Submitted:
19 March 2025
Posted:
20 March 2025
You are already at the latest version
Abstract

Keywords:
1. Introduction
2. Materials and Methods
3. Results
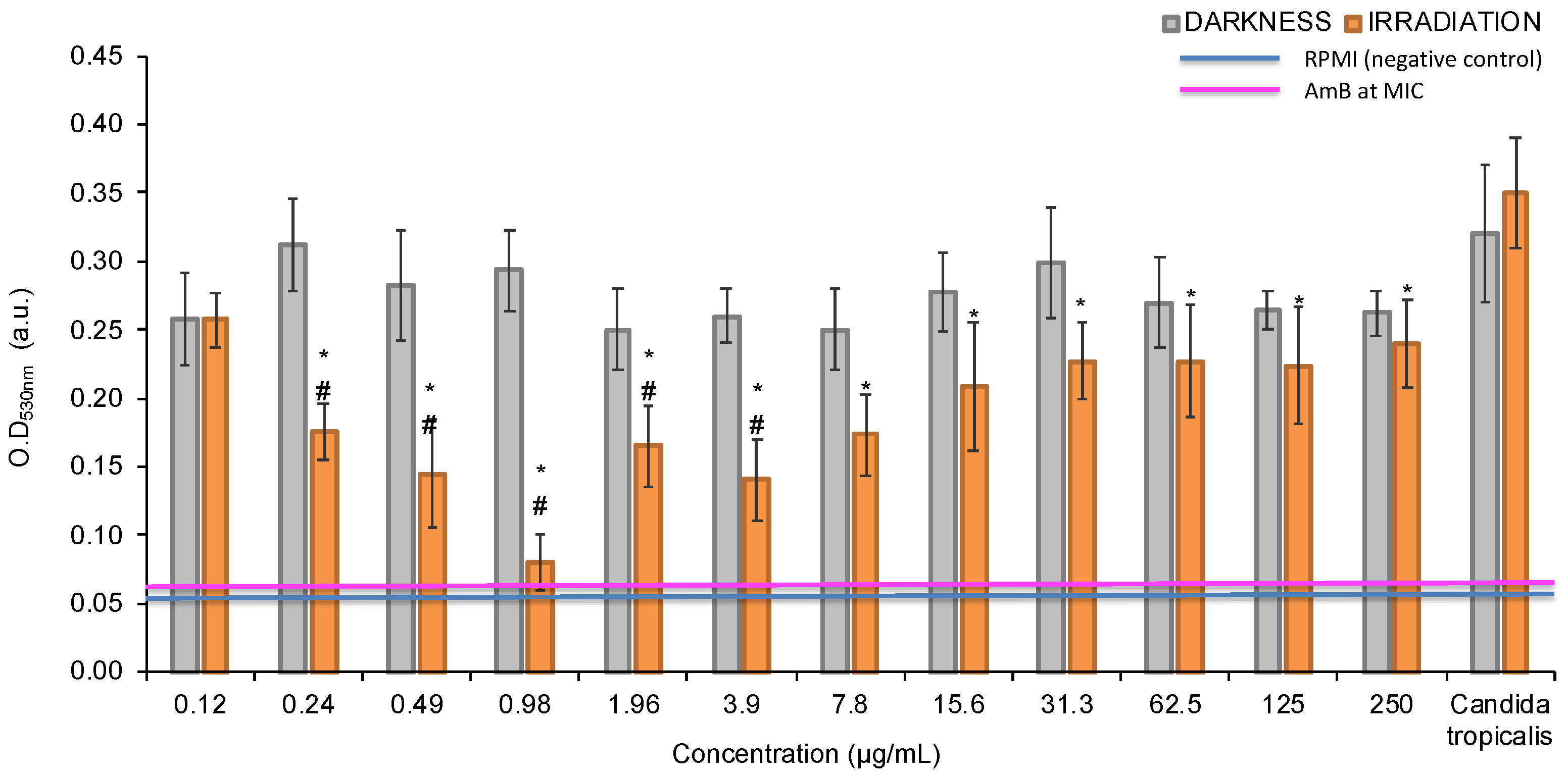
3.1. Photoactive Minimum Inhibitory Concentration (pMIC)
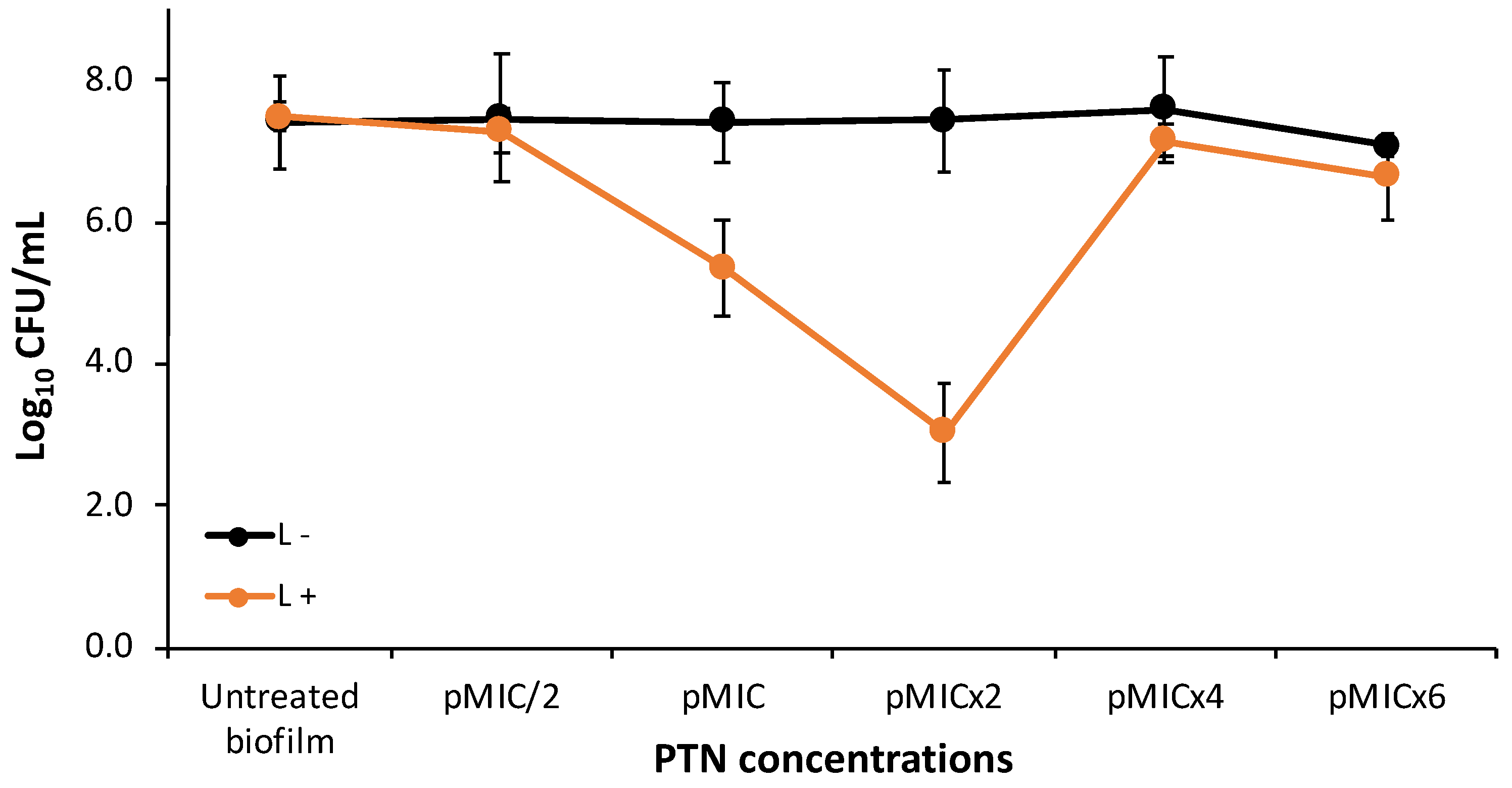
3.2. PTN Antimicrobial Photodynamic Therapy (APDT)
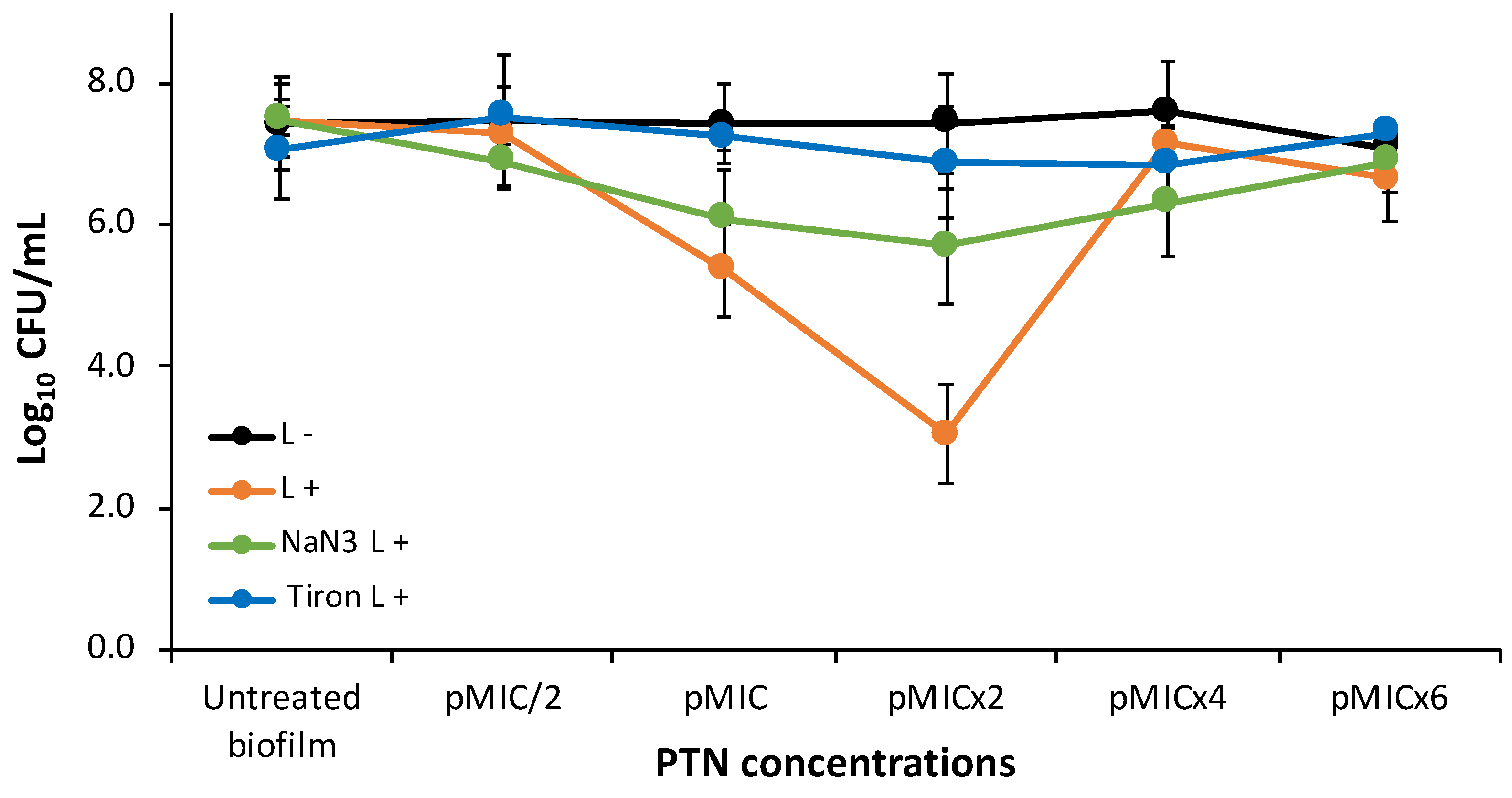
3.2.1. Photodynamic Mechanismon Analysis after PTN-APDT
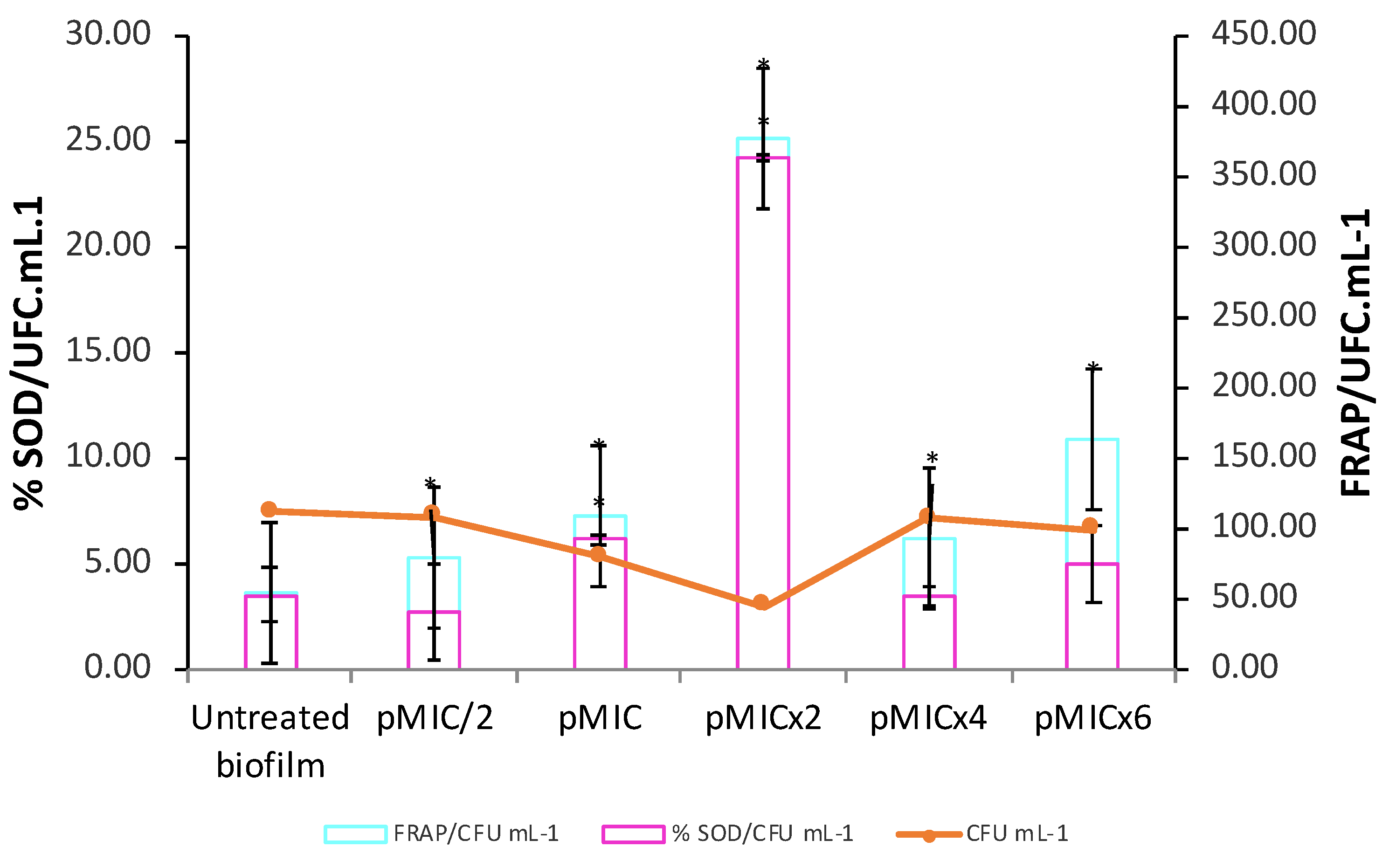
3.2.2. Biofilm Stress Response After PTN-APDT
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| ΦΔ | Quantum yield of singlet oxygen |
| +C | Positive control |
| 1O2 | Singlet oxygen |
| 3O2 | Molecular oxygen |
| AmB | Amphotericin B |
| aPDI | Antimicrobial photodynamic inactivation |
| aPDT | Antimicrobial Photodynamic Therapy |
| AQ/s | Anthraquinone/s |
| ATM | Antimicrobial |
| CFU | Colony forming units |
| CLSI | Clinical and Laboratory Standards Institute |
| EtOH | Ethanol |
| FeSO4 | Ferrous sulfate |
| FRAP | Ferric Reducing Antioxidant Potency assay |
| H2O2 | Hydrogen peroxide |
| HO∙ | Hydroxyl radical |
| MIC | Minimal Inhibitory Concentration |
| MOPS | Morpholine propane sulfonic acid |
| NaN3 | Sodium azide |
| NaNO2 | Sodium nitrate |
| NaOH | Sodium hydroxide |
| NBT | Nitro BlueTetrazolium |
| NCPF | National Collection of Pathogenic Fungi |
| NO· | Nitric oxide |
| O2•- | Superoxide radical anion |
| OD | Optical density |
| PBS | Phosphate Buffered Saline |
| pMIC | Photoactive Minimal Inhibitory Concentration |
| PS | Photosensitizer |
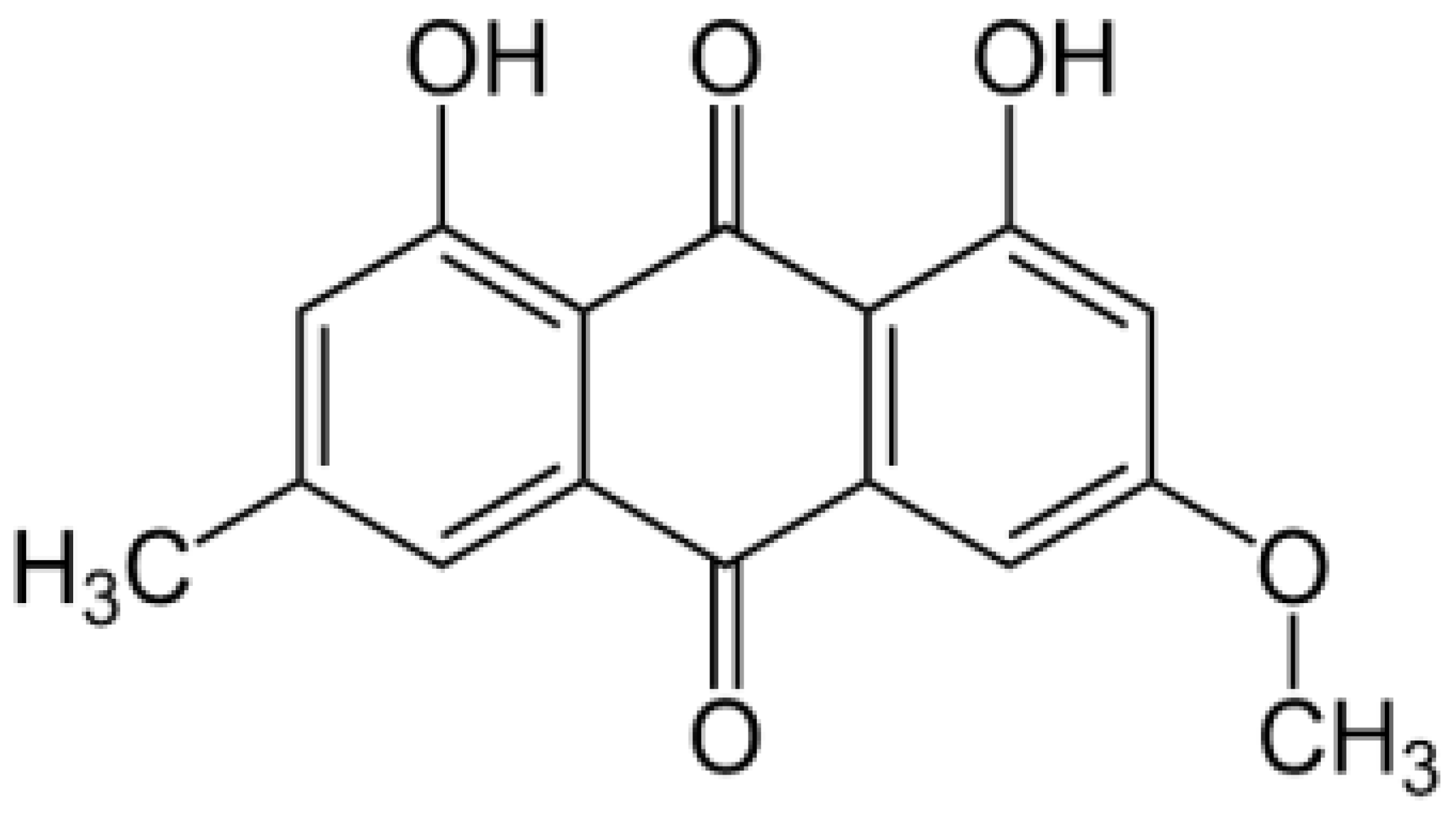
| PTN | Parietin |
| RNI | Reactive nitrogen intermediates |
| ROS | Reactive oxygen species |
| RPMI | Roswell Park Memorial Institute 1640 |
| SDA | Sabouraud Dextrose Agar |
| SDB | Sabouraud Dextrose Broth |
| SOD | Superoxide dismutase |
| MFC | Minimum Fungicidal Concentration |
| MTT | 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide |
| OH | Hydroxyl groups |
| DNA | Deoxyribonucleic acid |
| ONOO⁻ | Peroxynitrite |
References
- Ahmed, S.K.; Hussein, S.; Qurbani, K.; Ibrahim, R.H.; Fareeq, A.; Mahmood, K.A.; Mohamed, M.G. Antimicrobial Resistance: Impacts, Challenges, and Future Prospects. J. Med. Surg. Public Health 2024, 2, 100081. [Google Scholar] [CrossRef]
- Riera, F.O.; Caeiro, J.P.; Angiolini, S.C.; Vigezzi, C.; Rodriguez, E.; Icely, P.A.; Sotomayor, C.E. Invasive Candidiasis: Update and Current Challenges in the Management of This Mycosis in South America. Antibiotics 2022, 11, 877. [Google Scholar] [CrossRef]
- Invasive Candidiasis | Nature Reviews Disease Primers. Nat. Rev. Dis. Primer.
- Cieplik, F.; Deng, D.; Crielaard, W.; Buchalla, W.; Hellwig, E.; Al-Ahmad, A.; Maisch, T. Antimicrobial Photodynamic Therapy – What We Know and What We Don’t. Crit. Rev. Microbiol. 2018, 44, 571–589. [Google Scholar] [CrossRef] [PubMed]
- Klausen, M.; Ucuncu, M.; Bradley, M. Design of Photosensitizing Agents for Targeted Antimicrobial Photodynamic Therapy. Molecules 2020, 25, 5239. [Google Scholar] [CrossRef] [PubMed]
- Aware, C.B.; Patil, D.N.; Suryawanshi, S.S.; Mali, P.R.; Rane, M.R.; Gurav, R.G.; Jadhav, J.P. Natural Bioactive Products as Promising Therapeutics: A Review of Natural Product-Based Drug Development. South Afr. J. Bot. 2022, 151, 512–528. [Google Scholar] [CrossRef]
- Lichens as a Potential Source of Bioactive Secondary Metabolites | SpringerLink.
- Thakur, M.; Chander, H. Potential of Lichens: A Review of Bioactive Compounds with Biological Activities.
- Christina Pires Gonçalves, L. Photophysical Properties and Therapeutic Use of Natural Photosensitizers. J. Photochem. Photobiol. 2021, 7, 100052. [Google Scholar] [CrossRef]
- Polat, E.; Kang, K. Natural Photosensitizers in Antimicrobial Photodynamic Therapy. Biomedicines 2021, 9, 584. [Google Scholar] [CrossRef]
- Fernández-Marín, B.; Artetxe, U.; Becerril, J.M.; Martínez-Abaigar, J.; Núñez-Olivera, E.; García-Plazaola, J.I. Can Parietin Transfer Energy Radiatively to Photosynthetic Pigments? Molecules 2018, 23, 1741. [Google Scholar] [CrossRef]
- Comini, L.R.; Morán Vieyra, F.E.; Mignone, R.A.; Páez, P.L.; Laura Mugas, M.; Konigheim, B.S.; Cabrera, J.L.; Núñez Montoya, S.C.; Borsarelli, C.D. Parietin: An Efficient Photo-Screening Pigment in Vivo with Good Photosensitizing and Photodynamic Antibacterial Effects in Vitro. Photochem. Photobiol. Sci. 2017, 16, 201–210. [Google Scholar] [CrossRef]
- Hurtado Bredda, F.J.; Nin Vaeza, N.; Rubbo Amonini, H. Estrés oxidativo y nitrosativo en la sepsis. Med. Intensiva 2005, 29, 159–165. [Google Scholar] [CrossRef]
- Baltazar, L.M.; Ray, A.; Santos, D.A.; Cisalpino, P.S.; Friedman, A.J.; Nosanchuk, J.D. Antimicrobial Photodynamic Therapy: An Effective Alternative Approach to Control Fungal Infections. Front. Microbiol. 2015, 6. [Google Scholar] [CrossRef] [PubMed]
- Ong, K.S.; Mawang, C.I.; Daniel-Jambun, D.; Lim, Y.Y.; Lee, S.M. Current Anti-Biofilm Strategies and Potential of Antioxidants in Biofilm Control. Expert Rev. Anti Infect. Ther. 2018, 16, 855–864. [Google Scholar] [CrossRef] [PubMed]
- M27-A2 Reference Method for Broth Dilution Antifungal Susceptibility Testing of Yeasts; Approved Standard—Second Edition.
- Mugas, M.L.; Calvo, G.; Marioni, J.; Céspedes, M.; Martinez, F.; Sáenz, D.; Di Venosa, G.; Cabrera, J.L.; Montoya, S.N.; Casas, A. Photodynamic Therapy of Tumour Cells Mediated by the Natural Anthraquinone Parietin and Blue Light. J. Photochem. Photobiol. B 2021, 214, 112089. [Google Scholar] [CrossRef] [PubMed]
- Marioni, J.; Arce, J.E.; Cabrera, J.L.; Paraje, M.G.; Núñez Montoya, S.C. Reduction of Candida Tropicalis Biofilm by Photoactivation of a Heterophyllaea Pustulata Extract. Pharm. Biol. 2016, 54, 2791–2801. [Google Scholar] [CrossRef]
- M27-A3: Reference Method for Broth Dilution Antifungal Susceptibility Testing of Yeasts; Approved Standard—Third Edition.
- Marioni, J.; Da Silva, M.A.; Cabrera, J.L.; Montoya, S.C.N.; Paraje, M.G. The Anthraquinones Rubiadin and Its 1-Methyl Ether Isolated from Heterophyllaea Pustulata Reduces Candida Tropicalis Biofilms Formation. Phytomedicine 2016, 23, 1321–1328. [Google Scholar] [CrossRef]
- Marioni, J.; Bresolí-Obach, R.; Agut, M.; Comini, L.R.; Cabrera, J.L.; Paraje, M.G.; Nonell, S.; Montoya, S.C.N. On the Mechanism of Candida Tropicalis Biofilm Reduction by the Combined Action of Naturally-Occurring Anthraquinones and Blue Light. PLOS ONE 2017, 12, e0181517. [Google Scholar] [CrossRef]
- Mattila, H.; Khorobrykh, S.; Havurinne, V.; Tyystjärvi, E. Reactive Oxygen Species: Reactions and Detection from Photosynthetic Tissues. J. Photochem. Photobiol. B 2015, 152, 176–214. [Google Scholar] [CrossRef]
- Tsikas, D. Analysis of Nitrite and Nitrate in Biological Fluids by Assays Based on the Griess Reaction: Appraisal of the Griess Reaction in the l-Arginine/Nitric Oxide Area of Research. J. Chromatogr. B 2007, 851, (1–2). [Google Scholar] [CrossRef]
- Beauchamp, C.; Fridovich, I. Superoxide Dismutase: Improved Assays and an Assay Applicable to Acrylamide Gels. Anal. Biochem. 1971, 44, 276–287. [Google Scholar] [CrossRef]
- Benzie, I.F.F.; Strain, J.J. The Ferric Reducing Ability of Plasma (FRAP) as a Measure of “Antioxidant Power”: The FRAP Assay. Anal. Biochem. 1996, 239, 70–76. [Google Scholar] [CrossRef]
- Pappas, P.G.; Lionakis, M.S.; Arendrup, M.C.; Ostrosky-Zeichner, L.; Kullberg, B.J. Invasive Candidiasis. Nat. Rev. Dis. Primer 2018, 4, 18026. [Google Scholar] [CrossRef]
- Youf, R.; Müller, M.; Balasini, A.; Thétiot, F.; Müller, M.; Hascoët, A.; Jonas, U.; Schönherr, H.; Lemercier, G.; Montier, T.; Le Gall, T. Antimicrobial Photodynamic Therapy: Latest Developments with a Focus on Combinatory Strategies. Pharmaceutics 2021, 13, 1995. [Google Scholar] [CrossRef] [PubMed]
- Fiala, J.; Roach, T.; Holzinger, A.; Husiev, Y.; Delueg, L.; Hammerle, F.; Armengol, E.S.; Schöbel, H.; Bonnet, S.; Laffleur, F.; Kranner, I.; Lackner, M.; Siewert, B. The Light-Activated Effect of Natural Anthraquinone Parietin against Candida Auris and Other Fungal Priority Pathogens. Planta Med. 2024, 90, 588–594. [Google Scholar] [CrossRef]
- Ma, W.; Liu, C.; Li, J.; Hao, M.; Ji, Y.; Zeng, X. The Effects of Aloe Emodin-Mediated Antimicrobial Photodynamic Therapy on Drug-Sensitive and Resistant Candida Albicans. Photochem. Photobiol. Sci. 2020, 19, 485–494. [Google Scholar] [CrossRef] [PubMed]
- Quishida, C.C.C.; De Oliveira Mima, E.G.; Jorge, J.H.; Vergani, C.E.; Bagnato, V.S.; Pavarina, A.C. Photodynamic Inactivation of a Multispecies Biofilm Using Curcumin and LED Light. Lasers Med. Sci. 2016, 31, 997–1009. [Google Scholar] [CrossRef]
- Alshehri, A.H. Mechanical and Antimicrobial Effects of Riboflavin-Mediated Photosensitization of in Vitro C. Albicans Formed on Polymethyl Methacrylate Resin. Photodiagnosis Photodyn. Ther. 2021, 36, 102488. [Google Scholar] [CrossRef]
- Janeczko, M.; Masłyk, M.; Kubiński, K.; Golczyk, H. Emodin, a Natural Inhibitor of Protein Kinase CK2, Suppresses Growth, Hyphal Development, and Biofilm Formation of Candida albicans . Yeast 2017, 34, 253–265. [Google Scholar] [CrossRef]
- Manoharan, R.K.; Lee, J.-H.; Kim, Y.-G.; Lee, J. Alizarin and Chrysazin Inhibit Biofilm and Hyphal Formation by Candida Albicans. Front. Cell. Infect. Microbiol. 2017, 7, 447. [Google Scholar] [CrossRef]
- Tsang, P.W.-K.; Bandara, H.M.H.N.; Fong, W.-P. Purpurin Suppresses Candida Albicans Biofilm Formation and Hyphal Development. PLoS ONE 2012, 7, e50866. [Google Scholar] [CrossRef]
- Galinari, C.B.; Biachi, T.D.P.; Gonçalves, R.S.; Cesar, G.B.; Bergmann, E.V.; Malacarne, L.C.; Kioshima Cotica, É.S.; Bonfim-Mendonça, P.D.S.; Svidzinski, T.I.E. Photoactivity of Hypericin: From Natural Product to Antifungal Application. Crit. Rev. Microbiol. 2023, 49, 38–56. [Google Scholar] [CrossRef]
- Łopaciński, M.; Fiegler-Rudol, J.; Niemczyk, W.; Skaba, D.; Wiench, R. Riboflavin- and Hypericin-Mediated Antimicrobial Photodynamic Therapy as Alternative Treatments for Oral Candidiasis: A Systematic Review. Pharmaceutics 2024, 17, 33. [Google Scholar] [CrossRef] [PubMed]
- Amorim, C.F.; Iglesias, B.A.; Pinheiro, T.R.; Lacerda, L.E.; Sokolonski, A.R.; Pedreira, B.O.; Moreira, K.S.; Burgo, T.A.L.; Meyer, R.; Azevedo, V.; Portela, R.W. Photodynamic Inactivation of Different Candida Species and Inhibition of Biofilm Formation Induced by Water-Soluble Porphyrins. Photodiagnosis Photodyn. Ther. 2023, 42, 103343. [Google Scholar] [CrossRef]
- Souza, S.O.; Raposo, B.L.; Sarmento-Neto, J.F.; Rebouças, J.S.; Macêdo, D.P.C.; Figueiredo, R.C.B.Q.; Santos, B.S.; Freitas, A.Z.; Cabral Filho, P.E.; Ribeiro, M.S.; Fontes, A. Photoinactivation of Yeast and Biofilm Communities of Candida Albicans Mediated by ZnTnHex-2-PyP4+ Porphyrin. J. Fungi 2022, 8, 556. [Google Scholar] [CrossRef] [PubMed]
- Bors, W. Pulse-Radiolytic Investigations of Catechols and Catecholamines II. Reactions of Tiron with Oxygen Radical Species. Biochim. Biophys. Acta BBA - Gen. Subj. 1979, 582, 537–542. [Google Scholar] [CrossRef]
- Snyder, J.W.; Skovsen, E.; Lambert, J.D.C.; Poulsen, L.; Ogilby, P.R. Optical Detection of Singlet Oxygen from Single Cells. Phys. Chem. Chem. Phys. 2006, 8, 4280. [Google Scholar] [CrossRef] [PubMed]
- Vatansever, F.; De Melo, W.C.M.A.; Avci, P.; Vecchio, D.; Sadasivam, M.; Gupta, A.; Chandran, R.; Karimi, M.; Parizotto, N.A.; Yin, R.; Tegos, G.P.; Hamblin, M.R. Antimicrobial Strategies Centered around Reactive Oxygen Species – Bactericidal Antibiotics, Photodynamic Therapy, and Beyond. FEMS Microbiol. Rev. 2013, 37, 955–989. [Google Scholar] [CrossRef]
- Elian, C.; Méallet, R.; Versace, D. Photoactive Dye-Loaded Polymer Materials: A New Cutting Edge for Antibacterial Photodynamic Therapy. Adv. Funct. Mater. 2024, 34, 2407228. [Google Scholar] [CrossRef]
- Wink, D.A.; Mitchell, J.B. Chemical Biology of Nitric Oxide: Insights into Regulatory, Cytotoxic, and Cytoprotective Mechanisms of Nitric Oxide. Free Radic. Biol. Med. 1998, 25, (4–5). [Google Scholar] [CrossRef]
- Fraix, A.; Sortino, S. Combination of PDT Photosensitizers with NO Photodononors. Photochem. Photobiol. Sci. 2018, 17, 1709–1727. [Google Scholar] [CrossRef]
- Zhao, Z.; Li, H.; Tao, X.; Xie, Y.; Yang, L.; Mao, Z.; Xia, W. Light-Triggered Nitric Oxide Release by a Photosensitizer to Combat Bacterial Biofilm Infections. Chem. – Eur. J. 2021, 27, 5453–5460. [Google Scholar] [CrossRef]
- Tavares, A.; Carvalho, C.M.B.; Faustino, M.A.; Neves, M.G.P.M.S.; Tomé, J.P.C.; Tomé, A.C.; Cavaleiro, J.A.S.; Cunha, Â.; Gomes, N.C.M.; Alves, E.; Almeida, A. Antimicrobial Photodynamic Therapy: Study of Bacterial Recovery Viability and Potential Development of Resistance after Treatment. Mar. Drugs 2010, 8, 91–105. [Google Scholar] [CrossRef] [PubMed]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).




