Submitted:
21 July 2025
Posted:
22 July 2025
You are already at the latest version
Abstract

Keywords:
1. Introduction
2. Materials and Methods
2.1. Plant Material
2.2. Experiment Design
2.3. The Extraction Methods
2.4. Measurement and Sampling
2.5. Vegetative Traits
2.6. Total Chlorophyll Content
2.7. Total Phenol and Flavonoid Content
2.8. Proline Content
2.9. Catalase (CAT) and Phenylalanine Ammonia-Lyase (PAL) Enzyme Activity
2.10. Antioxidant Activity by DPPH and FRAP Methods
2.11. Sodium and Potassium Content
2.12. Identification of Essential Oil Compounds with GC-MS
2.14. Data Analysis and Statistical Calculations
3. Results
3.1. Vegetative Traits
3.2. Total Chlorophyll
3.3. Sodium (Na) and Potassium (K) Content
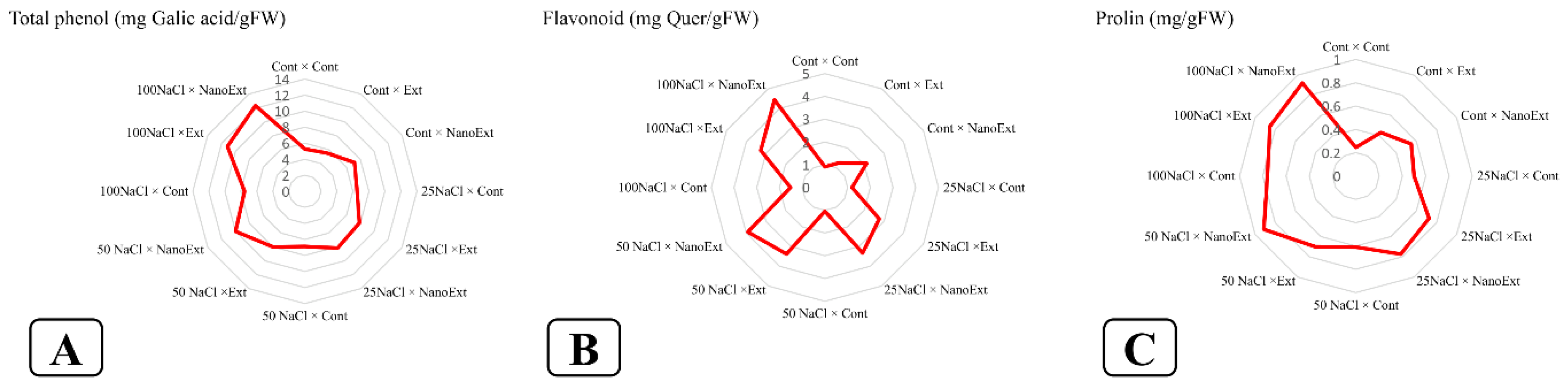
3.4. Total Phenols and Flavonoid Content
3.5. Proline
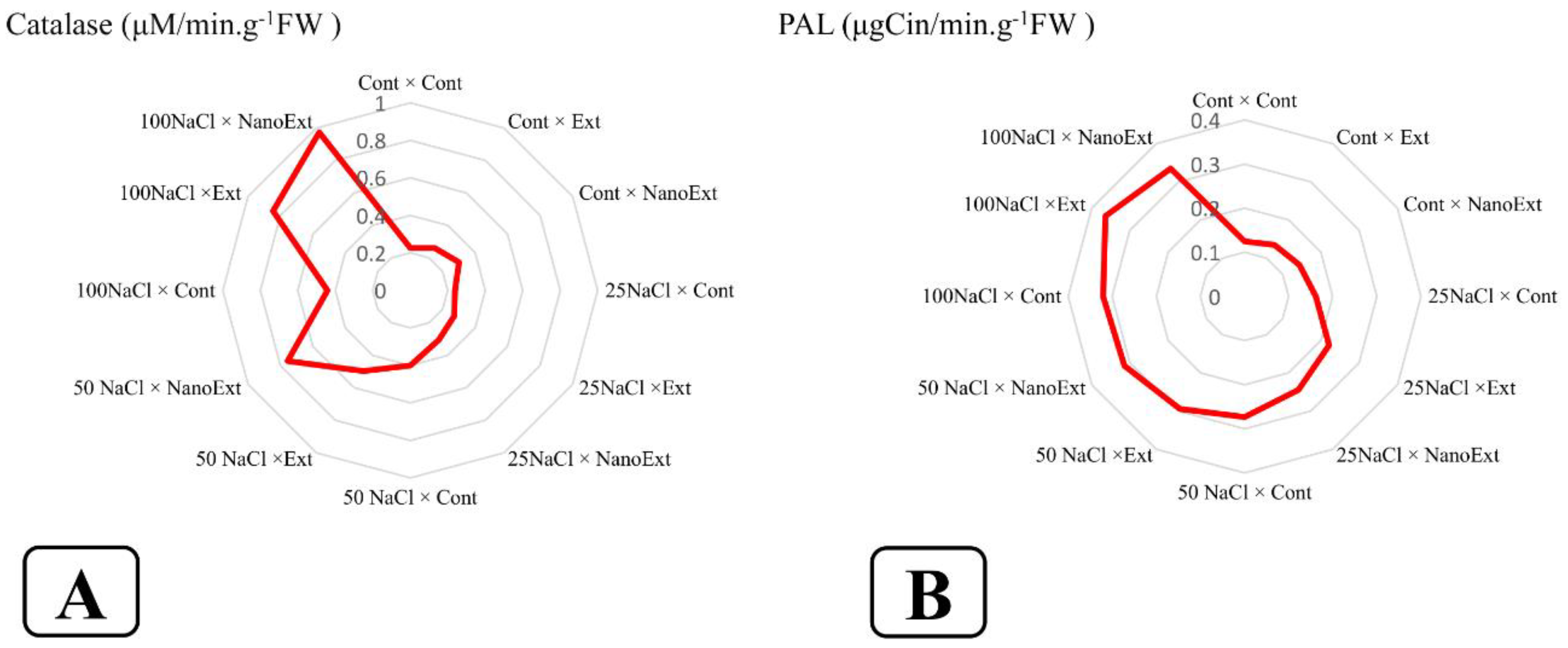
3.6. Catalase Enzyme and Phenolalanine Ammonialyase (PAL) Enzyme
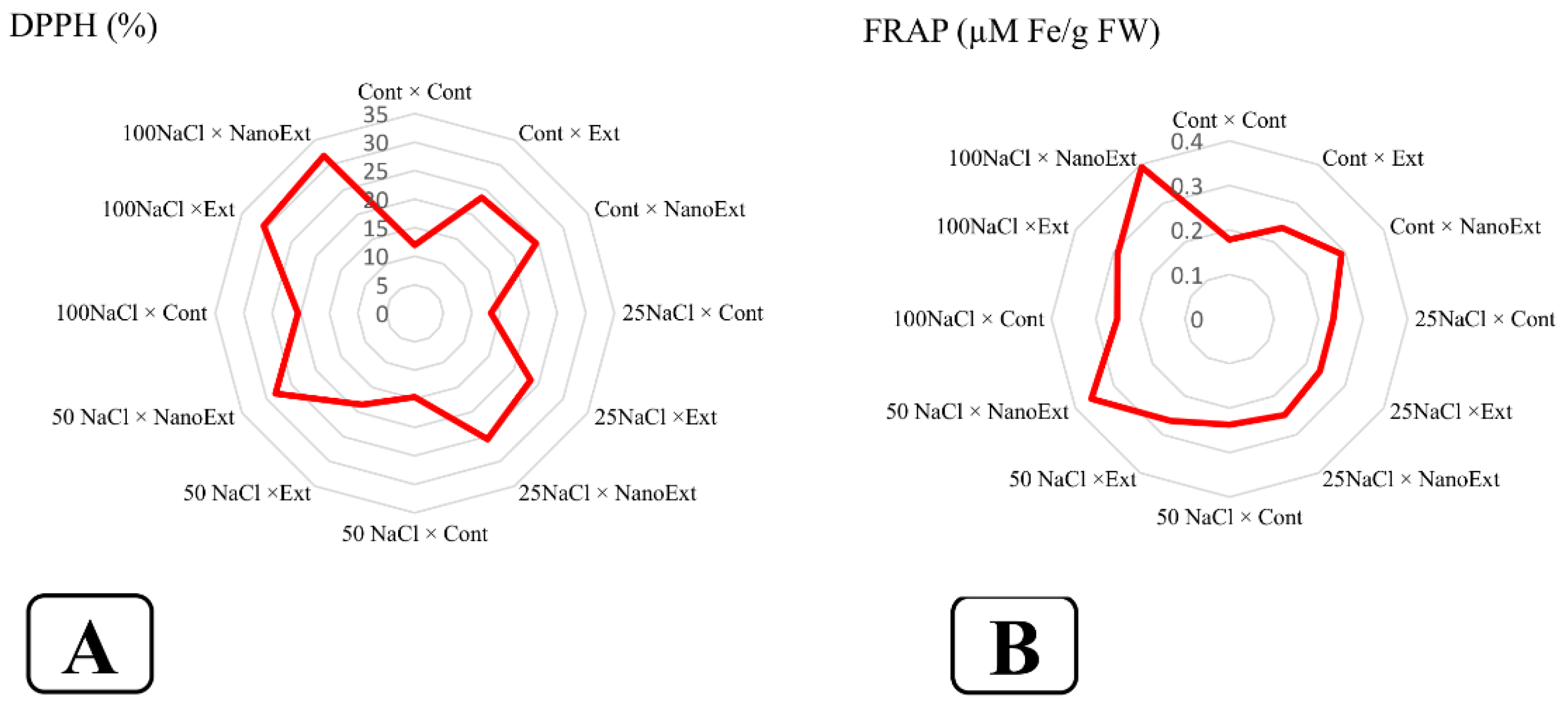
3.7. Antioxidant Activity (DPPH and FARP)
3.8. Identification of Essential Oil Compounds with GC-MS
3.9. Correlation and Principal Component Analysis (PCA)
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| PAL | Phenol Alanine Ammonia Lyase |
| ROS | Reactive Oxygen Species |
| CAT | Catalase enzyme |
| MDA | Malondialdehyde |
References
- Formisano, L.; Ciriello, M.; E.l-Nakhel, C.; Kyriacou, M.C.; Rouphael, Y. Successive harvests modulate the productive and physiological behavior of three genovese pesto basil cultivars. Agronomy. 2021, 11, 560. [CrossRef]
- Makri, O.; Kintzios, S. Ocimum sp. (basil): Botany, cultivation, pharmaceutical properties, and biotechnology. J. Herbs Spices Med. Plants. 2008, 13, 123–150. [Google Scholar] [CrossRef]
- Onofrei, V.; Benchennouf, A.; Jancheva, M.; Loupassaki, S.; Ouaret, W.; Burducea, M.; Lobiuc, A.; Teliban, G.C.; Robu, T. Ecological foliar fertilization effects on essential oil composition of sweet basil (Ocimum basilicum L.) cultivated in a field system. Sci. Hortic. 2018, 239, 104–113. [Google Scholar] [CrossRef]
- Shabani, E.; Bolandnazar, S.; Tabatabaei, S.J. Magnetized nutrient solution and arbuscular mycorrhizal affect essential oil and physiological aspects of sweet basil (Ocimum basilicum L.) grown in various P concentrations. J. Plant Nutr. 2022, 45, 883–895. [Google Scholar] [CrossRef]
- Jakovljević, D.; Momčilović, J.; Bojović, B.; Stanković, M. The Short-Term Metabolic Modulation of Basil (Ocimum basilicum L. cv.’Genovese’) after Exposure to Cold or Heat. Plants. 2021, 10, 590. [Google Scholar] [CrossRef] [PubMed]
- Dudai, N.; Belanger, F.C. Aroma as a factor in the Breeding Process of Fresh Herbs---The case of basil. Biotechnology in Flavor Production. 2009, 161–184. [Google Scholar] [CrossRef]
- Bahcesular, B.; Yildirim, E.D.; Karaçocuk, M.; Kulak, M.; Karaman, S. Seed priming with melatonin effects on growth, essential oil compounds and antioxidant activity of basil (Ocimum basilicum L.) under salinity stress. Ind Crops Prod. 2020, 146, 112165. [CrossRef]
- Maggio, A.; Roscigno, G.; Bruno, M.; De Falco, E.; Senatore, F. Essential-Oil Variability in a Collection of Ocimum basilicum L. Cultivars. Chem Biodivers. 2016, 13, 1357–1368. [Google Scholar] [CrossRef]
- Ciriello, M.; Formisano, L.; El-Nakhel, C.; Kyriacou, M.C.; Soteriou, G.A.; Pizzolongo, F.; Romano, R.; De Pascale, S.; Rouphael, Y. Genotype and successive harvests interaction affects phenolic acids and aroma profile of genovese basil for pesto sauce production. Foods. 2021, 10, 278. [Google Scholar] [CrossRef]
- Trettel, J.R.; Nascimento, A.B.; Barbosa, L.N.; Magalhaes, H.M. ‘In vitro’organogenesis and growth of’ Ocimum basilicum’’Genovese’(basil) cultivated with growth regulators. Aust. J. Crop Sci. 2019, 13, 1131–1140. [Google Scholar] [CrossRef]
- Bauer, K.; Garbe, D.; Surburg, H. Common fragrance and flavor materials: Preparation, properties and uses. John Wiley and Sons, 2008.
- Park, M.J.; Gwak, K.S.; Yang, I.; Kim, K.W.; Jeung, E.B.; Chang, J.W.; Choi, I.G. ; Effect of citral, eugenol, nerolidol and α-terpineol on the ultrastructural changes of Trichophyton rubrum. Fitoterapia. 2012, 83, 410–416. [Google Scholar] [CrossRef]
- Saia, S.; Corrado, G.; Vitaglione, P.; Colla, G.; Bonini, P.; Giordano, M.; Fiorillo, A.; Rouphael, Y. An Endophytic Fungi-Based Biostimulant Modulates Volatile and Non-Volatile Secondary Metabolites and Yield of Greenhouse Basil (Ocimum basilicum L.) through Variable Mechanisms Dependent on Salinity Stress Level. Pathogens. 2021, 10, 797. [Google Scholar] [CrossRef]
- Kanber, R.; Elhindi, K.M.; Alotaibi, M.A. Silicon supplementation mitigates salinity stress on Ocimum basilicum L. via improving water balance, ion homeostasis, and antioxidant defense system. cotoxicol. Environ. Saf 2020, 206, 111396. [Google Scholar] [CrossRef]
- .
- Kulak, M.; Gul, F.; Sekeroglu, N. Changes in growth parameter and essential oil composition of sage (Salvia officinalis L.) leaves in response to various salt stresses. Ind Crops Prod. 2020, 145, 112078. [Google Scholar] [CrossRef]
- Dias, N.S.; Fernandes, C.S.; Sousa-Neto, O.N.; Silva, C.R.; Ferreira, J.F.S.; Sa, F.V.S.; Cosme, C.R.; Souza, A.C.M.S.; Oliveira, A.M.; Batista, C.N.O. Potential agricultural use of reject brine from desalination plants in family farming areas. Saline and Alkaline Soils in Latin America. Natural Resources, Management and Productive Alternatives; 2021, 101–118. [CrossRef]
- Ghaemi, A.A.; Salimi, M.H.; Tabarza, A. The interaction of fishery effluent and plant residues on the yield and water consumption efficiency of cherry tomatoes under drip irrigation system in the greenhouse. J. Plant Interact. 2016, 8, 41–49. [Google Scholar] [CrossRef]
- Alexopoulos, A.A.; Marandos, E.; Assimakopoulou, A.; Vidalis, N.; Petropoulos, S.A.; Karapanos, I.C. Effect of nutrient solution pH on the growth, yield, and quality of Taraxacum officinale and Reichardia picroides in a floating hydroponic system. Agronomy. 2021, 11, 1118. [Google Scholar] [CrossRef]
- Sardare, M.D.; Admane, S.A. Review on plant without soil-hydroponics. IJRET. 2013, 02, 299–304. [Google Scholar]
- Liu, J.; Wang, F.; Liu, W.; Tang, C.; Wu, C.; Wu, Y. Nutrient removal by up-scaling a hybrid floating treatment bed (HFTB) using plant and periphyton: From laboratory tank to polluted river. Bioresour. Technol. 2016, 207, 142–149. [Google Scholar] [CrossRef] [PubMed]
- Wang, P.H. Nitrogen removal during the cold season by constructed floating wetlands planted with Oenanthe javanica. Mar. Freshw. Res. 2018, 69, 635–647. [Google Scholar] [CrossRef]
- Ge, Z. .G.; Feng, C.M.; Wang, X.P.; Zhang, J.B. Seasonal applicability of three vegetation constructed floating treatment wetlands for nutrient removal and harvesting strategy in urban stormwater retention ponds. INT BIODETER BIODEGR. 2016, 112, 80–87. [Google Scholar] [CrossRef]
- Headley, T.R.; Tanner, C.C. Constructed wetlands with floating emergent macrophytes: An innovative stormwater treatment technology. rit Rev Environ Sci Technol. 2012, 42, 2261–2310. [Google Scholar] [CrossRef]
- Giménez, A.; Fernández, J.A.; Pascual, J.A.; Ros, M.; López-Serrano, M.; Egea-Gilabert, C. An agroindustrial compost as alternative to peat for production of baby leaf red lettuce in a floating system. Scientia Horticulturae. 2019, 246, 907–915. [Google Scholar] [CrossRef]
- Khater, E.S.; Bahnasawy, A.; Abass, W.; Morsy, O.; El-Ghobashy, H.; Shaban, Y.; Egela, M. Production of basil (Ocimum basilicum L.) under different soilless cultures. Adv. Environ. Sci. Technol. 2021, 11, 12754. [Google Scholar] [CrossRef]
- Moncada, A.; Miceli, A.; Vetrano, F. Use of plant growth-promoting rhizobacteria (PGPR) and organic fertilization for soilless cultivation of basil. Sci. Hortic. 2021, 275, 109733. [Google Scholar] [CrossRef]
- Rakocy, J.; Shultz, R.C.; Bailey, D.S.; Thoman, E.S. Aquaponic production of tilapia and basil: Comparing a batch and staggered cropping system. SPSCC. 2003, 648, 63–69. [Google Scholar] [CrossRef]
- Stoia, M.; Oancea, S. Low-molecular-weight synthetic antioxidants: Classification, pharmacological profile, effectiveness and trends. Antioxidants. 2022, 11, 638. [Google Scholar] [CrossRef]
- Rathee, P.; Sehrawat, R.; Rathee, P.; Khatkar, A.; Akkol, E.K.; Khatkar, S.; Sobarzo-Sánchez, E. Polyphenols: Natural preservatives with promising applications in food, cosmetics and pharma industries; problems and toxicity associated with synthetic preservatives; impact of misleading advertisements; recent trends in preservation and legislation. Materials. 2023, 16, 4793. [Google Scholar] [CrossRef] [PubMed]
- Rathee-Gomes, E.A.; del, C.; Mejia-da-Silva, L.; Pérez-Mora, L.S.; Matsudo, M.C.; Ferreira-Camargo, L.S.; Singh, A.K.; de Carvalho, J.C.M. Potential of microalgae carotenoids for industrial application. Appl. Biochem. Biotechnol. 2019, 188, 602–634. [Google Scholar] [CrossRef] [PubMed]
- Shahid, I.; Mehnaz, S. Microbial secondary metabolites: Effectual armors to improve stress survivability in crop plants. In Microbial Services in Restoration Ecology; 2020, pp. 47–70. [CrossRef]
- Dutta, S.; Ray, S. Comparative assessment of total phenolic content and in vitro antioxidant activities of bark and leaf methanolic extracts of Manilkara hexandra (Roxb.) Dubard. J. King Saud Univ. Sci. 2020, 32, 643–647. [Google Scholar] [CrossRef]
- Agarwal, S.; Pandey, V. Antioxidant enzyme responses to NaCl stress in Cassia angustifolia. Biol. Plant. 2004, 48, 555–560. [Google Scholar] [CrossRef]
- Kumaran, A. Antioxidant and free radical scavenging activity of an aqueous extract of Coleus aromaticus. Food Chem. 2006, 97, 109–114. [Google Scholar] [CrossRef]
- Stoilova, I.; Krastanov, A.; Stoyanova, A.; Denev, P.; Gargova, S. Antioxidant activity of a ginger extract (Zingiber officinale). Food Chem. 2007, 102, 764–770. [Google Scholar] [CrossRef]
- Ali, E.; Hassan, F.; Elgimabi, M. Improving the growth, yield and volatile oil content of Pelargonium graveolens L. Herit by foliar application with moringa leaf extract through motivating physiological and biochemical parameters. S Afr J Bot. 2018, 119, 383–389. [Google Scholar] [CrossRef]
- Diab, F.; Khalil, M.; Lupidi, G.; Zbeeb, H.; Salis, A.; Damonte, G.; Bramucci, M.; Portincasa, P.; Vergani, L. Influence of simulated in vitro gastrointestinal digestion on the phenolic profile, antioxidant, and biological activity of Thymbra Spicata L. extracts. Antioxidants. 2022, 11, 1778. [Google Scholar] [CrossRef] [PubMed]
- Solgi, M.; Bagnazari, M.; Mohammadi, M.; Azizi, A. Thymbra spicata extract and arbuscular mycorrhizae improved the morphophysiological traits, biochemical properties, and essential oil content and composition of Rosemary (Rosmarinus officinalis L.) under salinity stress. BMC Plant Biol. 2025, 25, 220. [Google Scholar] [CrossRef] [PubMed]
- Zheng, W.; Wang, S.Y. Antioxidant activity and phenolic compounds in selected herbs. JAFC. 2001, 49, 5165–5170. [Google Scholar] [CrossRef] [PubMed]
- Bonesi, M.; Loizzo, M.R.; Acquaviva, R.; Malfa, G.A.; Aiell, F.; Tundis, R. Anti-inflammatory and antioxidant agents from Salvia genus (Lamiaceae): An assessment of the current state of knowledge. Anti-Inflamm. Anti-Allergy Agents Med. Chem. 2017, 16, 70–86. [CrossRef]
- Bonesi, S.; Pimoradloo, E.; Bonesi, M.; Vessal, M. Evaluation of antioxidant potentials and α-amylase inhibition of different fractions of labiatae plants extracts: As a model of antidiabetic compounds properties. BioMed Research International. 2017, 2017, 7319507. [Google Scholar] [CrossRef]
- Mimica-Dukic, N.; Bozin, B. Mentha L. species (Lamiaceae) as promising sources of bioactive secondary metabolites. Current Pharmaceutical Design. 2008, 14, 3141–3150. [Google Scholar] [CrossRef]
- Ahari, H.; Yousefi, S. Green synthesis of three-component Ag/AgCl/TiO2 nanocomposite using Zataria Multiflora plant. Journal of food science and technology (Iran). 2023, 20, 94–112. [Google Scholar] [CrossRef]
- Golkar, P.; Mosavat, N.; Jalali, S.A.H. Essential oils, chemical constituents, antioxidant, antibacterial and in vitro cytotoxic activity of different Thymus species and Zataria multiflora collected from Iran. S Afr J Bot. 2020, 130, 250–258. [Google Scholar] [CrossRef]
- Akrami, F.; Rodríguez-Lafuente, A.; Bentayeb, K.; Pezo, D.; Ghalebi, S.R.; Nerín, C. Antioxidant and antimicrobial active paper based on Zataria (Zataria multiflora) and two cumin cultivars (Cuminum cyminum). LWT - Food Sci. Technol. 2015, 60, 929–933. [Google Scholar] [CrossRef]
- Pandey, G. Agri-Nanotechnology for sustainable agriculture, in: J. Sustain. Agric. 2020, 229–249. [Google Scholar] [CrossRef]
- Hofmann, T.; Lowry, G.V.; Ghoshal, S.; Tufenkji, N.; Brambilla, D.; Dutcher, J.R.; Wilkinson, K.J. Technology readiness and overcoming barriers to sustainably implement nanotechnology-enabled plant agriculture. Nat. Food. 2020, 1, 416–425. [Google Scholar] [CrossRef]
- Abdel Latef, A.A.H.; Srivastava, A.K.; Elsadek, M.S.A.; Kordrostami, M.; Tran, L.S.P. Titanium dioxide nanoparticles improve growth and enhance the tolerance of broad bean plants under saline soil conditions. LAND DEGRAD DEV. 2018, 29, 1065–1073. [Google Scholar] [CrossRef]
- Soleymanzadeh, R.; Iranbakhsh, A.; Habibi, G.; Ardebili, Z.O. Selenium nanoparticle protected strawberry against salt stress through modifications in salicylic acid, ion homeostasis, antioxidant machinery, and photosynthesis performance. ACTA BIOL CRACOV BOT. 2020, 62, 33–42. [Google Scholar] [CrossRef]
- Arora, S.; Sharma, P.; Kumar, S.; Nayan, R.; Khanna, P.K.; Zaidi, M.G.H. Gold-nanoparticle induced enhancement in growth and seed yield of Brassica juncea. Plant Growth Regul. 2012, 66, 303–310. [Google Scholar] [CrossRef]
- Manke, A.; Wang, L.; Rojanasakul, Y. Mechanisms of nanoparticle-induced oxidative stress and toxicity. Biomed Res. Int. 2013, 2013, 942916. [Google Scholar] [CrossRef]
- Caser, M.; Lovisolo, C.; Scariot, V. The influence of water stress on growth, ecophysiology and ornamental quality of potted Primula vulgaris ‘Heidy’ plants. New insights to increase water use efficiency in plant production. J. Plant Biochem Physiol. 2024, 196, 111–122. [Google Scholar] [CrossRef]
- El-Berawey, D.Y.; Eldebawy, E.M.M. The effects of Marrubium alysson and Torilis arvensis natural and nano extracts on priming of wheat seeds in response to drought. Cereal Res. Commun. 2025, 53, 275–289. [Google Scholar] [CrossRef]
- Ghazy, O.A.; Fouad, M.T.; Saleh, H.H.; Kholif, A.E.; Morsy, T.A. Ultrasound-assisted preparation of anise extract nanoemulsion and its bioactivity against different pathogenic bacteria. Food Chem. 2021, 341, 128259. [Google Scholar] [CrossRef]
- Arnon, A.N. Method of extraction of chlorophyll in the plants. Agronomy journal. 1967, 23, 112–121. [Google Scholar]
- Pandjaitan, N.; Howard, L.R.; Morelock, T.; Gil, M.I. Antioxidant capacity and phenolic content of spinach as affected by genetics and maturation. J Agric Food Chem. 2005, 53, 8618–8623. [Google Scholar] [CrossRef]
- Krizek, D.T.; Britz, S.J.; Mirecki, R.M. Inhibitory effects of ambient levels of solar UV-A and UV-B radiation on growth of cv. New Red Fire lettuce. Physiol. Plant. 1998, 103, 1–7. [Google Scholar] [CrossRef]
- Bates, L.S.; Waldren, R.P.A.; Teare, I.D. Rapid determination of free proline for water-stress studies. J Plant Nutr Soil Sci. 1973, 39, 205–207. [Google Scholar] [CrossRef]
- Chance, B.; Maehly, A.C. Assay of catalases and peroxidases. METHOD ENZYMOL. 1955, 2, 764–775. [Google Scholar] [CrossRef]
- Wang, J.; Zheng, L.; W.u, J.; Tan, R. Involvement of nitric oxide in oxidative burst, phenylalanine ammonia-lyase activation and Taxol production induced by low-energy ultrasound in Taxus yunnanensis cell suspension cultures. Nitric Oxide. 2006, 15, 351–358. [CrossRef]
- Akowuah, G.A.; Ismail, Z.; Norhayati, I.; Sadikun, A. The effects of different extraction solvents of varying polarities on polyphenols of Orthosiphon stamineus and evaluation of the free radical-scavenging activity. Food Chem. 2005, 93, 311–317. [Google Scholar] [CrossRef]
- Benzie, I.F.F.; Strain, J.J. The ferric reducing ability of plasma (FRAP) as a measure of “antioxidant power”: The FRAP assay. Analytical biochemistry. 1996, 239, 70–76. [Google Scholar] [CrossRef] [PubMed]
- Tandon, H.L.S. Methods of analysis of soils, plants, water andfertilizers. FDCO, New Delhi; 1995.
- Adams, R.P. Identification of essential oil components by gas chromatography/mass spectrometry, ed. 4.1. Allured publishing corporation; 2007.
- Pirbalouti, A.G.; Hashemi, M.; Ghahfarokhi, F.T. Essential oil and chemical compositions of wild and cultivated Thymus daenensis Celak and Thymus vulgaris L. Ind Crops Prod. 2013, 48, 43–48. [Google Scholar] [CrossRef]
- Rezende, R.A.L.S.; Rodrigues, F.A.; Soares, J.D.R.; Silveira, H.R.O.D.; Pasqual, M.; Dias, G.M.G. Nitrogen removal during the cold season by constructed floating wetlands planted with Oenanthe javanica. Mar. Freshw. Res. 2018, 69, 635–647. [Google Scholar] [CrossRef]
- Abdel-Latif, A.; El-Demerdash, F.M. The ameliorative effects of silicon on salt-stressed sorghum seedlings and its influence on the activities of sucrose synthase and PEP carboxylase. Physiol. Plant Pathol. 2017, 5, 2–8. [Google Scholar] [CrossRef]
- Kalteh, M.; Alipour, Z.T.; Ashraf, S.; Marashi, A.M.; Falah, N.A. Effect of silica nanoparticles on basil (Ocimum basilicum) under salinity stress. J. Chem. Health Risks. 2014, 4, 49–55. [Google Scholar]
- Chehregani Rad, A.; Khorzaman, N.; LariYazdi, H.; Shirkhani, Z. Changes in growth characteristics and physiological indices in Zn-Stressed Phaseolus vulgaris plants on hydroponic medium. J. Dev. Biol. 2016, 8, 31–39. [Google Scholar]
- Mahlooji, M. Effects of salinity stress and Zinc application and some physiological traits on grain filling of three barley cultivars. Journal of Plant Process and Function. 2022, 11, 211–227. [Google Scholar]
- Zulfiqar, F.; Ashraf, M. Nanoparticles potentially mediate salt stress tolerance in plants. Plant Physiol. Biochem. 2021, 160, 257–268. [Google Scholar] [CrossRef] [PubMed]
- Hniličková, H.; Hnilička, F.; Martinkova, J.; Kraus, K. Effects of salt stress on water status, photosynthesis and chlorophyll fluorescence of rocket. Plant Soil Environ. 2017, 63, 362–367. [Google Scholar] [CrossRef]
- Sofy, M.R.; Elhawat, N.; Alshaal, T. Glycine betaine counters salinity stress by maintaining high K+/Na+ ratio and antioxidant defense via limiting Na+ uptake in common bean (Phaseolus vulgaris L.). Ecotoxicol. Environ. Saf. 2020, 200, 110732. [Google Scholar] [CrossRef]
- Summart, J.; Thanonkeo, P.; Panichajakul, S.; Prathepha, P.; McManus, M.T. Effect of salt stress on growth, inorganic ion and proline accumulation in Thai aromatic rice, Khao Dawk Mali 105, callus culture. Afr. J. Biotechnol. 2010, 9, 145–152. [Google Scholar]
- Shabala, S.; Demidchik, V.; Shabala, L.; Cuin, T.A.; Smith, S.J.; Miller, A.J.; Davies, J.M.; Newman, I.A. Extracellular Ca2+ ameliorates NaCl-induced K+ loss from Arabidopsis root and leaf cells by controlling plasma membrane K+-permeable channels. J. Plant Physiol. 2006, 141, 1653–1665. [Google Scholar] [CrossRef]
- Carmona, F.J.; Guagliardi, A.; Masciocchi, N. Nanosized calcium phosphates as novel macronutrient nano-fertilizers. J. Nanomater. 2022, 12, 2709. [Google Scholar] [CrossRef]
- Das, K.; Roychoudhury, A. Reactive oxygen species (ROS) and response of antioxidants as ROS-scavengers during environmental stress in plants. Front. environ. sci. 2014, 2, 53. [Google Scholar] [CrossRef]
- Waskiewicz, A.; Muzolf-Panek, M.; Golinski, P. Phenolic content changes in plants under salt stress. In: J. Plant Stress Physiol. 2013, 283–314. [CrossRef]
- Firoozeh, R.; Khavarinejad, R.; Najafi, F.; Saadatmand, S. Effects of gibberellin on contents of photosynthetic pigments, proline, phenol and flavonoid in savory plants (Satureja hortensis L.) under salt stress. Iran. J. Plant Physiol. 2019, 31, 894–908. [Google Scholar]
- Isah, T. Stress and defense responses in plant secondary metabolites production. J. Biol. Res. 2019, 52, 39. [Google Scholar] [CrossRef]
- Amist, N.; Singh, N.B. Responses of enzymes involved in proline biosynthesis and degradation in wheat seedlings under stress. J. Biol. Res. 2017, 42, 195–206. [Google Scholar] [CrossRef]
- Hmidi, D.; Abdelly, C.; Athar, H.U.R.; Ashraf, M.; Messedi, D. Effect of salinity on osmotic adjustment, proline accumulation and possible role of ornithine-δ-ami notransferase in proline biosynthesis in Cakile maritima. Physiol. Mol. Biol. Plants. 2018, 24, 1017–1033. [Google Scholar] [CrossRef] [PubMed]
- Sarkar, M.M.; Pradhan, N.; Subba, R.; Saha, P.; Roy, S. Sugar-terminated carbon-nanodots stimulate osmolyte accumulation and ROS detoxification for the alleviation of salinity stress in Vigna radiata. Sci. Rep. 2022, 12, 17567. [Google Scholar] [CrossRef] [PubMed]
- Khan, M.N.; Siddiqui, M.H.; Mohammad, F.; Naeem, M. Interactive role of nitric oxide and calcium chloride in enhancing tolerance to salt stress. Nitric Oxide. 2012, 27, 210–218. [Google Scholar] [CrossRef] [PubMed]
- Shao, H.B.; Chu, L.Y.; Shao, M.A.; Jaleel, C.A.; Hong-mei, M. Higher plant antioxidants and redox signaling under environmental stresses. C R Biol. 2008, 331, 433–441. [Google Scholar] [CrossRef]
- Wen, P.F.; Chen, J.Y.; Wan, S.B.; Kong, W.F.; Zhang, P.; Wang, W.; Huang, W.D. Salicylic acid activates phenylalanine ammonia-lyase in grape berry in response to high temperature stress. J. Plant Growth Regul. 2008, 55, 1–10. [Google Scholar] [CrossRef]
- Rezaie, R.; Abdollahi Mandoulakani, B.; Fattahi, M. Cold stress changes antioxidant defense system, phenylpropanoid contents and expression of genes involved in their biosynthesis in Ocimum basilicum L. Sci. Rep. 2020, 10, 5290. [Google Scholar] [CrossRef]
- Valifard, M.; Mohsenzadeh, S.; Kholdebarin, B.; Rowshan, V.; Niazi, A.; Moghadam, A. Effect of salt stress on terpenoid biosynthesis in Salvia mirzayanii: From gene to metabolite. J. Hortic. Sci. Biotechnol. 2019, 94, 389–399. [Google Scholar] [CrossRef]
- El Amerany, F. The role of terpenoids in plant development and stress tolerance. In: Molecular and Physiological Insights into Plant Stress Tolerance and Applications in Agriculture—Part 2. Bentham Science Publishers; 2024, 71–98. [CrossRef]
- Selmar, D.; Kleinwächter, M. Stress enhances the synthesis of secondary plant products: The impact of stress-related over-reduction on the accumulation of natural products. Plant Cell Physiol. 2013, 54, 817–826. [Google Scholar] [CrossRef]
- Rafeie, M.; Shabani, L.; Sabzalian, M.R.; Gharibi, S. Pretreatment with LEDs regulates antioxidant capacity and polyphenolic profile in two genotypes of basil under salinity stress. Protoplasma. 2022, 259, 1567–1583. [Google Scholar] [CrossRef]
- Lo, Presti.; M., Ragusa.; S., Trozzi.; A., Dugo.; P., Visinoni.; F., Fazio.; A., Dugo.; G., Mondello.; L. A comparison between different techniques for the isolation of rosemary essential oil. J. Sep. Sci. 2005, 28, 273–280. [CrossRef]
- Gachkar, L.; Yadegari, D.; Rezaei, M.B.; Taghizadeh, M.; Astaneh, S.A.; Rasooli, I. Chemical and biological characteristics of Cuminum cyminum and Rosmarinus officinalis essential oils. Food Chem. 2007, 102, 898–904. [Google Scholar] [CrossRef]
- Karray-Bouraoui, N.; Rabhi, M.; Neffati, M.; Baldan, B.; Ranieri, A.; Marzouk, B.; Lachaal, M.; Smaoui, A. Salt effect on yield and composition of shoot essential oil and trichome morphology and density on leaves of Mentha pulegium. IND CROP PROD. 2009, 30, 338–343. [Google Scholar] [CrossRef]
- Bernstein, N.; Kravchik, M.; Dudai, N. Salinity-induced changes in essential oil, pigments and salts accumulation in sweet basil (Ocimum basilicum) in relation to alterations of morphological development. Ann. Appl. Biol. 2010, 156, 167–177. [Google Scholar] [CrossRef]
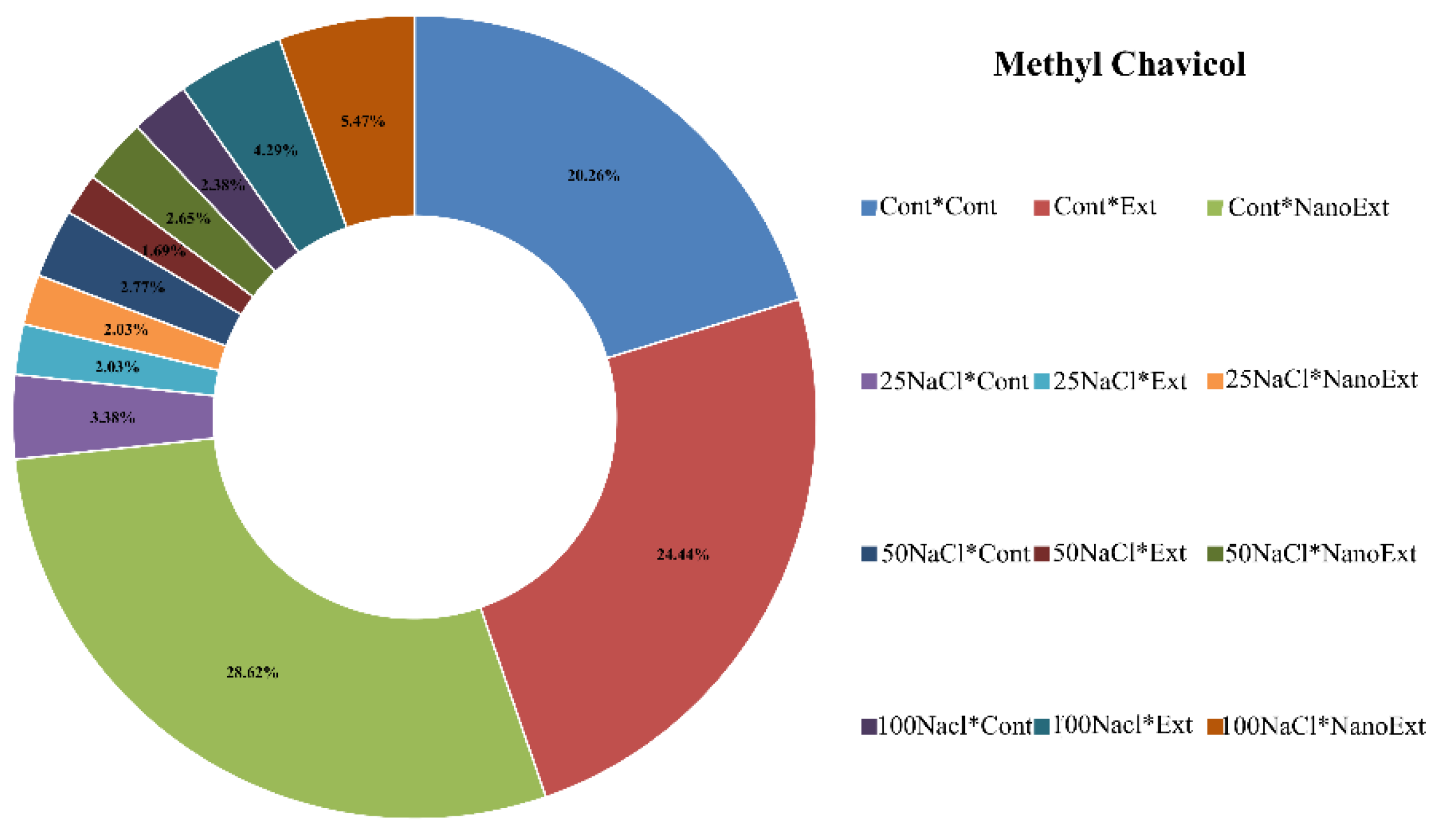
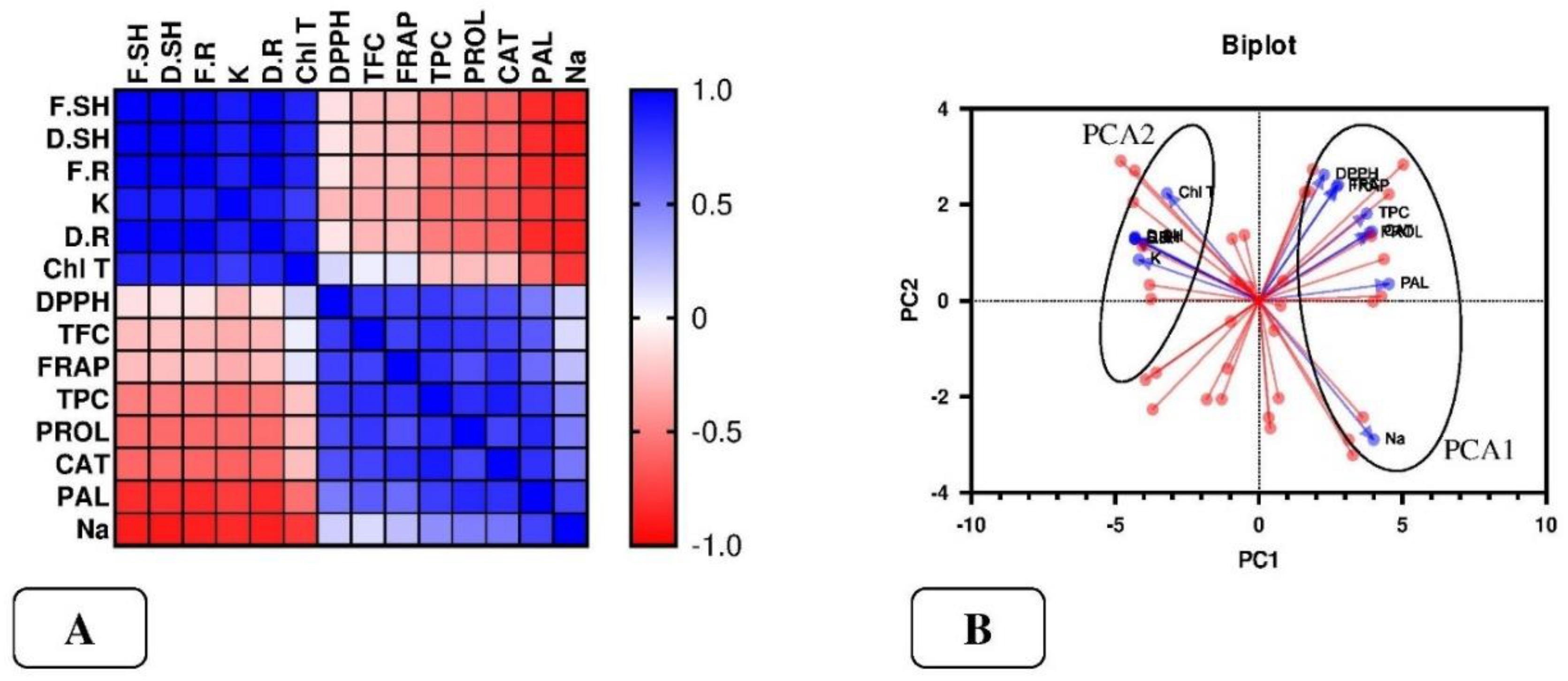
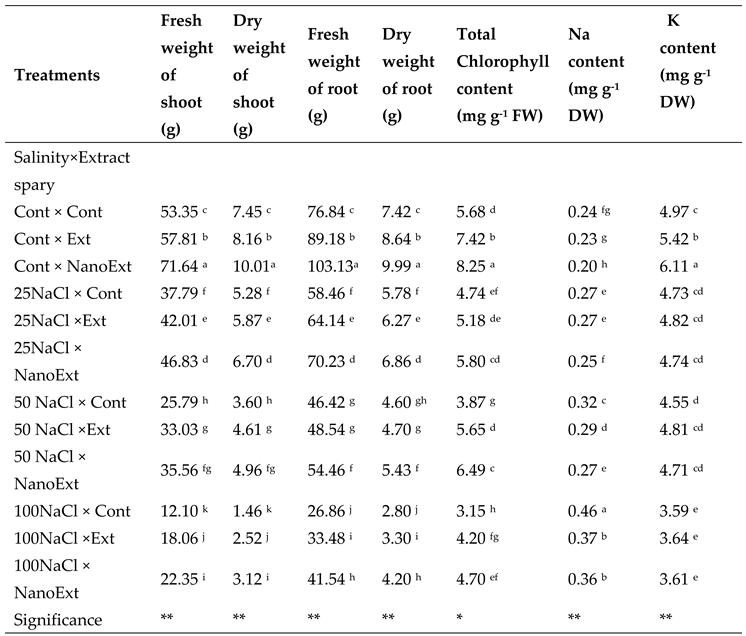 |
| Treatment | df | Phenol | Flavonoid | Proline | Catalase | Phenolalanine Ammonialyase | DPPH | FRAP |
|---|---|---|---|---|---|---|---|---|
| Block | 2 | 2.05 ** | 0.03 NS | 0.00 NS | 0.006 ** | 0.00 NS | 18.16 * | 0.002 * |
| Salinity (S) | 3 | 29.27 ** | 4.65 ** | 0.31 ** | 0.50 ** | 0.07 ** | 119.25 ** | 0.01 ** |
| Error a | 4 | 0.46 | 0.07 | 0.00 | 0.001 | 0.00 | 3.95 | 0.00 |
| Extract spray (E) | 2 | 11.26 ** | 10.07 ** | 0.19 ** | 0.06 ** | 0.005 ** | 378.77 ** | 0.01 ** |
| S×E | 4 | 1.23 ** | 0.86 ** | 0.007 ** | 0.02 ** | 0.00 * | 19.81 * | 0.002 * |
| Error b | 18 | 0.31 | 0.08 | 0.0002 | 0.00 | 0.00 | 4.68 | 0.00 |
| CV(%) | - | 6.93 | 11.90 | 2.38 | 0.02 | 0.01 | 9.74 | 10.01 |
| Cont × Cont | Cont × Ext | Cont × NanoExt | 25NaCl × Cont | 25NaCl ×Ext | 25NaCl × NanoExt | 50 NaCl × Cont | 50 NaCl ×Ext | 50 NaCl × NanoExt | 100NaCl × Cont | 100NaCl ×Ext | 100NaCl × NanoExt | ||
| Compound | KI | Area% | Area% | Area% | Area% | Area% | Area% | Area% | Area% | Area% | Area% | Area% | Area% |
| a-Pinene | 939 | 0.14 | 0.32 | 0.45 | 0.61 | 0.85 | 0.54 | 1.25 | 1.12 | 1.36 | 0.63 | 0.85 | 0.93 |
| Sabinene | 975 | 0.97 | 1.12 | 1.36 | 1.49 | 1.64 | 1.96 | 2.36 | 1.39 | 0.48 | 0.63 | 0.78 | 0.96 |
| Myrcene | 989 | 0.41 | 0.36 | 0.89 | 0.92 | 1.26 | 0.15 | 0.54 | 0.64 | 0.78 | 0.37 | 0.54 | 0.24 |
| 1,8-Cineol | 1030 | 1.52 | 1.45 | 2.58 | 3.26 | 2.31 | 2.13 | 3.79 | 2.15 | 1.65 | 2.98 | 2.45 | 2.47 |
| Linalool | 1088 | 12.3 | 13.25 | 15.3 | 22.64 | 18.26 | 21.26 | 22.25 | 23.13 | 19.94 | 21.47 | 22.68 | 23.91 |
| Eugenol | 1355 | 2.4 | 3.4 | 3.85 | 3.95 | 5.84 | 6.84 | 7.26 | 7.96 | 5.95 | 4.78 | 4.55 | 4.15 |
| Camphor | 1145 | 0.74 | 0.89 | 0.65 | 0.74 | 0.61 | 0.64 | 0.97 | 1.14 | 8.56 | 5.14 | 6.37 | 6.29 |
| Terpinen-4-ol | 1177 | 0.41 | 0.61 | 0.74 | 0.85 | 0.45 | 0.79 | 0.85 | 1.29 | 2.14 | 1.34 | 2.78 | 2.18 |
| a-Terpineol | 1188 | 1.54 | 1.2 | 2.3 | 3.2 | 3.28 | 2.15 | 1.02 | 2.46 | 3.26 | 2.96 | 3.79 | 2.65 |
| Cis-Carveol | 1229 | 0.65 | 0.64 | 0.78 | 0.64 | 0.79 | 0.75 | 0.63 | 0.98 | 1.24 | 2.78 | 2.36 | 2.06 |
| Geraniol | 1265 | 0.41 | 0.18 | 0.52 | 0.56 | 0.14 | 0.62 | 2.36 | 0 | 0 | 0 | 0 | 0 |
| Cubenol | 1511 | 2.7 | 1.36 | 1.25 | 1.39 | 2.31 | 1.12 | 0 | 0 | 0 | 0 | 0 | 0 |
| Epi-a-Muurolol | 1642 | 1.47 | 1.78 | 1.97 | 3.24 | 5.6 | 2.03 | 0 | 0 | 0 | 0 | 0 | 0 |
| E-B-Ocimene | 1045 | 2.1 | 1.2 | 2.5 | 2.6 | 3.1 | 1.46 | 1.25 | 2.15 | 1.02 | 3.98 | 3.94 | 4.18 |
| Carvone | 1243 | 15.9 | 16.6 | 16.4 | 18.23 | 9.24 | 12.4 | 14.26 | 13.6 | 7.6 | 7.63 | 7.37 | 7.7 |
| 6-Methyl-5-hepten-2-one | 989 | 0.35 | 0.21 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Methyl Chavicol | 1196 | 12.6 | 15.2 | 17.8 | 2.1 | 1.26 | 1.26 | 1.72 | 1.05 | 1.65 | 1.48 | 2.67 | 3.4 |
| Nero | 1230 | 0.62 | 0.85 | 1.25 | 1.24 | 2.12 | 1.02 | 2.36 | 3.65 | 2.15 | 1.96 | 1.64 | 1.85 |
| Neral | 1242 | 0.52 | 0.62 | 0.65 | 0.89 | 3.16 | 0.96 | 0.89 | 0.85 | 0.89 | 0.52 | 0.53 | 0.63 |
| A-Humulene | 1456 | 10.2 | 8.25 | 5.12 | 4.29 | 2.12 | 2.31 | 1.56 | 1.36 | 2.16 | 3.46 | 2.98 | 3.26 |
| Actanol acetate | 1213 | 0.5 | 0.52 | 0.62 | 0.89 | 1.21 | 0 | 0 | 0 | 0.39 | 0 | 0 | 0 |
| 1-Octen-3-ol | 980 | 0.35 | 0.41 | 0.23 | 0.27 | 0.36 | 0 | 0 | 0 | 0.46 | 0 | 0 | 0 |
| Geranial | 1272 | 6.2 | 0.74 | 0.84 | 0.82 | 0.8 | 1.96 | 1.36 | 1.96 | 0.52 | 0.45 | 0.63 | 0.59 |
| E-Caryophyllene | 1418 | 0.5 | 0.65 | 0.79 | 0.8 | 0.75 | 0.61 | 0.98 | 1.97 | 0.17 | 0.52 | 0.78 | 0.64 |
| Isopulego | 1150 | 0.41 | 0.12 | 0.15 | 0 | 0 | 0.45 | 0.45 | 0.89 | 0.61 | 0.61 | 0.96 | 0.79 |
| Italicence ether | 1537 | 0.65 | 0.87 | 1.25 | 1.24 | 1.25 | 2.89 | 1.26 | 1.52 | 0.36 | 0.89 | 1.02 | 1.36 |
| B-Pinene | 979 | 1.4 | 2.14 | 1.36 | 4.58 | 3.46 | 2.16 | 0.84 | 0.94 | 0.34 | 0.98 | 2.16 | 1.03 |
| Menthol | 1171 | 4.2 | 5.6 | 6.2 | 6.85 | 9.03 | 9.25 | 10.39 | 11.26 | 10.15 | 11.57 | 12.48 | 13.62 |
| DeltGuaie | 1025 | 0.95 | 0.89 | 0.65 | 0.45 | 0 | 0.45 | 0.65 | 0.64 | 0.12 | 1.36 | 1.65 | 2.49 |
| Cabbenol | 1136 | 1.14 | 2.15 | 0.46 | 0.35 | 0 | 0 | 0.45 | 0.35 | 0 | 0 | 0 | 0 |
| y-Eudesmol | 1145 | 0 | 0 | 0 | 0.12 | 0 | 0 | 0 | 0.6 | 0 | 0.78 | 1.79 | 0 |
| tau.-Cadinol | 1026 | 0 | 0 | 0 | 0 | 0.23 | 0 | 0 | 0 | 0 | 0.25 | 1.25 | 0.37 |
| y-Cadinene | 856 | 0 | 0 | 0 | 0 | 0.56 | 0 | 0 | 0 | 0 | 0 | 0 | 0.28 |
| Isothymol methyl ether | 1234 | 0 | 0 | 0 | 0 | 1.07 | 0 | 0 | 0 | 0 | 0 | 0 | 0.96 |
| (+)-2-Carene | 1126 | 0 | 0 | 0 | 0 | 0.21 | 1.63 | 0 | 0 | 0 | 0.16 | 0 | 0.19 |
| 6-Cadinene, (+)- | 975 | 0 | 0 | 0 | 0 | 0 | 1.3 | 1.25 | 0.48 | 2.03 | 1.89 | 0.48 | 0 |
| endo-Borneol | 1596 | 0 | 0 | 0 | 0 | 0 | 0 | 3.64 | 0.41 | 2.67 | 1.96 | 0.52 | 0.97 |
| Caryophyllene oxide | 1138 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0.18 | 2.65 | 1.12 | 0.32 | 0.89 |
| 84.25 | 83.58 | 88.91 | 89.21 | 83.27 | 81.09 | 86.59 | 86.12 | 81.3 | 84.65 | 90.32 | 91.04 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





