Introduction
Alzheimer's disease (AD) currently represents one of the biggest medical and social problems in modern healthcare. This chronic progressive neurodegenerative disease is the main cause of dementia in the elderly, accounting for about 60–70% of all cognitive disorder cases [
1,
2,
3]. According to the latest WHO data, the total number of patients with Alzheimer's disease worldwide exceeds 55 million, with about 10 million new cases being reported annually. Epidemiological studies predict a three-fold increase in the prevalence of AD by 2050, which would create an unprecedented burden on health and social protection systems around the world. A tendency to "rejuvenation" of this disease causes particular concern: more and more often the first symptoms appear at the age of 50–60, which radically changes approaches to diagnosis and prevention [
4,
5].
The latest concepts of Alzheimer's disease pathogenesis point to several interrelated pathological processes [
6,
7]. The central one is the theory of the amyloid cascade, according to which the key event is the accumulation of β-amyloid peptides (Aß42) in brain tissue accompanied by the formation of senile plaques [
8]. In parallel, there develops a process of hyperphosphorylation of tau protein, which results in the disintegration of microtubules and the formation of neurofibrillary tangles. These changes are accompanied by the activation of neuroinflammatory processes, oxidative stress, mitochondrial dysfunction, and apoptosis of neurons [
9,
10,
11]. It is worth noting that such pathological changes begin 15–20 years before the first clinical symptoms appear, creating a critically important "therapeutic window” for early intervention.
Modern methods of diagnosing Alzheimer's disease are based on the criteria provided by the National Institute on Aging and the Alzheimer's Association, which include three groups of biomarkers. The first group reflects amyloid pathology (a decrease of Aß42 and an increase of the Aß42/Aß40 ratio in cerebrospinal fluid, positive PET-amyloid imaging). The second group characterizes tau pathology (an increase in total and phosphorylated tau protein in cerebrospinal fluid, positive PET-tau imaging) [
12,
13]. The third group of markers provides evidence of neurodegeneration (an increase in neurofilament light chain protein (NFL), a decrease in hippocampal volume on MRI). Still, these methods have significant limitations due to the invasiveness of lumbar puncture, high costs of PET imaging, limited availability of specialized equipment, and the need for a complex interpretation of the results.
In recent years, special attention has been paid to searching for molecular biomarkers that could become the basis for the development of non-invasive methods for the diagnosis and monitoring of Alzheimer's disease [
14,
15]. According to recent studies, such processes as β-amyloid accumulation, tau protein hyperphosphorylation, neuroinflammation, and oxidative stress play a pivotal role in the pathogenesis of the disease. However, the identification of most of existing biomarkers, such as β-amyloid and tau protein in cerebrospinal fluid, requires invasive sampling procedures, which restricts their widespread use in clinical practice.
Therefore, a relevant line of research is looking for biomarkers in easily accessible biological fluids and tissues, such as blood, saliva, or buccal epithelium [
16,
17,
18]. Recent studies show the viability of using buccal epithelium for identifying various pathological markers. Buccal epithelial cells have high metabolic activity and express multiple neuron-specific proteins. Moreover, in epithelial cells it is possible to verify key signaling molecules involved in the mechanisms of systemic pathological processes, including oxidative stress, inflammation, and mitochondrial dysfunction, which play an important role in the pathogenesis of neurodegenerative diseases [
19].
The purpose of this study is to develop non-invasive method, and a diagnostically meaningful integrated panel of molecular biomarkers in the buccal epithelium for screening and differential diagnosis of AD.
Materials and Methods
An immunocytochemical method was used to investigate the expression of signaling molecules involved in the neurodegeneration process (molecular markers) in buccal epithelial cells in patients with dementia caused by AD, vascular dementia, and in volunteers of the appropriate age without such pathologies. The total number of examined persons was 203, of whom 53 (26.1%) were male and 150 (73.9%) were female. The average age of the examined patients in all groups was 84.6±7.6 (with AD – 77±12.2 years, with vascular dementia – 79.7±9.8 years, and volunteers – 61.7±7.6). All the subjects were divided into 3 groups:
- (1)
patients with clinically diagnosed AD (57 persons, of whom 21% were male and 79% were female);
- (2)
patients with clinically diagnosed vascular dementia (100 persons, of whom 26% were male and 74% were female);
- (3)
volunteers without clinical manifestations of neuropsychiatric disorders (46 persons, of whom 32.6% were male and 67.4% were female).
The dynamic cohort method was used, which provides for the formation and reformation of groups and subgroups in the study based on the variation of cognitive dysfunctions and the presence of comorbidity, which allows to carry out a correlation analysis of participants with different mixed cognitive disorders. Study participants were selected based on an expert assessment of medical records (including analysis of diagnoses, anamnestic data, results of biochemical, functional, imaging examinations, psychometric tests), their geriatric status based on a comprehensive geriatric assessment, and, where necessary, additional follow-up examination and testing based on experts’ recommendations.
Cases of dementia, including AD and vascular dementia, were identified based on the analysis of medical records using rigorous diagnostic algorithms in accordance with current European (
EFNS-ENS Guidelines on diagnosis and management of disorders associated with dementia) and national guidelines [
19].
Additionally, the diagnosis of vascular dementia was based on the criteria of the VICCCS (Vascular Impairment of Cognition Classification Consensus Study) [
20], which provides for a comprehensive assessment of clinical syndromes, neuroimaging signs of cerebrovascular disease and their causal link with cognitive decline.
While selecting control group participants, a psychometric examination was also carried out, since they could have pre-clinical cognitive disorders in the absence of complaints and clinically significant manifestations, which prevented them from being included in the control group.
The following inclusion criteria for the study were determined: age over 50 years, clinical manifestations of AD or vascular dementia. The exclusion criteria for the main and control groups are: oncological or hematological diseases with no remission, conditions requiring emergency care; refusal of study participants or their legal guardians to participate in the study.
The object of the study were buccal epithelium samples. Sterile cytobrushes were used to take buccal epithelium samples: after mouth rinsing, buccal smears were collected from the inner surface of the cheeks, which were then fixed in a buffer solution and stored at +2–8 °C for <14 days. The liquid-based cytology method was used to create a monolayer of cells on the stage of microscope.
Monolayer cytopreparations of buccal epithelium are obtained on glasses with an adhesive coating of L-polylysine using a CytoPrep-4 cytocentrifuge at a centrifugation mode of 1000 rpm for 6 minutes. The prepared cytopreparations are stored at a temperature of 4° C for no more than 30 days before molecular microscopic examination.
For immunocytochemical analysis (ICC test), we used monoclonal antibodies (all Abcam) to the following key signaling molecules (biomarkers) that are involved in the process of neurodegeneration: β-amyloid, NF-κB, tau protein, S100 protein, claudin, synuclein, RAGE, and PTEN-induced kinase 1 (PINK1). To visualize the ICC test, a reagent kit based on the NovoLink polymer and peroxidase, RE7150-K (NovoCastra) was used.
Images of buccal epithelium preparations with a magnification of 400x were obtained using computer analysis systems for microscopic images, included the Olympus BX46 microscope, VideoZavrStandartVZ-18C23-B digital camera, PC (AMD Ryzen 3 3200G), and VideoZavrCatalog software.
The number of immunopositive cells and the total number of cells were estimated manually. The so-called "cell specific weight" (the ratio of the number of immunopositive cells to the number of all cells in the field of view expressed as a percentage) was determined with a total number of cells in preparation > 100.
Using the ImageJ application, we can calculate the area occupied by immunopositive cells, which makes it possible to calculate the area of protein expression and find correlations of marker expression.
The Shapiro-Wilk test was used in statistical data processing to verify the normality of the distribution. The results were described using parametric and nonparametric methods. If the data matched the normal distribution (only for age in the comparison groups), the typical value was presented as the mean and standard deviation (M±SD), and the groups were compared by using Student's t-test. If the data did not match the normal distribution (all extensive indicators characterizing the specific weight of cells with marker expression), the typical value was presented as a median, the deviation was characterized by an interquartile range of Ме (Q1–Q3), and the 95% confidence interval was calculated by using the Wilson method. The overall confidence interval was calculated with the Wilson formula.
The groups were compared by using the Mann-Whitney U-test. In a paired comparison, the null hypothesis was rejected at a significance level less than 0.05. In a multiple pairwise comparison, the null hypothesis was rejected at a significance level less than 0.01.
Results and Discussion
Our findings confirm the possibility, in principle, of identifying specific molecular biomarkers of brain pathology in peripheral tissue accessible for intra-vital sampling — the buccal epithelium. It is especially significant that non-invasive sampling of the buccal epithelium, opens a new prospective for the development of easy and safe methods for assessment of patients.
An important outcome of this study was the identification of clear distinctions in the expression of a number of biomarkers between patients with AD and the control group.
In particular, a statistically significant decrease was found in the expression of the following markers in the buccal epithelium in AD: β-amyloid, NF-kB transcription factor, PINK1, RAGE, α-synuclein, and S100 protein.
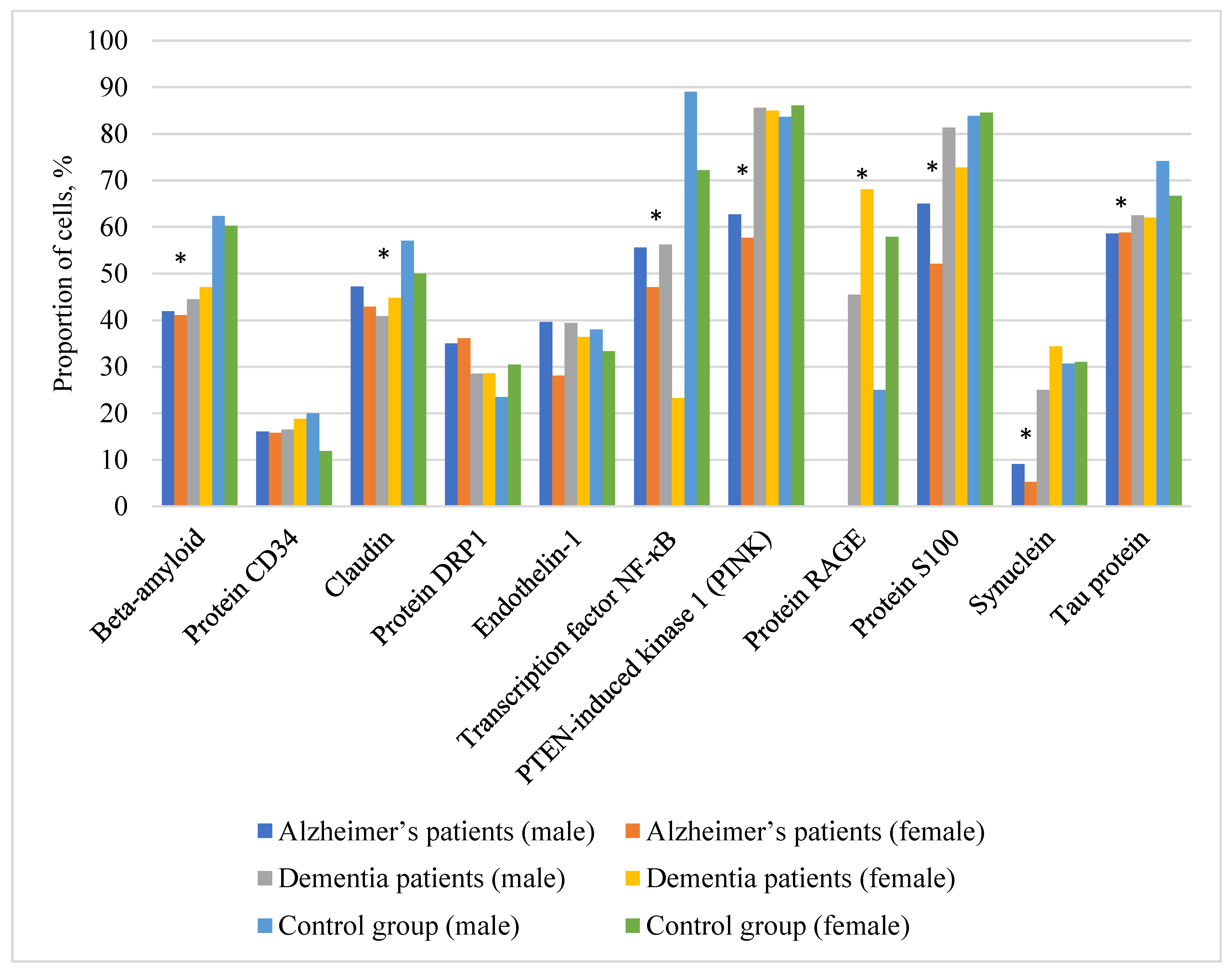
Table 1 and
Figure 1 represent the comparative analysis data regarding the level of 11 biomarkers in the buccal epithelium in patients with AD, patients with clinically diagnosed vascular dementia, and in healthy controls, divided by gender.
Special attention should be drawn to the results concerning the differential diagnosis of AD. Our study revealed a unique combination of markers that allows to clearly distinguish AD from other types of dementia.
Among the most indicative changes were the following: a statistically significant decrease in the expression of RAGE, a marked decrease in PINK1 and α-synuclein in the buccal epithelium. At the same time, the expression of phosphorylated tau protein in the buccal epithelium was statistically significantly higher in patients with AD than in patients with vascular dementia and in volunteers. It is these changes that may be considered as the specific molecular profile of AD. The obtained results confirm the current understanding of the key pathogenetic mechanisms of AD, including the dysregulated metabolism of β-amyloid and tau protein, along with the involvement of neuroinflammation and oxidative stress.
The analysis of gender differences in the studied biomarkers, as presented below, did not reveal statistically significant changes in the figures both in healthy volunteers and in patients with AD (p>0.05).
The most significant changes were observed for β-amyloid, the average level of which in patients with AD was significantly lower than in controls — 41.9% (35–57.1%) in male against 62.4% (50.5–69.9%) in the control group, 41.1% (26.1–54.3%) in female against 60.3% (50–67.7%) in the control group.
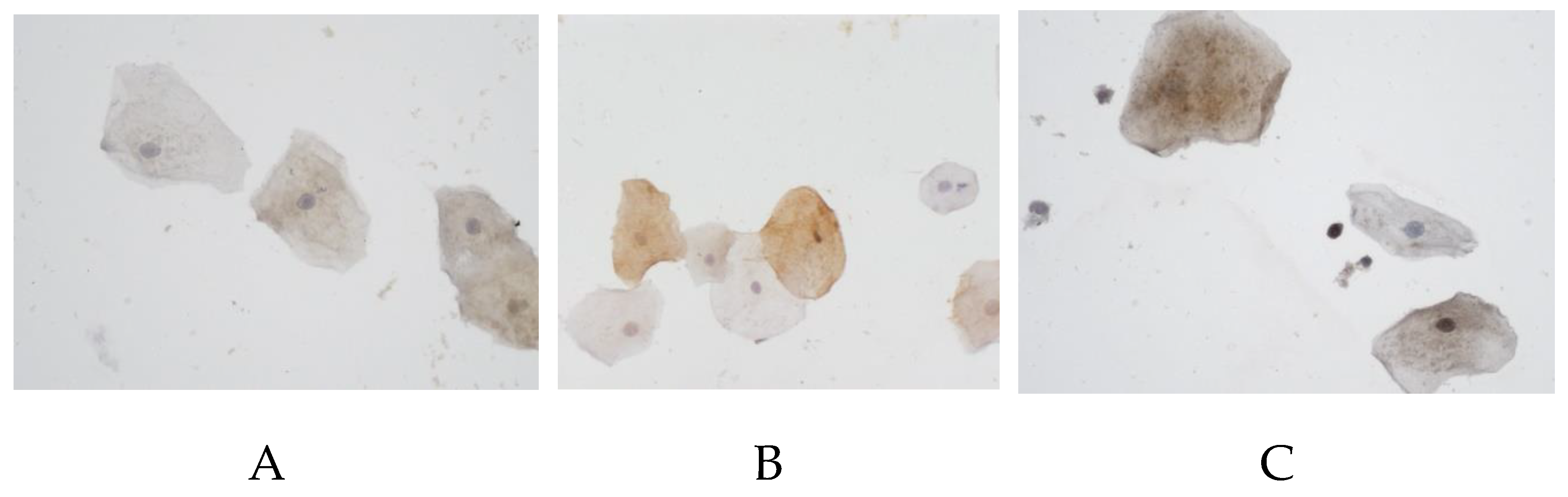
Particularly remarkable is an almost complete absence of RAGE protein expression in patients with AD — 0% (0–6.6%) in male and 0% (0–15%) in female, whereas in the control group its level was 25% (0–88.9%) and 57.9% (10-70.8%) respectively (
Figure 2).
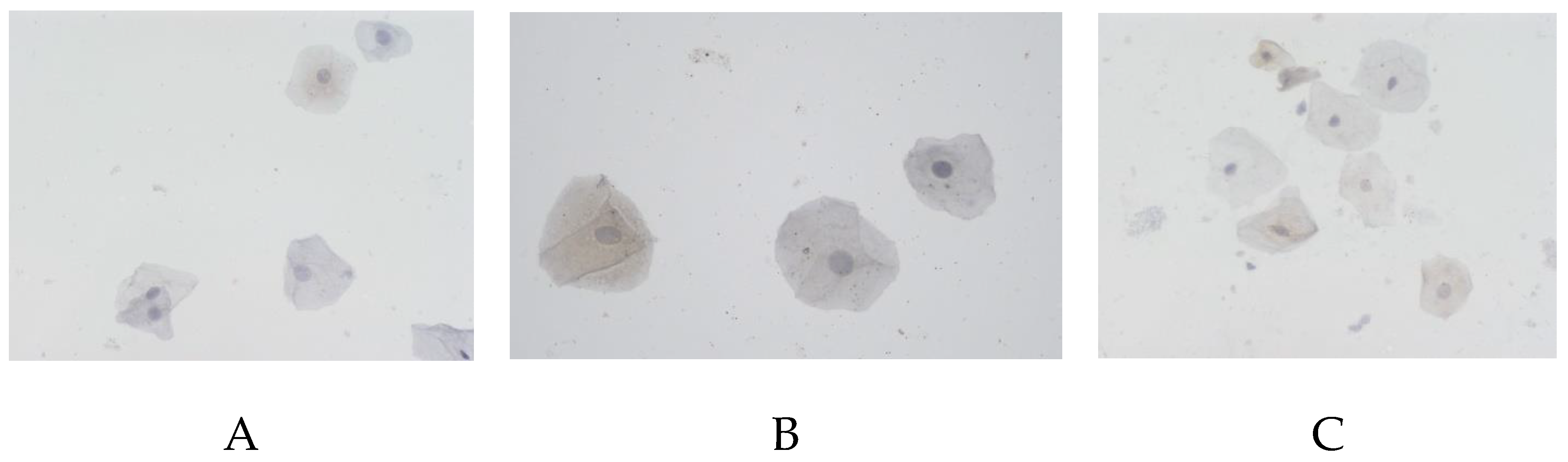
The tau protein also demonstrated statistically significantly lower rates in AD (
Figure 3): 58.6% (44.4–72.9%) in male against 74.2% (47.4–89.7%) in the control, 58.8% (50–67.9%) in female against 66.7 (53.3–85.7%) in the control.
A similar pattern was observed for α-synuclein: 9.1% (0–15.8%) in male with AD against 30.7% (17.2–44.2%) in the control, 5.3% (0–10%) in females with AD against 31% (14–50%) in the control (
Figure 4).
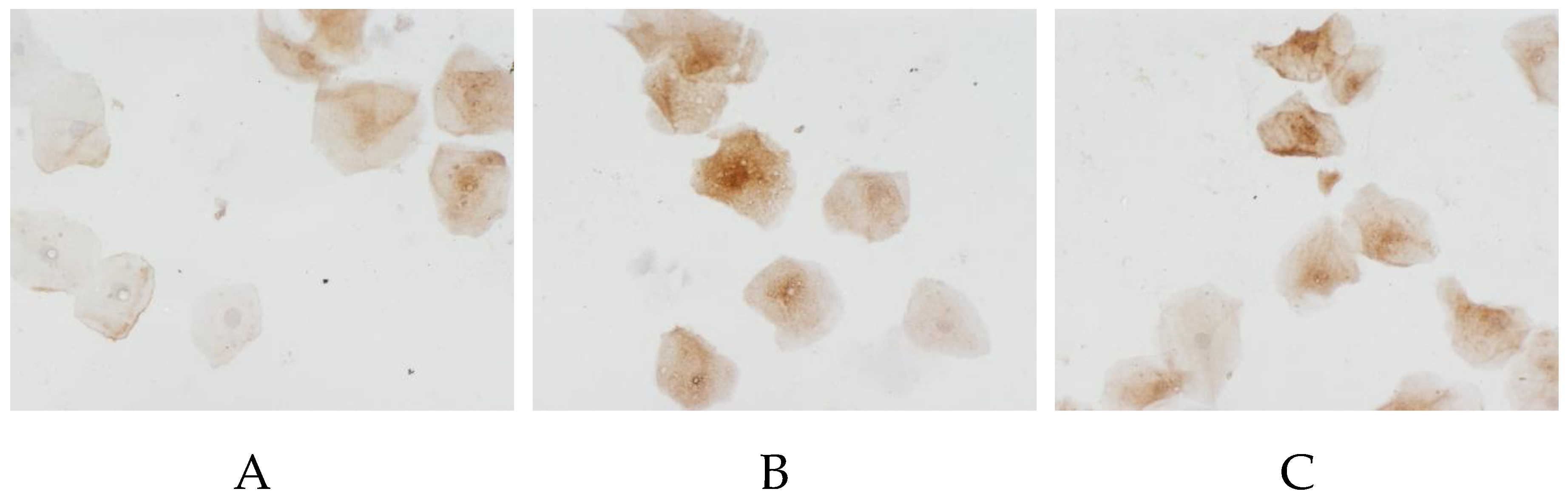
PTEN-induced kinase 1 (PINK1) also showed a significant decrease in expression in AD patients (
Figure 5): 62.7% (14.5–83.4%) in male against 83.7% (77.8–89.7%) in the control, and 57.7% (25–84%) in female against 86.1% (81.3–91.7%) in the control.
Interestingly, the DRP1 protein, on the contrary, shows higher values in AD – 35% (12.5–46.9%) in male against 23.5% (16.7–40%) in the control, and 36.1% (25.3–46.9%) in female against 30.5 (23.8–50%) in the control.
This data gives evidence that there occur significant changes in the profile of biomarker expression in the buccal epithelium in AD and confirms the viability of using a combination of biomarkers (RAGE, α-synuclein, PINK1, and tau protein) to diagnose this neurodegenerative disease.
The non-invasive nature of buccal epithelium sampling makes this approach particularly promising for clinical practice.
Based on the overall results obtained in this study, a panel was developed for the intra-vital molecular diagnosis of AD (including both screening and differentiating this pathology from vascular dementia) by analyzing the expression of α-synuclein, RAGE, PINK1, and phosphorylated tau protein in the buccal epithelium (
Table 2).
To improve the intra-vital diagnosis and monitoring of AD, it appears promising to further elaborate a personalized predictive algorithm that will account for the personal significance of each molecular biomarker based on additional mathematical analysis. This approach will not only improve diagnosis, but also enable us to predict the dynamics of disease development in specific patients.
Conclusion
For the differential diagnosis of AD from vascular dementia, it is advisable to use a panel of biomarkers for verifying the expression of α-synuclein, RAGE, PINK, and phosphorylated tau protein in the buccal epithelium.
The development of this diagnostic panel is an example that can become the basis for creating an accessible, non-invasive and highly informative method for early diagnostics of socially significant diseases. This is especially relevant in connection with the need to develop new pathogenetically substantiated (targeted) treatment methods that are most effective at preclinical stages of the disease.
To optimize the intra-vital diagnosis and monitoring of AD, it appears reasonable to create a personalized algorithm for predicting its occurrence and development based on additional mathematical analysis to evaluate the informativeness of each molecular biomarker.
References
- Bondi MW, Edmonds EC, Salmon DP. Alzheimer's Disease: Past, Present, and Future. J Int Neuropsychol Soc. 2017;23(9-10):818-831. [CrossRef]
- Kurmyshev M.V., Ivko O.M., Ponomarev A.S., Gavrilova A.A., Fesenko E.V., Prashchayeu K.I., Litvinov M.S. Clinical detenminants for the targeted effects of neurocognitive rehabilitation in elderly and senile patients with mild cognitive impairment. Adv. Geront. 2024. Vol. 37, № 6. P. 731–736. [CrossRef]
- Klyucherev TO, Olszewski P, Shalimova AA, Chubarev VN, Tarasov VV, Attwood MM, Syvänen S, Schiöth HB. Advances in the development of new biomarkers for Alzheimer's disease. Transl. Neurodegener. 2022 Apr 21;11(1):25. PMID: 35449079; PMCID: PMC9027827. [CrossRef]
- Knopman DS, Amieva H, Petersen RC, Chételat G, Holtzman DM, Hyman BT, Nixon RA, Jones DT. Alzheimer disease. Nat Rev Dis Primers. 2021 May 13;7(1):33. PMID: 33986301; PMCID: PMC8574196. [CrossRef]
- Beata BK, Wojciech J, Johannes K, Piotr L, Barbara M. Alzheimer's Disease-Biochemical and Psychological Background for Diagnosis and Treatment. Int J Mol Sci. 2023 Jan 5;24(2):1059. PMID: 36674580; PMCID: PMC9866942. [CrossRef]
- Ferrari C, Sorbi S. The complexity of Alzheimer's disease: an evolving puzzle. Physiol Rev. 2021 Jul 1;101(3):1047-1081. Epub 2021 Jan 21. PMID: 33475022. [CrossRef]
- Blackman J, Swirski M, Clynes J, Harding S, Leng Y, Coulthard E. Pharmacological and non-pharmacological interventions to enhance sleep in mild cognitive impairment and mild Alzheimer's disease: A systematic review. J Sleep Res. 2021 Aug;30(4):e13229. Epub 2020 Dec 2. PMID: 33289311; PMCID: PMC8365694. [CrossRef]
- Bonomi CG, Assogna M, Di Donna MG, Bernocchi F, De Lucia V, Nuccetelli M, Fiorelli D, Loizzo S, Mercuri NB, Koch G, Martorana A, Motta C. Cerebrospinal Fluid sTREM-2, GFAP, and β-S100 in Symptomatic Sporadic Alzheimer's Disease: Microglial, Astrocytic, and APOE Contributions Along the Alzheimer's Disease Continuum. J Alzheimers Dis. 2023;6. [CrossRef]
- Scheltens P, De Strooper B, Kivipelto M, Holstege H, Chételat G, Teunissen CE, Cummings J, van der Flier WM. ALZHEIMER'S DISEASE: Lancet. 2021;397(10284):1577-1590. [CrossRef]
- Nazzi C, Avenanti A, Battaglia S. The Involvement of Antioxidants in Cognitive Decline and Neurodegeneration: Mens Sana in Corpore Sano. Antioxidants (Basel). 2024 Jun 7;13(6):701. PMID: 38929140; PMCID: PMC11200558. [CrossRef]
- Zheng Q, Wang X. Alzheimer's disease: insights into pathology, molecular mechanisms, and therapy. Protein Cell. 2025 Feb 1;16(2):83-120. PMID: 38733347; PMCID: PMC11786724. [CrossRef]
- Wegmann S, Biernat J, Mandelkow E. A current view on Tau protein phosphorylation in Alzheimer's disease. Curr Opin Neurobiol. 2021;69:131-138. [CrossRef]
- Gonzalez LL, Garrie K, Turner MD. Role of S100 proteins in health and disease. Biochim Biophys Acta Mol Cell Res. 2020;1867(6):118677. [CrossRef]
- Sadick JS, O'Dea MR, Hasel P, Dykstra T, Faustin A, Liddelow SA. Astrocytes and oligodendrocytes undergo subtype-specific transcriptional changes in Alzheimer's disease. Neuron. 2022;110(11):1788-1805.e10. [CrossRef]
- Watanabe Y, Taguchi K, Tanaka M. Ubiquitin, Autophagy and Neurodegenerative Diseases. Cells. 2020;9(9):2022. [CrossRef]
- Kvetnoy IM, Hernandez-Yago J, Kvetnaia TV, Khavinson VK, Malinin VV, Yarilin AA, Sharova NI, Blesa JR, Anisimov VN, Lenskaia LV, Sluchevskaia SF, Chekalina SI, Tokarev OY, Yuzhakov VV. Tau-protein expression in human blood lymphocytes: a promising marker and suitable sample for life-time diagnosis of Alzheimer's disease. Neuroendocrinol Lett. 2000;21(4):313-318. PMID: 11455366.
- François M, Fenech MF, Thomas P, Hor M, Rembach A, Martins RN, Rainey-Smith SR, Masters CL, Ames D, Rowe CC, Macaulay SL, Hill AF, Leifert WR, The Australian Imaging Biomarkers And Lifestyle Study Research Group. High Content, Multi-Parameter Analyses in Buccal Cells to Identify Alzheimer's Disease. Curr Alzheimer Res. 2016;13(7):787-99. PMID: 26975368. [CrossRef]
- Arredondo LF, Aranda-Romo S, Rodríguez-Leyva I, Chi-Ahumada E, Saikaly SK, Portales-Pérez DP, González-Amaro R, Salgado-Bustamante M, Enriquez-Macias L, Eng W, Norman RA, Jimenez-Capdeville ME. Tau Protein in Oral Mucosa and Cognitive State: A Cross-sectional Study. Front Neurol. 2017 Oct 13;8:554. PMID: 29081764; PMCID: PMC5645496. [CrossRef]
- Sorbi S, Hort J, Erkinjuntti T, Fladby T, Gainotti G, Gurvit H, Nacmias B, Pasquier F, Popescu BO, Rektorova I, Religa D, Rusina R, Rossor M, Schmidt R, Stefanova E, Warren JD, Scheltens P; EFNS Scientist Panel on Dementia and Cognitive Neurology. EFNS-ENS Guidelines on the diagnosis and management of disorders associated with dementia. Eur J Neurol. 2012 Sep;19(9):1159-79. PMID: 22891773. [CrossRef]
- Skrobot OA, O'Brien J, Black S, Chen C, DeCarli C, Erkinjuntti T, Ford GA, Kalaria RN, Pantoni L, Pasquier F, Roman GC, Wallin A, Sachdev P, Skoog I; VICCCS group; Ben-Shlomo Y, Passmore AP, Love S, Kehoe PG. The Vascular Impairment of Cognition Classification Consensus Study. Alzheimers Dement. 2017 Jun;13(6):624-633. PMID: 27960092. [CrossRef]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).