Submitted:
14 May 2025
Posted:
14 May 2025
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Materials and Methods
2.1. Plant Materials and Surface Disinfection
2.2. Effect of PGRs on Adventitious Shoot Regeneration
2.3. Effect of Combinations of 2-IP and NAA on Shoot Multiplication and Shoot Elongation
2.4. Effect of IBA and NAA on Rooting
2.5. Acclimatization
2.6. Statistical Analysis
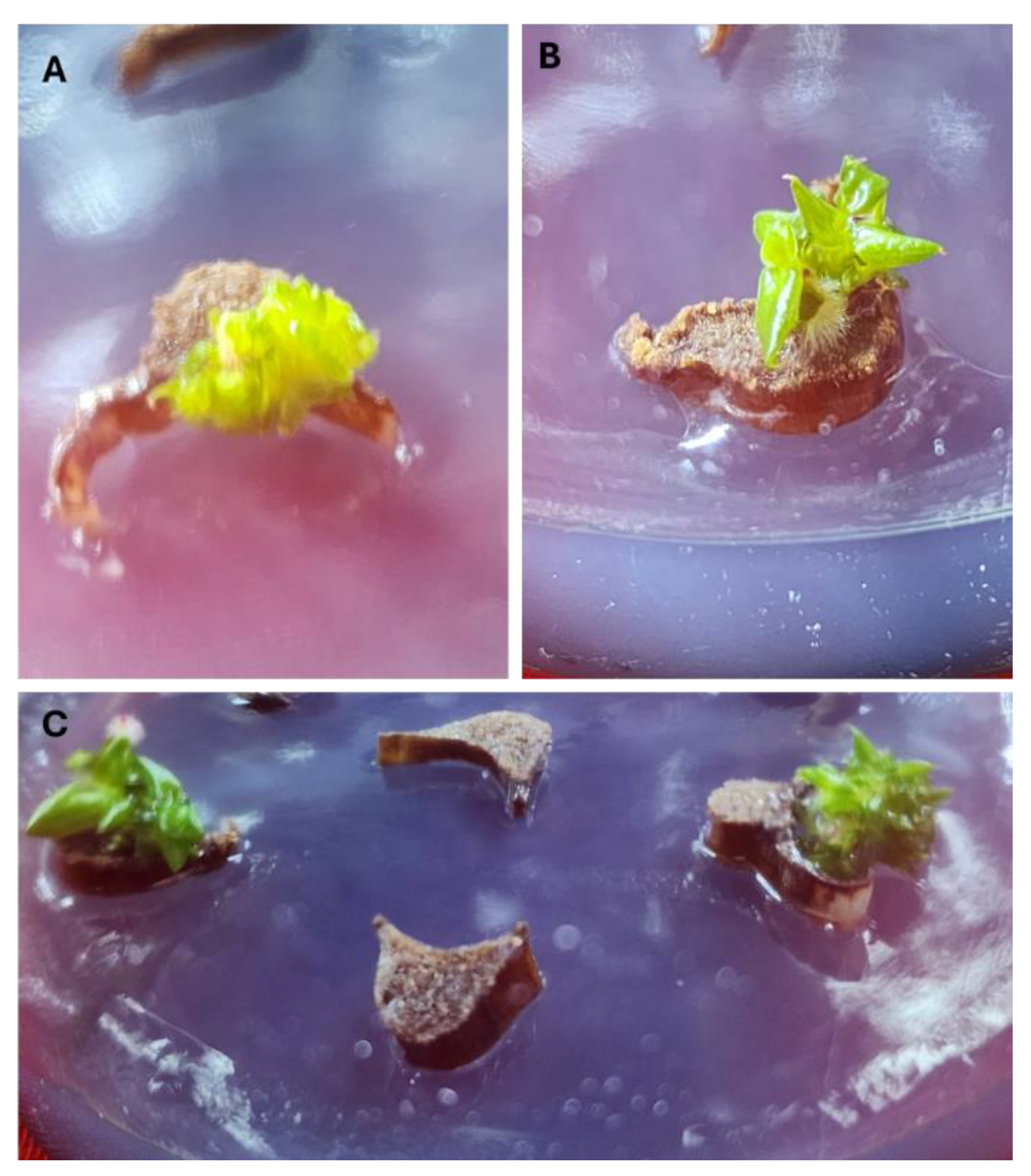
3. Results and Discussion
3.1. Impact of Ag NPs on the Surface Disinfection of Philodendron ‘White Knight’ Petiole Explants
3.2. Influence of PGRs on Adventitious Shoot Regeneration from Petiole Explants of Philodendron ‘White Knight’
3.2.1. Combination of 2-IP and Auxins (NAA and IBA)
3.2.2. Combination of BA and Auxins (NAA and IBA)
3.3. Combination of 2-IP and NAA on Philodendron ‘White Knight’ Shoot Multiplication
3.4. Effect of IBA and NAA on Rooting
3.5. Acclimatization
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| Ag NPs | silver nanoparticles |
| MS-B | Murashige and Skoog shoot multiplication B |
| 2-IP | 2-isopentenyl adenine |
| IBA | indole-3-butyric acid |
| NAA | naphthalene acetic acid |
| BA | benzyladenine |
| PPFD | photosynthetic photon flux density |
References
- International Plant Names Index. Royal Botanic Gardens, Kew Names and Taxonomic Backbone. 2025. https://powo.science.kew.org/taxon/urn:lsid:ipni.org:names:326132-2#source-KB. Retrieved 08 May 2025.
- Chen, F.C.; Wang, C.Y.; Fang, J.Y. Micropropagation of self-heading Philodendron via direct shoot regeneration. Sci. Hortic. 2012, 141, 23–29. [Google Scholar] [CrossRef]
- Sreekumar, S.; Mukunthakumar, S.; Seeni, S. Morphogenetic response of six Philodendron cultivars in vitro. Indian J. Exp. Biol. 2001, 39, 1280–1287. [Google Scholar]
- Klanrit, P.; Kitwetcharoen, H.; Thanonkeo, P.; Thanonkeo, S. In Vitro Propagation of Philodendron erubescens ‘Pink Princess’ and Ex Vitro Acclimatization of the Plantlets. Horticulturae 2023, 9, 688. [Google Scholar] [CrossRef]
- Debergh, P.; Maene, L. A scheme for commercial propagation of ornamental plants by tissue culture. Sci. Hortic. 1981, 14, 335–345. [Google Scholar] [CrossRef]
- Maikaeo, L.; Puripunyavanich, V.; Limtiyayotin, M.; Orpong, P.; Kongpeng, C. Micropropagation and gamma irradiation mutagenesis in Philodendron billietiae. Thai J. Agric. Sci. 2024, 57, 11–19. [Google Scholar]
- Alawaadh, A.A.; Dewir, Y.H.; Alwihibi, M.S.; Aldubai, A.A.; El-Hendawy, S.; Naidoo, Y. Micropropagation of lacy tree philodendron (Philodendron bipinnatifidum Schott ex Endl.). Hortscience 2020, 55, 294–299. [Google Scholar] [CrossRef]
- Kumar, D.; Tiwari, J.P.; Singh, R. In-vitro clonal propagation of Philodendron pertusum. Ind. J. Hortic. 1998, 55, 340–343. [Google Scholar]
- Seliem, M.K.; El-Mahrouk, M.E.; El-Banna, A.N.; Hafez, Y.M.; Dewir, Y.H. Micropropagation of Philodendron selloum: Influence of copper sulfate on endophytic bacterial contamination, antioxidant enzyme activity, electrolyte leakage, and plant survival. South Afr. J. Bot. 2021, 139, 230–240. [Google Scholar] [CrossRef]
- Jambor-Benczur, E.; Marta-Riffer, A. In vitro propagation of Philodendron tuxilanum Bunting with benzylaminopurine. Acta Agron. Hung. 1990, 39, 341–348. [Google Scholar]
- Akramian, M.; Khaleghi, A.R.; Salehi-Arjmand, H. Optimization of plant growth regulators for in vitro mass propagation of Philodendron cv. Birkin through shoot tip culture. Greenh. Plant Prod. J. 2024, 1, 55–62. [Google Scholar] [CrossRef]
- Chun, S.C.; Seob, P.K.; Kang, K.W.; Sivanesan, I. Micropropagation of Ardisia mamillata Hance through axillary shoot multiplication. Propag. Ornam. Plants 2024, 24, 63–70. [Google Scholar]
- Gangopadhyay, G.; Bandyopadhyay, T.; Gangopadhyay, S.B.; Mukherjee, K.K. Luffa sponge – a unique matrix for tissue culture of Philodendron. Curr. Sci. 2004, 86, 315–319. [Google Scholar]
- Kim, D.H.; Gopal, J.; Iyyakkannu Sivanesan, I. Nanomaterials in plant tissue culture: The disclosed and undisclosed. RSC Adv. 2017, 7, 36492–36505. [Google Scholar] [CrossRef]
- Ochatt, S.; Abdollahi, M.R.; Akin, M.; Bello Bello, J.J.; Eimert, K.; Faisal, M.; Nhut, D.T. Application of nanoparticles in plant tissue cultures: minuscule size but huge effects. Plant Cell Tiss. Organ Cult. 2023, 155, 323–326. [Google Scholar] [CrossRef]
- Singh, Y.; Kumar, U.; Panigrahi, S.; Balyan, P.; Mehla, S.; Sihag, P.; Sagwal, V.; Singh, K.P.; White, J.C.; Dhankher, O.P. Nanoparticles as novel elicitors in plant tissue culture applications: Current status and future outlook. Plant Physiol. Biochem. 2023, 203, 108004. [Google Scholar] [CrossRef]
- Lara-Ascencio, M.; Andrade-Rodriguez, M.; Guillen-Sanchez, D.; Sotelo-Nava, H.; Villegas-Torres, O.G. Establishment of in vitro aseptic culture of Philodendron xanadu Croat. Rev. Cienc. Agron. 2021, 52, e20197034. [Google Scholar] [CrossRef]
- Murashige, T.; Skoog, F. A revised medium for rapid growth and bioassays with tobacco tissue cultures. Physiol. Plant. 1962, 15, 473–497. [Google Scholar] [CrossRef]
- Tung, H.T.; Van, H.T.; Bao, H.G.; Bien, L.H.; Khai, H.D.; Luan, V.Q.; Cuong, D.M.; Phong, T.H.; Nhut, D.T. Silver nanoparticles enhanced efficiency of explant surface disinfection and somatic embryogenesis in Begonia tuberous via thin cell layer culture. Vietnam J. Biotechnol. 2021, 19, 337–347. [Google Scholar] [CrossRef]
- Cuong, D.M.; Mai, N.T.; Tung, H.T.; Khai, H.D.; Luan, V.Q.; Phong, T.H.; Van The Vinh, B.; Phuong, H.T.; Van Binh, N.; Tan Nhut, D. Positive effect of silver nanoparticles in micropropagation of Limonium sinuatum (L.) Mill. ‘White’. Plant Cell Tissue Organ Cult. 2023, 22, 417–432. [Google Scholar] [CrossRef]
- Sivanesan, I.; Lee, Y.K.; Kang, K.W.; Park, H.Y. Micropropagation of Monstera delicosa Liebm. ‘Thai Constellation’. Propag. Ornam. Plants.
- Nartop, P. Effects of surface sterilisation with green synthesized silver nanoparticles on Lamiaceae seeds. IET Nanobiotechnol. 2018, 12, 663–668. [Google Scholar] [CrossRef]
- Park, H.-Y.; Kim, K.-S.; Ak, G.; Zengin, G.; Cziáky, Z.; Jekő, J.; Adaikalam, K.; Song, K.; Kim, D.-H.; Sivanesan, I. Establishment of a Rapid Micropropagation System for Kaempferia parviflora Wall. Ex Baker: Phytochemical Analysis of Leaf Extracts and Evaluation of Biological Activities. Plants 2021, 10, 698. [Google Scholar] [CrossRef]
- Yang, H.; Yang, Y.; Wang, Q.; He, J.; Liang, L.; Qiu, H.; Wang, Y.; Zou, L. Adventitious Shoot Regeneration from Leaf Explants in Sinningia Hybrida ‘Isa’s Murmur’. Plants 2022, 11, 1232. [Google Scholar] [CrossRef]
- Pérez-Caselles, C.; Faize, L.; Burgos, L.; Alburquerque, N. Improving Adventitious Shoot Regeneration and Transient Agrobacterium-Mediated Transformation of Apricot (Prunus armeniaca L.) Hypocotyl Sections. Agronomy 2021, 11, 1338. [Google Scholar] [CrossRef]
- Klimaszewska, K. Plant regeneration from petiole segments of some species in tissue culture. Acta Agrobotanica 1981, 34, 5–28. [Google Scholar] [CrossRef]
- El-Gedawey, H.I.; Hussein, S.E. Micropropagation of Aglonema ‘Lady Valentine’ by axillary shoots explants. Egyptian Academic Journal of Biological Sciences, H. Botany 2022, 13, 129–42. [Google Scholar] [CrossRef]
- Zhang, H.; Wang, G.; Qiao, Y.; Chen, C. Plant regeneration from root segments of Anthurium andraeanum and assessment of genetic fidelity of in vitro regenerates. In Vitro Cell. Dev. Biol. Plant 2021, 57, 954–964. [Google Scholar] [CrossRef]
- Qu, L.; Chen, J.; Henny, R.J.; Huang, Y.; Caldwell, R.D.; Robinson, C.A. Thidiazuron promotes adventitious shoot regeneration from pothos (Epipremnum aureum) leaf and petiole explants. Vitr. Cell Dev. Biol. Plant 2014, 50, 561–567. [Google Scholar] [CrossRef]
- Chun, S.C.; Seob, P.K.; Kang, K.W.; Sivanesan, I. In vitro propagation of Spathiphyllum wallsii Regel ‘Domino’ through adventitious shoot regeneration and somatic embryogenesis. Propag. Ornam. Plants 2024, 24, 55–62. [Google Scholar]
- Pourhassan, A.; Kaviani, B.; Kulus, D.; Miler, N.; Negahdar, N. A Complete Micropropagation Protocol for Black-Leaved Zamioculcas zamiifolia (Lodd.) Engl. ‘Dowon’. Horticulturae 2023, 9, 422. [Google Scholar] [CrossRef]
- Mohammed, A.; Chiruvella, K.K.; Namsa, N.D.; Ghanta, R.G. An efficient in vitro shoot regeneration from leaf petiolar explants and ex vitro rooting of Bixa orellana L.—A dye yielding plant. Physiol. Mol. Biol. Plants 2015, 21, 417–424. [Google Scholar] [CrossRef]
- Chiewchan, N.; Saetiew, K.; Teerarak, M. The effect of BA on inducing shoots of Philodendron erubescent ‘Pink Princes’ in vitro. International Journal of Agricultural Technology 2023, 19, 2385–2398. [Google Scholar]
- Bhojwani, S.S. In vitro propagation of garlic by shoot proliferation. Sci. Hortic. 1980, 13, 47–52. [Google Scholar] [CrossRef]
- Irawati, I. Diferensiasi Berbagai Macam Eksplan Pada Perbanyakan Philodendron Goeldii (Araceae) Secara In-vitro*[differentiation of Several Explants on In-vitro Propagation of Philodendron Goeldii (Araceae)]. Berita Biologi 2000, 5, 67669. [Google Scholar]
- Fang, J.Y.; Hsul, Y.R.; Chen, F.C. Development of an efficient micropropagation procedure for Aglaonema ‘Lady Valentine’ through adventitious shoot induction and proliferation. Plant Biotechnol. 2013, 30, 423–431. [Google Scholar] [CrossRef]


| Ag NPs (mg L-1) | Soaking Period (min) | Aseptic Explant (%) | Survival (%) |
|---|---|---|---|
| Control | 0 | 25.9 ± 1.6 h | 100 ± 0.0 a |
| 10 | 30 | 29.3 ± 1.2 h | 100 ± 0.0 a |
| 20 | 30 | 34.8 ± 1.2 g | 100 ± 0.0 a |
| 40 | 30 | 47.8 ± 1.3 e | 100 ± 0.0 a |
| 80 | 30 | 54.7 ± 1.7 d | 100 ± 0.0 a |
| 10 | 60 | 40.1 ± 1.4 f | 100 ± 0.0 a |
| 20 | 60 | 72.1 ± 2.1 c | 100 ± 0.0 a |
| 40 | 60 | 100 ± 0.0 a | 100 ± 0.0 a |
| 80 | 60 | 100 ± 0.0 a | 77.0 ± 3.0 d |
| 10 | 90 | 51.4 ± 1.5 d | 100 ± 0.0 a |
| 20 | 90 | 81.2 ± 1.9 b | 92.7 ± 1.5 b |
| 40 | 90 | 100 ± 0.0 a | 84.9 ± 2.4 c |
| 80 | 90 | 100 ± 0.0 a | 68.2 ± 2.3 e |
| PGRs (µM) | Shoot Induction (%) | Number of Shoots/Explant | ||
|---|---|---|---|---|
| 2-IP | NAA | IBA | ||
| 0 | 0.0 | 0.0 | 0.0 ± 0.0 i | 0.0 ± 0.0 h |
| 5 | 2.5 | 0.0 | 0.0 ± 0.0 i | 0.0 ± 0.0 h |
| 10 | 2.5 | 0.0 | 15.0 ± 1.9 h | 2.8 ± 0.4 fg |
| 20 | 2.5 | 0.0 | 38.9 ± 1.7 bc | 7.0 ± 0.7 bc |
| 30 | 2.5 | 0.0 | 33.0 ± 1.4 de | 4.7 ± 0.3 de |
| 5 | 5.0 | 0.0 | 0.0 ± 0.0 i | 0.0 ± 0.0 h |
| 10 | 5.0 | 0.0 | 30.0 ± 2.2 ef | 4.2 ± 0.7 def |
| 20 | 5.0 | 0.0 | 52.6 ± 2.0 a | 13.9 ± 1.0 a |
| 30 | 5.0 | 0.0 | 42.2 ± 2.5 b | 8.4 ± 1.0 b |
| 5 | 0.0 | 2.5 | 0.0 ± 0.0 i | 0.0 ± 0.0 h |
| 10 | 0.0 | 2.5 | 14.5 ± 1.3 h | 1.9 ± 0.4 g |
| 20 | 0.0 | 2.5 | 21.2 ± 1.9 g | 5.4 ± 0.7 cd |
| 30 | 0.0 | 2.5 | 17.8 ± 1.8 gh | 3.5 ± 0.6 efg |
| 5 | 0.0 | 5.0 | 0.0 ± 0.0 i | 0.0 ± 0.0 h |
| 10 | 0.0 | 5.0 | 20.6 ± 1.2 g | 2.2 ± 0.4 g |
| 20 | 0.0 | 5.0 | 34.9 ± 2.0 cd | 6.3 ± 0.6 c |
| 30 | 0.0 | 5.0 | 28.2 ± 2.1 f | 3.4 ± 0.5 efg |
| PGRs (µM) | Shoot Induction (%) | Number of Shoots/Explant | ||
|---|---|---|---|---|
| BA | NAA | IBA | ||
| 0 | 0.0 | 0.0 | 0.0 ± 0.0 i | 0.0 ± 0.0 g |
| 5 | 2.5 | 0.0 | 0.0 ± 0.0 i | 0.0 ± 0.0 g |
| 10 | 2.5 | 0.0 | 22.8 ± 1.9 d | 2.2 ± 0.3 de |
| 20 | 2.5 | 0.0 | 38.8 ± 2.0 b | 4.8 ± 0.4 b |
| 30 | 2.5 | 0.0 | 30.9 ± 1.2 c | 2.9 ± 0.4 cd |
| 5 | 5.0 | 0.0 | 0.0 ± 0.0 i | 0.0 ± 0.0 g |
| 10 | 5.0 | 0.0 | 10.8 ± 1.3 gh | 1.6 ± 0.2 ef |
| 20 | 5.0 | 0.0 | 16.7 ± 1.7 ef | 3.0 ± 0.4 cd |
| 30 | 5.0 | 0.0 | 8.3 ± 1.2 h | 1.2 ± 0.2 f |
| 5 | 0.0 | 2.5 | 0.0 ± 0.0 i | 0.0 ± 0.0 g |
| 10 | 0.0 | 2.5 | 18.9 ± 1.4 e | 1.8 ± 0.2 ef |
| 20 | 0.0 | 2.5 | 24.8 ± 1.6 d | 2.7 ± 0.2 cd |
| 30 | 0.0 | 2.5 | 13.9 ± 1.4 fg | 1.4 ± 0.2 ef |
| 5 | 0.0 | 5.0 | 0.0 ± 0.0 i | 0.0 ± 0.0 g |
| 10 | 0.0 | 5.0 | 32.2 ± 2.2 c | 3.3 ± 0.4 c |
| 20 | 0.0 | 5.0 | 46.1 ± 1.8 a | 7.1 ± 0.6 a |
| 30 | 0.0 | 5.0 | 39.3 ± 1.5 b | 4.6 ± 0.4 b |
| 2-IP (µM) | NAA (µM) | Number of Shoots/Explant | Shoot Length (cm) |
|---|---|---|---|
| 0.0 | 0.0 | 4.9 ± 0.7 d | 6.4 ± 0.7 a |
| 2.5 | 2.5 | 11.2 ± 1.0 c | 5.1 ± 0.7 ab |
| 5.0 | 2.5 | 34.0 ± 2.4 a | 3.7 ± 0.6 bc |
| 10.0 | 2.5 | 21.4 ± 1.5 b | 2.9 ± 0.5 c |
| Auxin (µM) | Rooting (%) | Number of Roots/Explant | Plantlet Height (cm) | |
|---|---|---|---|---|
| IBA | NAA | |||
| 0.0 | 0.0 | 18.9 ± 2.0 f | 2.2 ± 0.1 d | 3.9 ± 0.3 ef |
| 2.5 | 0.0 | 52.2 ± 2.1 e | 3.6 ± 0.4 cd | 5.8 ± 0.2 cd |
| 5.0 | 0.0 | 75.1 ± 3.1 c | 4.7 ± 0.4 bc | 7.3 ± 0.4 b |
| 10.0 | 0.0 | 100 ± 0.0 a | 8.2 ± 0.7 a | 9.1 ± 0.3 a |
| 20.0 | 0.0 | 87.2 ± 2.6 b | 3.1 ± 0.3 d | 6.4 ± 0.3 c |
| 0.0 | 2.5 | 55.8 ± 3.2 e | 2.7 ± 0.4 d | 5.3 ± 0.5 d |
| 0.0 | 5.0 | 91.7 ± 1.7 b | 5.2 ± 0.3 b | 4.3 ± 0.2 e |
| 0.0 | 10.0 | 74.4 ± 1.8 c | 3.2 ± 0.5 d | 3.6 ± 0.2 ef |
| 0.0 | 20.0 | 65.9 ± 2.1 d | 2.9 ± 0.4 d | 3.1 ± 0.2 f |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).




