Submitted:
04 August 2025
Posted:
05 August 2025
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Materials and Methods
2.1. Patients (Recipients)
2.2. Perioperative UTI
2.3. Immunosuppressive Therapy Regimens
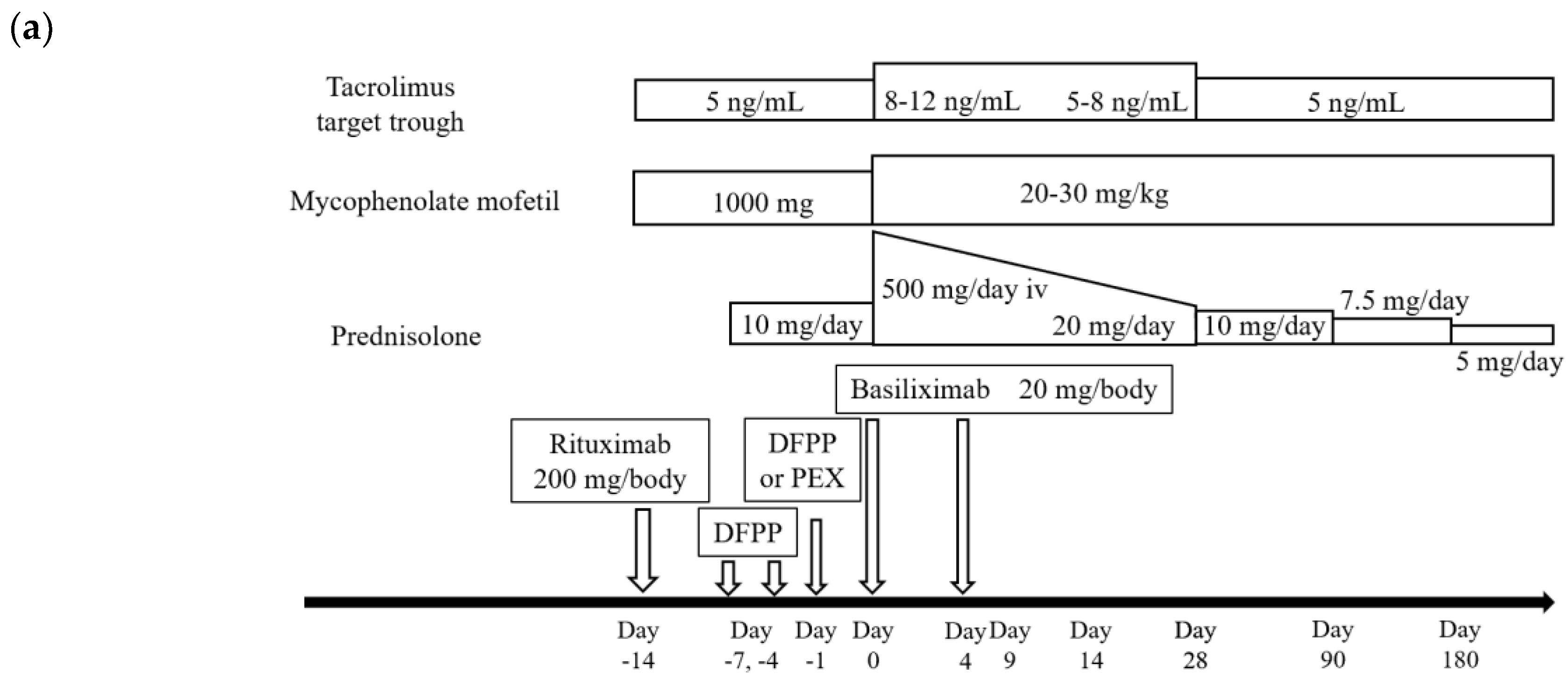
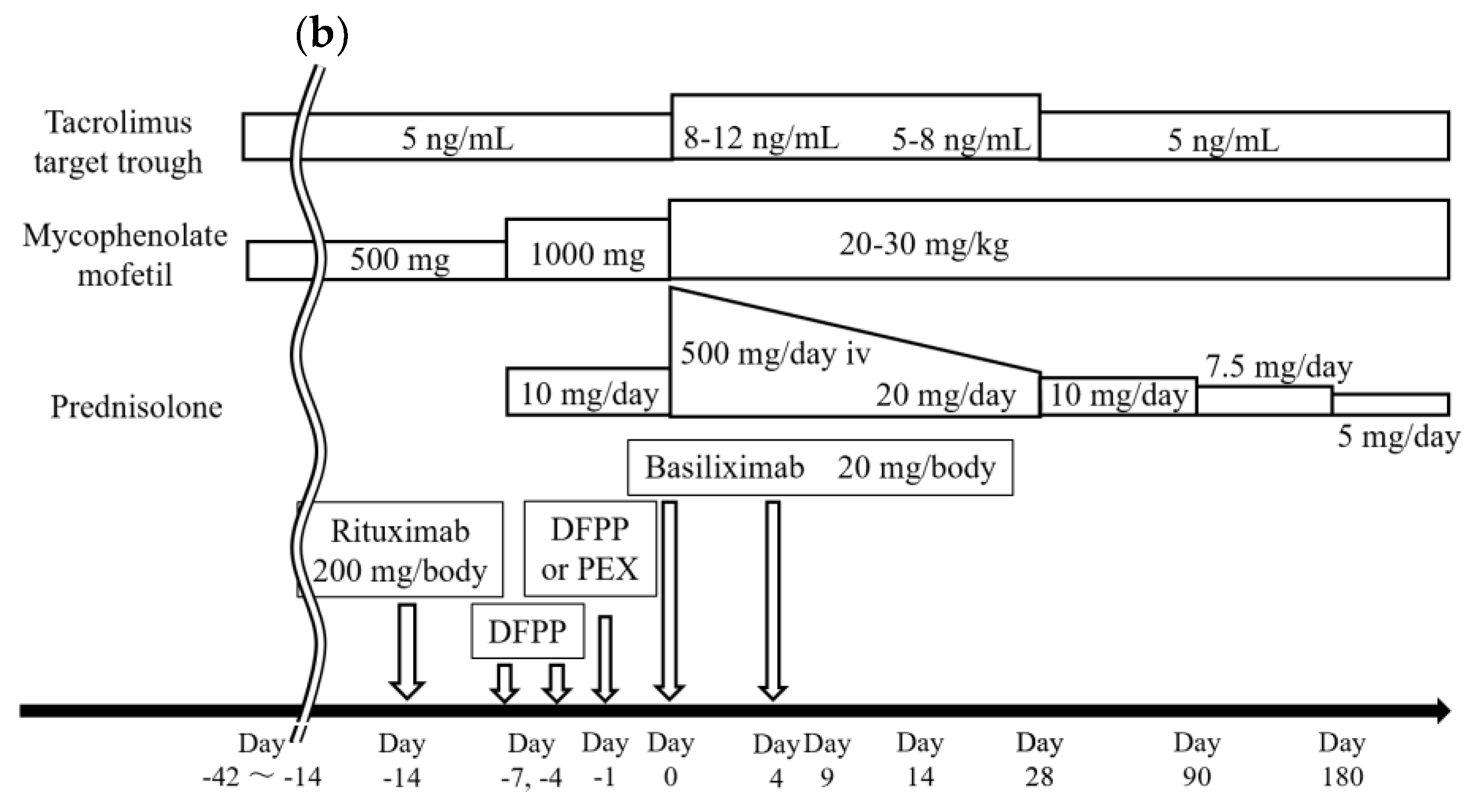
2.4. Standard Procedures of LDKT
2.5. Bacteriological Examination and Prophylactic Antimicrobial Agents
2.6. Statistical Methods and Analyses
2.7. Ethics
3. Results
3.1. Recipient Characteristics
3.2. Antimicrobial Prophylaxis and Results of the Procedure
3.3. Perioperative UTIs and Outcomes
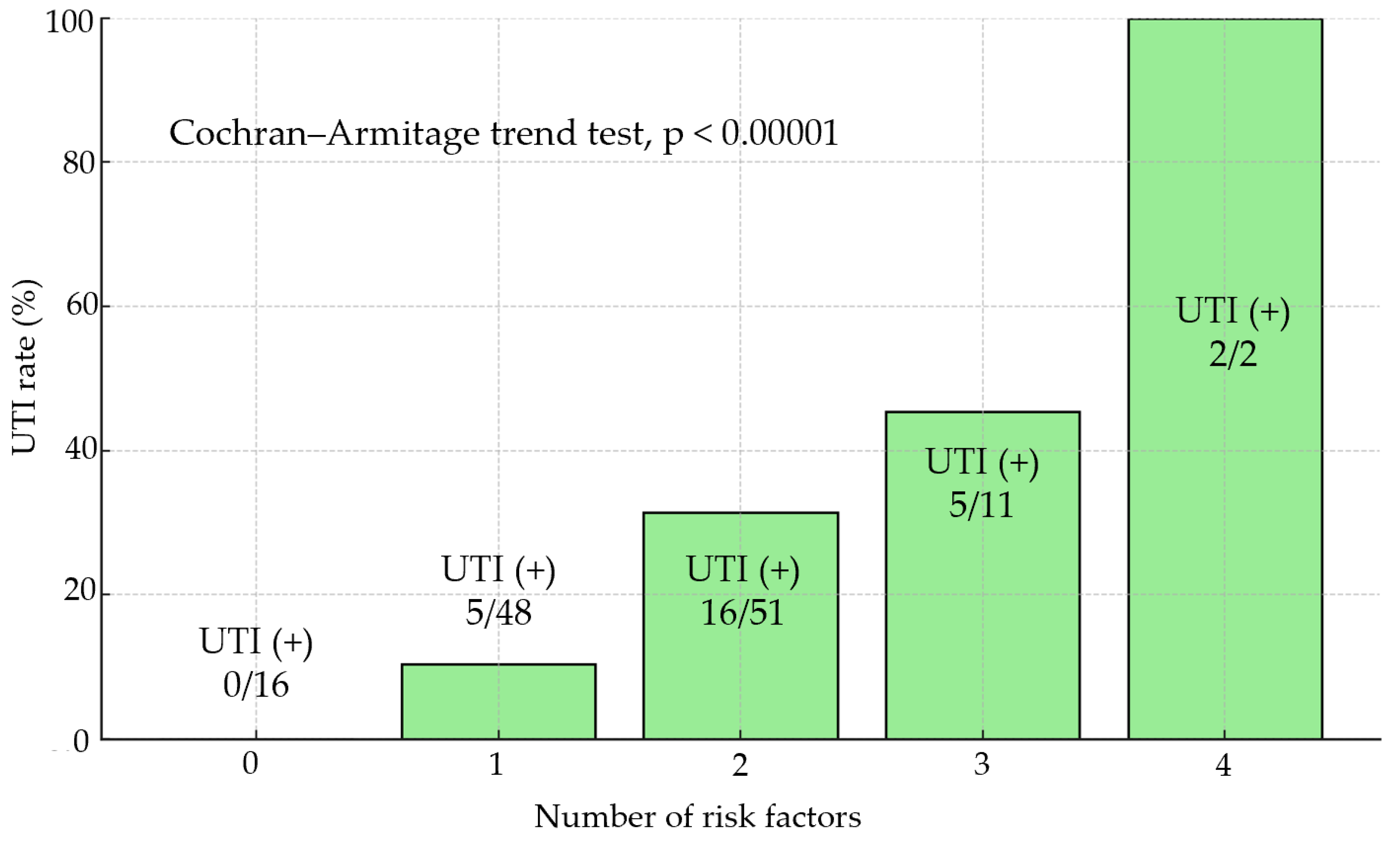
3.4. Risk Factors for Perioperative UTIs
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| UTI | Urinary Tract Infection |
| LDKT | living donor kidney transplantation |
| DSA | donor-specific antibody |
| NLR | neutrophil-to-lymphocyte ratio |
| WIT | warm ischemic time |
| BMI | body mass index |
| HLA | human leukocyte antigen |
| CNI | calcineurin inhibitor |
| MMF | mycophenolate mofetil |
| DFPP | double filtration plasmapheresis |
| PEX | plasma exchange |
| FSGS | focal segmental glomerulosclerosis |
| IVIG | intravenous immunoglobulin |
| DM | diabetes mellitus |
| ESBL | extended-spectrum beta-lactamase |
| AUC | area under the curve |
| ASA | American Society of Anesthesiologists |
| ECD | expanded criteria donors |
| DGF | delayed graft function |
| SSI | Surgical Site Infection |
| CKD | Chronic Kidney Disease |
| VUR | vesicoureteral reflux |
| DIC | disseminated intravascular coagulation |
| RI | Remote Infection |
| AMR | antimicrobial resistance |
| POD | postoperative day |
| TMP-SMX | trimethoprim-sulfamethoxazole |
References
- Lee JR, Bang H, Dadhania D, Hartono C, Aull MJ, Satlin M, et al. Independent risk factors for urinary tract infection and for subsequent bacteremia or acute cellular rejection: a single-center report of 1166 kidney allograft recipients. Transplantation. 2013;96(8):732-8. [CrossRef]
- Takahashi, K. Recent findings in ABO-incompatible kidney transplantation: classification and therapeutic strategy for acute antibody-mediated rejection due to ABO-blood-group-related antigens during the critical period preceding the establishment of accommodation. Clin Exp Nephrol. 2007;11(2):128-41. [CrossRef]
- de Weerd A, Vonk A, van der Hoek H, van Groningen M, Weimar W, Betjes M, et al. Late antibody-mediated rejection after ABO-incompatible kidney transplantation during Gram-negative sepsis. BMC Nephrol. 2014;15:31. [CrossRef]
- Pellé G, Vimont S, Levy PP, Hertig A, Ouali N, Chassin C, et al. Acute pyelonephritis represents a risk factor impairing long-term kidney graft function. Am J Transplant. 2007;7(4):899-907. [CrossRef]
- Nishi SP, Valentine VG, Duncan S. Emerging bacterial, fungal, and viral respiratory infections in transplantation. Infect Dis Clin North Am. 2010;24(3):541-55. [CrossRef]
- Adamska Z, Karczewski M, Cichańska L, Więckowska B, Małkiewicz T, Mahadea D, et al. Bacterial Infections in Renal Transplant Recipients. Transplant Proc. 2015;47(6):1808-12. [CrossRef]
- Rabkin DG, Stifelman MD, Birkhoff J, Richardson KA, Cohen D, Nowygrod R, et al. Early catheter removal decreases incidence of urinary tract infections in renal transplant recipients. Transplant Proc. 1998;30(8):4314-6. [CrossRef]
- Capocasale E, De Vecchi E, Mazzoni MP, Dalla Valle R, Pellegrino C, Ferretti S, et al. Surgical site and early urinary tract infections in 1000 kidney transplants with antimicrobial perioperative prophylaxis. Transplant Proc. 2014;46(10):3455-8. [CrossRef]
- Orlando G, Manzia TM, Sorge R, Iaria G, Angelico R, Sforza D, et al. One-shot versus multidose perioperative antibiotic prophylaxis after kidney transplantation: a randomized, controlled clinical trial. Surgery. [CrossRef]
- The Japan Society for Transplantation. “Transplantation Fact Book 2023,” (in Japanese). Available from: http://www.asas.or.jp/jst/pdf/factbook/factbook2023.pdf.
- Takahashi K, Saito K, Takahara S, Fuchinoue S, Yagisawa T, Aikawa A, et al. Results of a multicenter prospective clinical study in Japan for evaluating efficacy and safety of desensitization protocol based on rituximab in ABO-incompatible kidney transplantation. Clin Exp Nephrol. 2017;21(4):705-13. [CrossRef]
- Morath C, Zeier M, Döhler B, Opelz G, Süsal C. ABO-Incompatible Kidney Transplantation. Front Immunol. 2017;8:234. [CrossRef]
- Yamamoto S, Shigemura K, Kiyota H, Wada K, Hayami H, Yasuda M, et al. Essential Japanese guidelines for the prevention of perioperative infections in the urological field: 2015 edition. Int J Urol. 2016;23(10):814-24. [CrossRef]
- Kanda, Y. Investigation of the freely available easy-to-use software 'EZR' for medical statistics. Bone Marrow Transplant. 2013;48(3):452-8. [CrossRef]
- Meena P, Bhargava V, Rana DS, Bhalla AK. Urinary tract infection in renal transplant recipient: A clinical comprehensive review. Saudi J Kidney Dis Transpl. 2021;32(2):307-17. [CrossRef]
- Bratzler DW, Dellinger EP, Olsen KM, Perl TM, Auwaerter PG, Bolon MK, et al. Clinical practice guidelines for antimicrobial prophylaxis in surgery. Am J Health Syst Pharm. 2013;70(3):195-283. [CrossRef]
- Kälble T, Lucan M, Nicita G, Sells R, Burgos Revilla FJ, Wiesel M. EAU guidelines on renal transplantation. Eur Urol. 2005;47(2):156-66. [CrossRef]
- Røine E, Bjørk IT, Oyen O. Targeting risk factors for impaired wound healing and wound complications after kidney transplantation. Transplant Proc. 2010;42(7):2542-6. [CrossRef]
- Trivin C, Tran A, Moulin B, Choukroun G, Gatault P, Courivaud C, et al. Infectious complications of a rituximab-based immunosuppressive regimen in patients with glomerular disease. Clin Kidney J. [CrossRef]
- Schrezenmeier E, Budde K, Staeck O, Lehner L, Duerr M, Khadzhynov D, et al. Incidence of Infectious Disease and Malignancies After Rituximab Therapy in Kidney Transplant Recipients: Results From a Cohort in Germany. Transplant Proc. 2017;49(10):2269-73. [CrossRef]
- Kamar N, Milioto O, Puissant-Lubrano B, Esposito L, Pierre MC, Mohamed AO, et al. Incidence and predictive factors for infectious disease after rituximab therapy in kidney-transplant patients. Am J Transplant. 2010;10(1):89-98. [CrossRef]
- Tydén G, Kumlien G, Efvergren M. Present techniques for antibody removal. Transplantation. 2007;84(12Suppl):S27-9. [CrossRef]
- Melexopoulou C, Filiopoulos V, Marinaki S. Therapeutic apheresis in renal transplantation: An update. Transfus Apher Sci. 2024;63(1):103844. [CrossRef]
- Chung BH, Yun JT, Ha SE, Kim JI, Moon IS, Choi BS, et al. Combined use of rituximab and plasmapheresis pre-transplant increases post-transplant infections in renal transplant recipients with basiliximab induction therapy. Transpl Infect Dis. 2013;15(6):559-68. [CrossRef]
- Lynch RJ, Ranney DN, Shijie C, Lee DS, Samala N, Englesbe MJ. Obesity, surgical site infection, and outcome following renal transplantation. Ann Surg. 2009;250(6):1014-20. [CrossRef]
- Semins MJ, Shore AD, Makary MA, Weiner J, Matlaga BR. The impact of obesity on urinary tract infection risk. Urology. 2012;79(2):266-9. [CrossRef]
- Lu JL, Kalantar-Zadeh K, Ma JZ, Quarles LD, Kovesdy CP. Association of body mass index with outcomes in patients with CKD. J Am Soc Nephrol. 2014;25(9):2088-96. [CrossRef]
- Yamamoto T, Nakayama M, Miyazaki M, Sato H, Matsushima M, Sato T, et al. Impact of lower body mass index on risk of all-cause mortality and infection-related death in Japanese chronic kidney disease patients. BMC Nephrol. 2020;21(1):244. [CrossRef]
- Ozato N, Saito S, Yamaguchi T, Katashima M, Tokuda I, Sawada K, et al. Association between Nutrients and Visceral Fat in Healthy Japanese Adults: A 2-Year Longitudinal Study Brief Title: Micronutrients Associated with Visceral Fat Accumulation. Nutrients. 2019;11(11). [CrossRef]
- Ogawa W, Hirota Y, Miyazaki S, Nakamura T, Ogawa Y, Shimomura I, et al. Definition, criteria, and core concepts of guidelines for the management of obesity disease in Japan. Endocr J. 2024;71(3):223-31. [CrossRef]
- World Health Organization. “Obesity and overweight”. Available from: https://www.who.int/newsroom/fact-sheets/detail/obesity-and-overweight.
- Sheetz KH, Zhao L, Holcombe SA, Wang SC, Reddy RM, Lin J, et al. Decreased core muscle size is associated with worse patient survival following esophagectomy for cancer. Dis Esophagus. 2013;26(7):716-22. [CrossRef]
- Lutz CT, Quinn LS. Sarcopenia, obesity, and natural killer cell immune senescence in aging: altered cytokine levels as a common mechanism. Aging (Albany NY). 2012;4(8):535-46. [CrossRef]
- Chen JL, Lee MC, Kuo HC. Reduction of cystometric bladder capacity and bladder compliance with time in patients with end-stage renal disease. J Formos Med Assoc. 2012;111(4):209-13. [CrossRef]
- Hotta K, Miura M, Wada Y, Fukuzawa N, Iwami D, Sasaki H, et al. Atrophic bladder in long-term dialysis patients increases the risk for urological complications after kidney transplantation. Int J Urol. 2017;24(4):314-9. [CrossRef]
- Cheng SF, Jiang YH, Kuo HC. Urothelial Dysfunction and Chronic Inflammation are Associated With Increased Bladder Sensation in Patients With Chronic Renal Insufficiency. Int Neurourol J. 2018;22(Suppl 1):S46-54. [CrossRef]
- Hosseinpour M, Pezeshgi A, Mahdiabadi MZ, Sabzghabaei F, Hajishah H, Mahdavynia S. Prevalence and risk factors of urinary tract infection in kidney recipients: a meta-analysis study. BMC Nephrol. 2023;24(1):284. [CrossRef]
- Pergialiotis V, Papalios T, Haidopoulos D, Papapanagiotou A, Vlachos A, Rodolakis A, et al. Pre-Operative Neutrophil-to-Lymphocyte Ratio as a Predictor of Post-Operative Infectious Morbidity in Gynecologic Oncology Patients. Surg Infect (Larchmt). 2023;24(4):390-6. [CrossRef]
- Mohri Y, Tanaka K, Toiyama Y, Ohi M, Yasuda H, Inoue Y, et al. Impact of Preoperative Neutrophil to Lymphocyte Ratio and Postoperative Infectious Complications on Survival After Curative Gastrectomy for Gastric Cancer: A Single Institutional Cohort Study. Medicine (Baltimore). 2016;95(11):e3125. [CrossRef]
- Lu H, Yu C, Maimaiti M, Li G. The predictive value of perioperative circulating markers on surgical complications in patients undergoing robotic-assisted radical prostatectomy. World J Surg Oncol. 2023;21(1):179. [CrossRef]
- Foley ME, Vinson AJ, Skinner TAA, Kiberd BA, Tennankore KK. The Impact of Combined Warm and Cold Ischemia Time on Post-transplant Outcomes. Can J Kidney Health Dis. 2023;10:20543581231178960. [CrossRef]
- Alshaikh EA, Astor BC, Muth B, Jorgenson M, Swanson K, Garg N, et al. Delayed Graft Function Among Kidney Transplant Recipients Is Associated With an Increased Risk of Urinary Tract Infection and BK Viremia. Transplant Direct. 2023;9(9):e1526. [CrossRef]
- Pontrelli P, Grandaliano G, Van Kooten C. Editorial: Kidney Transplantation and Innate Immunity. Front Immunol. 2020;11:603982. [CrossRef]
- Faba OR, Boissier R, Budde K, Figueiredo A, Hevia V, García EL, et al. European Association of Urology Guidelines on Renal Transplantation: Update 2024. Eur Urol Focus. 2024. [CrossRef]
- Lightner DJ, Wymer K, Sanchez J, Kavoussi L. Best Practice Statement on Urologic Procedures and Antimicrobial Prophylaxis. J Urol. 2020;203(2):351-6. [CrossRef]
- Mitsui M, Sadahira T, Nagasaki N, Maruyama Y, Sekito T, Iwata T, et al. Postoperative infections after robotic-assisted radical prostatectomy in a single large institution: Effect of type and duration of prophylactic antibiotic administration. Int J Urol. 2025;32(3):258-63. [CrossRef]
- Rosado-Canto R, Parra-Avila I, Tejeda-Maldonado J, Kauffman-Ortega C, Rodriguez-Covarrubias FT, Trujeque-Matos M, et al. Perioperative fosfomycin disodium prophylaxis against urinary tract infection in renal transplant recipients: a randomized clinical trial. Nephrol Dial Transplant. 2020;35(11):1996-2003. [CrossRef]
- Velioglu A, Guneri G, Arikan H, Asicioglu E, Tigen ET, Tanidir Y, et al. Incidence and risk factors for urinary tract infections in the first year after renal transplantation. PLoS One. 2021;16(5):e0251036. [CrossRef]
- Sakaeda K, Sadahira T, Maruyama Y, Iwata T, Watanabe M, Wada K, et al. The Genotypic and Phenotypic Characteristics Contributing to Flomoxef Sensitivity in Clinical Isolates of ESBL-Producing E. coli Strains from Urinary Tract Infections. Antibiotics (Basel). 2023;12(3). [CrossRef]
| N | 128 | |||
| Median age (IQR)a | 43 | (32.8-57) | ||
| Median BMIb, kg/m2 (IQR) | 21.7 | (19.2-24.4) | ||
| Median duration of dialysis before LDKTc (n=91), year (IQR) | 1.3 | (0.5-3) | ||
| Pre-LDKT s-albumin,d g/dL (IQR) | 3.9 | (3.5-4.1) | ||
| Pre-LDKT Neutrophil-to-lymphocyte ratio (NLR), (IQR) | 2.7 | (2-3.8) | ||
| Sex, n (%) | ||||
| male | 86 | (67) | ||
| female | 42 | (33) | ||
| ASAe physical status classification, n (%) | ||||
| 2 | 5 | (4) | ||
| 3 | 119 | (93) | ||
| 4 | 4 | (3) | ||
| Diabetes Mellitus, n (%) | 27 | (21) | ||
| Diabetic nephropathy, n (%) | 24 | (19) | ||
| ABO-incompatible, n (%) | 49 | (38) | ||
| Donor-specific antibody, n (%) | 25 | (20) | ||
| Administration of rituximab, n (%) | 90 | (70) | ||
| Plasmapheresis + rituximab before LDKT, n (%) | 74 | (58) | ||
| Pre-emptive kidney transplantation, n (%) | 37 | (29) | ||
| Positive urine culture before surgery, n (%) | 20 | (16) | ||
| Donor | ||||
| Median age (IQR) | 60 | (53-67) | ||
| Expanded criteria donor, n (%) | 69 | (54) |
| N | 128 | ||||
| Type of prophylactic antimicrobials, cases (%) | |||||
| Ampicillin/sulbactam | 9 | (7) | |||
| Cefazolin | 111 | (87) | |||
| Cefotiam | 1 | (1) | |||
| Cefmetazole | 1 | (1) | |||
| Flomoxef | 3 | (2) | |||
| Levofloxacin | 3 | (2) | |||
| Duration of prophylactic antimicrobials, cases (%) | |||||
| Single-dose | 86 | (67) | |||
| Within 72 hours (up to PODa 2) | 42 | (33) | |||
| Median operative time, min (IQR) | 490 | (427-559) | |||
| Median EBLb, mL (IQR) | 178 | (100-318) | |||
| Median WITc, min (IQR) | 5 | (3.3-6.7) | |||
| With retroperitoneal drain, cases (%) | 128 | (100) | |||
| Median duration of placement, days (IQR) | 5 | (4-7) | |||
| Urethral catheter, cases (%) | 128 | (100) | |||
| Median duration of placement, days (IQR) | 7 | (7-8) | |||
| Subcutaneous drain placement, cases (%) | 37 | (29) | |||
| Median duration of placement, days (IQR) | 6 | (4-9) | |||
| Surgical site infection, n (%) | 0 | (0) | |||
| Urinary tract infection, n (%) | 28 | (22) | |||
| Lymphocele, n (%) | 5 | (4) | |||
| Viral infection, n (%) | 2 | (2) | |||
| Fungal infection, n (%) | 1 | (1) | |||
| Delayed graft function, n (%) | 1 | (1) | |||
| Acute rejection within 1-month, n (%) | 2 | (2) | |||
| Graft loss within 1-month, n (%) | 0 | (0) | |||
| 1-month postoperative serum-Creatinine, mg/dl (IQR) | 1.3 | (1.1-1.7) | |||
| Urine samples | Number of isolates | |||
| Escherichia coli | 10 | |||
| Escherichia coli (QRECa) | 4 | |||
| Escherichia coli (ESBLb) | 5 | |||
| Klebsiella pneumoniae | 3 | |||
| Enterobactor cloacae | 3 | |||
| Proteus mirabilis | 2 | |||
| Pseudomonas aeruginosa | 1 | |||
| Citrobactor speies | 1 | |||
| CNSc | 2 |
| Univariate analysis | |||||
|---|---|---|---|---|---|
| Variable | ORa | 95% CIb | P | ||
| Age ( < 45 ) | 0.85 | 0.37-1.97 | 0.708 | ||
| Sex (Female) | 1.18 | 0.49-2.85 | 0.712 | ||
| BMI ( < 20, or 25 < , kg/m2 ) | 3.14 | 1.32-7.46 | 0.01 | ||
| Duration of dialysis before LDKT ( 2.5 < , years) | 2.71 | 1.07-6.89 | 0.036 | ||
| Serum-albumin (< 3.9 , g/dL) | 3.9 | 0.35-1.85 | 0.603 | ||
| ASA ( 3 < ) | 3.77 | 0.51-28.1 | 0.195 | ||
| Diabetes Mellitus | 1.71 | 0.65-4.45 | 0.276 | ||
| Pre-LDKT NLR ( 3≦ ) | 2.63 | 1.11-6.22 | 0.028 | ||
| Administration of rituximab | 2.27 | 0.79-6.49 | 0.128 | ||
| Desensitization ( plasmapheresis + rituximab ) | 3.38 | 1.26-9.06 | 0.015 | ||
| Positive urine culture before LDKT | 0.59 | 0.16-2.16 | 0.422 | ||
| Administration of cefazolin | 5.14 | 0.65-40.6 | 0.12 | ||
| Duration of prophylactic antimicrobials (single-dose) | 0.62 | 0.24-1.6 | 0.322 | ||
| Donor Age (60<) | 2.11 | 0.87-5.11 | 0.098 | ||
| Expanded criteria donor | 1.73 | 0.73-4.11 | 0.215 | ||
| WIT (7.8≦, min) | 2.93 | 1.06-8.12 | 0.038 | ||
| Duration of retroperitoneal drain placement ( 10 < , days) | 1.45 | 0.47-4.5 | 0.515 | ||
| Duration of urethral catheter placement ( 7 < , days) | 0.86 | 0.31-2.38 | 0.777 | ||
| Duration of double-J ureter stent placement ( 18 <, days) | 0.59 | 0.23-1.53 | 0.279 | ||
| Multivariate analysis | ||||||
|---|---|---|---|---|---|---|
| Variable | OR | 95% CI | P | |||
| ModelⅠ | BMI ( < 20, or 25 < , kg/m2 ) | 3 | 1.18-7.63 | 0.021 | ||
| Duration of dialysis before LDKT ( 2.5 < , years) | 2.88 | 1.04-8 | 0.042 | |||
| Desensitization ( plasmapheresis + rituximab ) | 3.54 | 1.24-10.1 | 0.018 | |||
| Pre-LDRT NLR ( 3≦ ) | 2.06 | 0.82-5.21 | 0.126 | |||
| AUC = 0.759 | ||||||
| ModelⅡ | BMI ( < 20, or 25 < , kg/m2 ) | 3.35 | 1.3-8.6 | 0.012 | ||
| Duration of dialysis before LDKT ( 2.5 < , years) | 3.15 | 1.1-9 | 0.031 | |||
| Desensitization ( plasmapheresis + rituximab ) | 4.31 | 1.46-12.8 | 0.008 | |||
| WIT (7.8≦, min) | 3.53 | 1.1-11.3 | 0.033 | |||
| AUC = 0.764 | ||||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





