.; Chamseddine, A.; Chkonia, E.; et al. Universal Patterns in Color-Emotion Associations Are Further Shaped by Linguistic and Geographic Proximity. Psychol. Sci. 2020, 31, 1245–1260. [Google Scholar] [CrossRef]
- Kourtesis, P.; Vizcay, S.; Marchal, M.; Pacchierotti, C.; Argelaguet, F. Action-Specific Perception & Performance on a Fitts’s Law Task in Virtual Reality: The Role of Haptic Feedback. IEEE Trans. Vis. Comput. Graph. 2022, 28, 3715–3726. [Google Scholar] [CrossRef]
- Weijs, M.L.; Jonauskaite, D.; Reutimann, R.; Mohr, C.; Lenggenhager, B. Effects of Environmental Colours in Virtual Reality: Physiological Arousal Affected by Lightness and Hue. R. Soc. Open Sci. 2023, 10, 230432. [Google Scholar] [CrossRef]
- Díaz-Barrancas, F.; Rodríguez, R.G.; Bayer, F.S.; Aizenman, A.; Gegenfurtner, K.R. High-Fidelity Color Characterization in Virtual Reality across Head Mounted Displays, Game Engines, and Materials. Opt. Express 2024, 32, 22388–22409. [Google Scholar] [CrossRef]
- Rizzo, A. “Skip”; Koenig, S.T. Is Clinical Virtual Reality Ready for Primetime? Neuropsychology 2017, 31, 877–899. [Google Scholar] [CrossRef] [PubMed]
- Scorgie, D.; Feng, Z.; Paes, D.; Parisi, F.; Yiu, T.W.; Lovreglio, R. Virtual Reality for Safety Training: A Systematic Literature Review and Meta-Analysis. Saf. Sci. 2024, 171, 106372. [Google Scholar] [CrossRef]
- Stockman, A.; Sharpe, L.T. The Spectral Sensitivities of the Middle- and Long-Wavelength-Sensitive Cones Derived from Measurements in Observers of Known Genotype. Vision Res. 2000, 40, 1711–1737. [Google Scholar] [CrossRef] [PubMed]
- Wyszecki, G.; Stiles, W.S. Color Science: Concepts and Methods, Quantitative Data and Formulae; John Wiley & Sons,, 2000; ISBN 978-0-471-39918-6. [Google Scholar]
- Kaiser, P.K.; Boynton, R.M. Human Color Vision; Optical Society of America, 1996; ISBN 978-1-55752-461-4. [Google Scholar]
- Gegenfurtner, K.R.; Kiper, D.C. COLOR VISION. Annu. Rev. Neurosci. 2003, 26, 181–206. [Google Scholar] [CrossRef] [PubMed]
- Conway, B.R. Color Vision, Cones, and Color-Coding in the Cortex. The Neuroscientist 2009, 15, 274–290. [Google Scholar] [CrossRef]
- Beams, R.; Kim, A.S.; Badano, A. Transverse Chromatic Aberration in Virtual Reality Head-Mounted Displays. Opt. Express 2019, 27, 24877–24884. [Google Scholar] [CrossRef]
- Gil Rodríguez, R.; Bayer, F.; Toscani, M.; Guarnera, D.; Guarnera, G.C.; Gegenfurtner, K.R. Colour Calibration of a Head Mounted Display for Colour Vision Research Using Virtual Reality. SN Comput. Sci. 2021, 3, 22. [Google Scholar] [CrossRef]
- Pardo, P.J.; Suero, M.I.; Pérez, Á.L. Correlation between Perception of Color, Shadows, and Surface Textures and the Realism of a Scene in Virtual Reality. JOSA A 2018, 35, B130–B135. [Google Scholar] [CrossRef]
- Sharma, G.; Wu, W.; Dalal, E.N. The CIEDE2000 Color-Difference Formula: Implementation Notes, Supplementary Test Data, and Mathematical Observations. Color Res. Appl. 2005, 30, 21–30. [Google Scholar] [CrossRef]
- Seetzen, H.; Makki, S.; Ip, H.; Wan, T.; Kwong, V.; Ward, G.; Heidrich, W.; Whitehead, L. Self-Calibrating Wide Color Gamut High-Dynamic-Range Display. In Proceedings of the Human Vision and Electronic Imaging XII; SPIE, February 12 2007; Vol. 6492, pp. 361–369. [Google Scholar]
- Billger, M.; Heldal, I.; Stahre, B.; Renstrom, K. Perception of Color and Space in Virtual Reality: A Comparison between a Real Room and Virtual Reality Models. In Proceedings of the Human Vision and Electronic Imaging IX; SPIE, June 7 2004; Vol. 5292, pp. 90–98. [Google Scholar]
- Xia, G.; Henry, P.; Li, M.; Queiroz, F.; Westland, S.; Yu, L. A Comparative Study of Colour Effects on Cognitive Performance in Real-World and VR Environments. Brain Sci. 2022, 12, 31. [Google Scholar] [CrossRef] [PubMed]
- Ou, L.-C.; Luo, M.R.; Woodcock, A.; Wright, A. A Study of Colour Emotion and Colour Preference. Part II: Colour Emotions for Two-Colour Combinations. Color Res. Appl. 2004, 29, 292–298. [Google Scholar] [CrossRef]
- Ou, L.-C.; Luo, M.R.; Woodcock, A.; Wright, A. A Study of Colour Emotion and Colour Preference. Part III: Colour Preference Modeling. Color Res. Appl. 2004, 29, 381–389. [Google Scholar] [CrossRef]
- Kourtesis, P.; MacPherson, S.E. How Immersive Virtual Reality Methods May Meet the Criteria of the National Academy of Neuropsychology and American Academy of Clinical Neuropsychology: A Software Review of the Virtual Reality Everyday Assessment Lab (VR-EAL). Comput. Hum. Behav. Rep. 2021, 4, 100151. [Google Scholar] [CrossRef]
- Allinson, C.W.; Hayes, J. The Cognitive Style Index: A Measure of Intuition-Analysis For Organizational Research. J. Manag. Stud. 1996, 33, 119–135. [Google Scholar] [CrossRef]
- Soto, C.J.; John, O.P. Short and Extra-Short Forms of the Big Five Inventory–2: The BFI-2-S and BFI-2-XS. J. Res. Personal. 2017, 68, 69–81. [Google Scholar] [CrossRef]
- Bradley, M.M.; Cuthbert, B.N.; Lang, P.J. Picture Media and Emotion: Effects of a Sustained Affective Context. Psychophysiology 1996, 33, 662–670. [Google Scholar] [CrossRef]
- Partala, T.; Surakka, V. Pupil Size Variation as an Indication of Affective Processing. Int. J. Hum.-Comput. Stud. 2003, 59, 185–198. [Google Scholar] [CrossRef]
- Dzedzickis, A.; Kaklauskas, A.; Bucinskas, V. Human Emotion Recognition: Review of Sensors and Methods. Sensors 2020, 20. [Google Scholar] [CrossRef]
- Kourtesis, P.; Linnell, J.; Amir, R.; Argelaguet, F.; MacPherson, S.E. Cybersickness in Virtual Reality Questionnaire (CSQ-VR): A Validation and Comparison against SSQ and VRSQ. Virtual Worlds 2023, 2, 16–35. [Google Scholar] [CrossRef]
-
R Core Team R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2022.
-
RStudio Team RStudio: Integrated Development Environment for R; RStudio, PBC: Boston, MA, 2022.
- Singmann, H.; Bolker, B.; Westfall, J.; Aust, F.; Ben-Shachar, M.S. Afex: Analysis of Factorial Experiments, 2021.
- Lenth, R.V. Emmeans: Estimated Marginal Means, Aka Least-Squares Means, 2022.
- Bates, D.; Mächler, M.; Bolker, B.; Walker, S. Fitting Linear Mixed-Effects Models Using Lme4. J. Stat. Softw. 2015, 67, 1–48. [Google Scholar] [CrossRef]
- Peterson, R.A.; Cavanaugh, J.E. Ordered Quantile Normalization: A Semiparametric Transformation Built for the Cross-Validation Era. J. Appl. Stat. 2020, 47, 2312–2327. [Google Scholar] [CrossRef]
- Elkin, L.A.; Kay, M.; Higgins, J.J.; Wobbrock, J.O. An Aligned Rank Transform Procedure for Multifactor Contrast Tests. In Proceedings of the Proceedings of the ACM Symposium on User Interface Software and Technology (UIST ’21); ACM Press: New York, 2021; pp. 754–768. [Google Scholar]
- Binda, P.; Murray, S.O. Keeping a Large-Pupilled Eye on High-Level Visual Processing. Trends Cogn. Sci. 2015, 19, 1–3. [Google Scholar] [CrossRef] [PubMed]
- Kaya, N. Relationship Betw Een Color and Em Otion: A Study of College St u d e n t s.
- Conway, B.R.; Tsao, D.Y. Color-Tuned Neurons Are Spatially Clustered According to Color Preference within Alert Macaque Posterior Inferior Temporal Cortex. Proc. Natl. Acad. Sci. 2009, 106, 18034–18039. [Google Scholar] [CrossRef] [PubMed]
- Aston-Jones, G.; Cohen, J.D. An Integrative Theory of Locus Coeruleus-Norepinephrine Function: Adaptive Gain and Optimal Performance. Annu. Rev. Neurosci. 2005, 28, 403–450. [Google Scholar] [CrossRef] [PubMed]
- Kourtesis, P.; Argelaguet, F.; Vizcay, S.; Marchal, M.; Pacchierotti, C. Electrotactile Feedback Applications for Hand and Arm Interactions: A Systematic Review, Meta-Analysis, and Future Directions. IEEE Trans. Haptics 2022, 15, 479–496. [Google Scholar] [CrossRef]
Figure 1.
A participant during the experimental task, wearing the Varjo Aero XR head-mounted display (HMD) and holding the HTC Vive Controller 2.0. Electrodermal activity was recorded via fingertip electrodes connected to the Biosignals hub on the desk.
Figure 1.
A participant during the experimental task, wearing the Varjo Aero XR head-mounted display (HMD) and holding the HTC Vive Controller 2.0. Electrodermal activity was recorded via fingertip electrodes connected to the Biosignals hub on the desk.
Figure 2.
Illustration of the Self-Assessment Manikin (SAM) used for affective ratings of Pleasure, Arousal, and Dominance. Participants selected a position on a 9-point bipolar scale (–4 to +4) that best represented their emotional state while viewing each colour stimulus. This non-verbal format combines iconic pictorial figures with dimensional labels, reducing reliance on verbal interpretation.
Figure 2.
Illustration of the Self-Assessment Manikin (SAM) used for affective ratings of Pleasure, Arousal, and Dominance. Participants selected a position on a 9-point bipolar scale (–4 to +4) that best represented their emotional state while viewing each colour stimulus. This non-verbal format combines iconic pictorial figures with dimensional labels, reducing reliance on verbal interpretation.
Figure 3.
Estimated marginal means (z-scaled) for Pleasure across colour hues. Error bars indicate 95% confidence intervals. Colours are presented in HSV-defined order and include the Baseline condition. Highest pleasure ratings were observed for Blue-Green, followed by Blue; Medium Grey and Yellow elicited the lowest ratings.
Figure 3.
Estimated marginal means (z-scaled) for Pleasure across colour hues. Error bars indicate 95% confidence intervals. Colours are presented in HSV-defined order and include the Baseline condition. Highest pleasure ratings were observed for Blue-Green, followed by Blue; Medium Grey and Yellow elicited the lowest ratings.
Figure 4.
Estimated marginal means (z-scaled) for Arousal across colour hues. Error bars indicate 95% confidence intervals. Highest arousal ratings were observed for Red, followed by Red-Purple; Medium Grey and Light Grey elicited the lowest ratings.
Figure 4.
Estimated marginal means (z-scaled) for Arousal across colour hues. Error bars indicate 95% confidence intervals. Highest arousal ratings were observed for Red, followed by Red-Purple; Medium Grey and Light Grey elicited the lowest ratings.
Figure 5.
Estimated marginal means (z-scaled) for Dominance across colour hues. Error bars indicate 95% confidence intervals. The highest dominance ratings were observed for both Red and Blue-Green, whose mean difference was not statistically significant (p = 1.000). Medium Grey and Yellow-Red elicited the lowest ratings.
Figure 5.
Estimated marginal means (z-scaled) for Dominance across colour hues. Error bars indicate 95% confidence intervals. The highest dominance ratings were observed for both Red and Blue-Green, whose mean difference was not statistically significant (p = 1.000). Medium Grey and Yellow-Red elicited the lowest ratings.
Figure 6.
Estimated marginal means (z-scaled) for Pupil Size across colour hues. Error bars indicate 95% confidence intervals. The largest pupil dilation was observed for Black, followed by Red. White elicited the smallest dilation.
Figure 6.
Estimated marginal means (z-scaled) for Pupil Size across colour hues. Error bars indicate 95% confidence intervals. The largest pupil dilation was observed for Black, followed by Red. White elicited the smallest dilation.
Table 1.
Descriptive labels, Munsell notations, and HSV coordinates for the fifteen colour stimuli used in the experiment.
Table 1.
Descriptive labels, Munsell notations, and HSV coordinates for the fifteen colour stimuli used in the experiment.
| Label |
Munsell Notation |
HSV Coordinates |
| Black |
N 0/0 |
0°, 0%, 0% |
| Dark Gray |
N 3/0 |
0°, 0%, 30% |
| Medium Gray |
N 5/0 |
0°, 0%, 50% |
| Light Gray |
N 7/0 |
0°, 0%, 70% |
| Red |
5R 5/6 |
0°, 75%, 60% |
| Yellow |
5Y 5/6 |
60°, 75%, 60% |
| Green |
5G 5/6 |
120°, 75%, 60%% |
| Blue |
5B 5/6 |
240°, 75%, 60% |
| Purple |
5P 5/6 |
300°, 75%, 60% |
| Yellow-Red |
5YR 5/6 |
30°, 75%, 60 |
| Green-Yellow |
5GY 5/6 |
90°, 75%, 60% |
| Blue-Green |
5BG 5/6 |
180°, 75%, 60% |
| Purple-Blue |
5PB 5/6 |
270°, 75%, 60% |
| Red-Purple |
5RP 5/6 |
330°, 75%, 60% |
| White |
5RP 5/6 |
0°, 0%, 100% |
Table 2.
Descriptive overview of all measures, instruments, and timing used in the experiment.
Table 2.
Descriptive overview of all measures, instruments, and timing used in the experiment.
| Domain |
Variable |
Instrument/Derivation |
Timing |
| Subjective Emotion |
Pleasure
Arousal
Dominance |
9-point-Self-Assestment Manikin sliders in VR Study Description |
After Each Colour |
| Ocular Physiology |
Pupil Diameter (mm) |
Mean Value During Final 20s of Colour Exposure |
During Exposure to
Colour |
| Electrodermal Activity |
Skin Conductance (μS) |
Mean & Peak Amplitude During Colour Exposure Minus Preceding White |
During Exposure to
Colour |
| Individual differences |
Cognitive Style Index Total; Big-Five inventory-2 Short Traits |
Paper Forms pre-VR |
Pre-Session |
Table 2.
Descriptive statistics for demographic, psychometric, and physiological measures.
Table 2.
Descriptive statistics for demographic, psychometric, and physiological measures.
| Variable |
M |
SD |
Min |
Max |
| Age (years) |
25.86 |
6.02 |
19.00 |
45.00 |
| Education (years) |
15.50 |
1.63 |
12.00 |
18.00 |
| CSI total score |
46.39 |
10.13 |
28.00 |
66.00 |
| Pleasure |
5.44 |
2.15 |
1.00 |
9.00 |
| Arousal |
5.10 |
2.20 |
1.00 |
9.00 |
| Dominance |
5.52 |
2.09 |
1.00 |
9.00 |
| Pupil size (mm) |
3.64 |
0.75 |
1.42 |
6.77 |
| Electrodermal activity (µS) |
5.16 |
3.23 |
0.05 |
16.40 |
| Extraversion |
20.44 |
4.06 |
13.00 |
29.00 |
| Agreeableness |
24.03 |
3.89 |
14.00 |
30.00 |
| Conscientiousness |
20.47 |
3.97 |
10.00 |
28.00 |
| Negative emotionality |
16.33 |
4.62 |
8.00 |
25.00 |
| Open-mindedness |
23.69 |
2.66 |
18.00 |
30.00 |
| Sociability |
6.94 |
1.92 |
3.00 |
10.00 |
| Assertiveness |
6.89 |
1.75 |
2.00 |
10.00 |
| Energy level |
6.61 |
1.71 |
3.00 |
10.00 |
| Compassion |
8.50 |
1.63 |
4.00 |
11.00 |
| Respectfulness |
5.56 |
1.24 |
3.00 |
9.00 |
| Trust |
7.22 |
1.62 |
2.00 |
10.00 |
| Organization |
6.42 |
2.15 |
2.00 |
10.00 |
| Productiveness |
7.11 |
1.66 |
2.00 |
10.00 |
| Responsibility |
6.97 |
1.17 |
5.00 |
9.00 |
| Anxiety |
6.19 |
2.19 |
2.00 |
10.00 |
| Depression |
4.75 |
1.89 |
2.00 |
10.00 |
| Emotional volatility |
6.06 |
1.87 |
2.00 |
10.00 |
| Aesthetic sensitivity |
7.11 |
1.63 |
4.00 |
10.00 |
| Intellectual curiosity |
8.47 |
1.26 |
5.00 |
10.00 |
| Creative imagination |
8.11 |
1.54 |
5.00 |
10.00 |
Table 3.
Means (M) and standard deviations (SD) for Pleasure, Arousal, Dominance, pupil diameter (mm), and skin conductance response (μS) across the 15 colour conditions.
Table 3.
Means (M) and standard deviations (SD) for Pleasure, Arousal, Dominance, pupil diameter (mm), and skin conductance response (μS) across the 15 colour conditions.
| Colour |
Pleasure M (SD) |
Arousal M (SD) |
Dominance M (SD) |
Pupil M (SD) |
GSR M (SD) |
Baseline
Black |
4.72 (2.28) 1
4.53 (1.75) |
4.25 (2.25)
5.11 (1.98) |
5.44 (1.98)
5.19 (2.65) |
3.04 (0.39)
5.17 (0.61 |
5.78 (4.07)
5.24 (3.26) |
Dark Gray
Medium Gray
Light Gray
Red
Yellow
Green
Blue
Purple
Yellow-Red
Green-Yellow
Blue-Green
Purple-Blue
Red-Purple
White |
4.50 (1.63)
4.14 (1.48)
4.72 (1.78)
6.08 (1.93)
4.25 (2.33)
6.25 (1.93)
6.75 (1.78
5.86 (1.84)
4.44 (2.49)
5.36 (2.27)
7.67 (1.35)
6.11 (2.16)
6.00 (1.71)
5.67 (1.79) |
4.06 (1.41)
4.03 (1.54)
3.89 (1.97)
7.25 (1.36)
4.56 (1.92)
5.53 (2.34)
5.39 (2.48)
6.28 (1.86)
4.44 (1.87)
4.50 (2.26)
5.61 (2.28)
5.56 (2.44)
6.42 (1.95)
4.67 (1.96) |
5.14 (2.13)
4.89 (1.69)
5.06 (1.84)
6.72 (1.91)
4.64 (2.26)
5.97 (1.84)
6.28 (2.11)
5.28 (1.95)
4.28 (1.73)
5.11 (2.31)
6.69 (1.69)
5.89 (1.89)
6.08 (1.90)
5.64 (1.94) |
4.04 (0.82)
3.68 (0.45)
3.16 (0.38)
4.06 (0.54)
3.43 (0.50)
3.51 (0.53)
3.72 (0.50)
3.54 (0.44)
3.78 (0.64
3.52 (0.57)
3.18 (0.53)
3.78 (0.57)
3.83 (0.45)
2.72 (0.52) |
5.01 (3.07)
5.20 (3.11)
5.02 (3.43)
5.01 (3.16)
5.41 (3.81)
5.23 (3.44)
5.41 (2.82)
4.77 (2.97)
5.17 (3.14)
5.25 (3.07)
4.73 (3.04)
5.12 (3.41)
5.08 (2.94)
5.09 (3.16) |
Table 4.
Bonferroni-adjusted Pearson correlations among main study variables.
Table 4.
Bonferroni-adjusted Pearson correlations among main study variables.
| Predictor/Outcome |
r |
p |
| Pleasure – Dominance |
.62 |
<.001 |
| Pleasure – Arousal |
.42 |
<.001 |
| Pupil size – Extraversion |
.18 |
.010 |
| Pupil size – Energy Level |
.18 |
<.001 |
| Pupil size – Trust |
−.20 |
<.001 |
| Openness – Pleasure (blue-green) |
~.30 |
.02 |
| Openness – Pleasure (purple) |
~.41 |
.04 |
| Cognitive Style Index total – Arousal |
.17 |
.010 |
| Cognitive Style Index total – Physiology |
n.s. |
1 |
Table 5.
ART factorial tests (Type III) for each dependent variable.
Table 5.
ART factorial tests (Type III) for each dependent variable.
| Dependent Variable |
Effect |
df |
Sum of squares |
F |
p |
| Pleasure |
Colour |
15 |
3756838.89 |
13.117 |
0.000 |
| Pleasure |
Cognitive Style |
1 |
659757.29 |
7.444 |
0.010 |
| Pleasure |
Interaction |
15 |
1019064.57 |
2.938 |
0.000 |
| Arousal |
Colour |
15 |
3331229.89 |
11.480 |
0.000 |
| Arousal |
Cognitive Style |
1 |
758661.66 |
8.133 |
0.007 |
| Arousal |
Interaction |
15 |
668401.30 |
1.919 |
0.019 |
| Dominance |
Colour |
15 |
2050373.67 |
6.652 |
0.000 |
| Dominance |
Cognitive Style |
1 |
263640.82 |
2.509 |
0.122 |
| Dominance |
Interaction |
15 |
969934.73 |
2.886 |
0.000 |
| Pupil size |
Colour |
15 |
7364736.28 |
77.662 |
0.000 |
| Pupil size |
Cognitive Style |
1 |
522173.90 |
1.896 |
0.178 |
| Pupil size |
Interaction |
15 |
289065.11 |
1.632 |
0.061 |
| Micro siemens |
Colour |
15 |
96452.22 |
0.990 |
0.465 |
| Micro siemens |
Cognitive Style |
1 |
267598.17 |
0.741 |
0.395 |
| Micro siemens |
Interaction |
15 |
151049.99 |
1.566 |
0.079 |
Table 6.
Highest and lowest ranked hues for Pleasure ratings, with descriptive statistics, number of significant contrasts, and p-value ranges.
Table 6.
Highest and lowest ranked hues for Pleasure ratings, with descriptive statistics, number of significant contrasts, and p-value ranges.
| Rank |
Colour |
M (SD) |
Significant pairwise
differences with…
|
Total colours |
p-value range |
| Highest |
Blue-Green |
7.67 (1.35) |
Baseline, Dark Grey, Green, Green-Yellow, Light Grey, Medium Grey, Purple-Blue, Purple, Red-Purple, Red, White, Yellow-Red, Yellow |
13 |
< .001 to .033 |
| 2nd highest |
Blue |
6.75 (1.78) |
Baseline, Dark Grey, Light Grey, Medium Grey, Yellow-Red, Yellow |
6 |
< .001 to .004 |
| 2nd lowest |
Yellow |
4.25 (2.33) |
Blue-Green, Blue, Green, Red-Purple, Red |
5 |
< .001 to .003 |
| Lowest |
Medium Grey |
4.14 (1.48) |
Blue-Green, Blue, Green, Purple-Blue, Purple, Red-Purple, Red, White |
8 |
< .001 to .012 |
Table 7.
Highest and lowest ranked hues for Arousal ratings, with descriptive statistics, number of significant contrasts, and p-value ranges.
Table 7.
Highest and lowest ranked hues for Arousal ratings, with descriptive statistics, number of significant contrasts, and p-value ranges.
| Rank |
Colour |
M (SD) |
Significant pairwise
differences with…
|
Total
colours
|
p-value
range
|
| Highest |
Red |
7.25 (1.36) |
Baseline, Black, Blue-Green, Blue, Dark Grey, Green, Green-Yellow, Light Grey, Medium Grey, Purple-Blue, White, Yellow-Red, Yellow |
13 |
< .001 to .011 |
| 2nd highest |
Red-Purple |
6.42 (1.95) |
Baseline, Dark Grey, Light Grey, Medium Grey, Yellow-Red, Yellow |
6 |
< .001 to .033 |
| 2nd lowest |
Medium Grey |
4.03 (1.54) |
Purple, Red-Purple, Red |
3 |
< .001 |
| Lowest |
Light Grey |
3.89 (1.78) |
Purple-Blue, Purple, Red-Purple, Red |
4 |
< .001 to .048 |
Table 8.
Highest and lowest ranked hues for Dominance ratings, with descriptive statistics, number of significant contrasts, and p-value ranges.
Table 8.
Highest and lowest ranked hues for Dominance ratings, with descriptive statistics, number of significant contrasts, and p-value ranges.
| Rank |
Colour |
M (SD) |
Significant pairwise
differences with…
|
Total colours |
p-value range |
| Highest |
Red |
6.72 (1.93) |
Green-Yellow, Medium Grey, Yellow-Red, Yellow |
4 |
.000 to .012 |
| Highest (tie) * |
Blue-Green |
6.69 (1.35) |
Green-Yellow, Light Grey, Medium Grey, Yellow-Red, Yellow |
5 |
.000 to .023 |
| 2nd lowest |
Yellow |
4.64 (2.33) |
Blue-Green, Blue, Red |
3 |
.005 to .039 |
| Lowest |
Yellow-Red |
4.28 (2.49) |
Blue-Green, Blue, Green, Purple-Blue, Red-Purple, Red |
6 |
.000 to .010 |
Table 9.
Post-hoc ranking of hues for Pupil Size, with mean (M), standard deviation (SD), and number of statistically significant pairwise differences relative to other colours.
Table 9.
Post-hoc ranking of hues for Pupil Size, with mean (M), standard deviation (SD), and number of statistically significant pairwise differences relative to other colours.
| Rank |
Colour |
M (SD) |
Significant pairwise
differences with…
|
Total
colours
|
p-value
range
|
| Highest |
Black |
5.17 (0.61) |
Baseline, Blue-Green, Blue, Dark Grey, Green, Green-Yellow, Light Grey, Medium Grey, Purple-Blue, Purple, Red-Purple, Red, White, Yellow-Red, Yellow |
15 |
< .001 |
| 2nd highest |
Red |
4.06 (0.54) |
Baseline, Green, Green-Yellow, Light Grey, Medium Grey, Purple, Red-Purple, White, Yellow |
9 |
< .001 to .002 |
| 2nd lowest |
Baseline |
3.04 (0.39) |
Black, Red, Dark Grey, Red-Purple, Purple-Blue, Yellow-Red, Blue, Medium Grey, Purple, Green-Yellow, Green, Yellow |
12 |
< .001 to .004 |
| Lowest |
White |
2.72 (0.52) |
Black, Blue-Green, Blue, Dark Grey, Green, Green-Yellow, Light Grey, Medium Grey, Purple-Blue, Purple, Red-Purple, Red, Yellow-Red, Yellow |
14 |
< .001 |
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).