Submitted:
11 July 2025
Posted:
14 July 2025
You are already at the latest version
Abstract
Keywords:
1. Introduction
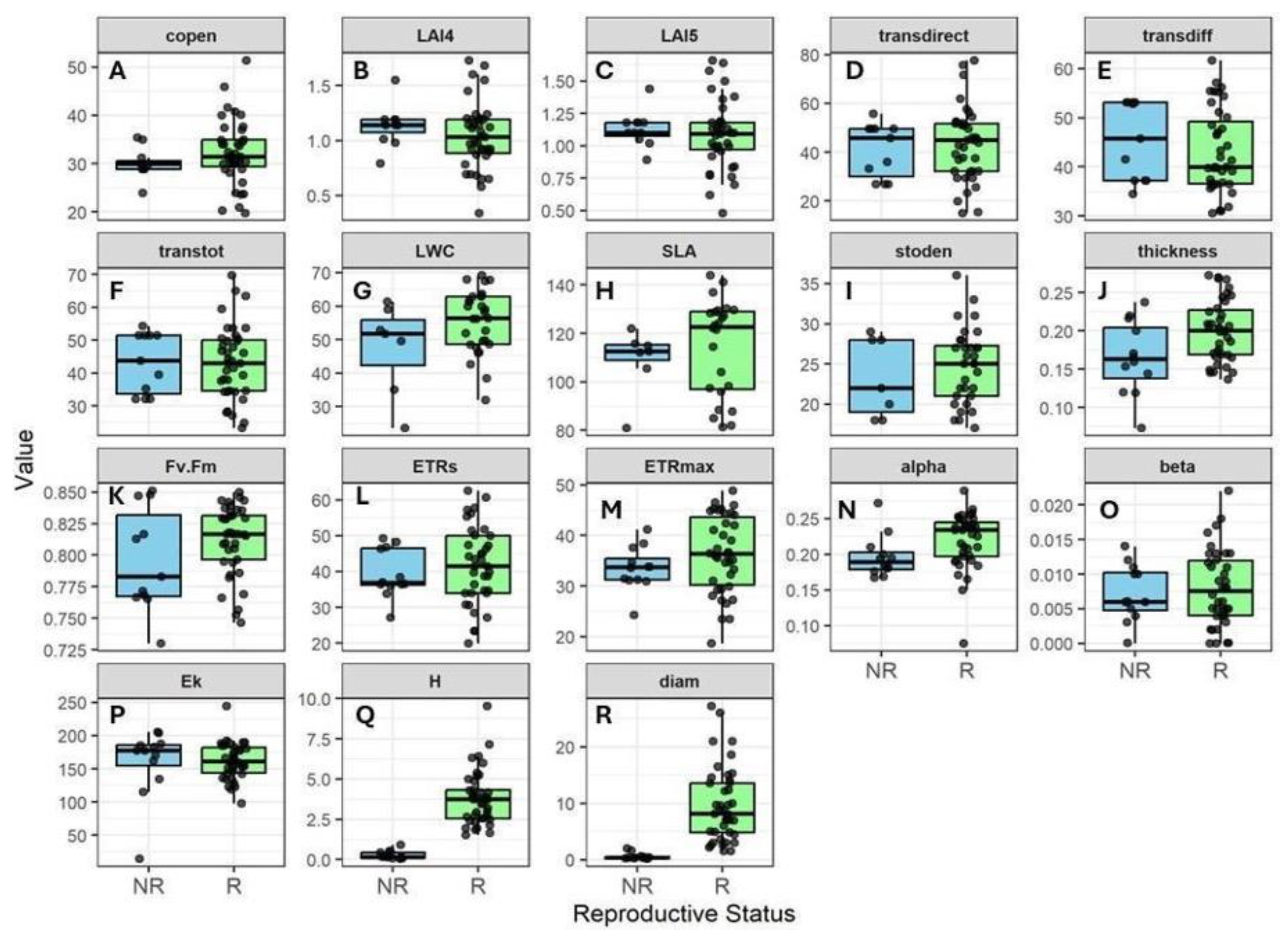
- Trait variation will correlate with plant size and reproductive status: Smaller, non-reproductive plants maximize light capture and growth by having thinner, larger leaves and a more acquisitive physiology (i.e., higher SLA, greater photosynthetic efficiency). As they grow larger and become reproductive, their strategy shifts toward a conservative and stress-tolerant strategy, developing traits that help them survive in a more variable and harsher environment (i.e., lower SLA, relatively lower photosynthetic efficiency), consistent with DRH.
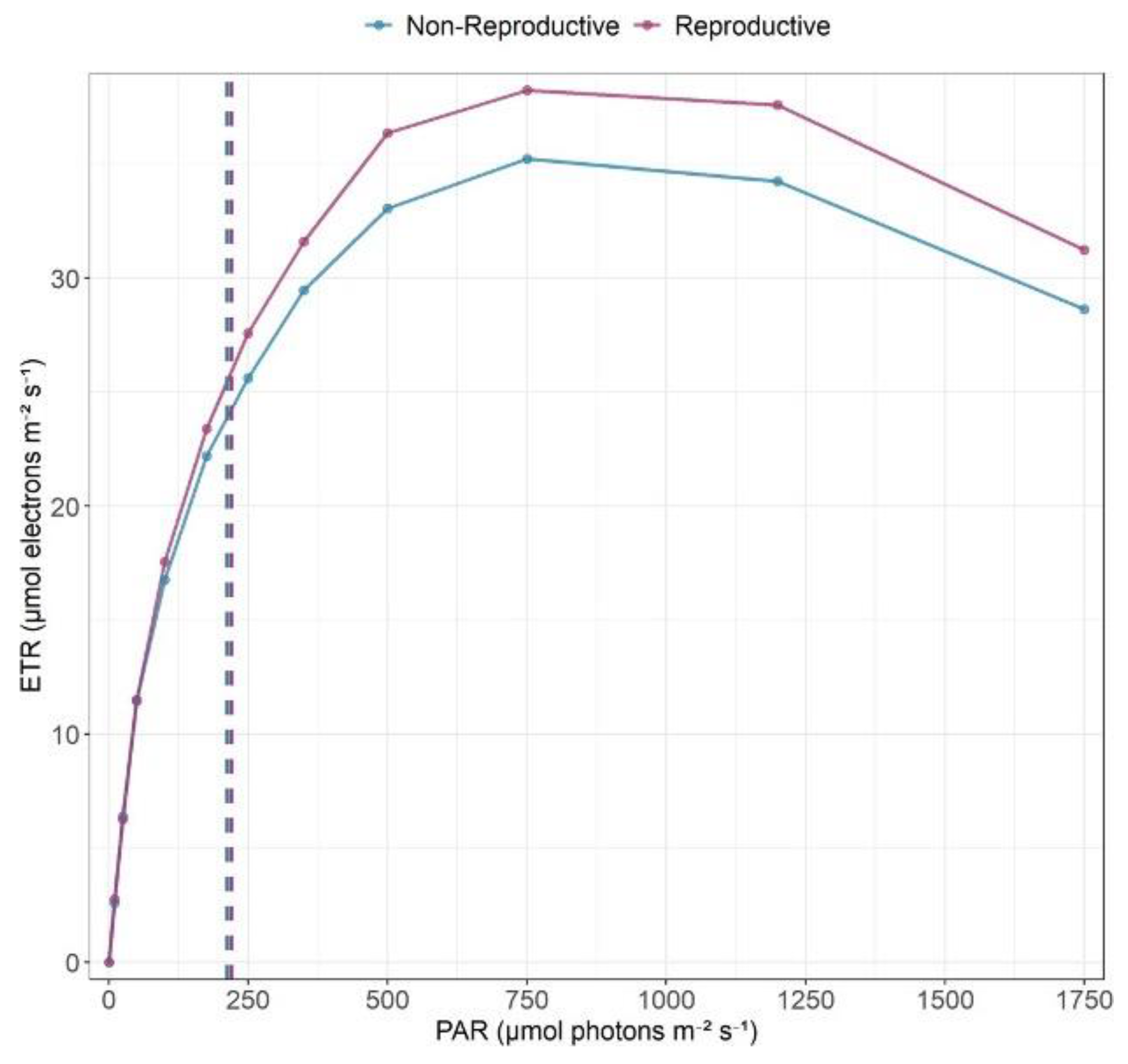
- Canopy openness will modulate trait expression (i.e., the light gradient hypothesis): in more open canopies, plants maximize light capture and show higher photochemical performance, while under more shaded canopies, traits become more conservative due to low light. This pattern would be especially pronounced in B. nervosa, whose inverted phenology and phreatophytic character mean light, not water, limits growth during the dry season. Therefore, individuals in more open canopies are expected to exhibit higher photochemical performance during this period.
2. Results
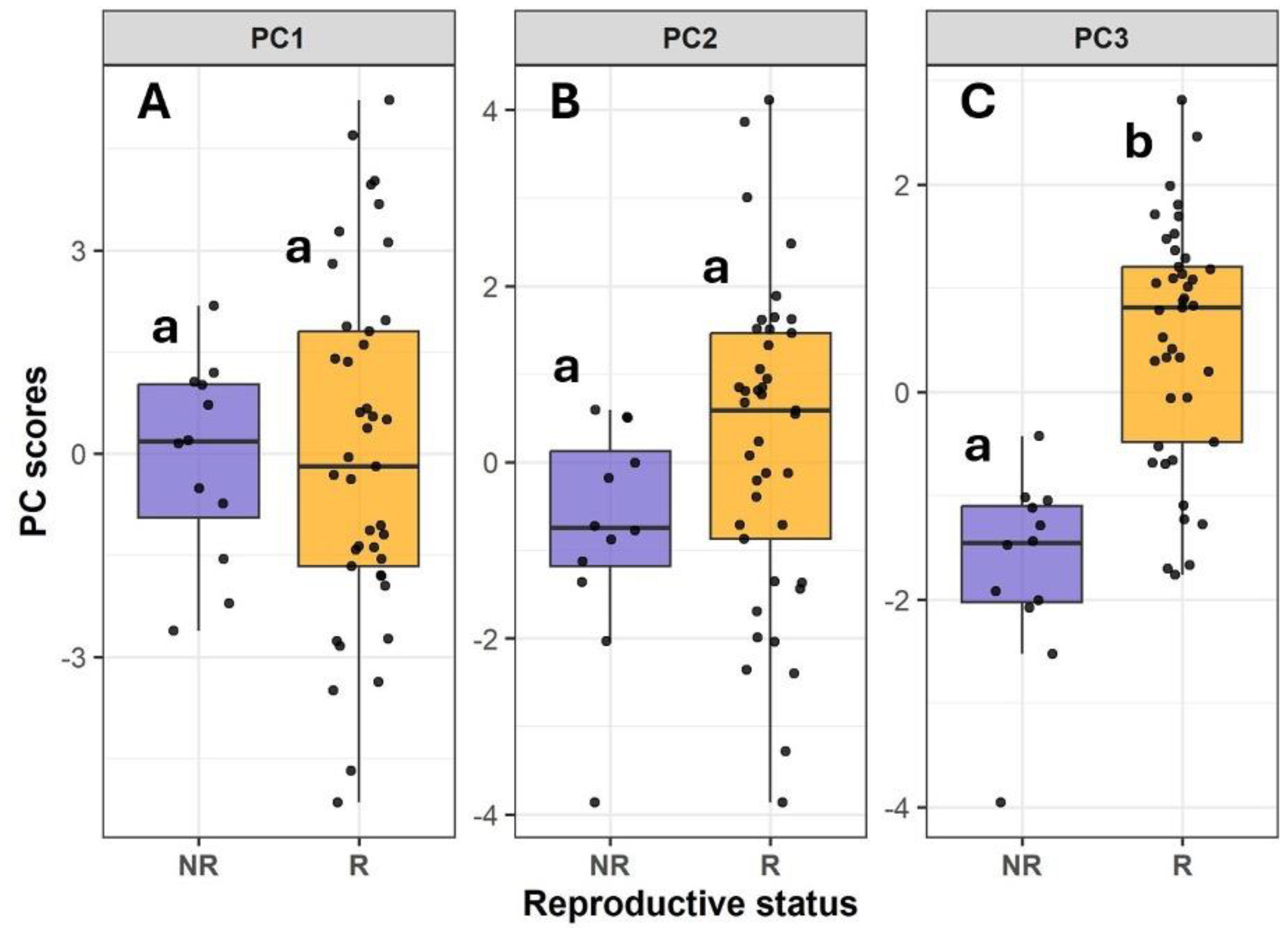
3.1. Definition of the Threshold for Reproductive Status
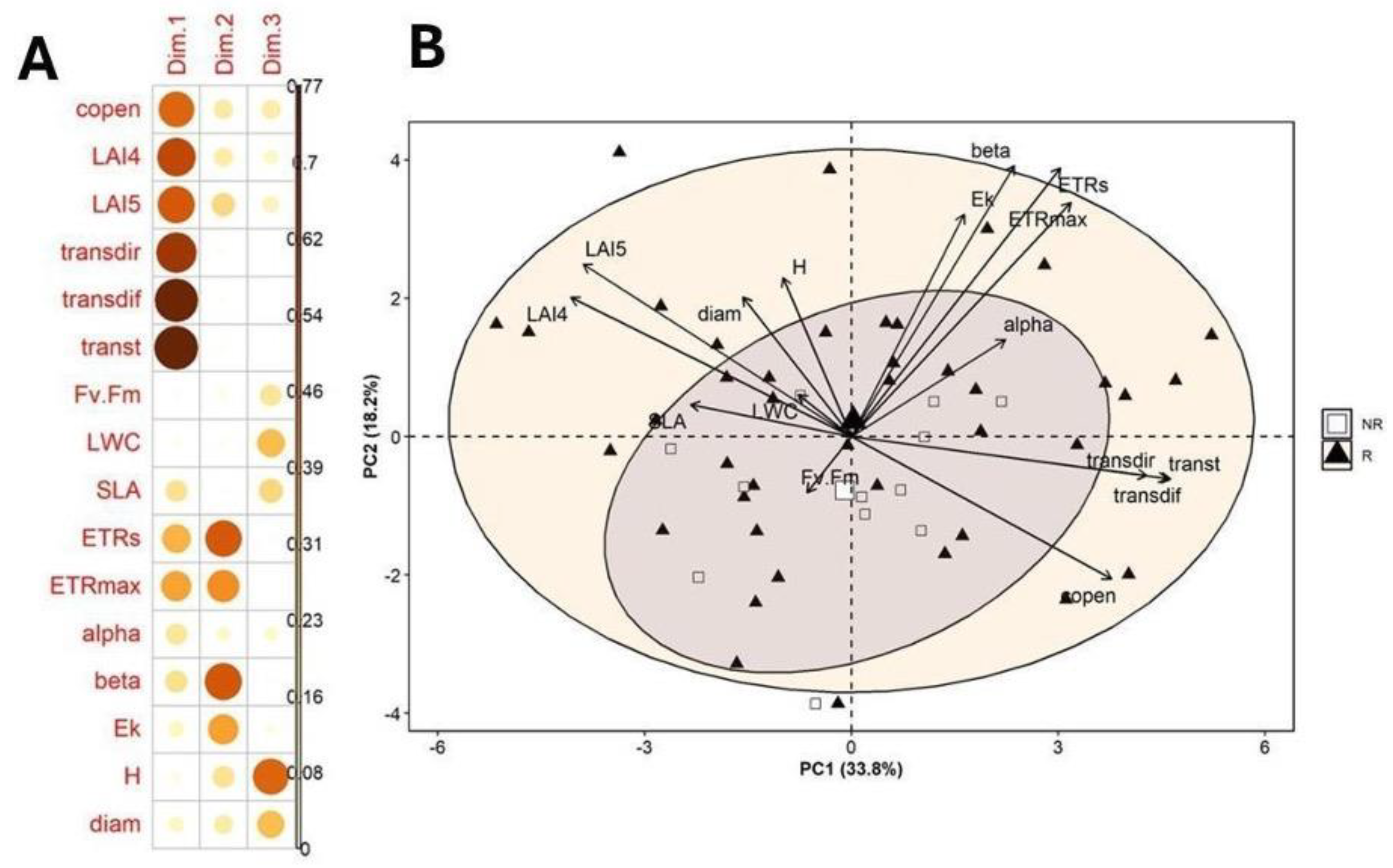
| Component | Eigenvalue | Percent | Cum Percent |
|---|---|---|---|
| 1 | 5.40 | 33.75 | 33.75 |
| 2 | 2.91 | 18.18 | 51.94 |
| 3 | 2.00 | 12.56 | 64.50 |
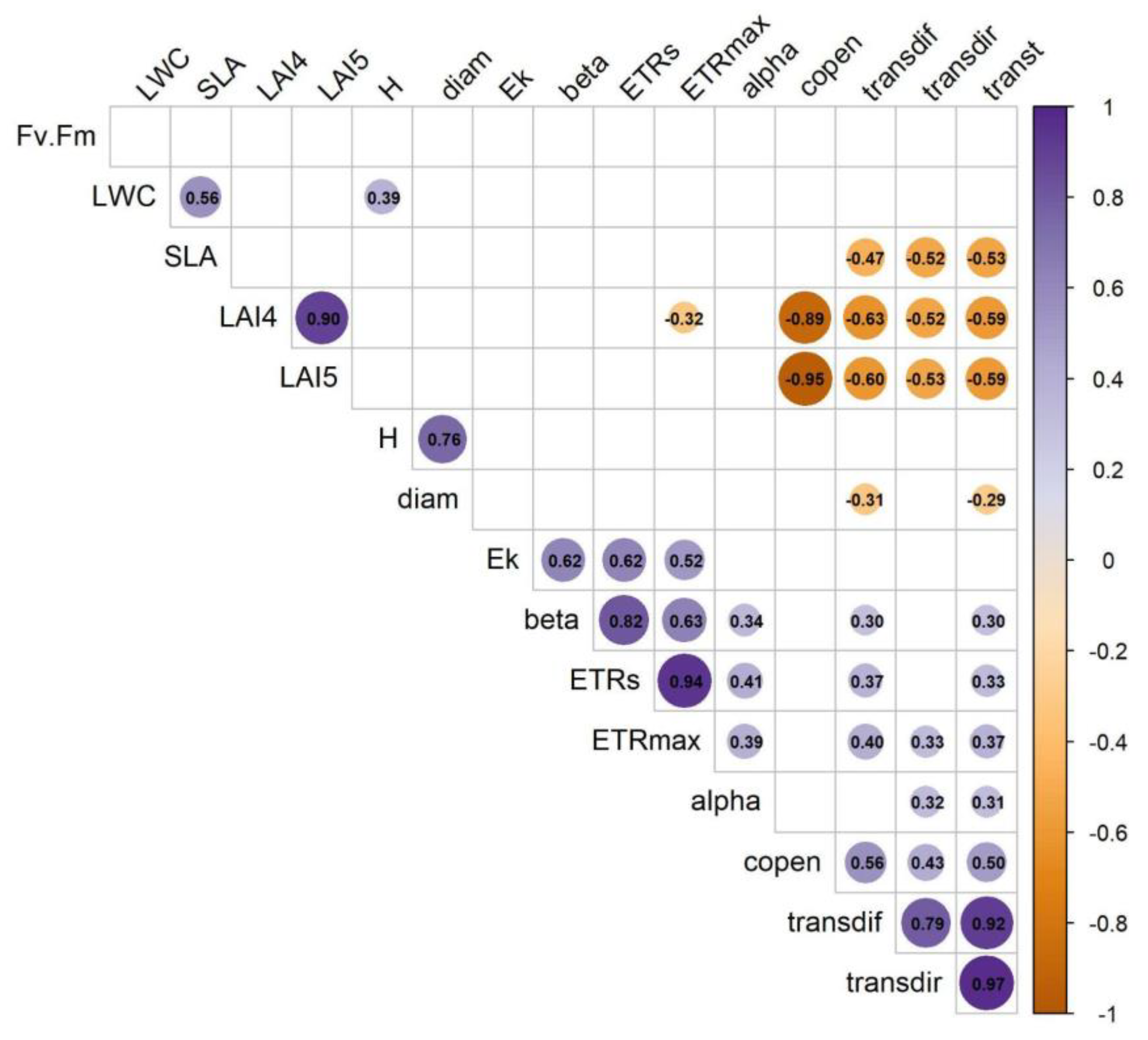
| Trait | PC1 | PC2 | PC3 |
| Canopy openness (copen, %) | 0.71 | -0.39 | 0.38 |
| LAI4 | -0.77 | 0.38 | -0.29 |
| LAI5 | -0.74 | 0.47 | -0.33 |
| Transmitted direct light (transdir, µmol·m⁻²·s⁻¹) | 0.81 | -0.10 | -0.01 |
| Transmitted diffuse light (transdif, µmol·m⁻²·s⁻¹) | 0.87 | -0.11 | -0.03 |
| Transmitted total light (transt, µmol·m⁻²·s⁻¹) | 0.88 | -0.11 | -0.02 |
| Fv/Fm | -0.12 | -0.15 | 0.43 |
| Leaf water content (LWC) | -0.14 | 0.11 | 0.55 |
| Specific leaf area (SLA, cm²/g) | -0.44 | 0.08 | 0.48 |
| Electron transport rate (ETR, µmol electrons m⁻² s⁻¹) | 0.58 | 0.73 | -0.05 |
| Maximum electron transport rate (ETRmax, µmol electrons m⁻² s⁻¹) | 0.60 | 0.64 | -0.04 |
| alpha | 0.42 | 0.26 | 0.26 |
| beta | 0.45 | 0.74 | -0.08 |
| Ek (µmol photons m⁻² s⁻¹) | 0.31 | 0.61 | -0.17 |
| Height (H, m) | -0.18 | 0.43 | 0.72 |
| Diameter (diam, cm) | -0.30 | 0.38 | 0.55 |
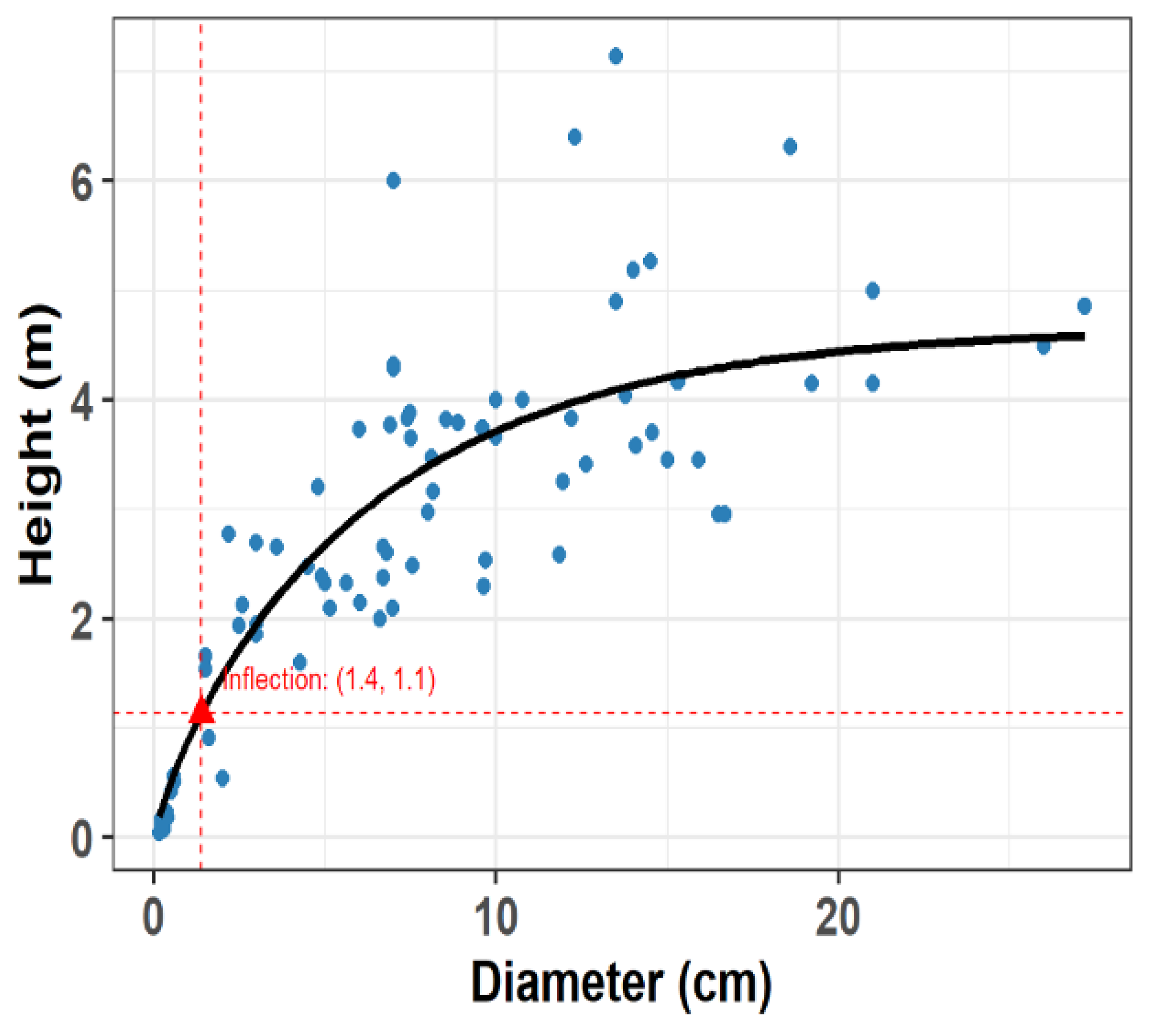
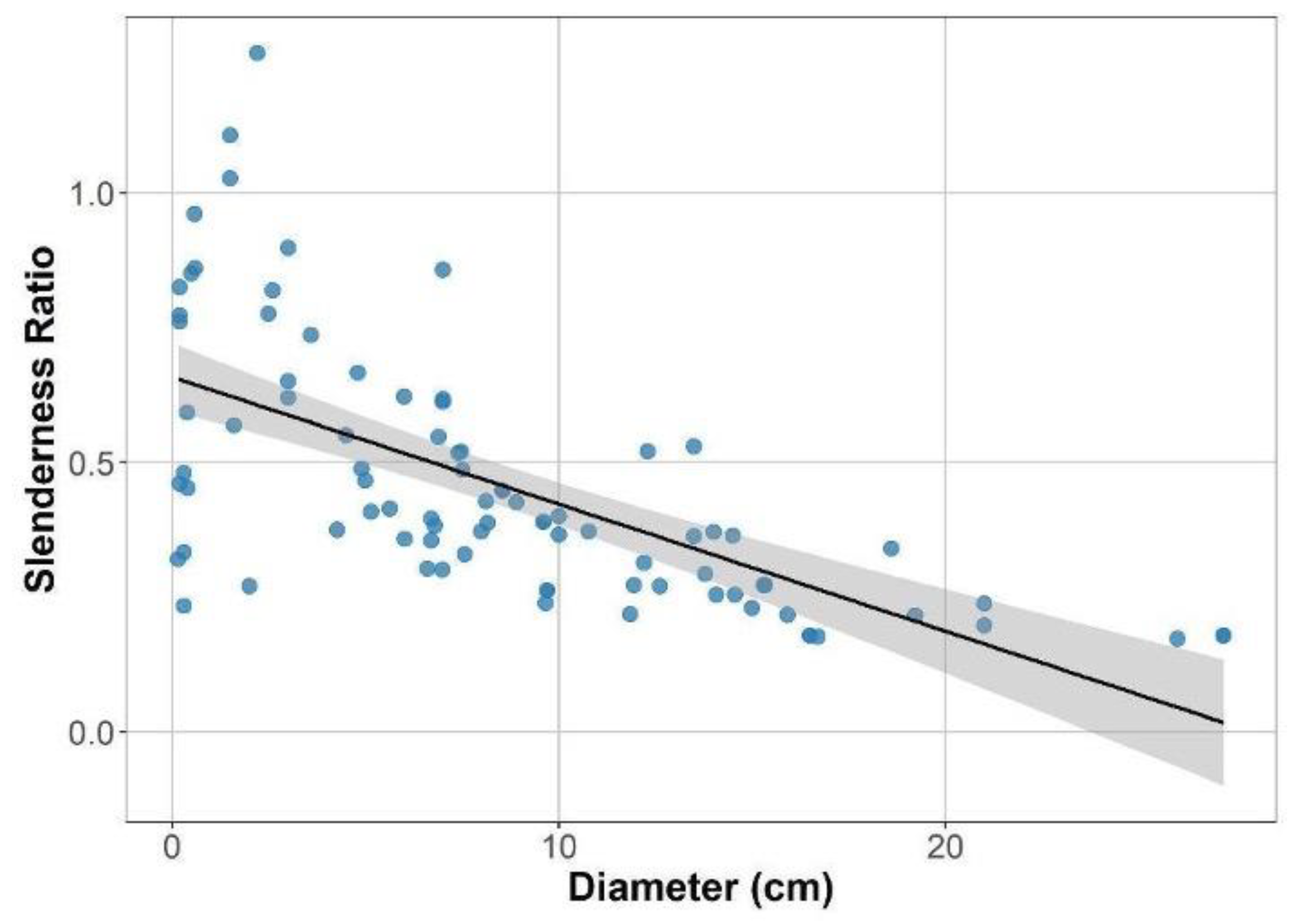
3.2. Variation in Canopy Structure and Functional Traits
3.3. Correlation of Canopy Structure and Functional Traits
3.4. Principal Component Analysis
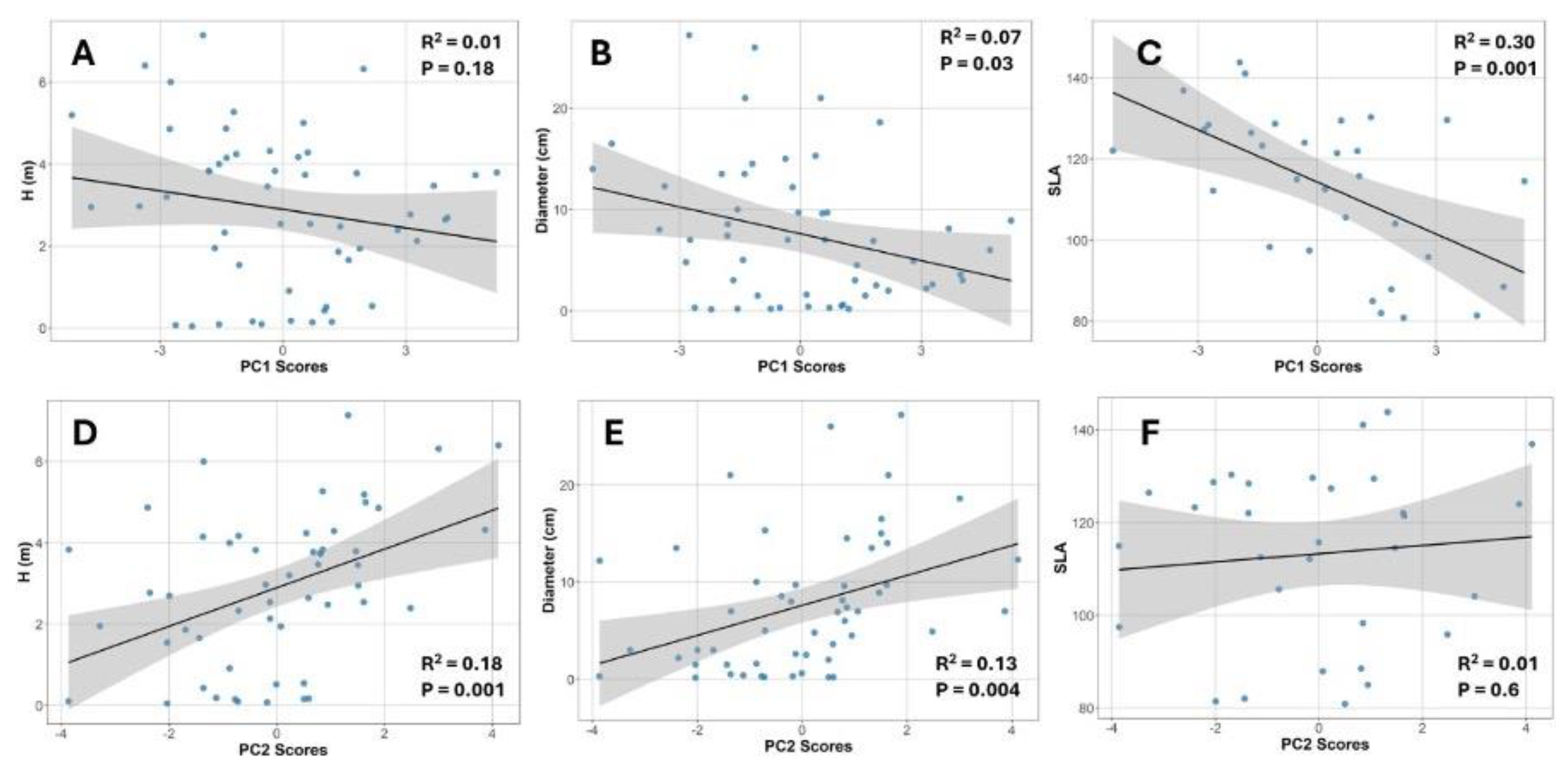
3.5. Relationships Among Canopy Structure, Plant Size, SLA, and Photosynthetic Performance
3.6. Photosynthetic Efficiency
4. Discussion
4.1. Canopy Structure Drives Leaf Trait Variation
4.2. SLA Was More Responsive to Light Variation Than Physiological Traits
4.3. Weak Support for the Diminishing Returns Hypothesis
4.4. Adaptive Significance of Reverse Phenology and Trait Conservatism
4.5. Implications for Dry Forest Ecology and Conservation
5. Materials and Methods
5.1. Study Site
5.2. Study Species
5.3. Height-Diameter Relationship
5.4. Measurement of Leaf Structural Traits
5.5. Photosynthetic Efficiency Analysis
5.6. Canopy Structure Analysis
5.7. Principal Component Analysis
5.8. Regression Between Size and Canopy Structure (Predictor Variables) and Morphological and Fluorescence Variables (Response Variables)
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| TDF | Tropical dry forest |
| SLA | Specific leaf area |
| LAI | Leaf area index |
| LES | Leaf economics spectrum |
| LL | Leaf lifespan |
| DRH | Diminishing returns hypothesis |
| ETR | Electron transport rate |
| RLC | Rapid light curve |
| PCA | Principal component analysis |
| PAR | Photosynthetically active radiation |
| SRNP | Santa Rosa National Park |
| DBH | Diameter at breast height |
References
- Janzen, D. H. Management of habitat fragments in a tropical dry forest: Growth. Ann. Mo. Bot. Gard. 1988, 75, 105–116. [CrossRef]
- Jiménez, Q., Carrillo, E., Kappelle, M. The Northern Pacific Lowland Seasonal Dry Forests of Guanacaste and the Nicoya Peninsula. In Costa Rican Ecosystems; Kappelle, M., Ed.; The University of Chicago Press, Chicago, Illinois, USA, 2016; pp 247–289.
- Meinzer, F. C., Andrade, J. L., Goldstein, G., Holbrook, N. M., Cavelier, J., & Wright, S. J. Partitioning of soil water among canopy trees in a seasonally dry tropical forest. Oecologia 1999, 121(3), 293–301. [CrossRef]
- Borchert, R. Soil and stem water storage determine phenology and distribution of tropical dry forest trees. Ecology 1994, 75(5), 1437-1449. [CrossRef]
- Sandoval-Granillo, V., & Meave, J. A. Leaf functional diversity and environmental filtering in a tropical dry forest: Comparison between two geological substrates. Ecol. Evol. 2023, 13(9), e10491. [CrossRef]
- Vargas Gutiérrez, G., Pérez-Aviles, D., Raczka, N., Pereira-Arias, D., Tijerín-Triviño, J., Pereira-Arias, L. D., ... & Powers, J. S. Throughfall exclusion and fertilization effects on tropical dry forest tree plantations, a large-scale experiment. Biogeosciences 2023, 20(11), 2143-2160. [CrossRef]
- Chaturvedi, R. K., Pandey, S. K., Tripathi, A., Goparaju, L., Raghubanshi, A. S., & Singh, J. S. (2024). Variations in the plasticity of functional traits indicate the differential impacts of abiotic and biotic factors on the structure and growth of trees in tropical dry forest fragments. Front. Plant Sci. 2024, 14, 1181293. [CrossRef]
- Ribeiro, D. R., Silva, J. L. A., do Nascimento, M. T., & Vitória, A. P. Leaf habits and their relationship with leaf and wood traits in tropical dry forests. Trees 2022, 36(1), 7-24. [CrossRef]
- Vargas G, G., Brodribb, T. J., Dupuy, J. M., González-M, R., Hulshof, C. M., Medvigy, D., ... & Powers, J. S. Beyond leaf habit: generalities in plant function across 97 tropical dry forest tree species. New Phytol. 2021, 232(1), 148-161. [CrossRef]
- Alvarado, M. V., & Terrazas, T. Tree species differ in plant economic spectrum traits in the tropical dry forest of Mexico. Plos One 2023, 18(11), e0293430. [CrossRef]
- Rodríguez-Alarcón, S., González-M, R., Carmona, C. P., & Tordoni, E. Trait–growth relationships in Colombian tropical dry forests: Incorporating intraspecific variation and trait interactions. J. Veg. Sci. 2024, 35(1), e13233. [CrossRef]
- Borchert, R. Climatic periodicity, phenology, and cambium activity in tropical dry forest trees. IAWA J. 1999, 20(3), 239-247. [CrossRef]
- Lebrija-Trejos, E., Pérez-García, E. A., Meave, J. A., Poorter, L., & Bongers, F. Environmental changes during secondary succession in a tropical dry forest in Mexico. J. Trop. Ecol. 2011, 27(5), 477-489. [CrossRef]
- Derroire, G. Powers, J. S., Hulshof, C. M.,Varela, L. E. C. & J. R. Healey. Contrasting patterns of leaf trait variation among and within species during tropical dry forest succession in Costa Rica. Sci. Rep. 2018, 8. [CrossRef]
- Werden, L. K., Calderón-Morales, E., Alvarado J, P., Gutiérrez L, M., Nedveck, D. A., & Powers, J. S. Using large-scale tropical dry forest restoration to test successional theory. Ecol. Appl. 2020, 30(6), e02116.
- Markesteijn, L., Poorter, L., & Bongers, F. Light-dependent leaf trait variation in 43 tropical dry forest tree species. Am. J. Bot. 2007, 94(4), 515-525. [CrossRef]
- Nascimento, A. R. T., Fagg, J. M. F., & Fagg, C. W. Canopy openness and LAI estimates in two seasonally deciduous forests on limestone outcrops in Central Brazil using hemispherical photographs. Rev. Árvore 2007, 31, 167-176. [CrossRef]
- Avalos, G., E. Marín Castillo, V. Acevedo Fernández, E. Zamora Villalobos, and T. Aguilar Bermúdez. Population Structure, Light Preferences, and late-wet season predispersal seed predation in the tropical dry forest understory shrub Bonellia nervosa. Rev. Biol. Trop. 2025a, Accepted.
- Chaves, Ó. M., & Avalos, G. Is the inverse leafing phenology of the dry forest understory shrub Jacquinia nervosa (Theophrastaceae) a strategy to escape herbivory?. Rev. Biol. Trop. 2006, 54(3), 951-963. [CrossRef]
- Janzen, D. H. Jacquinia pungens, a heliophile from the understory of tropical deciduous forest. Biotropica 1970, 112-119. [CrossRef]
- Janzen, D. H., & Wilson, D. E. The cost of being dormant in the tropics. Biotropica 1974, 260-262. [CrossRef]
- Chaves, Ó. M., & Avalos, G. Do seasonal changes in light availability influence the inverse leafing phenology of the neotropical dry forest understory shrub Bonellia nervosa (Theophrastaceae)?. Rev. Biol. Trop. 2008, 56(1), 257-268. [CrossRef]
- Sánchez, O., Quesada, M., Dirzo, R., & Schlichting, C. D. A field experiment to determine the effect of dry-season irrigation on vegetative and reproductive traits in the wet-deciduous tree Bonellia nervosa. J. Trop. Ecol. 2020, 36(1), 29-35. [CrossRef]
- Pineda-García, F., Paz, H., & Meinzer, F. C. Drought resistance in early and late secondary successional species from a tropical dry forest: the interplay between xylem resistance to embolism, sapwood water storage and leaf shedding. Plant, Cell & Environ. 2013, 36(2), 405-418. [CrossRef]
- Markesteijn, L., Poorter, L., Bongers, F., Paz, H. & L. Sack. Hydraulics and life history of tropical dry forest tree species: coordination of species’ drought and shade tolerance. New Phytologist. 2011, 191(2), 480-495. [CrossRef]
- Hulshof, C. M. & N. G. Swenson. Variation in leaf functional trait values within and across individuals and species: an example from a Costa Rican dry forest. 2010, Funct. Ecol. 24(1), 217-223. [CrossRef]
- Hulshof, C. M., Martínez-Yrízar, A., Burquez, A., Boyle, B., & Enquist, B. J. Plant functional trait variation in tropical dry forests: A review and synthesis. In Tropical dry forests in the Americas: ecology, conservation, and management; Sanches-Azofeifa, A., Powers, J.S., Fernandes, G.W., Quesada, M., Eds; CRC Press: Boca Raton, Florida, USA, 2013, pp. 129-140.
- Wright, I. J., Reich, P. B., Westoby, M., Ackerly, D. D., Baruch, Z., Bongers, F., ... & Villar, R. The worldwide leaf economics spectrum. Nature 2004, 428(6985), 821-827. [CrossRef]
- Funk, J. L., Larson, J. E., Ames, G. M., Butterfield, B. J., Cavender-Bares, J., Firn, J., ... & Wright, J. Revisiting the Holy Grail: using plant functional traits to understand ecological processes. Biol. Rev. 2017, 92(2), 1156-1173. [CrossRef]
- Sobrado, M. A. Cost-benefit relationships in deciduous and evergreen leaves of tropical dry forest. Funct. Ecol. 1991, 5(5), 608-616. [CrossRef]
- McGill, B. J., Enquist, B. J., Weiher, E. & Westoby, M. Rebuilding community ecology from functional traits. Trends Ecol. Evol. 2006, 21(4), 178-185. [CrossRef]
- Yang, J., Cao, M., & Swenson, N. G. Why functional traits do not predict tree demographic rates. Trends Ecol. Evol. 2018, 33(5), 326-336. [CrossRef]
- Avalos, G. Specific leaf area (SLA) serves as a proxy to predict total carbon content in understory individuals of the neotropical canopy palm Socratea exorrhiza. Trees 2023, 37(6), 1831-1840. [CrossRef]
- de Freitas, G. V., Silva, J. L. A., Ribeiro, D. R., Simioni, P., Campbell, G., Pireda, S., ... & Vitória, A. P. Functional trait patterns: investigating variation-covariation relationships and the importance of intraspecific variability along distinct vegetation types. Community Ecol. 2024, 25(2), 221-236. [CrossRef]
- Avalos, G., Frazer, K., & Le Gall, H. Plant size influences specific leaf area in palms: a case for the diminishing returns hypothesis. Oecologia 2025b, 207(4), 56. [CrossRef]
- Albert, C. H., Thuiller, W., Yoccoz, N. G., Douzet, R., Aubert, S. & S Lavorel. (2010). A multi-trait approach reveals the structure and the relative importance of intra- vs. interspecific variability in plant traits. J. of Ecol. 24(6), 1192–1201.
- Fajardo, A. & Siefert, A. Intraspecific trait variation and the leaf economics spectrum across resource gradients and levels of organization. Ecology 2018, 99(5), 1024-1030. [CrossRef]
- Siefert, A., Violle, C., Chalmandrier, L., Albert, C., H., Taudiere, A., Fajardo, A.… & Wardle DA. A global meta-analysis of the relative extent of intraspecific trait variation in plant communities. Ecology 2015, 18(12),1406-19. [CrossRef]
- Niklas, K. J., & Cobb, E. D. Evidence for "diminishing returns" from the scaling of stem diameter and specific leaf. Am. J. Bot. 2008, 95(5), 549-557.
- Falster, D. S., Duursma, R. A., & FitzJohn, R. G. How functional traits influence plant growth and shade tolerance across the life cycle. Proc. Natl. Acad. Sci. U.S.A. 2018, 115(29), E6789-E6798. [CrossRef]
- Kitajima, K., & Poorter, L. Functional basis for resource niche partitioning by tropical trees. In Tropical Forest Community Ecology; Carson, W.P., Schnitzer S.A., Eds.; John Wiley & Sons: Hoboken, New Jersey, USA, 2011; pp. 160-181.
- Dayrell, R. L., Arruda, A. J., Pierce, S., Negreiros, D., Meyer, P. B., Lambers, H., & Silveira, F. A. Ontogenetic shifts in plant ecological strategies. Funct. Ecol. 2018, 32(12), 2730-2741. [CrossRef]
- Sendall, K. M., Lusk, C. H. & Reich, P. B. Becoming less tolerant with age: Sugar maple, shade, and ontogeny. Oecologia 2015, 179, 1011–1021. [CrossRef]
- Ralph, P. J., & Gademann, R. Rapid light curves: A powerful tool to assess photosynthetic activity. Aquat. Bot. 2005, 82(3), 222-237. [CrossRef]
- Mishra, A.N. Chlorophyll Fluorescence: A Practical Approach to Study Ecophysiology of Green Plants. In Advances in Plant Ecophysiology Techniques; Sánchez-Moreiras, A., Reigosa, M., Eds.; Springer, Cham, Switzerland, 2018; pp. 77-97.
- Pleban, J. R., Guadagno, C. R., Mackay, D. S., Weinig, C., & Ewers, B. E. Rapid chlorophyll a fluorescence light response curves mechanistically inform photosynthesis modeling. Plant Physiol. 2020, 183(2), 602-619. [CrossRef]
- Guimarães, G. C., de Souza, J. P., Silva, L. C., & Franco, A. C. Leaf economic spectrum and photosynthetic performance in Neotropical savanna tree species. Funct. Plant Biol. 2021, 48(2), 133–143.
- Liu, Y. H., Liu, F. L., Long, B., Zhou, X. L., Zhang, X., Zhang, Y., Wang, W. L. & S. K. Shen. Chlorophyll fluorescence characteristics and rapid light response curves of alpine rhododendron species across elevation gradients. Hort. Sci. and Tech. 2019, 37(4), 463-472. [CrossRef]
- Demmig-Adams, B., & Adams, W. W. Photoprotection and other responses of plants to high light stress. Annu. Rev. Plant Physiol. Plant Mol. Biol. 1992, 43, 599–626. [CrossRef]
- Swoczyna, T., Kalaji, H. M., Bussotti, F., Mojski, J., & Pollastrini, M. Environmental stress-what can we learn from chlorophyll a fluorescence analysis in woody plants? A review. Front Plant Sci. 2022, 13, 1048582. [CrossRef]
- White, A. J., & Critchley, C. Rapid light curves: a new fluorescence method to assess the state of the photosynthetic apparatus. Photosynth. Res. 1999, 59, 63-72. [CrossRef]
- Liu, Y. H., Liu, F. L., Long, B., Zhou, X. L., Zhang, X., Zhang, Y., ... & Shen, S. K. Chlorophyll fluorescence characteristics and rapid light response curves of alpine rhododendron species across elevation gradients. Hortic. Sci. Technol. 2019, 37(4), 463-472. [CrossRef]
- Tripathi, S., Bhadouria, R., Srivastava, P., Devi, R. S., Chaturvedi, R., & Raghubanshi, A. S. Effects of light availability on leaf attributes and seedling growth of four tree species in tropical dry forest. Ecol. Process. 2020, 9, 1-16. [CrossRef]
- Opler, P. A., Frankie, G. W., & Baker, H. G. Comparative phenological studies of treelet and shrub species in tropical wet and dry forests in the lowlands of Costa Rica. J. Ecol. 1980, 167-188. [CrossRef]
- Westoby, M., Falster, D. S., Moles, A. T., Vesk, P. A., & Wright, I. J. Plant ecological strategies: some leading dimensions of variation between species. Annu. Rev. Ecol. Evol. Syst. 2002, 33, 125-159. [CrossRef]
- Sterck, F., Markesteijn, L., Schieving, F., & Poorter, L. Functional traits determine trade-offs and niches in a tropical forest community. Proc. Natl. Acad. Sci. U.S.A. 2011, 108(51), 20627-20632. [CrossRef]
- Díaz, S., Kattge, J., Cornelissen, J. H., Wright, I. J., Lavorel, S., Dray, S., ... & Zotz, G. The global spectrum of plant form and function: enhanced species-level trait dataset. Sci. Data 2022, 9(1), 755. [CrossRef]
- Santiago, L. S., Kitajima, K., Wright, S. J., & Mulkey, S. S. Coordinated changes in photosynthesis, water relations and leaf nutritional traits of canopy trees along a precipitation gradient in lowland tropical forest. Oecologia 2004, 139, 495-502. [CrossRef]
- Nathan, J., Osem, Y., Shachak, M. & E. Meron. Linking functional diversity to resource availability and disturbance: a mechanistic approach for water-limited plant communities. J. Ecol. 2016, (104)2, 419-429. [CrossRef]
- Liu, Y., Dawson, W., Prati, D., Haeuser, E., Feng, Y., & van Kleunen, M. Does greater specific leaf area plasticity help plants to maintain a high performance when shaded?. Ann. Bot. 2016, 118, 1329–1336. [CrossRef]
- Holdridge, L. R., Grenke, W. C., Hatheway, W. H., Liang, T., & Tosi, J. A. Forest Environments in Tropical Life Zones: A Pilot Study; Pergamon Press: Oxford, UK, 1971.
- Ståhl, B. A synopsis of Central American Theophrastaceae. Nord. J. Bot. 1989, 9(1), 15-30. [CrossRef]
- Ståhl, B., & Källersjö, M. Reinstatement of Bonellia (Theophrastaceae). Novon 2004, 14(1), 115–118.
- Morales, J. F. Theophrastaceae. In Manual de plantas de Costa Rica; Hammel, B.E., Grayum, M.H., Herrera, C., Zamora, N. Eds.; Monographs in Systematic Botany from the Missouri Botanical Garden, St. Louis, Missouri, USA, 2015; Volume 8; Dicotiledóneas (Sabiaceae–Zygophyllaceae), pp. 415-420.
- Domínguez-Calleros, P.A., Rodríguez-Flores, F.D.J., Lizárraga-Mendiola, L., Jiménez-Gómez, M.A. & J. Navar. Aplicaciones y ejemplos de modelos de crecimiento diamétrico para árboles tropicales. Ecosist. Recur. Agropec. 2017, 4(11), 265–274. [CrossRef]
- Platt, T., Gallegos, C.L. & W.G. Harrison. Photoinhibition of photosynthesis in natural assemblages of marine phytoplankton. J. Mar. Res. 1980, 57, 341–345.
- R Core Team (2025). R: A language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria. https://www.R-project.org/.
- Elzhov, T., V., Mullen, K.M., Spiess, A., Bolker, B. minpack.lm: R Interface to the Levenberg-Marquardt Nonlinear Least-Squares Algorithm Found in MINPACK, Plus Support for Bounds. R package version 1.2-4, 2023. Available online: https://CRAN.R-project.org/package=minpack.lm.
- Wickham, H. ggplot2: Elegant Graphics for Data Analysis. Springer-Verlag: New York, New York, USA, 2016. Available online: https://ggplot2.tidyverse.org.
- Wickham, H., Vaughan, D., Girlich, M. tidyr: Tidy Messy Data. R package version 1.3.1, 2024. Available online: https://github.com/tidyverse/tidyr. https://tidyr.tidyverse.org.
- Stenberg, P., Kangas, T., Smolander, H., & Linder, S. Shoot structure, canopy openness, and light interception in Norway spruce. Plant Cell Environ. 1999, 22(9), 1133-1142. [CrossRef]
- JMP Statistical Discovery LLC. JMP® (Version 18.2.1). JMP Statistical Discovery LLC: North Carolina, USA, 2025. Available online: https://www.jmp.com/.
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).





