Submitted:
23 July 2025
Posted:
24 July 2025
You are already at the latest version
Abstract
Keywords:
Introduction
2. Materials and Methods
2.1. Human Subjects
2.2. Polyclonal B cell Stimulation
2.3. Recombinant Proteins
2.4. B cell ImmunoSpot® Assays
2.4.1. Multiplexed antigen-specific FluoroSpot assays with affinity capture coating
2.4.2. Multiplexed pan Ig class and subclass detection
2.4.3. Single-color inverted FluoroSpot assays for detection of SARS-CoV-2 S-reactive IgG+ ASC
2.4.4. FluoroSpot Image Acquisition and SFU Counting
2.5. Statistical Methods
3. Results and Discussion
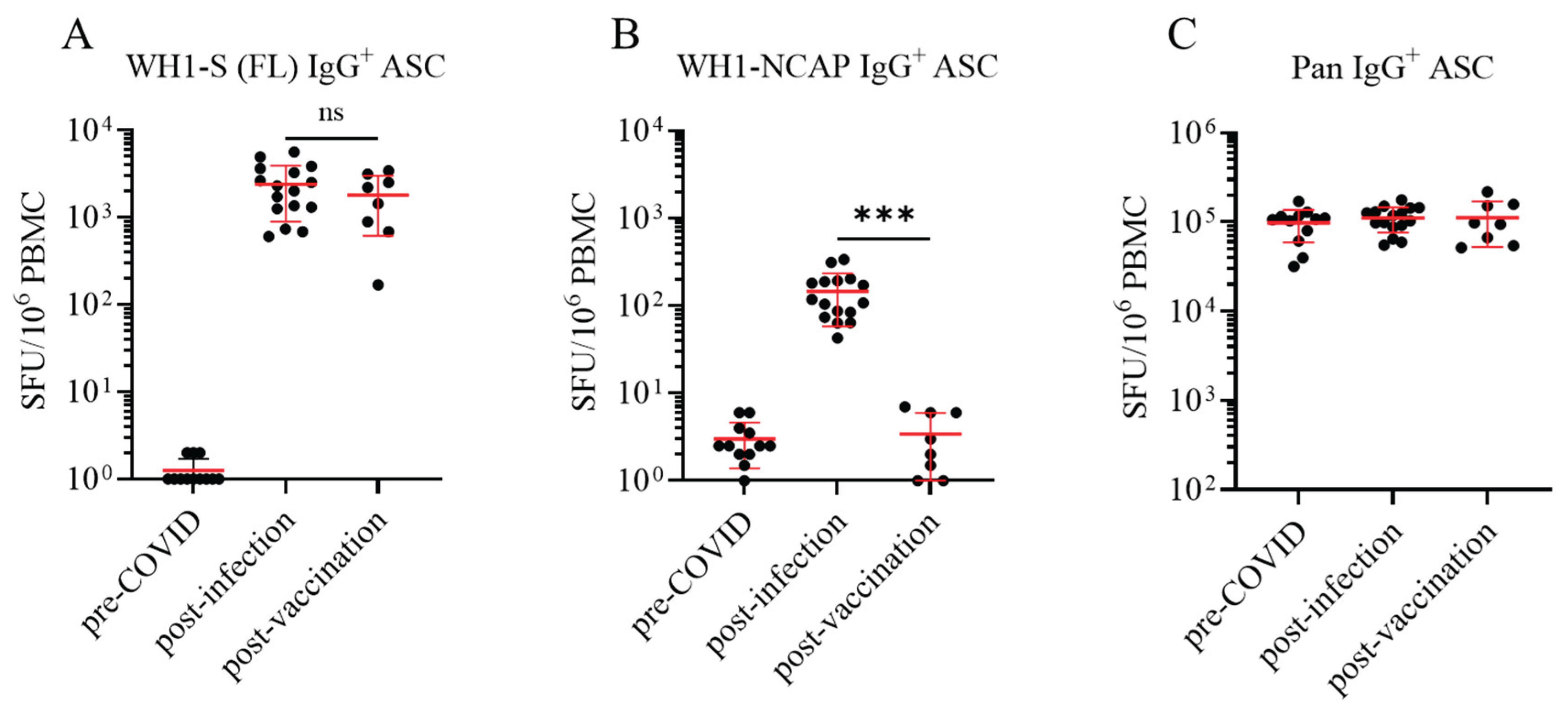
3.1. Establishing the frequency of SARS-CoV-2 Spike (S)- and Nucleocapsid (NCAP)-reactive IgG+ Bmem in defined PBMC cohorts
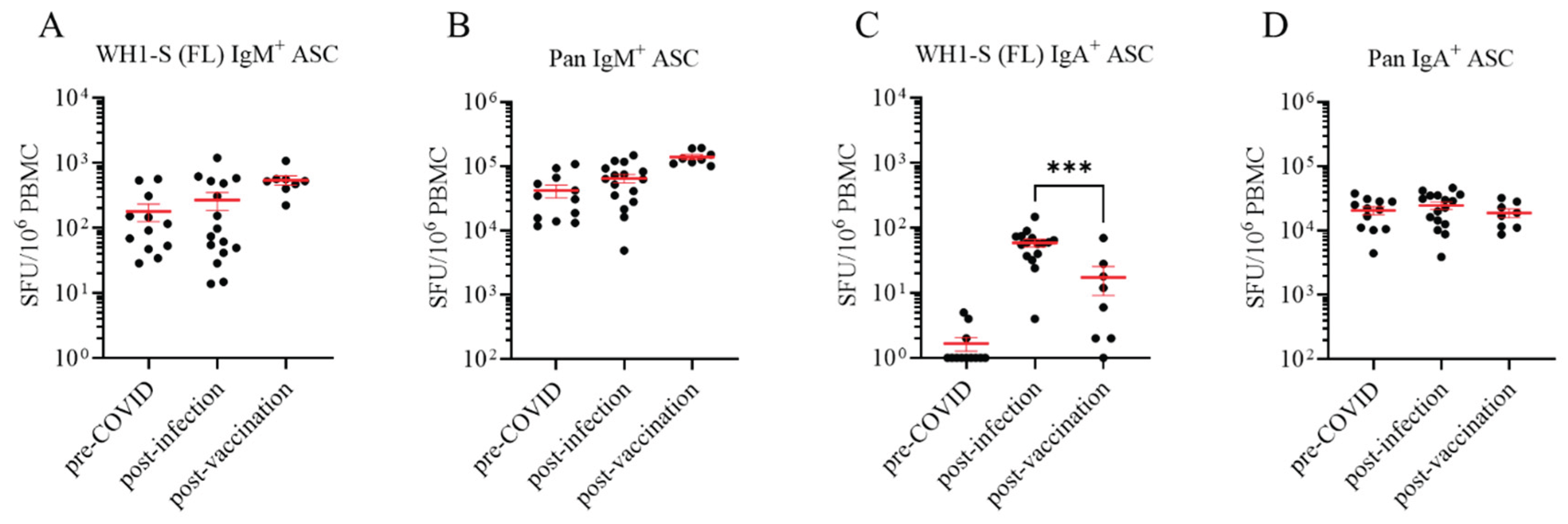
3.2. Defining Ig classes of Bmem elicited by natural infection vs. vaccination
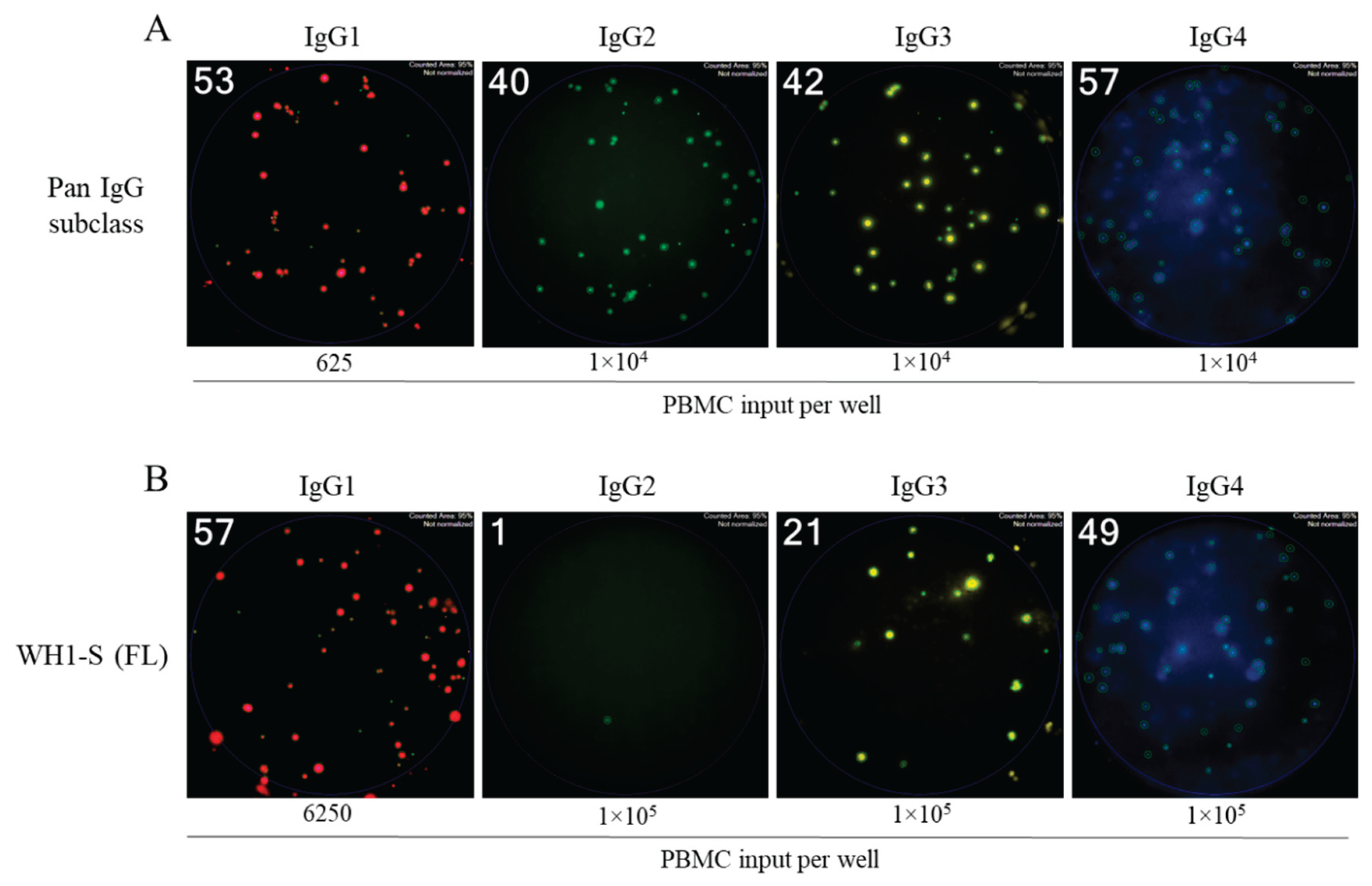
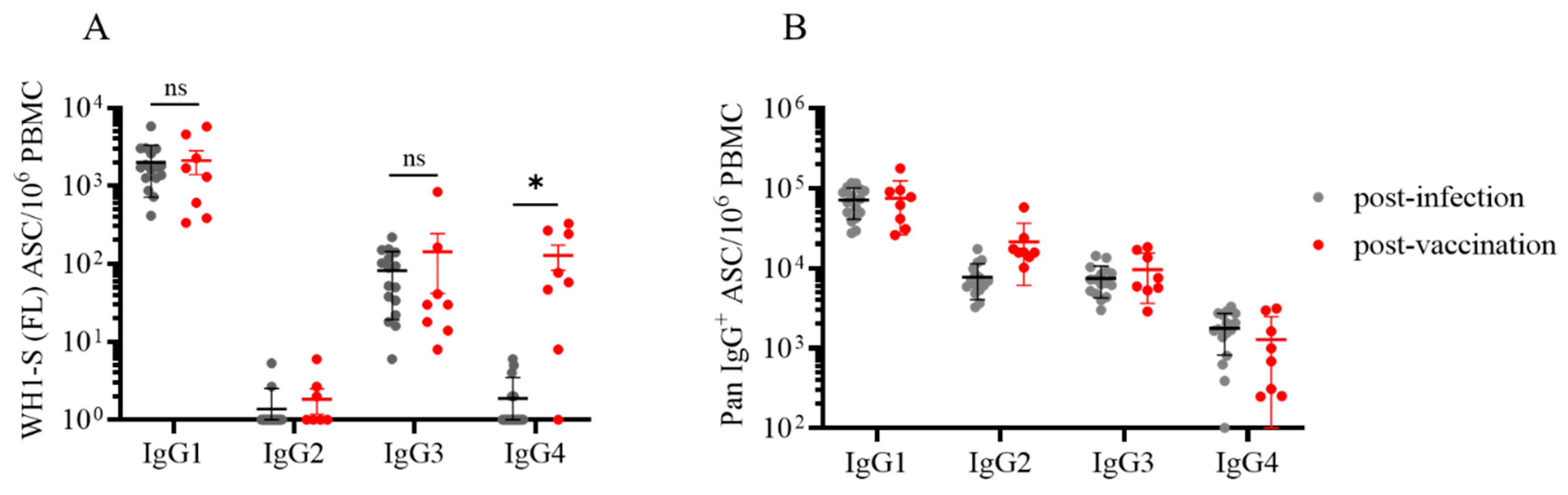
3.3. Dissecting IgG subclass utilization of WH1-S (FL)-reactive Bmem in infected or vaccinated subjects
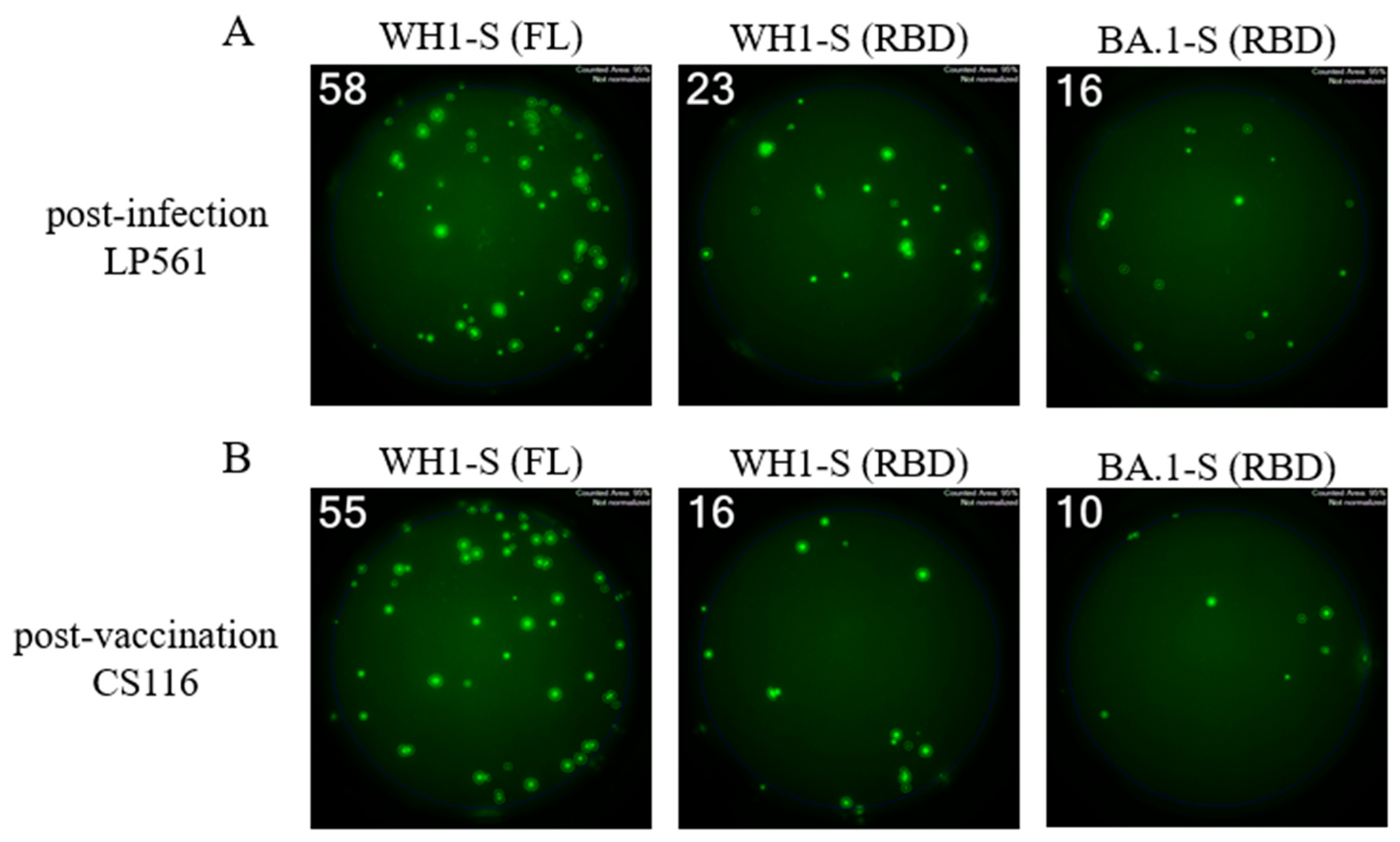
3.4. Characterization of RBD-reactive Bmem induced in infected or vaccinated subjects
3.5. Comparing Omicron cross-reactive Bmem in WH1 vaccinated vs. WH1 infected individuals
4. Summary and conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Randall, T.D. and R.E. Mebius, The development and function of mucosal lymphoid tissues: a balancing act with micro-organisms. Mucosal Immunol, 2014. 7(3): p. 455-66. [CrossRef]
- Bemark, M. and D. Angeletti, Know your enemy or find your friend?-Induction of IgA at mucosal surfaces. Immunol Rev, 2021. 303(1): p. 83-102. [CrossRef]
- Giri, S., et al., Quantity of Vaccine Poliovirus Shed Determines the Titer of the Serum Neutralizing Antibody Response in Indian Children Who Received Oral Vaccine. J Infect Dis, 2018. 217(9): p. 1395-1398. [CrossRef]
- Long, Q.X., et al., Antibody responses to SARS-CoV-2 in patients with COVID-19. Nat Med, 2020. 26(6): p. 845-848. [CrossRef]
- Pichilingue-Reto, P., et al., Serum IgG Profiling of Toddlers Reveals a Subgroup with Elevated Seropositive Antibodies to Viruses Correlating with Increased Vaccine and Autoantigen Responses. J Clin Immunol, 2021. 41(5): p. 1031-1047. [CrossRef]
- Vidarsson, G., G. Dekkers, and T. Rispens, IgG subclasses and allotypes: from structure to effector functions. Front Immunol, 2014. 5: p. 520. [CrossRef]
- Duchemin, M., et al., Antibody-Dependent Cellular Phagocytosis of HIV-1-Infected Cells Is Efficiently Triggered by IgA Targeting HIV-1 Envelope Subunit gp41. Front Immunol, 2020. 11: p. 1141. [CrossRef]
- Russell, M.W. and B. Mansa, Complement-fixing properties of human IgA antibodies. Alternative pathway complement activation by plastic-bound, but not specific antigen-bound, IgA. Scand J Immunol, 1989. 30(2): p. 175-83. [CrossRef]
- Steffen, U., et al., IgA subclasses have different effector functions associated with distinct glycosylation profiles. Nat Commun, 2020. 11(1): p. 120. [CrossRef]
- Hufnagl, K., et al., Intranasal tolerance induction with polypeptides derived from 3 noncross-reactive major aeroallergens prevents allergic polysensitization in mice. J Allergy Clin Immunol, 2005. 116(2): p. 370-6. [CrossRef]
- Tsitoura, D.C., et al., Intranasal exposure to protein antigen induces immunological tolerance mediated by functionally disabled CD4+ T cells. J Immunol, 1999. 163(5): p. 2592-600. [CrossRef]
- Unger, W.W., et al., Nasal tolerance induces antigen-specific CD4+CD25- regulatory T cells that can transfer their regulatory capacity to naive CD4+ T cells. Int Immunol, 2003. 15(6): p. 731-9. [CrossRef]
- Tordesillas, L. and M.C. Berin, Mechanisms of Oral Tolerance. Clin Rev Allergy Immunol, 2018. 55(2): p. 107-117. [CrossRef]
- Butcher, M.J. and J. Zhu, Recent advances in understanding the Th1/Th2 effector choice. Fac Rev, 2021. 10: p. 30. [CrossRef]
- Duan, T., et al., Toll-Like Receptor Signaling and Its Role in Cell-Mediated Immunity. Front Immunol, 2022. 13: p. 812774. [CrossRef]
- Forsthuber, T., H.C. Yip, and P.V. Lehmann, Induction of TH1 and TH2 immunity in neonatal mice. Science, 1996. 271(5256): p. 1728-30. [CrossRef]
- Gottwein, J.M., et al., Protective anti-Helicobacter immunity is induced with aluminum hydroxide or complete Freund’s adjuvant by systemic immunization. J Infect Dis, 2001. 184(3): p. 308-14. [CrossRef]
- Stavnezer, J. and C.E. Schrader, IgH chain class switch recombination: mechanism and regulation. J Immunol, 2014. 193(11): p. 5370-8. [CrossRef]
- de Taeye, S.W., et al., FcgammaR Binding and ADCC Activity of Human IgG Allotypes. Front Immunol, 2020. 11: p. 740. [CrossRef]
- Koenig, J.F.E., et al., Type 2-polarized memory B cells hold allergen-specific IgE memory. Sci Transl Med, 2024. 16(733): p. eadi0944. [CrossRef]
- Viana, R., et al., Rapid epidemic expansion of the SARS-CoV-2 Omicron variant in southern Africa. Nature, 2022. 603(7902): p. 679-686. [CrossRef]
- Berber, E. and T.M. Ross, Factors Predicting COVID-19 Vaccine Effectiveness and Longevity of Humoral Immune Responses. Vaccines (Basel), 2024. 12(11). [CrossRef]
- Post, N., et al., Antibody response to SARS-CoV-2 infection in humans: A systematic review. PLoS One, 2020. 15(12): p. e0244126. [CrossRef]
- Akkaya, M., K. Kwak, and S.K. Pierce, B cell memory: building two walls of protection against pathogens. Nat Rev Immunol, 2020. 20(4): p. 229-238. [CrossRef]
- Palm, A.E. and C. Henry, Remembrance of Things Past: Long-Term B Cell Memory After Infection and Vaccination. Front Immunol, 2019. 10: p. 1787. [CrossRef]
- Matz, H.C., K.M. McIntire, and A.H. Ellebedy, ‘Persistent germinal center responses: slow-growing trees bear the best fruits’. Curr Opin Immunol, 2023. 83: p. 102332. [CrossRef]
- Kirchenbaum, G.A., G. Pawelec, and P.V. Lehmann, The Importance of Monitoring Antigen-Specific Memory B Cells, and How ImmunoSpot Assays Are Suitable for This Task. Cells, 2025. 14(3). [CrossRef]
- Smith, S.A. and J.E. Crowe, Jr., Use of Human Hybridoma Technology To Isolate Human Monoclonal Antibodies. Microbiol Spectr, 2015. 3(1): p. AID-0027-2014. [CrossRef]
- Boonyaratanakornkit, J. and J.J. Taylor, Techniques to Study Antigen-Specific B Cell Responses. Front Immunol, 2019. 10: p. 1694. [CrossRef]
- Koppert, S., et al., Affinity Tag Coating Enables Reliable Detection of Antigen-Specific B Cells in Immunospot Assays. Cells, 2021. 10(8). [CrossRef]
- Lehmann, P.V., et al., Theoretical and practical considerations for validating antigen-specific B cell ImmunoSpot assays. J Immunol Methods, 2025. 537: p. 113817. [CrossRef]
- Becza, N., et al., Optimizing PBMC Cryopreservation and Utilization for Immunospot® Analysis of Antigen-Specific Memory B Cells. Vaccines, 2025. 13(7): p. 765. [CrossRef]
- Yao, L., et al., Four-Color ImmunoSpot((R)) Assays Requiring Only 1-3 mL of Blood Permit Precise Frequency Measurements of Antigen-Specific B Cells-Secreting Immunoglobulins of All Four Classes and Subclasses. Methods Mol Biol, 2024. 2768: p. 251-272. [CrossRef]
- Pinna, D., et al., Clonal dissection of the human memory B-cell repertoire following infection and vaccination. Eur J Immunol, 2009. 39(5): p. 1260-70. [CrossRef]
- Hsieh, C.L., et al., Structure-based design of prefusion-stabilized SARS-CoV-2 spikes. Science, 2020. 369(6510): p. 1501-1505. [CrossRef]
- Resources, B., Vector pCAGGS Containing the SARS-Related Coronavirus 2, Wuhan-Hu-1 Spike Glycoprotein Receptor Binding Domain (RBD), NR-52309.
- Karulin, A.Y., et al., Artificial Intelligence-Based Counting Algorithm Enables Accurate and Detailed Analysis of the Broad Spectrum of Spot Morphologies Observed in Antigen-Specific B-Cell ELISPOT and FluoroSpot Assays. Methods Mol Biol, 2024. 2768: p. 59-85. [CrossRef]
- Gaunt, E.R., et al., Epidemiology and clinical presentations of the four human coronaviruses 229E, HKU1, NL63, and OC43 detected over 3 years using a novel multiplex real-time PCR method. J Clin Microbiol, 2010. 48(8): p. 2940-7. [CrossRef]
- Wolf, C., et al., Antibody Levels Poorly Reflect on the Frequency of Memory B Cells Generated following SARS-CoV-2, Seasonal Influenza, or EBV Infection. Cells, 2022. 11(22). [CrossRef]
- Holshue, M.L., et al., First Case of 2019 Novel Coronavirus in the United States. N Engl J Med, 2020. 382(10): p. 929-936. [CrossRef]
- Vettermann, C. and M.S. Schlissel, Allelic exclusion of immunoglobulin genes: models and mechanisms. Immunol Rev, 2010. 237(1): p. 22-42. [CrossRef]
- Rispens, T. and M.G. Huijbers, The unique properties of IgG4 and its roles in health and disease. Nat Rev Immunol, 2023. 23(11): p. 763-778. [CrossRef]
- Uversky, V.N., et al., IgG4 Antibodies Induced by Repeated Vaccination May Generate Immune Tolerance to the SARS-CoV-2 Spike Protein. Vaccines (Basel), 2023. 11(5). [CrossRef]
- Aurelia, L.C., et al., Increased SARS-CoV-2 IgG4 has variable consequences dependent upon Fc function, Fc receptor polymorphism, and viral variant. Sci Adv, 2025. 11(9): p. eads1482. [CrossRef]
- Gelderloos, A.T., et al., Repeated COVID-19 mRNA vaccination results in IgG4 class switching and decreased NK cell activation by S1-specific antibodies in older adults. Immun Ageing, 2024. 21(1): p. 63. [CrossRef]
- Martin Perez, C., et al., Post-vaccination IgG4 and IgG2 class switch associates with increased risk of SARS-CoV-2 infections. J Infect, 2025. 90(4): p. 106473. [CrossRef]
- Murin, C.D., I.A. Wilson, and A.B. Ward, Antibody responses to viral infections: a structural perspective across three different enveloped viruses. Nat Microbiol, 2019. 4(5): p. 734-747. [CrossRef]
- Pantaleo, G., et al., Antibodies to combat viral infections: development strategies and progress. Nat Rev Drug Discov, 2022. 21(9): p. 676-696. [CrossRef]
- Morales-Nunez, J.J., et al., Overview of Neutralizing Antibodies and Their Potential in COVID-19. Vaccines (Basel), 2021. 9(12). [CrossRef]
- Lan, J., et al., Structure of the SARS-CoV-2 spike receptor-binding domain bound to the ACE2 receptor. Nature, 2020. 581(7807): p. 215-220. [CrossRef]
- Walls, A.C., et al., Structure, Function, and Antigenicity of the SARS-CoV-2 Spike Glycoprotein. Cell, 2020. 181(2): p. 281-292 e6. [CrossRef]
- Farrell, A.G., et al., Receptor-Binding Domain (RBD) Antibodies Contribute More to SARS-CoV-2 Neutralization When Target Cells Express High Levels of ACE2. Viruses, 2022. 14(9). [CrossRef]
- Suthar, M.S., et al., Rapid Generation of Neutralizing Antibody Responses in COVID-19 Patients. Cell Rep Med, 2020. 1(3): p. 100040. [CrossRef]
- Ferrari, D., et al., Evaluation of antibody titer kinetics and SARS-CoV-2 infections in a large cohort of healthcare professionals ten months after administration of the BNT162b2 vaccine. J Immunol Methods, 2022. 506: p. 113293. [CrossRef]
- Jo, D.H., et al., Rapidly Declining SARS-CoV-2 Antibody Titers within 4 Months after BNT162b2 Vaccination. Vaccines (Basel), 2021. 9(10). [CrossRef]
- Levin, E.G., et al., Waning Immune Humoral Response to BNT162b2 Covid-19 Vaccine over 6 Months. N Engl J Med, 2021. 385(24): p. e84. [CrossRef]
- Seow, J., et al., Longitudinal observation and decline of neutralizing antibody responses in the three months following SARS-CoV-2 infection in humans. Nat Microbiol, 2020. 5(12): p. 1598-1607. [CrossRef]
- Xiang, T., et al., Declining Levels of Neutralizing Antibodies Against SARS-CoV-2 in Convalescent COVID-19 Patients One Year Post Symptom Onset. Front Immunol, 2021. 12: p. 708523. [CrossRef]
- Evans, J.P., et al., Neutralization of SARS-CoV-2 Omicron sub-lineages BA.1, BA.1.1, and BA.2. Cell Host Microbe, 2022. 30(8): p. 1093-1102 e3. [CrossRef]
- Kumar, S., K. Karuppanan, and G. Subramaniam, Omicron (BA.1) and sub-variants (BA.1.1, BA.2, and BA.3) of SARS-CoV-2 spike infectivity and pathogenicity: A comparative sequence and structural-based computational assessment. J Med Virol, 2022. 94(10): p. 4780-4791. [CrossRef]
- Kaku, C.I., et al., Recall of preexisting cross-reactive B cell memory after Omicron BA.1 breakthrough infection. Sci Immunol, 2022. 7(73): p. eabq3511. [CrossRef]
- Quandt, J., et al., Omicron BA.1 breakthrough infection drives cross-variant neutralization and memory B cell formation against conserved epitopes. Sci Immunol, 2022. 7(75): p. eabq2427. [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).




