Submitted:
14 June 2025
Posted:
17 June 2025
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Materials and Methods
2.1. The Human Protein Atlas, TNM Plot, and Kaplan–Meier Plot Analysis
2.2. Cell Culture
2.4. MTT Cell Viability Assay
2.5. Cell Cycle Analysis
2.6. Hanging Drop Assay for 3D Sphere Formation
2.7. Cell Migration Assay
2.8. Total RNA Extraction and Quantitative Real-Time PCR
2.9. Western Blotting
2.10. In-Silico Protein Interaction Network Analysis
2.11. Statistical Analysis
3. Results
3.1. SDC3 Is Overexpressed in Breast Cancer
3.2. SDC3 Expression Affects the Prognosis and Survival of Breast Cancer Patients
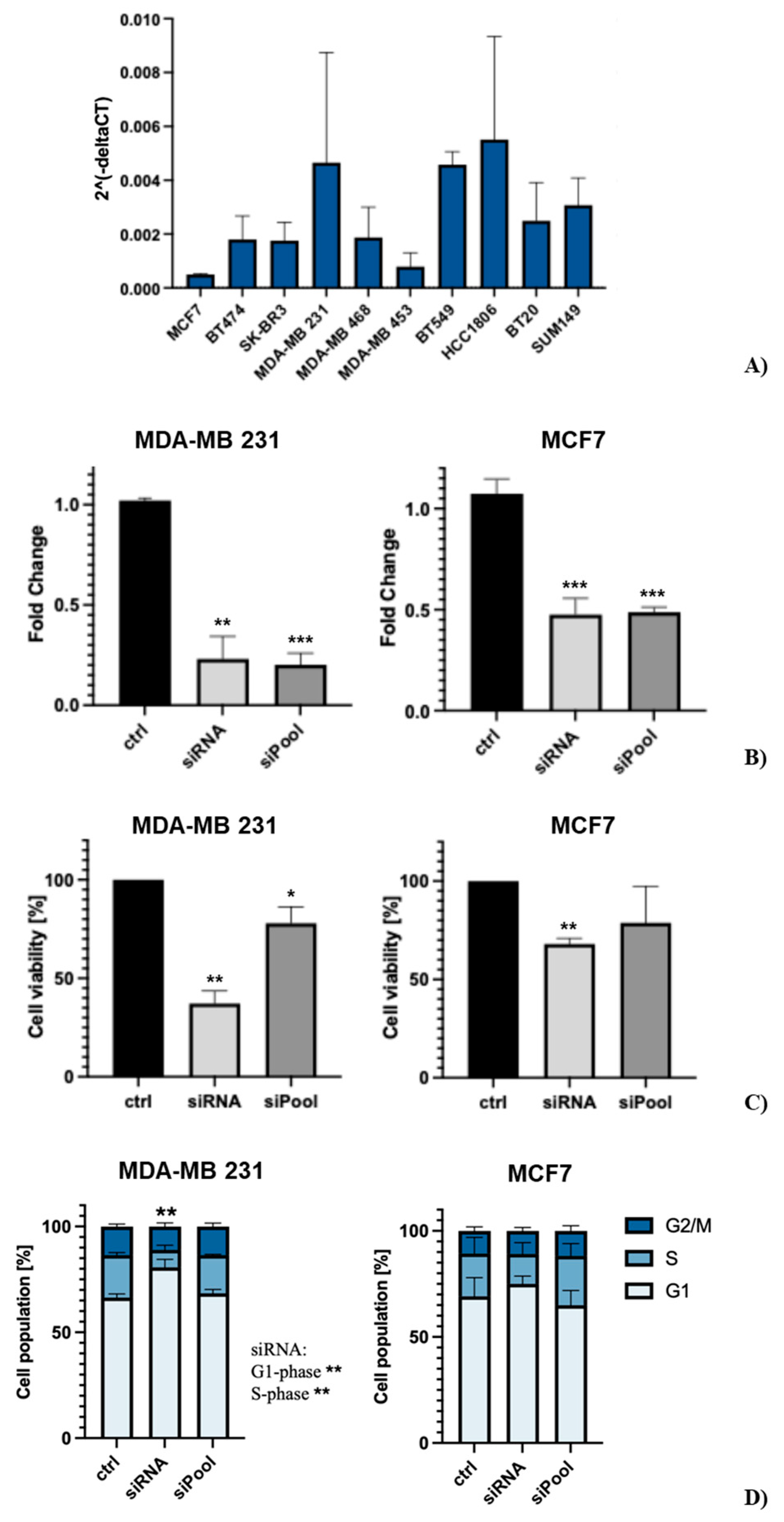
3.3. SDC3 RNA Expression Varies in Breast Cancer Cell Lines of Different Classification
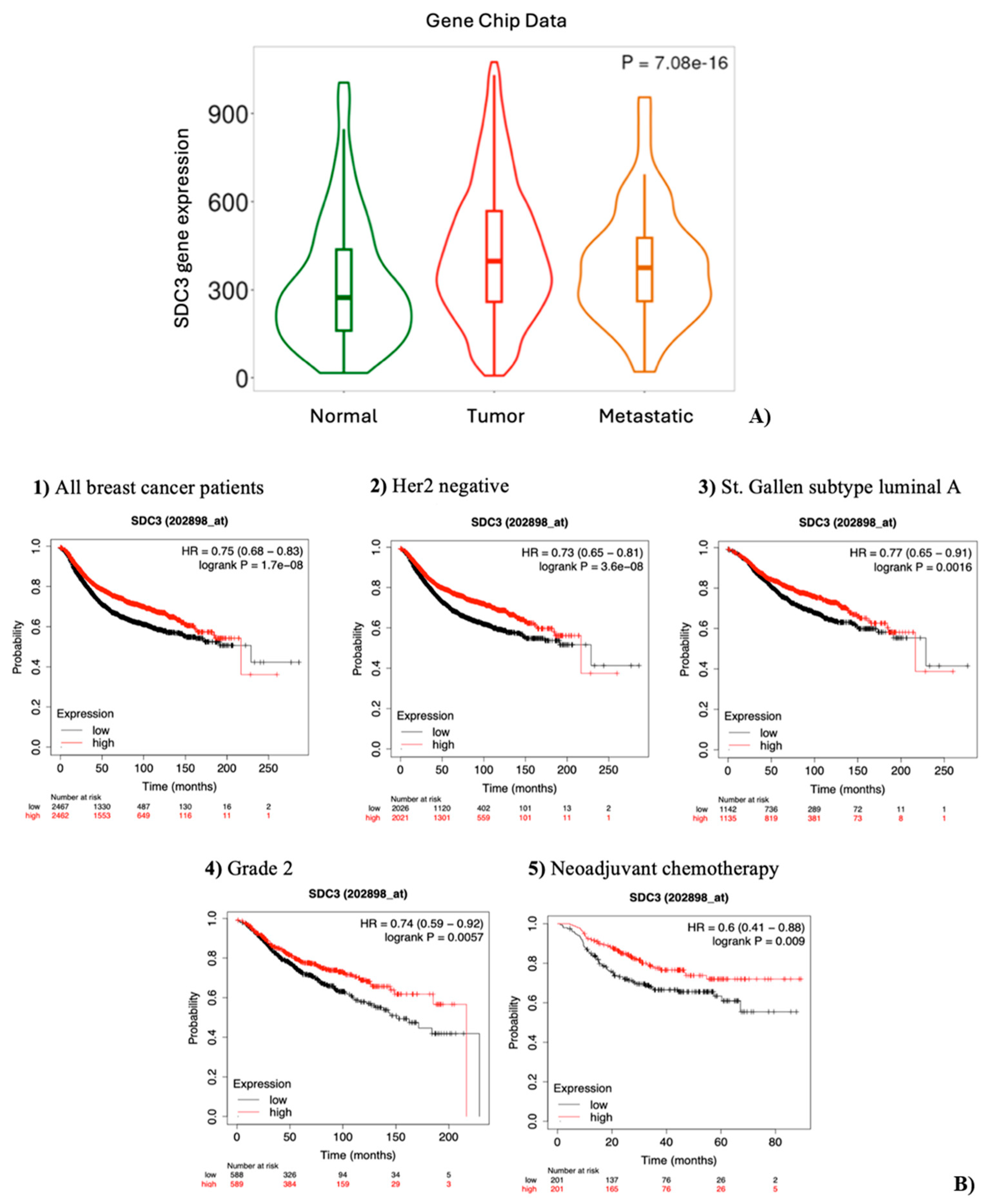
| Classification | Status | Cases | Hazard Ratio | P value |
|---|---|---|---|---|
| All breast cancer patients | 4929 | 0.75 (0.68 - 0.83) | Log-rank P = 1.7e-08 | |
| Estrogen receptor (ER) | Positive | 2561 | 0.87 (0.75 – 1.02) | Log-rank P = 0.079 |
| Negative | 796 | 0.81 (0.64 – 1.03) | Log-rank P =0.086 | |
| Progesterone receptor (PR) | Positive | 926 | 0.95 (0.71 – 1.26) | Log-rank P = 0.72 |
| Negative | 925 | 0.91 (0.72 – 1.14) | Log-rank P = 0.41 | |
| Her2 | Positive | 882 | 0.85 (0.68 – 1.06) | Log-rank P = 0.15 |
| Negative | 4047 | 0.73 (0.65 – 0.81) | Log-rank P = 3.6e-08 | |
| ER, PR, Her2 | Negative | 392 | 0.9 (0.63 – 1.29) | Log-rank P = 0.56 |
| St. Gallen subtype | Luminal A | 2277 | 0.77 (0.65 – 0.91) | Log-rank P = 0.0016 |
| Luminal B | 1419 | 0.71 (0.59 – 0.85) | Log-rank P = 0.00015 | |
| Her2 positive | 315 | 0.9 (0.64 – 1.29) | Log-rank P = 0.6 | |
| Basal | 846 | 0.72 (0.58 – 0.9) | Log-rank P = 0.004 | |
| PAM50 subtype | Luminal A | 1809 | 0.88 (0.72 – 1.08) | Log-rank P = 0.23 |
| Luminal B | 1353 | 0.83 (0.7 – 0.99) | Log-rank P = 0.038 | |
| Her2 positive | 695 | 0.86 (0.68 – 1.1) | Log-rank P = 0.23 | |
| Basal | 953 | 0.69 (0.56 – 0.86) | Log-rank P = 7e-04 | |
| Lymph node | Positive | 1656 | 0.97 (0.82 – 1.15) | Log-rank P = 0.75 |
| Negative | 2368 | 0.74 (0.63 – 0.87) | Log-rank P = 0.00027 | |
| Grade | 1 | 397 | 1.12 (0.73 – 2) | Log-rank P = 0.47 |
| 2 | 1177 | 0.74 (0.59 – 0.92) | Log-rank P = 0.0057 | |
| 3 | 1300 | 0.88 (0.73 – 1.06) | Log-rank P = 0.16 | |
| Treatment with chemotherapy | Neoadjuvant | 402 | 0.6 (0.41 – 0.88) | Log-rank P = 0.009 |
| Adjuvant | 458 | 0.93 (0.67 – 1.3) | Log-rank P = 0.68 | |
| Treatment with endocrine therapy | 867 | 0.86 (0.66 – 1.12) | Log-rank P = 0.25 | |
| Treatment with chemotherapy and endocrine therapy | 510 | 1.22 (0.81 – 1.83) | Log-rank P = 0.34 |
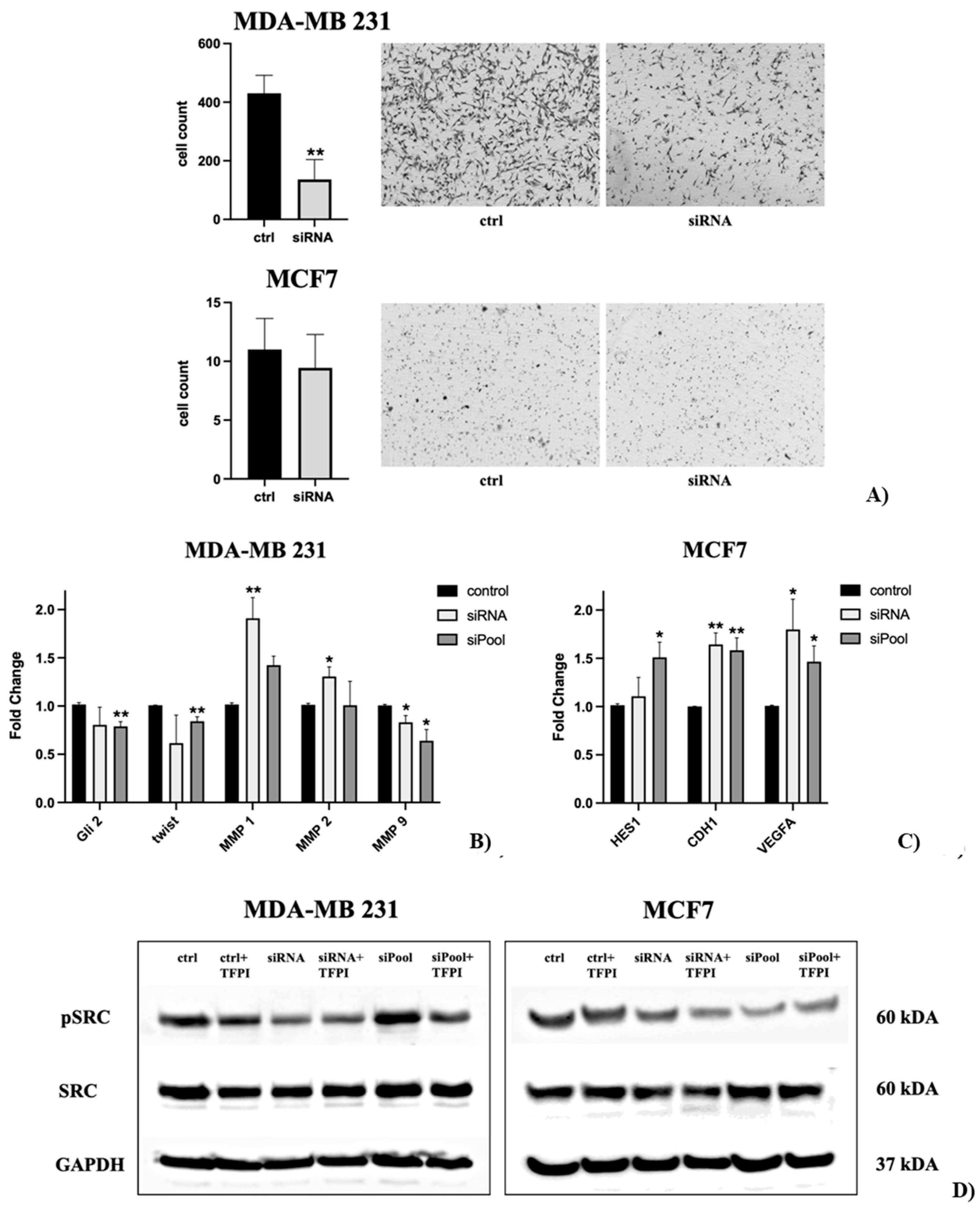
3.4. SDC3 Depletion Affects the Metabolic Activity and Cell Cycle of Human MDA-MB-231 and MCF-7 Breast Cancer Cells
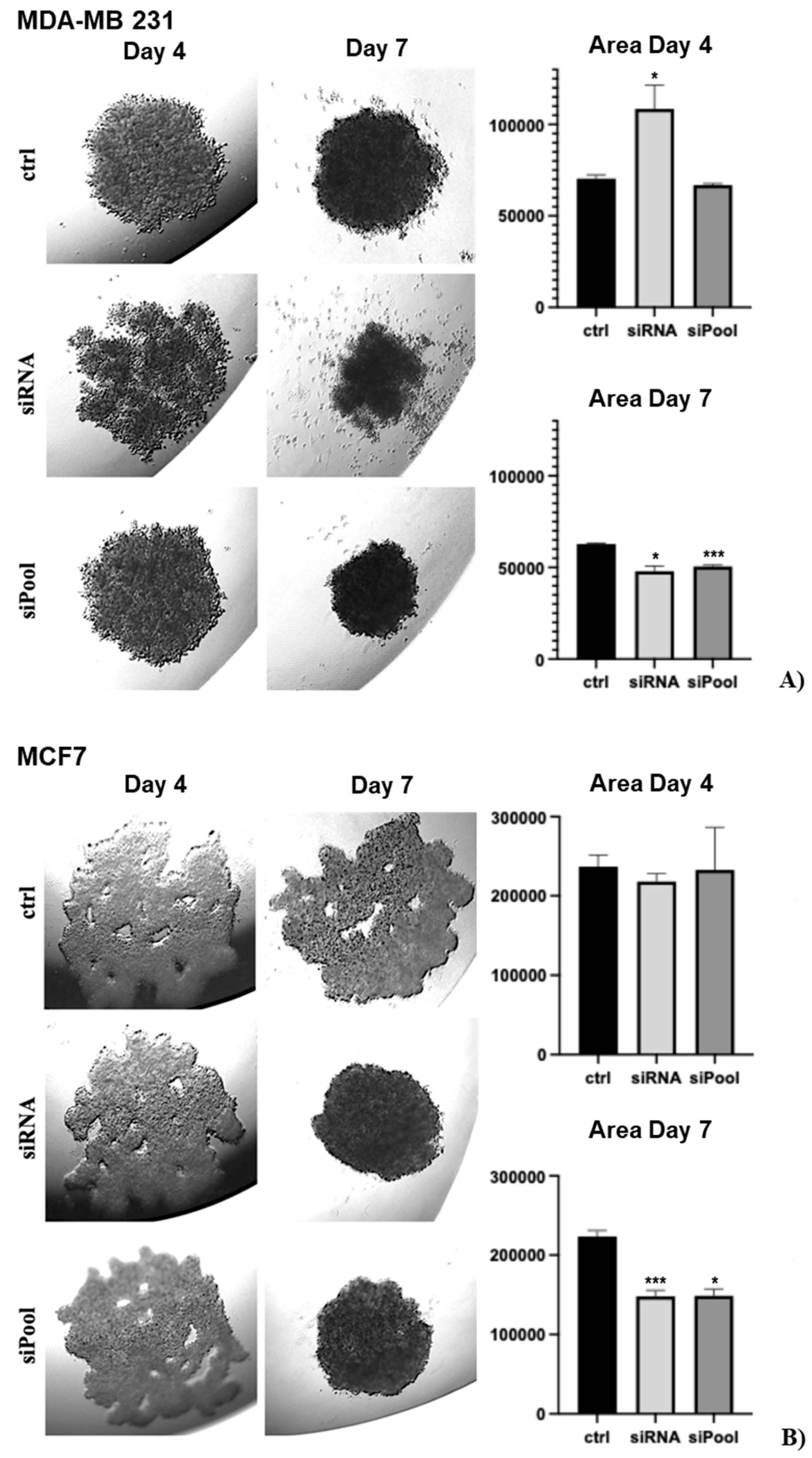
3.5. SDC3 Depletion Affects Three-Dimensional Spheroid Growth of Human MDA-MB-231 and MCF-7 Breast Cancer Cells
3.6. SDC3 Depletion Affects Cell Migration of Human MDA-MB-231 and MCF-7 Breast Cancer Cells
3.7. SDC3 Depletion Affects the RNA Expression of Target Genes Associated with Relevant Signaling Pathways in Breast Cancer
3.8. SDC3 Depletion Affects the Activation of SRC of Human MDA-MB-231 and MCF-7 Breast Cancer Cells
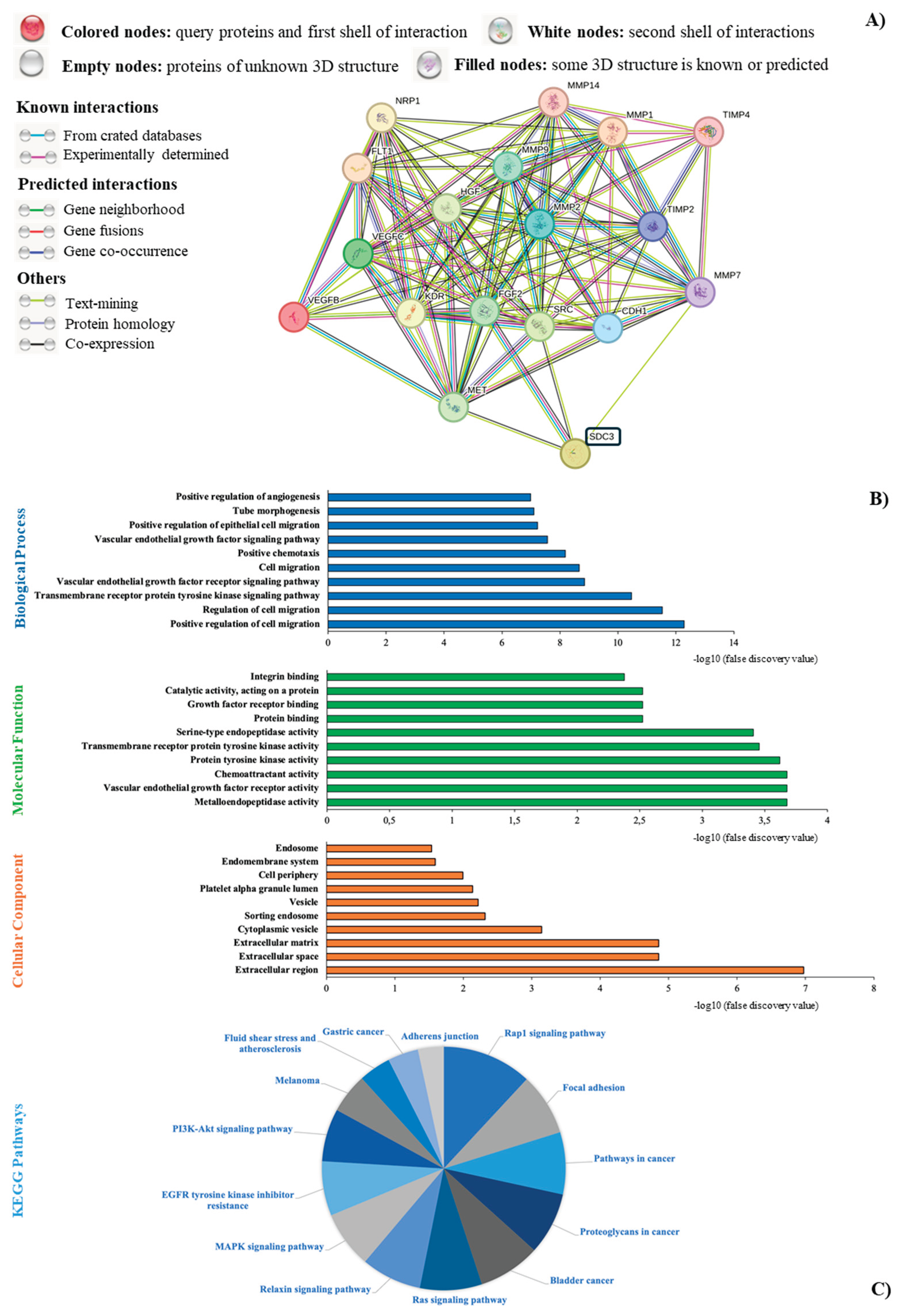
3.9. STRING Functional Enrichment Analysis
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Bray, F.; Laversanne, M.; Sung, H.; Ferlay, J.; Siegel, R.L.; Soerjomataram, I.; Jemal, A. Global cancer statistics 2022: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA: A Cancer Journal for Clinicians 2024, 74, 229–263. [Google Scholar] [CrossRef] [PubMed]
- Łukasiewicz, S.; Czeczelewski, M.; Forma, A.; Baj, J.; Sitarz, R.; Stanisławek, A. Breast Cancer - Epidemiology, Risk Factors, Classification, Prognostic Markers, and Current Treatment Strategies - An Updated Review. Cancers 2021, 13, 1–30. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X. Molecular Classification of Breast Cancer Relevance and Challenges. Archives of Pathology and Laboratory Medicine 2023, 147, 46–51. [Google Scholar] [CrossRef]
- Smolarz, B.; Nowak, A.Z.; Romanowicz, H. Breast Cancer—Epidemiology, Classification, Pathogenesis and Treatment (Review of Literature). Cancers 2022, 14. [Google Scholar] [CrossRef]
- Karamanos, N.K.; Piperigkou, Z.; Passi, A.; Götte, M.; Rousselle, P.; Vlodavsky, I. Extracellular matrix-based cancer targeting. Trends in Molecular Medicine 2021, 27, 1000–1013. [Google Scholar] [CrossRef]
- Theocharis, A.D.; Skandalis, S.S.; Gialeli, C.; Karamanos, N.K. Extracellular matrix structure. Advanced Drug Delivery Reviews 2016, 97, 4–27. [Google Scholar] [CrossRef]
- Pickup, M.W.; Mouw, J.K.; Weaver, V.M. The extracellular matrix modulates the hallmarks of cancer. EMBO reports 2014, 15, 1243–1253. [Google Scholar] [CrossRef]
- Lu, P.; Weaver, V.M.; Werb, Z. The extracellular matrix: A dynamic niche in cancer progression. Journal of Cell Biology 2012, 196, 395–406. [Google Scholar] [CrossRef]
- Schaefer, L.; Schaefer, R.M. Proteoglycans: from structural compounds to signaling molecules. Cell and Tissue Research 2010, 339, 237–246. [Google Scholar] [CrossRef]
- Wei, J.; Hu, M.; Huang, K.; Lin, S.; Du, H. Molecular Sciences Roles of Proteoglycans and Glycosaminoglycans in Cancer Development and Progression. International Journal of Molecular Sciences 2020, 21, 59–83. [Google Scholar] [CrossRef]
- Espinoza-Sánchez, N.A.; Götte, M. Role of cell surface proteoglycans in cancer immunotherapy. Seminars in Cancer Biology 2020, 62, 48–67. [Google Scholar] [CrossRef] [PubMed]
- Mouw, J.K.; Ou, G.; Weaver, V.M. Extracellular matrix assembly: a multiscale deconstruction. Nature Reviews Molecular Cell Biology 2014, 15, 771–785. [Google Scholar] [CrossRef] [PubMed]
- Bernfield, M.; Götte, M.; Park, P.W.; Reizes, O.; Fitzgerald, M.L.; Lincecum, J.; Zako, M. Functions of Cell Surface Heparan Sulfate Proteoglycans. Annual Review of Biochemistry 1999, 68, 729–777. [Google Scholar] [CrossRef] [PubMed]
- Hassan, N.; Greve, B.; Espinoza-Sánchez, N.A.; Götte, M. Cell-surface heparan sulfate proteoglycans as multifunctional integrators of signaling in cancer. Cellular Signalling 2021, 77, 1–21. [Google Scholar] [CrossRef]
- Piperigkou, Z.; Mohr, B.; Karamanos, N.; Götte, M. Shed proteoglycans in tumor stroma. Cell and Tissue Research 2016, 365, 643–655. [Google Scholar] [CrossRef]
- Motta, J.M.; Hassan, H.; Ibrahim, S.A. Revisiting the Syndecans: Master Signaling Regulators with Prognostic and Targetable Therapeutic Values in Breast Carcinoma. Cancers 2023, 15. [Google Scholar] [CrossRef]
- Hillemeyer, L.; Espinoza-Sanchez, N.A.; Greve, B.; Hassan, N.; Chelariu-Raicu, A.; Kiesel, L.; Götte, M. The Cell Surface Heparan Sulfate Proteoglycan Syndecan-3 Promotes Ovarian Cancer Pathogenesis. International Journal of Molecular Sciences 2022, 23. [Google Scholar] [CrossRef]
- Yao, J.; Zhang, L.L.; Huang, X.M.; Li, W.Y.; Gao, S.G. Pleiotrophin and N-syndecan promote perineural invasion and tumor progression in an orthotopic mouse model of pancreatic cancer. World Journal of Gastroenterology 2017, 23, 3907–3914. [Google Scholar] [CrossRef]
- Yamada, Y.; Arai, T.; Kojima, S.; Sugawara, S.; Kato, M.; Okato, A.; … Seki, N. Regulation of antitumor miR-144-5p targets oncogenes: Direct regulation of syndecan-3 and its clinical significance. Cancer Science 2018, 109, 2919–2936. [Google Scholar] [CrossRef]
- Digre, A.; Lindskog, C. The Human Protein Atlas—Spatial localization of the human proteome in health and disease. Protein Science 2021, 30, 218–233. [Google Scholar] [CrossRef]
- Bartha, Á.; Győrffy, B. TNMplot.com: A Web Tool for the Comparison of Gene Expression in Normal, Tumor and Metastatic Tissues. International Journal of Molecular Sciences 2021, 22, 1–22. [Google Scholar] [CrossRef] [PubMed]
- Győrffy, B. Survival analysis across the entire transcriptome identifies biomarkers with the highest prognostic power in breast cancer. Computational and Structural Biotechnology Journal 2021, 19, 4101–4109. [Google Scholar] [CrossRef] [PubMed]
- Stejskalová, A.; Fincke, V.; Nowak, M.; Schmidt, Y.; Borrmann, K.; von Wahlde, M.-K.; … Götte, M. Collagen I triggers directional migration, invasion and matrix remodeling of stroma cells in a 3D spheroid model of endometriosis. Scientific Reports 2021, 11, 4115. [Google Scholar] [CrossRef] [PubMed]
- Rao, X.; Huang, X.; Zhou, Z.; Lin, X. An improvement of the 2ˆ(-delta delta CT) method for quantitative real-time polymerase chain reaction data analysis. Biostat Bioinforma Biomath 2013, (3), 71–85. [Google Scholar]
- Szklarczyk, D.; Kirsch, R.; Koutrouli, M.; Nastou, K.; Mehryary, F.; Hachilif, R.; … von Mering, C. The STRING database in 2023: protein–protein association networks and functional enrichment analyses for any sequenced genome of interest. Nucleic Acids Research 2023, 51, 638–646. [Google Scholar] [CrossRef]
- Dai, X.; Cheng, H.; Bai, Z.; Li, J. Breast Cancer Cell Line Classification and Its Relevance with Breast Tumor Subtyping. Journal of Cancer 2017, 8, 3131–3141. [Google Scholar] [CrossRef]
- Holliday, D.L.; Speirs, V. Choosing the right cell line for breast cancer research. Breast Cancer Research 2011, 13. [Google Scholar] [CrossRef]
- Banerjee, M.; Bhonde, R.R. Application of hanging drop technique for stem cell differentiation and cytotoxicity studies. Cytotechnology 2006, 51, 1–5. [Google Scholar] [CrossRef]
- Kurosawa, H. Methods for inducing embryoid body formation: in vitro differentiation system of embryonic stem cells. Journal of Bioscience and Bioengineering 2007, 103, 389–398. [Google Scholar] [CrossRef]
- Vitale, D.; Kumar Katakam, S.; Greve, B.; Jang, B.; Oh, E.; Alaniz, L.; Götte, M. Proteoglycans and glycosaminoglycans as regulators of cancer stem cell function and therapeutic resistance. The FEBS Journal 2019, 286, 2870–2882. [Google Scholar] [CrossRef]
- Kuehn, J.; Espinoza-Sanchez, N.A.; Teixeira, F.C.O.B.; Pavão, M.S.G.; Kiesel, L.; Győrffy, B. , … Götte, M. Prognostic significance of hedgehog signaling network-related gene expression in breast cancer patients. Journal of Cellular Biochemistry 2021, 122, 577–597. [Google Scholar] [CrossRef] [PubMed]
- Corti, F.; Ristori, E.; Rivera-Molina, F.; Toomre, D.; Zhang, J.; Mihailovic, J.; … Simons, M. Syndecan-2 selectively regulates VEGF-induced vascular permeability. Nature Cardiovascular Research 2022, 1, 518–528. [Google Scholar] [CrossRef] [PubMed]
- Jang, B.; Kim, A.; Hwang, J.; Song, H.-K.; Kim, Y.; Oh, E.-S. Emerging Role of Syndecans in Extracellular Matrix Remodeling in Cancer. Journal of Histochemistry & Cytochemistry 2020, 68, 863–870. [Google Scholar] [CrossRef]
- Gopal, S. Syndecans in Inflammation at a Glance. Frontiers in Immunology 2020, 11. [Google Scholar] [CrossRef]
- Huang, X.; Reye, G.; Momot, K.I.; Blick, T.; Lloyd, T.; Tilley, W.D. , … Hugo, H.J. Heparanase Promotes Syndecan-1 Expression to Mediate Fibrillar Collagen and Mammographic Density in Human Breast Tissue Cultured ex vivo. Frontiers in Cell and Developmental Biology 2020, 8. [CrossRef]
- Hassan, N.; Bückreiß, N.; Efing, J.; Schulz-Fincke, M.; König, P.; Greve, B.; … Götte, M. The Heparan Sulfate Proteoglycan Syndecan-1 Triggers Breast Cancer Cell-Induced Coagulability by Induced Expression of Tissue Factor. Cells 2023, 12, 910. [Google Scholar] [CrossRef]
- Tinholt, M.; Stavik, B.; Louch, W.; Carlson, C.R.; Sletten, M.; Ruf, W. , … Iversen, N. Syndecan-3 and TFPI Colocalize on the Surface of Endothelial-, Smooth Muscle-, and Cancer Cells. PLOS ONE 2015, 10. [CrossRef]
- Czarnowski, D. Syndecans in cancer: A review of function, expression, prognostic value, and therapeutic significance. Cancer Treatment and Research Communications 2021, 27. [Google Scholar] [CrossRef]
- Prieto-Fernández, E.; Egia-Mendikute, L.; Bosch, A.; García del Río, A.; Jimenez-Lasheras, B.; Antoñana-Vildosola, A.; … Palazon, A. Hypoxia Promotes Syndecan-3 Expression in the Tumor Microenvironment. Frontiers in Immunology 2020, 11. [Google Scholar] [CrossRef]
- Lee, S.Y.; Prieto-Fernández, E.; Egia-Mendikute, L.; Antoñana-Vildosola, A.; Velasco-Beltrán, P.; Bosch, A. , … Pérez-Gutiérrez, L. Syndecan-3 positively regulates the pro-inflammatory function of macrophages. Cellular and Molecular Life Sciences 2025, 82, 145. [Google Scholar] [CrossRef]
- Wu, Z.-S.; Pandey, V.; Wu, W.-Y.; Ye, S.; Zhu, T.; Lobie, P.E. Prognostic significance of the expression of GFRα1, GFRα3 and Syndecan-3, proteins binding ARTEMIN, in mammary carcinoma. BMC Cancer 2013, 13. [Google Scholar] [CrossRef]
- Jiang, F.; Wu, C.; Wang, M.; Wei, K.; Wang, J. Identification of novel cell glycolysis related gene signature predicting survival in patients with breast cancer. Scientific Reports 2021, 11. [Google Scholar] [CrossRef]
- Watanabe, H.; Fukuda, A.; Ikeda, N.; Sato, M.; Hashimoto, K.; Miyamoto, Y. Syndecan-3 regulates the time of transition from cell cycle exit to initial differentiation stage in mouse cerebellar granule cell precursors. Brain Research 2023, 1807, 148317. [Google Scholar] [CrossRef] [PubMed]
- Pisconti, A.; Cornelison, D.D. W.; Olguín, H.C.; Antwine, T.L.; Olwin, B.B. Syndecan-3 and Notch cooperate in regulating adult myogenesis. Journal of Cell Biology 2010, 190, 427–441. [Google Scholar] [CrossRef]
- Ordaz-Ramos, A., Tellez-Jimenez, O., & Vazquez-Santillan, K. (2023). Signaling pathways governing the maintenance of breast cancer stem cells and their therapeutic implications. [CrossRef]
- 46. Katakam, S.K.; Pelucchi, P.; Cocola, C.; Reinbold, R.; Vlodavsky, I.; Greve, B.; Götte, M. Syndecan-1-Dependent Regulation of Heparanase Affects Invasiveness, Stem Cell Properties, and Therapeutic Resistance of Caco2 Colon Cancer Cells. Frontiers in Oncology 2020, 10. [Google Scholar] [CrossRef] [PubMed]
- Valla, S.; Hassan, N.; Vitale, D.L.; Madanes, D.; Spinelli, F.M.; Teixeira, F.C.O.B. , … Götte, M. Syndecan-1 depletion has a differential impact on hyaluronic acid metabolism and tumor cell behavior in luminal and triple-negative breast cancer cells. International Journal of Molecular Sciences 2021, 22. [Google Scholar] [CrossRef]
- Teixeira, F.C.O. B.; Vijaya Kumar, A.; Kumar Katakam, S.; Cocola, C.; Pelucchi, P.; Graf, M., … Götte, M. The Heparan Sulfate Sulfotransferases HS2ST1 and HS3ST2 Are Novel Regulators of Breast Cancer Stem-Cell Properties. Frontiers in Cell and Developmental Biology 2020, 8. [CrossRef]
- Jones, F.K.; Stefan, A.; Kay, A.G.; Hyland, M.; Morgan, R.; Forsyth, N.R. , … Kehoe, O. Syndecan-3 regulates MSC adhesion, ERK and AKT signalling in vitro and its deletion enhances MSC efficacy in a model of inflammatory arthritis in vivo. Scientific Reports 2020, 10. [Google Scholar] [CrossRef]
- Inatani, M.; Haruta, M.; Honjo, M.; Oohira, A.; Kido, N.; Takahashi, M.; … Tanihara, H. Upregulated expression of N-syndecan, a transmembrane heparan sulfate proteoglycan, in differentiated neural stem cells. Brain Research 2001, 920(1–2), 217–221. [CrossRef]
- Edwards, A.; Brennan, K. Notch Signalling in Breast Development and Cancer. Frontiers in Cell and Developmental Biology 2021, 9, 1–24. [Google Scholar] [CrossRef]
- Depau, L.; Brunetti, J.; Falciani, C.; Mandarini, E.; Zanchi, M.; Paolocci, M.F., … Bracci, L. Targeting heparan sulfate proteoglycans as an effective strategy for inhibiting cancer cell migration and invasiveness compared to heparin. Frontiers in Cell and Developmental Biology 2025, 12. [CrossRef]
- Hienola, A.; Tumova, S.; Kulesskiy, E.; Rauvala, H. N-syndecan deficiency impairs neural migration in brain. The Journal of Cell Biology 2006, 174, 569–580. [Google Scholar] [CrossRef]
- Bespalov, M.M.; Sidorova, Y.A.; Tumova, S.; Ahonen-Bishopp, A.; Magalhães, A.C.; Kulesskiy, E. , … Saarma, M. Heparan sulfate proteoglycan syndecan-3 is a novel receptor for GDNF, neurturin, and artemin. Journal of Cell Biology 2011, 192, 153–169. [Google Scholar] [CrossRef]
- Eustace, A.D.; McNaughton, E.F.; King, S.; Kehoe, O.; Kungl, A.; Mattey, D., … Middleton, J. Soluble syndecan-3 binds chemokines, reduces leukocyte migration in vitro and ameliorates disease severity in models of rheumatoid arthritis. Arthritis Research & Therapy 2019, 21. [CrossRef]
- Eustace, A.D.; McNaughton, E.F.; King, S.; Kehoe, O.; Kungl, A.; Mattey, D., … Middleton, J. Soluble syndecan-3 binds chemokines, reduces leukocyte migration in vitro and ameliorates disease severity in models of rheumatoid arthritis. Arthritis Research and Therapy 2019, 21. [CrossRef]
- Felipe Lima, J.; Nofech-Mozes, S.; Bayani, J.; Bartlett, J. EMT in Breast Carcinoma - A Review. Journal of Clinical Medicine 2016, 5, 1–14. [Google Scholar] [CrossRef]
- Kessenbrock, K.; Plaks, V.; Werb, Z. Matrix Metalloproteinases: Regulators of the Tumor Microenvironment. Cell 2010, 141, 52–67. [Google Scholar] [CrossRef] [PubMed]
- Dufour, A.; Sampson, N.S.; Zucker, S.; Cao, J. Role of the hemopexin domain of matrix metalloproteinases in cell migration. Journal of Cellular Physiology 2008, 217, 643–651. [Google Scholar] [CrossRef] [PubMed]
- Asundi, V.K.; Erdman, R.; Stahl, R.C.; Carey, D.J. Matrix metalloproteinase-dependent shedding of syndecan-3, a transmembrane heparan sulfate proteoglycan, in Schwann cells. Journal of Neuroscience Research 2003, 73, 593–602. [Google Scholar] [CrossRef] [PubMed]
- Arokiasamy, S.; Balderstone, M.J. M.; De Rossi, G.; Whiteford, J.R. Syndecan-3 in Inflammation and Angiogenesis. Frontiers in Immunology 2020, 10. [Google Scholar] [CrossRef]
- Bertrand, J.; Bollmann, M. Soluble syndecans: biomarkers for diseases and therapeutic options. British Journal of Pharmacology 2019, 176, 67–81. [Google Scholar] [CrossRef]
- Onyeisi, J.O. S.; El-Shorafa, H.M.; Greve, B.; Götte, M. Role of syndecan-4 in angiogenesis and vasculogenic mimicry in triple negative breast cancer cells. Matrix Biology 2025, 136, 127–133. [Google Scholar] [CrossRef]
- Nassar, E.; Hassan, N.; El-Ghonaimy, E.A.; Hassan, H.; Abdullah, M.S.; Rottke, T.V. , … Götte, M. Syndecan-1 Promotes Angiogenesis in Triple-Negative Breast Cancer through the Prognostically Relevant Tissue Factor Pathway and Additional Angiogenic Routes. Cancers 2021, 13. [Google Scholar] [CrossRef]
- De Rossi, G.; Whiteford, J.R. A novel role for syndecan-3 in angiogenesis. F1000Research 2013, 2. [Google Scholar] [CrossRef]
- Jannaway, M.; Yang, X.; Meegan, J.E.; Coleman, D.C.; Yuan, S.Y. Thrombin-cleaved syndecan-3/-4 ectodomain fragments mediate endothelial barrier dysfunction. PLOS ONE 2019, 14. [Google Scholar] [CrossRef]
- Liang, H.; Xiao, J.; Zhou, Z.; Wu, J.; Ge, F.; Li, Z.; … Chen, C. Hypoxia induces miR-153 through the IRE1α-XBP1 pathway to fine tune the HIF1α/VEGFA axis in breast cancer angiogenesis. Oncogene 2018, 37, 1961–1975. [Google Scholar] [CrossRef]
- Kinnunen, T.; Kaksonen, M.; Saarinen, J.; Kalkkinen, N.; Peng, H.B.; Rauvala, H. Cortactin-Src Kinase Signaling Pathway Is Involved in N-syndecan-dependent Neurite Outgrowth. Journal of Biological Chemistry 1998, 273, 10702–10708. [Google Scholar] [CrossRef] [PubMed]
- Luo, J.; Zou, H.; Guo, Y.; Tong, T.; Ye, L.; Zhu, C.; … Li, P. SRC kinase-mediated signaling pathways and targeted therapies in breast cancer. Breast Cancer Research 2022, 24. [Google Scholar] [CrossRef] [PubMed]
- Jiang, B.-H.; Agani, F.; Passaniti, A.; Semenza, G.L. V-SRC Induces Expression of Hypoxia-inducible Factor 1 (HIF-1) and Transcription of Genes Encoding Vascular Endothelial Growth Factor and Enolase 1: Involvement of HIF-1 in Tumor Progression. Cancer Research 1997, 23, 5328–5335. [Google Scholar]
- Johnson, D.; Agochiya, M.; Samejima, K.; Earnshaw, W.; Frame, M.; Wyke, J. Regulation of both apoptosis and cell survival by the v-Src oncoprotein. Cell Death & Differentiation 2000, 7, 685–696. [Google Scholar] [CrossRef]
- Zhang, S.; Yu, D. Targeting Src family kinases in anti-cancer therapies: turning promise into triumph. Trends in Pharmacological Sciences 2012, 33, 122–128. [Google Scholar] [CrossRef]
- Hanna, S.C.; Krishnan, B.; Bailey, S.T.; Moschos, S.J.; Kuan, P.-F.; Shimamura, T. , … Kim, W.Y. HIF1α and HIF2α independently activate SRC to promote melanoma metastases. Journal of Clinical Investigation 2013, 123, 2078–2093. [Google Scholar] [CrossRef]
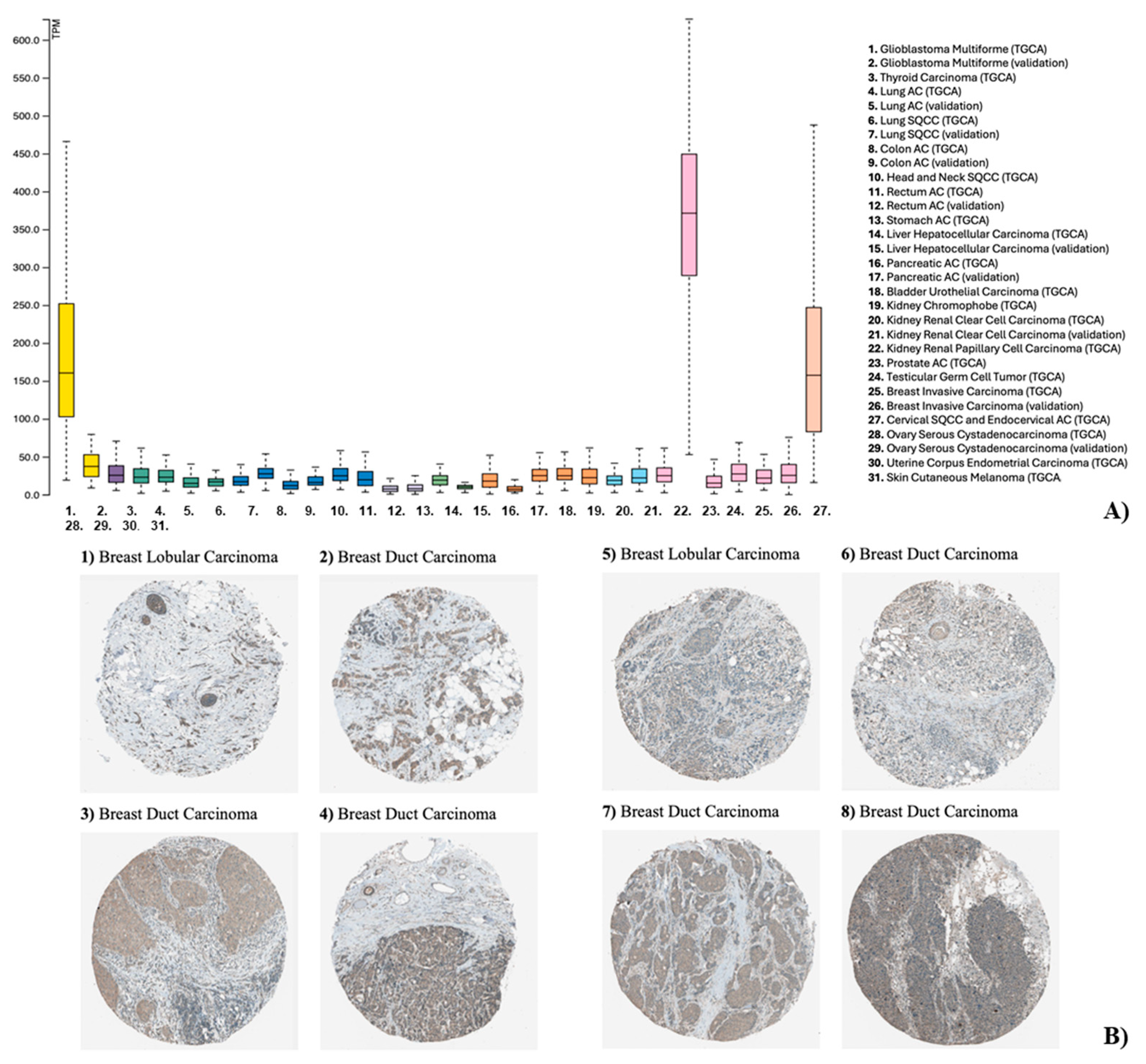
| Sample | Patient ID | Staining | Intensity | Quantity | Location |
|---|---|---|---|---|---|
| 1 | #2898 | medium | moderate | >75% | Cytoplasmic, membranous |
| 2 | #3257 | medium | moderate | ||
| 3 | #2392 | medium | moderate | ||
| 4 | #1939 | medium | moderate | ||
| 5 | #2805 | low | weak | ||
| 6 | #1874 | low | weak | ||
| 7 | #2428 | low | weak | ||
| 8 | #2174 | low | weak |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





