Submitted:
28 February 2025
Posted:
03 March 2025
You are already at the latest version
Abstract
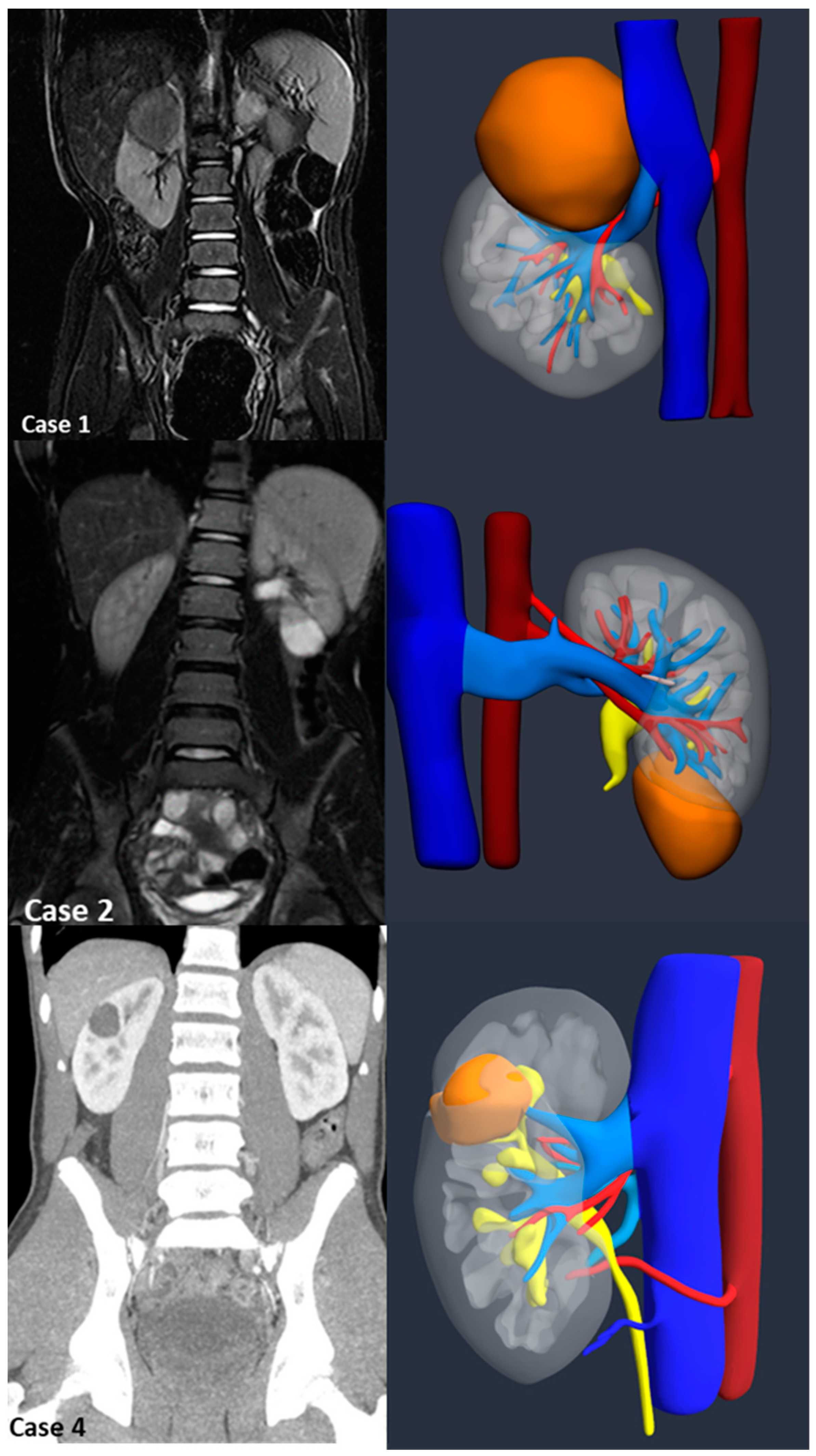
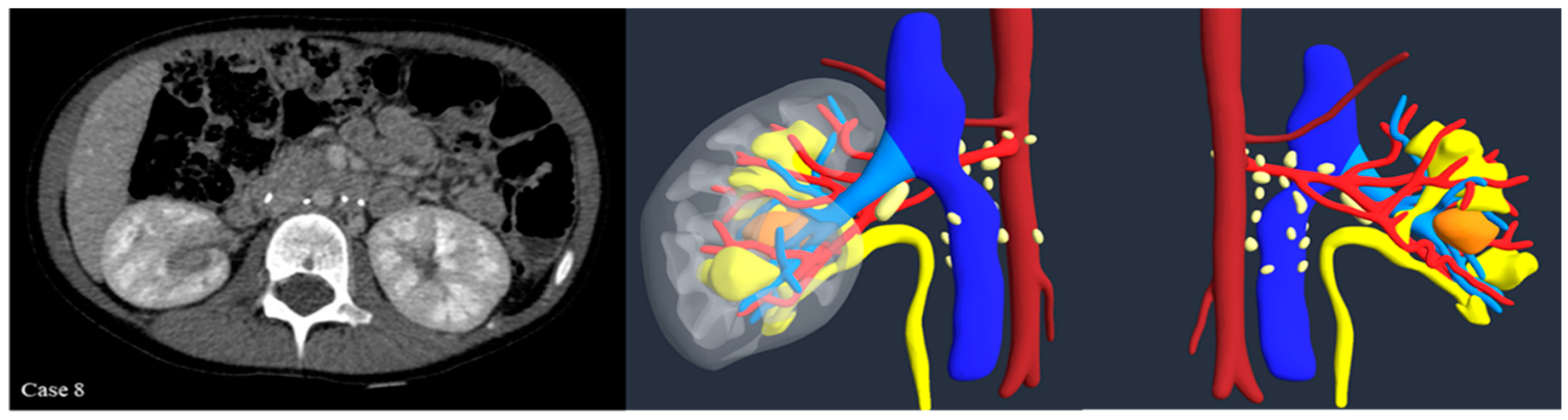
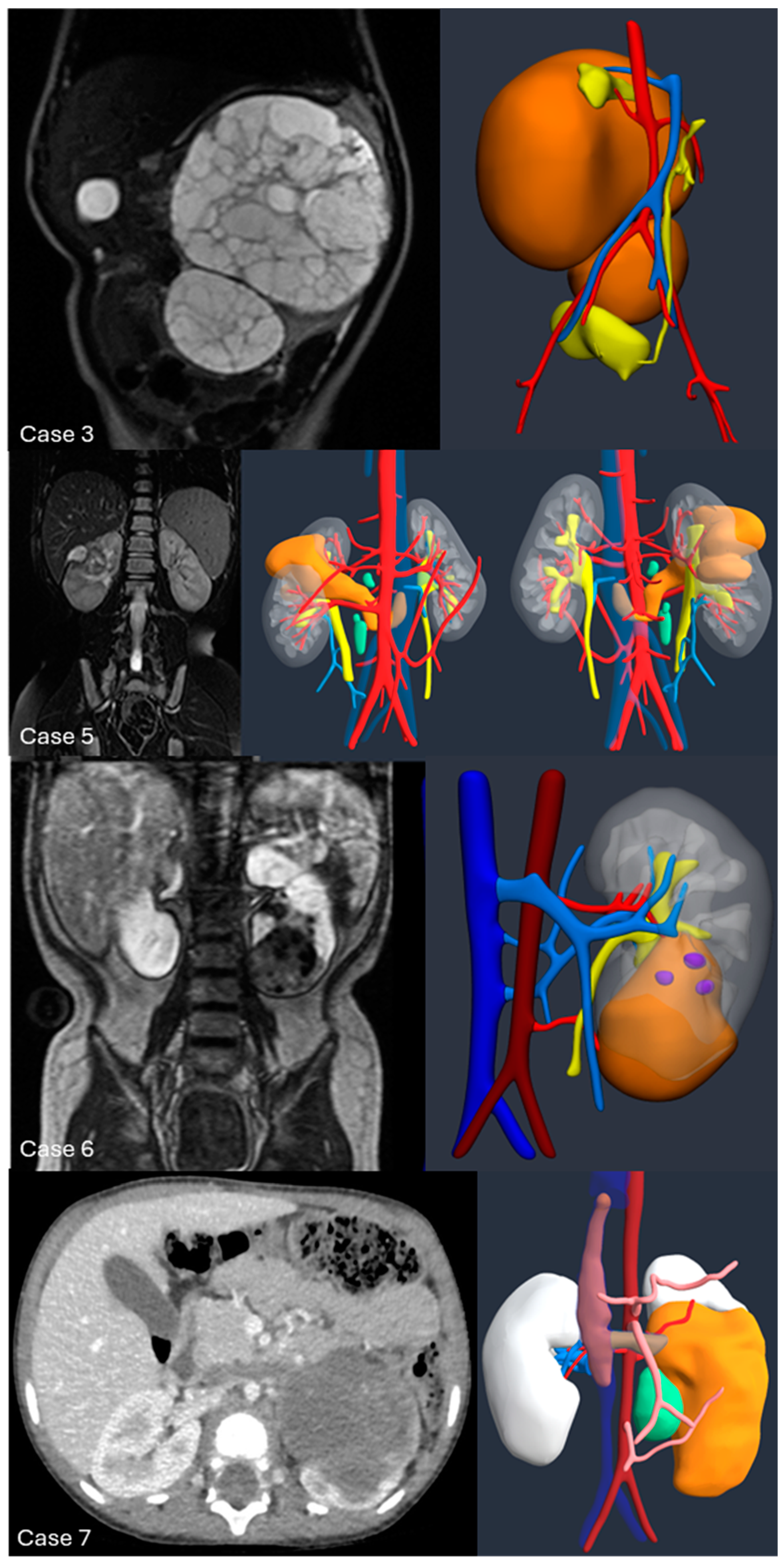
Introduction:The pre-operative planning for complex renal masses in children can be challenging, especially when nephron-sparing surgery (NSS) is recommended. We re-port our experience with the use of 3D-virtual reconstruction (3DVR) and its impact on surgical decision making. Materials and methods: Patients with complex renal masses underwent preoperative 3DVR. DICOM data were obtained from abdominal MRI and/or CT scans. 2D images segmentation was then performed. Three oncology surgeons were asked to individually evaluate each patient's preoperative MRI, CT and 3DVR. A questionnaire regarding the quality of conventional imaging compared to 3DVR was completed following surgery. Results: 8 patients (4♂,4♀) were included: Cases 1,2 and 4 were circumscribed tumours in the right upper pole, left lower pole (Bosniak cyst IV) and right mid-upper pole (Bosniak cyst IIF) respectively; Case 3 was a large hilar mass involving the whole kidney, unresponsive to chemotherapy; Case 5 and 7 were stage IV Wilms' tumour with venous thrombosis; case 6 was a left mid lower renal mass in a patient with WAGR syndrome and acute lymphoblastic leukae-mia (ALL) and case 8 was a recurrent central right WT after previous NSS in a child with Beckwith-Wiedemann syndrome(BWS). Four radical nephrectomies and three NSS were performed. In comparison to conventional imaging, the 3DVR models were judged to be superior by the expert reviewers for all anatomical structures except the urinary tract(p< 0.05). Conclusions: Our study suggests that 3DVR can be considered a useful tool in the pre-operative evaluation of children with complex renal masses and can facilitate NSS in selected patients.
Keywords:
1. Introduction
2. Materials and Methods
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgements
Conflicts of Interest
References
- Han Q, Li K, Dong K, Xiao X, Yao W, Liu G. Clinical features, treatment, and outcomes of bilateral Wilms' tumor: A systematic review and meta-analysis. J Pediatr Surg. 2018 Dec;53(12):2465-2469. Epub 2018 Sep 1. [CrossRef] [PubMed]
- Roy P, van Peer SE, de Witte MM, Tytgat GAM, Karim-Kos HE, van Grotel M, van de Ven CP, Mavinkurve-Groothuis AMC, Merks JHM, Kuiper RP, Hol JA, Janssens GOR, de Krijger RR, Jongmans MCJ, Drost J, van der Steeg AFW, Littooij AS, Wijnen MHWA, van Tinteren H, van den Heuvel-Eibrink MM. Characteristics and outcome of children with renal tumors in the Netherlands: The first five-year's experience of national centralization. PLoS One. 2022 Jan 13;17(1):e0261729. [CrossRef] [PubMed] [PubMed Central]
- De Kraker J, Graf N, van Tinteren H, Pein F, Sandstedt B, Godzinski J, Tournade MF; SIOP. Reduction of postoperative chemotherapy in children with stage I intermediate-risk and anaplastic Wilms' tumour (SIOP 93-01 trial): a randomised controlled trial. Lancet. 2004 Oct 2-8;364(9441):1229-35. [CrossRef] [PubMed]
- Theilen TM, Braun Y, Bochennek K, Rolle U, Fiegel HC, Friedmacher F. Multidisciplinary Treatment Strategies for Wilms Tumor: Recent Advances, Technical Innovations and Future Directions. Front Pediatr. 2022 Jul 14;10:852185. [CrossRef] [PubMed] [PubMed Central]
- Pereira HR, Barzegar M, Hamadelseed O, Esteve AV, Munuera J. 3D surgical planning of pediatric tumors: a review. Int J Comput Assist Radiol Surg. 2022 Apr;17(4):805-816. Epub 2022 Jan 18. [CrossRef] [PubMed]
- Günther P, Schenk JP, Wunsch R, Tröger J, Waag KL. Abdominal tumours in children: 3-D visualisation and surgical planning. Eur J Pediatr Surg. 2004 Oct;14(5):316-21. [CrossRef] [PubMed]
- Chepelev L, Wake N, Ryan J, Althobaity W, Gupta A, Arribas E, Santiago L, Ballard DH, Wang KC, Weadock W, Ionita CN, Mitsouras D, Morris J, Matsumoto J, Christensen A, Liacouras P, Rybicki FJ, Sheikh A; RSNA Special Interest Group for 3D Printing. Radiological Society of North America (RSNA) 3D printing Special Interest Group (SIG): guidelines for medical 3D printing and appropriateness for clinical scenarios. 3D Print Med. 2018 Nov 21;4(1):11. [CrossRef] [PubMed] [PubMed Central]
- Daum R, Roth H, Zachariou Z. Tumor infiltration of the vena cava in nephroblastoma. Eur J Pediatr Surg. 1994 Feb;4(1):16-20. [CrossRef] [PubMed]
- Owens CM, Brisse HJ, Olsen ØE, Begent J, Smets AM. Bilateral disease and new trends in Wilms tumour. Pediatr Radiol. 2008 Jan;38(1):30-9. Epub 2007 Nov 17. [CrossRef] [PubMed]
- Van den Heuvel-Eibrink MM, Hol JA, Pritchard-Jones K, van Tinteren H, Furtwängler R, Verschuur AC, Vujanic GM, Leuschner I, Brok J, Rübe C, Smets AM, Janssens GO, Godzinski J, Ramírez-Villar GL, de Camargo B, Segers H, Collini P, Gessler M, Bergeron C, Spreafico F, Graf N; International Society of Paediatric Oncology — Renal Tumour Study Group (SIOP–RTSG). Position paper: Rationale for the treatment of Wilms tumour in the UMBRELLA SIOP-RTSG 2016 protocol. Nat Rev Urol. 2017 Dec;14(12):743-752. Epub 2017 Oct 31. [CrossRef] [PubMed]
- Ritchey ML, Shamberger RC, Haase G, Horwitz J, Bergemann T, Breslow NE. Surgical complications after primary nephrectomy for Wilms' tumor: report from the National Wilms' Tumor Study Group. J Am Coll Surg. 2001 Jan;192(1):63-8; quiz 146. [CrossRef] [PubMed]
- Green, DM. Evaluation of renal function after successful treatment for unilateral, non-syndromic Wilms tumor. Pediatr Blood Cancer. 2013 Dec;60(12):1929-35. Epub 2013 Aug 19. [CrossRef] [PubMed]
- Cozzi DA, Ceccanti S, Cozzi F. Renal function up to the 5th decade of life after nephrectomy in childhood: A literature review. Nephrology (Carlton). 2018 May;23(5):397-404. [CrossRef] [PubMed]
- Long CJ, Mittal S, Kolon TF. Expanding the Use of Nephron-Sparing Surgery for Wilms Tumor. J Natl Compr Canc Netw. 2022 Feb 17;20(5):540-546. [CrossRef] [PubMed]
- Wilde JC, Aronson DC, Sznajder B, Van Tinteren H, Powis M, Okoye B, Cecchetto G, Audry G, Fuchs J, Schweinitz DV, Heij H, Graf N, Bergeron C, Pritchard-Jones K, Van Den Heuvel-Eibrink M, Carli M, Oldenburger F, Sandstedt B, De Kraker J, Godzinski J. Nephron sparing surgery (NSS) for unilateral wilms tumor (UWT): the SIOP 2001 experience. Pediatr Blood Cancer. 2014 Dec;61(12):2175-9. Epub 2014 Aug 23. [CrossRef] [PubMed]
- Klatte T, Ficarra V, Gratzke C, Kaouk J, Kutikov A, Macchi V, Mottrie A, Porpiglia F, Porter J, Rogers CG, Russo P, Thompson RH, Uzzo RG, Wood CG, Gill IS. A Literature Review of Renal Surgical Anatomy and Surgical Strategies for Partial Nephrectomy. Eur Urol. 2015 Dec;68(6):980-92. Epub 2015 Apr 22. [CrossRef] [PubMed] [PubMed Central]
- Schima W, Böhm G, Rösch CS, Klaus A, Függer R, Kopf H. Mass-forming pancreatitis versus pancreatic ductal adenocarcinoma: CT and MR imaging for differentiation. Cancer Imaging. 2020 Jul 23;20(1):52. [CrossRef] [PubMed] [PubMed Central]
- Pandey P, Lewis H, Pandey A, Schmidt C, Dillhoff M, Kamel IR, Pawlik TM. Updates in hepatic oncology imaging. Surg Oncol. 2017 Jun;26(2):195-206. Epub 2017 Apr 6. [CrossRef] [PubMed]
- Privitera L, Paraboschi I, Cross K, Giuliani S. Above and Beyond Robotic Surgery and 3D Modelling in Paediatric Cancer Surgery. Front Pediatr. 2021 Dec 20;9:777840. [CrossRef] [PubMed] [PubMed Central]
- Bianchi L, Schiavina R, Bortolani B, Cercenelli L, Gaudiano C, Carpani G, Rustici A, Droghetti M, Mottaran A, Boschi S, Salvador M, Chessa F, Cochetti G, Golfieri R, Bertaccini A, Marcelli E. Interpreting nephrometry scores with three-dimensional virtual modelling for better planning of robotic partial nephrectomy and predicting complications. Urol Oncol. 2021 Dec;39(12):836.e1-836.e9. Epub 2021 Sep 14. [CrossRef] [PubMed]
- Porpiglia F, Fiori C, Checcucci E, Amparore D, Bertolo R. Hyperaccuracy Three-dimensional Reconstruction Is Able to Maximize the Efficacy of Selective Clamping During Robot-assisted Partial Nephrectomy for Complex Renal Masses. Eur Urol. 2018 Nov;74(5):651-660. Epub 2018 Jan 6. [CrossRef] [PubMed]
- Amparore D, Pecoraro A, Checcucci E, Piramide F, Verri P, De Cillis S, Granato S, Angusti T, Solitro F, Veltri A, Fiori C, Porpiglia F. Three-dimensional Virtual Models' Assistance During Minimally Invasive Partial Nephrectomy Minimizes the Impairment of Kidney Function. Eur Urol Oncol. 2022 Feb;5(1):104-108. Epub 2021 Apr 24. [CrossRef] [PubMed]
- Wang Z, Qi L, Yuan P, Zu X, Chen W, Cao Z, Li Y, Wang L. Application of Three-Dimensional Visualization Technology in Laparoscopic Partial Nephrectomy of Renal Tumor: A Comparative Study. J Laparoendosc Adv Surg Tech A. 2017 May;27(5):516-523. Epub 2017 Feb 10. [CrossRef] [PubMed]
- Lupulescu C, Sun Z. A Systematic Review of the Clinical Value and Applications of Three-Dimensional Printing in Renal Surgery. J Clin Med. 2019 Jul 8;8(7):990. [CrossRef] [PubMed] [PubMed Central]
- Porpiglia F, Checcucci E, Amparore D, Piramide F, Volpi G, Granato S, Verri P, Manfredi M, Bellin A, Piazzolla P, Autorino R, Morra I, Fiori C, Mottrie A. Three-dimensional Augmented Reality Robot-assisted Partial Nephrectomy in Case of Complex Tumours (PADUA ≥10): A New Intraoperative Tool Overcoming the Ultrasound Guidance. Eur Urol. 2020 Aug;78(2):229-238. Epub 2019 Dec 30. [CrossRef] [PubMed]
- Pereira HR, Barzegar M, Hamadelseed O, Esteve AV, Munuera J. 3D surgical planning of pediatric tumors: a review. Int J Comput Assist Radiol Surg. 2022 Apr;17(4):805-816. Epub 2022 Jan 18. [CrossRef] [PubMed]
- Krauel L, Fenollosa F, Riaza L, Pérez M, Tarrado X, Morales A, Gomà J, Mora J. Use of 3D Prototypes for Complex Surgical Oncologic Cases. World J Surg. 2016 Apr;40(4):889-94. [CrossRef] [PubMed]
- Souzaki R, Kinoshita Y, Ieiri S, Kawakubo N, Obata S, Jimbo T, Koga Y, Hashizume M, Taguchi T. Preoperative surgical simulation of laparoscopic adrenalectomy for neuroblastoma using a three-dimensional printed model based on preoperative CT images. J Pediatr Surg. 2015 Dec;50(12):2112-5. Epub 2015 Sep 2. [CrossRef] [PubMed]
- Schenk JP, Waag KL, Graf N, Wunsch R, Jourdan C, Behnisch W, Tröger J, Günther P. 3-D-Visualisierung in der MRT zur Operationsplanung von Wilms-Tumoren [3D-visualization by MRI for surgical planning of Wilms tumors]. Rofo. 2004 Oct;176(10):1447-52. German. [CrossRef] [PubMed]
- Girón-Vallejo Ó, García-Calderón D, Ruiz-Pruneda R, Cabello-Laureano R, Doménech-Abellán E, Fuster-Soler JL, Ruiz-Jiménez JI. Three-dimensional printed model of bilateral Wilms tumor: A useful tool for planning nephron sparing surgery. Pediatr Blood Cancer. 2018 Apr;65(4). Epub 2017 Dec 12. [CrossRef] [PubMed]
- Sánchez-Sánchez Á, Girón-Vallejo Ó, Ruiz-Pruneda R, Fernandez-Ibieta M, García-Calderon D, Villamil V, Giménez-Aleixandre MC, Montoya-Rangel CA, Hernández Bermejo JP. Three-Dimensional Printed Model and Virtual Reconstruction: An Extra Tool for Pediatric Solid Tumors Surgery. European J Pediatr Surg Rep. 2018 Jan;6(1):e70-e76. Epub 2018 Oct 18. [CrossRef] [PubMed] [PubMed Central]
- Fuchs J, Warmann SW, Szavay P, Kirschner HJ, Schäfer JF, Hennemuth A, Scheel-Walter HG, Bourquain H, Peitgen HO. Three-dimensional visualization and virtual simulation of resections in pediatric solid tumors. J Pediatr Surg. 2005 Feb;40(2):364-70. [CrossRef] [PubMed]
- Van der Zee JM, Fitski M, Simonis FFJ, van de Ven CP, Klijn AJ, Wijnen MHWA, van der Steeg AFW. Virtual Resection: A New Tool for Preparing for Nephron-Sparing Surgery in Wilms Tumor Patients. Curr Oncol. 2022 Feb 1;29(2):777-784. [CrossRef] [PubMed] [PubMed Central]
- Davidoff AM, Fernandez-Pineda I. Complications in the surgical management of children with malignant solid tumors. Semin Pediatr Surg. 2016 Dec;25(6):395-403. Epub 2016 Oct 31. [CrossRef] [PubMed]
- Vinit N, Blanc T, Bloch I, Pio L, Kassir R, La Barbera G, Bonnot E, Gori P, Goulin J, Pire A, Boddaert N, Lozach C, Sarnacki S. Robotics and 3D modeling for precision surgery in pediatric oncology. EJC Paediatric Oncology 2024 Vol. 4. p.100181. [CrossRef]
- Durso TA, Carnell J, Turk TT, Gupta GN. Three-dimensional reconstruction volume: a novel method for volume measurement in kidney cancer. J Endourol. 2014 Jun;28(6):745-50. Epub 2014 Feb 27. [CrossRef] [PubMed]
- Chaussy Y, Vieille L, Lacroix E, Lenoir M, Marie F, Corbat L, Henriet J, Auber F. 3D reconstruction of Wilms' tumor and kidneys in children: Variability, usefulness and constraints. J Pediatr Urol. 2020 Dec;16(6):830.e1-830.e8. Epub 2020 Aug 28. [CrossRef] [PubMed]
| IMAGING EVALUATION QUESTIONNAIRE (Likert scale 1-2-3-4-5) |
| Is it possible to clearly see the extent of the tumour and its characteristics? |
| Is it possible to clearly identify the arterial vasculature and its intraparenchymal branches? |
| Can the venous vasculature, intraparenchymal branches and thrombosis presence be clearly identified? |
| Is it possible to assess how the ureteral collecting system relates to the tumour and its possible invasion? |
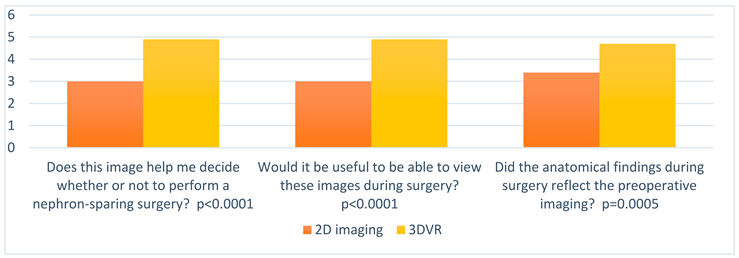
| Does this image help me decide whether or not to perform a nephron-sparing surgery? |
| Would it be useful to be able to view these images during surgery? |
| Did the anatomical findings during surgery reflect the preoperative imaging? |
| Case | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 |
| Sex (M/F) | F | M | M | M | F | F | M | F |
| Age (months) | 48 | 180 | 14 | 178 | 50 | 42 | 28 | 52 |
| Weight (kg) | 15 | 50 | 9.4 | 70 | 18 | 10.8 | 13 | 17 |
| Side | Right | Left | Left | Right | Right | Left | Left | Right |
| Position | UP | LP | UP+MP+LP | MP+UP | UP | MP+LP | LP | LP |
| Volume (ml) at surgery | 33 | 24 | 597 | 23 | 421 | 63 | 136 | - |
| Syndrome | - | - | - | - | - | WAGR | - | BWS |
| MRI | X | X | X | X | X | X | X | X |
| CT scan | X | X | - | X | X | X | X | X |
| Vein Thrombosis | - | - | - | - | X | - | X | - |
| UCSI | - | - | - | - | - | X | - | - |
| Surgery | RAL NSS | RAL NSS |
Open nephrectomy | RAL NSS | Open Nephrectomy | Open Nephrectomy | Open Nephrectomy | - |
| Operative time (minutes) | 210 | 200 | 240 | 150 | 450 | 310 | 320 | - |
| Histology | WT | TRCC | CN | CN | WT | WT | WT | - |
| Anatomical structure | 2D imaging | 3DVR | P-value |
| Tumor | 3.4 (3-4) | 4.8 (4-5) | < 0.0001 |
| Arteries | 3.6 (3-4) | 4.9 (4-5) | <0.0001 |
| Veins | 3.6 (3-4) | 4.8 (4-5) | .0004 |
| Urinary collecting system | 2.9 (2-4) | 3.5 (3-5) | .0961 |
 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





