1. Introduction
Robotic assistance has revolutionized abdominal surgery by addressing the limitations of conventional laparoscopic techniques. Key advantages include enhanced visualization, superior dexterity, and improved precision, which contribute to its growing adoption in urological procedures [
1]. Multiple studies have demonstrated that robotic surgeries achieve outcomes comparable, and sometimes superior, to conventional or laparoscopic approaches, with potential benefits such as shorter warm ischemia times and lower complication rates [
1,
2,
3]. Laparoscopic partial nephrectomy (LAPN) is a safe alternative to open partial nephrectomy (PN) with comparable oncological outcomes, better cosmetic and functional outcomes, and shorter hospital stays, but it carries increased risks of operative complications, requires advanced laparoscopic skills, and prolonged warm ischemia during tumor excision and renorrhaphy [
4]. Although robotic assistance offers technical advantages in managing complex renal tumours, its broader adoption is often tempered by concerns regarding cost. A study comparing robotic-assisted and laparoscopic radical nephrectomy found that the robotic approach increased total direct hospital costs, primarily due to higher operating room expenses [
5]. Despite these economic considerations, the benefits of robotic surgery for complex renal tumours, such as improved surgical precision and potentially better perioperative outcomes, warrant further investigation to determine its cost-effectiveness and clinical utility.
There are several nephrometry scoring systems (NSS) which are used to assess renal tumours prior to surgery. Among the NSS are the Preoperative Aspects and Dimensions Used for Anatomical (PADUA) classification; the Radius, Exophytic/Endophytic, Nearness, Anterior/Posterior, Location (RENAL) nephrometry score, the centrality index (C-index), diameter-axial-polar (DAP) Nephrometry and the Arterial-Based Complexity (ABC) scoring system have been developed to classify renal tumours according to anatomical distributions [
6]. The most used classification systems to localize renal carcinomas are the RENAL and PADUA [
7]. In this study, we employed the RENAL nephrometry score, which assesses five components: tumour Radius (maximum diameter), Exophytic/endophytic properties, Nearness of the tumour to the collecting system or sinus, Anterior/posterior location, and the Location relative to polar lines. Higher scores (≥9) indicate increased surgical complexity due to factors such as larger tumour size, central or hilar location, proximity to vital renal structures, or endophytic growth patterns that limit visibility and access during surgery.
Radical nephrectomy has been the standard treatment of localised renal tumors, however, utilization of nephron-sparing surgery or partial nephrectomy was recommended in smaller tumors due to equivalent oncological outcomes with reduced incidence of adverse events in terms of loss of renal function and cardiovascular risk [
8]. Robotic-assisted partial nephrectomy (RAPN) is an advanced surgical method that provides a minimally invasive option to traditional open or laparoscopic techniques for the management of renal tumours. RAPN as a minimally invasive surgical treatment for localised renal tumours has been gaining popularity [
9]. Its growing adoption is attributed to improved perioperative outcomes and enhanced preservation of renal function [
5,
10]. While the outcomes of RAPN for localized renal tumours are well-documented, its application in managing complex renal tumours, characterized by a RENAL nephrometry score of 9 or higher, remains relatively unexplored [
11,
12]. Existing studies on RAPN have exclusively concentrated on the perioperative metrics such as warm ischemia time, predicted blood loss, and complication rates. These studies emphasize the ability of the robotic platform to improve surgical results, primarily in cases that are technically demanding [
13,
14]. Although RAPN has been widely studied for small or moderately complex renal tumours, its use in highly complex cases (defined by a RENAL nephrometry score of 9 or higher) remains under investigated [
15].
Evidence on RAPN for highly complex renal tumors is particularly limited in Asian countries. Sharma
, et al. [
16] conducted a systematic review and meta-analysis, which included 22 studies that only focused on partial nephrectomy for complex kidney tumours. The findings showed that only four Asian countries; India, China, South Korea, and Japan have published studies, indicating the lack of available evidence [
16]. For instance, a retrospective study from China had constructed the R.O.A.D score to determine the workability of nephron-sparing surgery for hilar kidney tumours [
6]. The existing literature remains sparse, especially regarding outcomes in the Southeast Asian context, where demographic, clinical, and healthcare system factors may differ from those of Western countries [
17]. This gap underscores the urgent need for studies focused on Southeast Asian populations to better understand the applicability and outcomes of RAPN in this specific context [
17,
18].
This retrospective study evaluates the outcomes of RAPN in managing complex renal tumours (RENAL nephrometry score ≥ 9) at a single tertiary care centre in Malaysia. Warm ischemic time perioperative blood loss, postoperative changes in renal function, length of hospital stay, and surgical margins are the key metrics analysed to establish the efficacy and safety of this method. The findings aim to contribute valuable insights into the feasibility and effectiveness of RAPN in managing complex renal tumours within this unique regional context. With growing experience in RAPN, its role in managing complex renal tumours should be further explored to justify its potential benefits. More robust data are needed to confirm the feasibility of RAPN as a first-line management option for complex renal tumours, potentially replacing radical nephrectomy.
2. Materials and Methods
This study involves a retrospective review of a database that has been prospectively maintained, assessing the results of RAPN conducted by a single surgeon at our institution from January 2023 to June 2024. All patients who underwent RAPN during this period were included, utilizing both the transperitoneal and retroperitoneal approaches.
2.1. Data Collection
Data collected included patient demographics, tumour characteristics (such as location, size, and complexity), perioperative parameters (including warm ischemia time and blood loss volume), and renal function metrics (both pre- and post-operative serum creatinine and estimated glomerular filtration rate [eGFR]). The difference between the post- and pre-operative values was used for the change in eGFR Meanwhile, the RENAL nephrometry scoring system was used to assess the tumour complexity, with scores ≥ 9 categorized as highly complex renal tumours. The evaluation of histopathological examination and surgical margin status were also carried out.
2.2. Surgical Techniques
All surgeries were performed by a single urologist with extensive experience in minimally invasive and robotic urologic procedures. This consistency helped standardize surgical technique and decision-making across all cases. For this study, both the transperitoneal and retroperitoneal approaches were used for RAPN, depending on the tumour location. The docking of the robotic system and surgical steps were standardized for all cases.
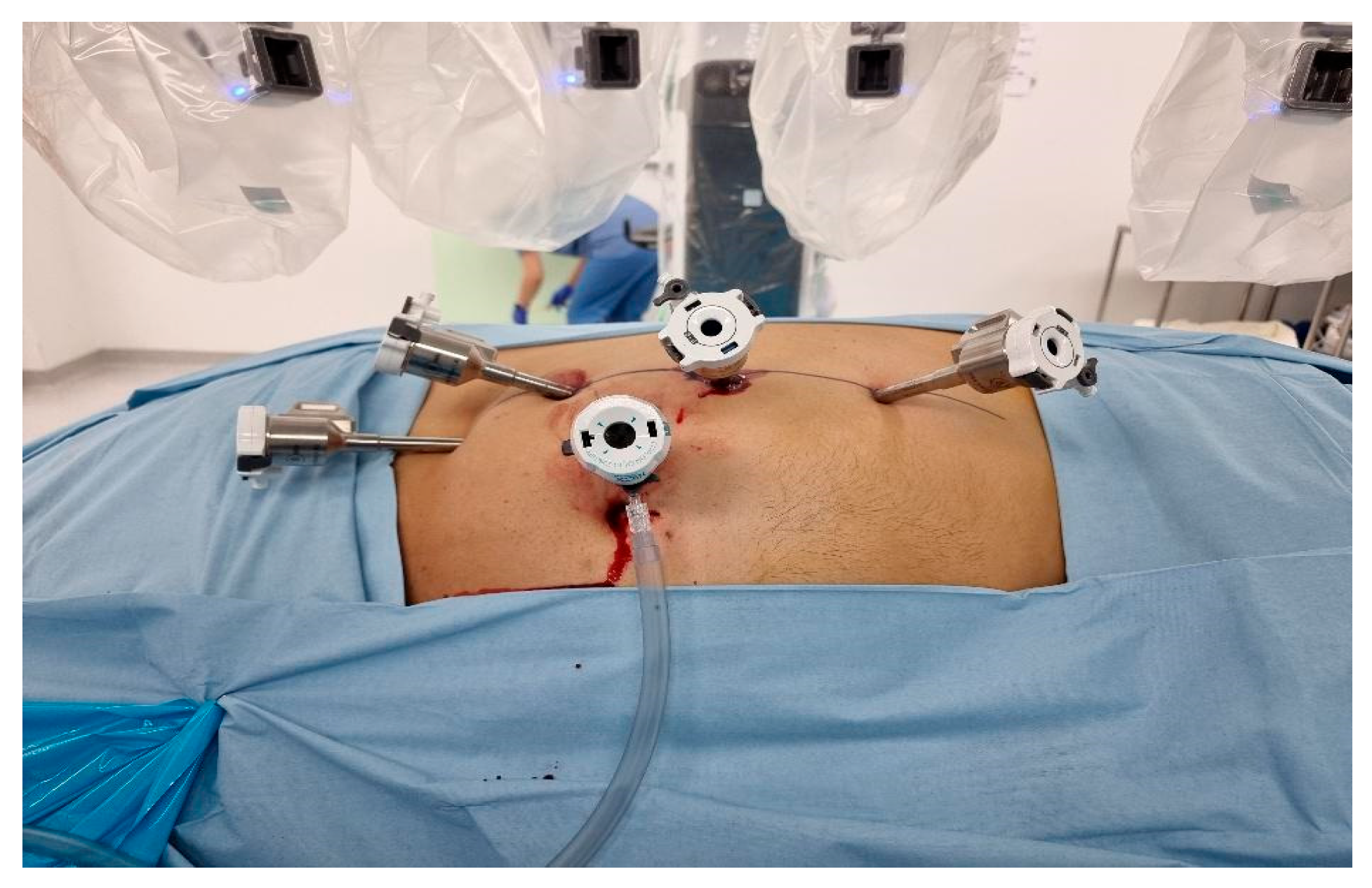
Transperitoneal Approach: Patients were positioned in a 70-degree decubitus posture with their flank extended. Pneumoperitoneum was created using a Veress needle, and trocars were placed under direct vision at 8 cm intervals along the semilunar line. The Da Vinci robotic system was docked with targeting. The robotic instruments used consisted of a bipolar grasper, monopolar scissors, ProGrasp forceps, and a large needle driver. Once docking is done, the colon was medially mobilized to reveal the kidney. The dissection of the renal hilum was done to isolate the renal artery and vein. Clamping of the artery was then done using a robotic bulldog clamp, followed by tumour excision using robotic scissors. Barbed sutures were used to repair the renal defect to achieve haemostasis and reconstruct the parenchyma (
Figure 1).
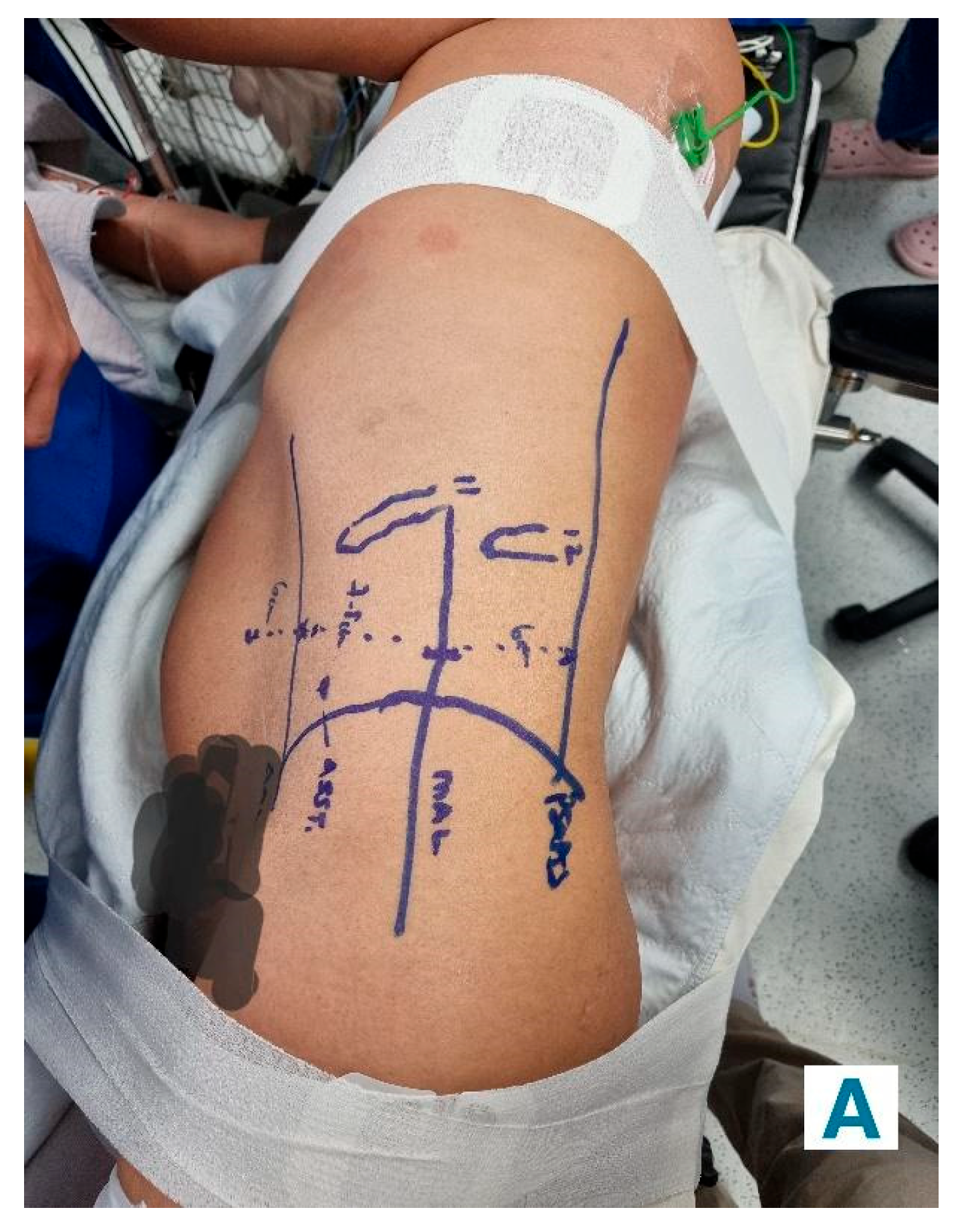
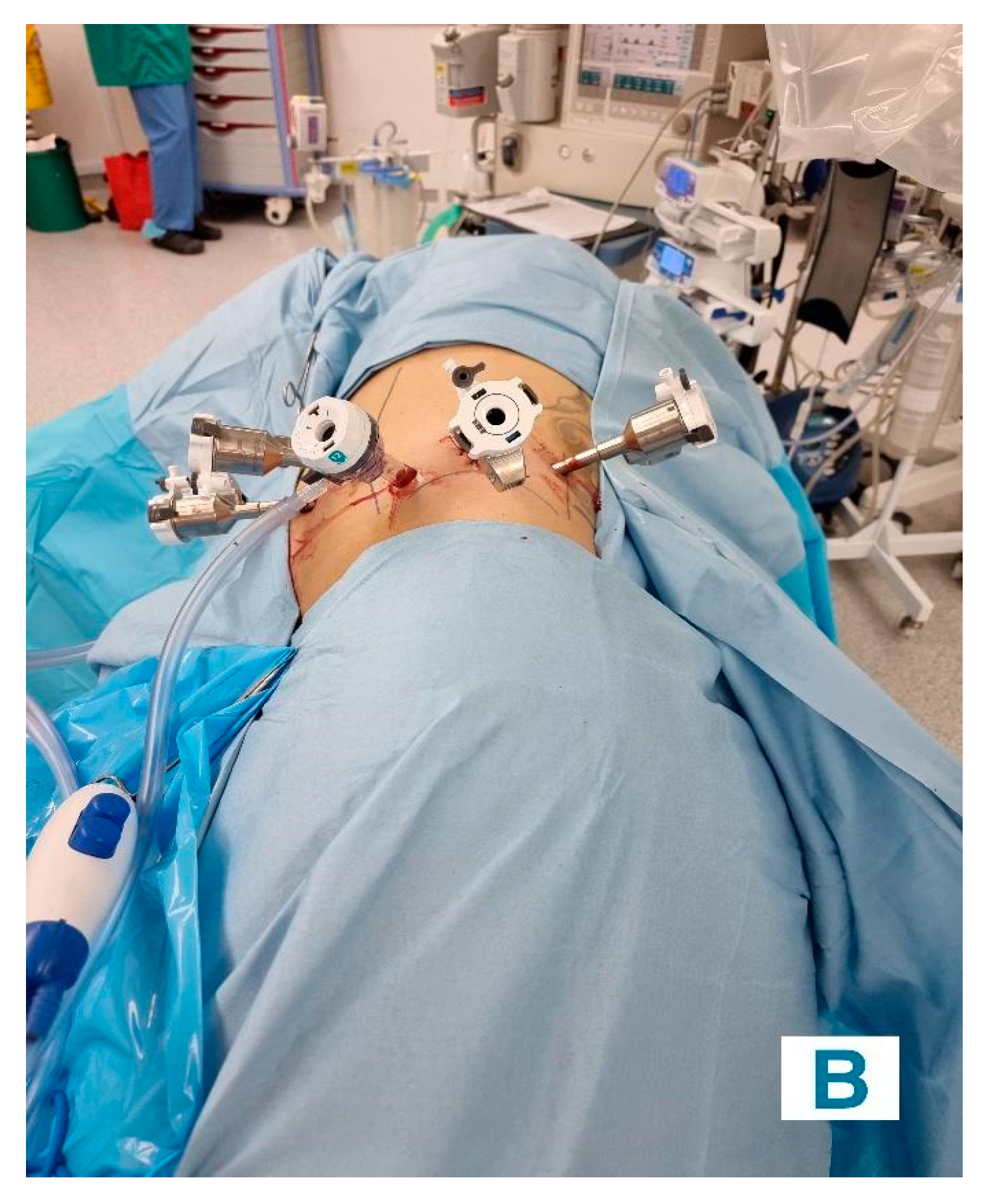
Retroperitoneal Approach: Patients were positioned in a 90-degree lateral position, and port sites were marked according to the selected approach. A balloon dissector was used to create retroperitoneal space, and trocars were placed at 6 cm intervals using finger guidance or direct vision. Docking of the Da Vinci robotic system was then done to provide an ideal working space to allow the robotic arms and camera accurate surgical access. The bipolar grasper, monopolar scissors, ProGrasp forceps, and a large needle driver were the robotic instruments used for this procedure. The retroperitoneal fat was mobilized, and the lateral coronal fascia was incised. The perirenal fat was then carefully dissected to expose the kidney tumour. The renal hilum was accessed directly, allowing for precise isolation of the renal artery, which was clamped as needed to control blood flow (
Figure 2A and
Figure 2B). The tumour was meticulously excised, and the renal defect was repaired in layers using barbed sutures to achieve haemostasis and reconstruct the parenchyma, following the same principles as the transperitoneal approach [
19].
Both approaches aim to minimize warm ischemia time (maintained under 30 minutes wherever possible), ensure complete tumour excision, and achieve secure haemostasis.
2.3. Statistical Analysis
SPSS version 26.0 for Windows was used to analyse the data. Descriptive statistics were utilized, with parametric data shown as the mean ± standard deviation, and non-parametric data displayed as the median with interquartile range. Normality of continuous variables was assessed using the Shapiro-Wilk test. For normally distributed continuous variables, Pearson’s correlation was used to assess linear relationships between tumour complexity and operative metrics (e.g., warm ischemia time, blood loss). Independent sample t-tests were employed to compare means between the transperitoneal and retroperitoneal surgical approaches, as these groups met assumptions for normality and homogeneity of variances. For categorical variables, Chi-square tests were used to assess associations. A p-value of less than 0.05 was considered statistically significant.
2.4. Ethics Statement
This study was carried out adhering to the ethical principles outlined in the Declaration of Helsinki (revised 2013). The study protocol was reviewed and approved by the Research Ethics Committee of The National University of Malaysia (IRB no. JEP-2024-804). Written informed consent for surgery was obtained from all patients prior to the procedure. The necessity to obtain additional consent for data publication was waived by the Institutional Review Board of the National University of Malaysia due to the retrospective study design.
2.5. Outcomes
The primary outcome of the study was achieving the "trifecta," defined as the absence of severe perioperative complications, negative surgical margins, and preservation of renal function. Secondary outcomes included operative time, warm ischemia time, estimated blood loss, length of hospital stay, and changes in renal function.
3. Results
A total of 35 patients, comprising 21 males and 14 females with a mean age of 53.37 ± 15.52 years, underwent RAPN during the study period (
Table 1). Tumour characteristics are summarized in
Table 2, and perioperative outcomes are detailed in
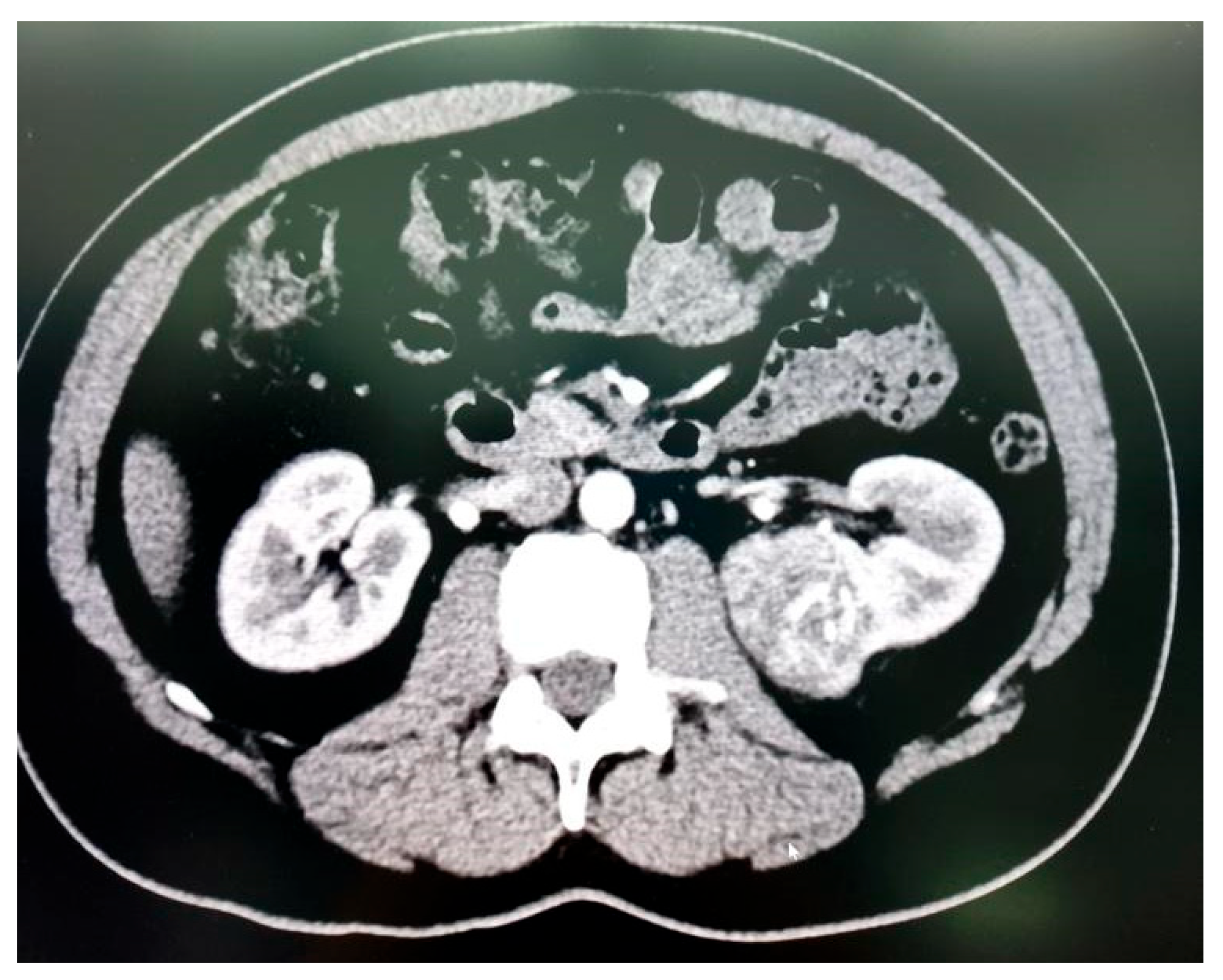
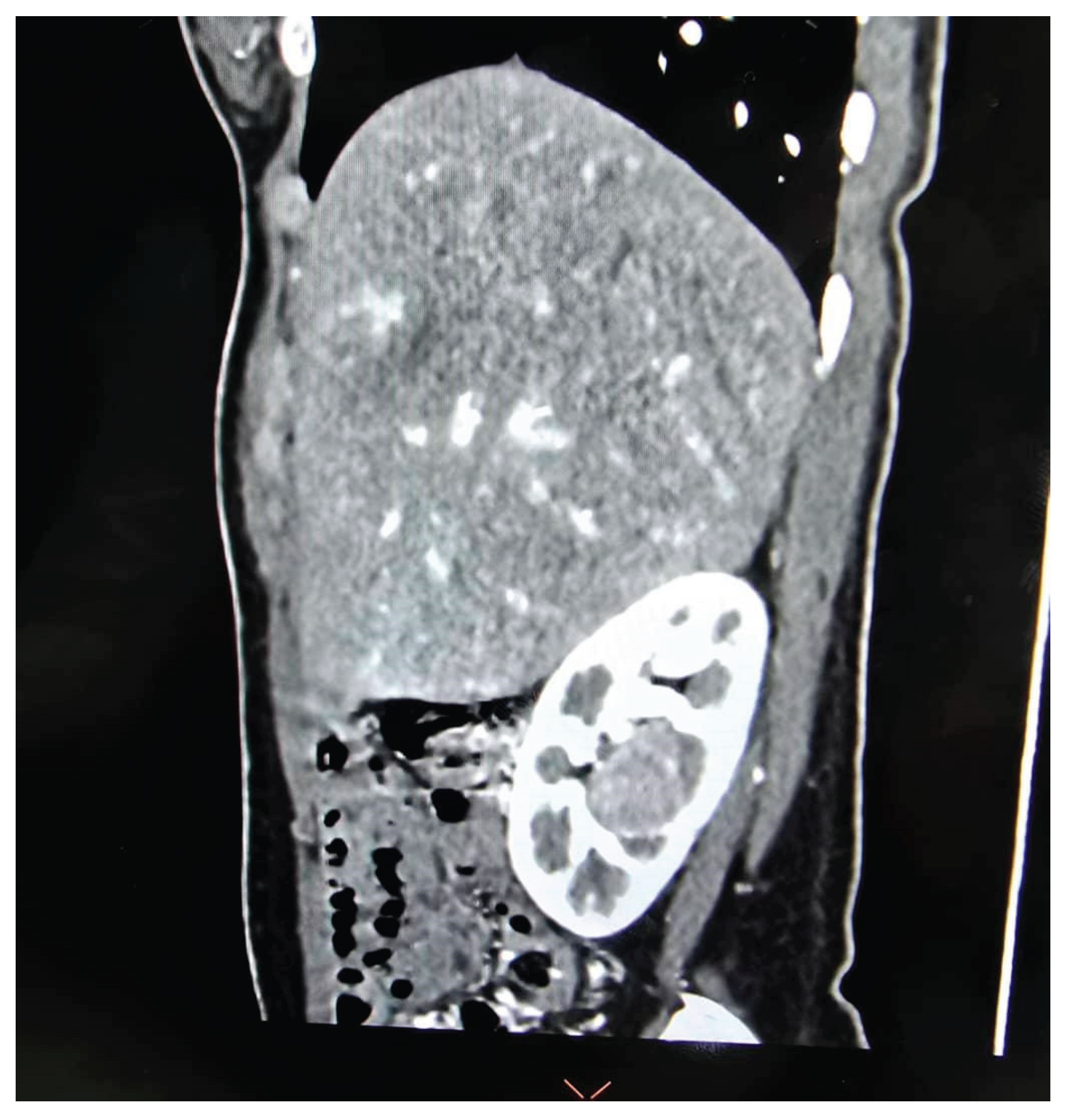
Table 3 and
Table 4. All patients had high-complexity tumours, with a median RENAL nephrometry score of 10 (
Figure 3A,
Figure 3B and
Figure 3C).
3.1. Correlation Analysis
Higher RENAL nephrometry scores were moderately associated with longer warm ischemia times (correlation coefficient: 0.35) and greater intraoperative blood loss (correlation coefficient: 0.47). A weak positive correlation was observed with length of hospital stay (correlation coefficient: 0.27), while changes in postoperative creatinine levels showed a negligible correlation (correlation coefficient: 0.07). These findings indicate that increased tumour complexity is associated with greater surgical challenges and marginally prolonged recovery times. Creatinine levels remain stable when warm ischemia time (WIT) is less than 20 minutes, and minimal normal parenchymal tissue is excised, as observed in most of our cases.
3.2. Comparative Analysis of Surgical Approaches
An independent sample t-test comparing the results of the transperitoneal and retroperitoneal approaches revealed no statistically significant difference in warm ischemia times (t = −0.85, p = 0.40). Nevertheless, the retroperitoneal approach showed significantly lower intraoperative blood loss (t = −2.37, p = 0.028). There were no notable differences shown in both the length of hospital stay (t = −0.95, p = 0.35) and the postoperative changes in serum creatinine levels (t = 1.27, p = 0.21).
3.3. Warm Ischemia Time and Renal Function
The relationship between WIT and the percentage change in serum creatinine revealed a negligible negative correlation (r = −0.04, p = 0.403). Regression analysis showed an R² value of 0.0016, indicating that less than 1% of the variance in creatinine changes could be attributed to WIT. These findings suggest that WIT has minimal impact on postoperative renal function, particularly when WIT is maintained at less than 20 minutes, as observed in most of our cases. Instead, outcomes are more likely influenced by other factors, such as the patient’s baseline renal reserve.
3.4. Operative Console Time and Tumour Complexity
The correlation observed between the RENAL nephrometry score and operative console time (correlation coefficient: 0.22) is a weak positive, indicating a slight increase in operative console time with higher tumour complexity. However, this relationship was not statistically significant (p = 0.206). Furthermore, the retroperitoneal and transperitoneal approaches indicated no significant difference in the operative console time (t = 0.28, p = 0.779).
3.5."Trifecta" Achievement and Complications
The "trifecta" outcome: absence of severe perioperative complications, negative surgical margins, and preservation of renal function, was achieved in 31 patients (88.6%). There were 4 cases (11.4%) of Clavien-Dindo Grade ≥ 2 complications (
Table 5). These included a postoperative fever requiring intravenous antibiotics, ileus managed conservatively, lung atelectasis treated with non-invasive ventilation, and a transient ischemic attack (TIA) requiring intensive care monitoring. All patients recovered without long-term complications and were discharged within one week.
3.6. Histopathological Findings
The histopathological findings revealed a diverse range of tumour types among the patients who underwent robotic-assisted partial nephrectomy. Clear cell renal cell carcinoma was the most common, identified in 22 patients, followed by angiomyolipoma in 7 patients. Additionally, 2 patients exhibited papillary RCC, and another 2 had multiloculated cystic RCC. Notably, a rare case of Ewing sarcoma of the kidney was also observed. Importantly, none of the cases showed a positive surgical margin, and no local recurrence or port-site metastases were detected in RCC patients during the mean follow-up period of 11.31 ± 5.78 months.
4. Discussion
This study demonstrates that RAPN is a safe and effective surgical option for managing complex renal tumours, defined by a RENAL nephrometry score of ≥ 9. The favourable perioperative and postoperative outcomes align with findings reported in the literature, emphasizing the role of RAPN in handling high-complexity renal tumours [
13,
14,
20].
The correlation analysis revealed that higher RENAL nephrometry scores were moderately associated with longer warm ischemia times and greater intraoperative blood loss. This finding aligns with the established challenges of managing complex renal tumours, which often require meticulous dissection and reconstruction. Longer ischemic durations are sometimes necessary to balance tumour resection and parenchymal preservation [
13,
14,
20]. The weak correlation with hospital stays duration and the negligible association with serum creatinine changes were expected, as most patients experienced early recovery due to the benefits of minimally invasive surgery. Notably, renal function is significantly affected when WIT is severely prolonged or when a significant amount of normal kidney tissue is resected. These findings highlight that tumour complexity impacts surgical parameters more than immediate recovery or renal function outcomes [
21]. Additionally, console time is often prolonged in cases with toxic renal fat, where more time is required to mobilize adhesive perirenal fat. In this study, WIT was consistently kept below 30 minutes, and there were no cases of severe WIT prolongation despite tumour complexity. Therefore, console time was not significantly correlated with the RENAL nephrometry score.
Our strategic approach of tailoring the surgical technique to tumour location likely contributed to the favourable outcomes observed. Generally, the retroperitoneal approach was preferred for upper pole, midline, and posteriorly located tumours, while the transperitoneal approach was favoured for lower pole and anteriorly located tumours. This selection allowed for direct access to the tumour, facilitating optimum excision and suturing. The retroperitoneal approach provides better access to the renal hilum and reduces blood loss compared to the transperitoneal approach. This finding aligns with studies that report reduced bleeding due to optimized visualization in retroperitoneal surgeries [
12,
14]. Notably, our tumour-tailored approach resulted in comparable warm ischemia times and hospital stays between the two approaches, suggesting that both techniques are viable options depending on tumour characteristics and surgical expertise.
The mean WIT of 15.03 ± 5.84 minutes in this study is consistent with reported averages for RAPN, typically ranging from 15 to 18 minutes [
19,
22]. Importantly, the negligible correlation between WIT and postoperative serum creatinine changes reinforces the notion that renal functional outcomes depend on factors such as baseline renal reserve rather than ischaemic duration alone [
13,
14]. Furthermore, renal function is well-preserved, particularly when WIT is kept below 20 minutes [
23].
The complete negative surgical margin rate and the absence of local recurrences or port-site metastases during a mean follow-up of 11.31 ± 5.78 months underscore the oncological safety of RAPN for managing complex renal tumours. These findings are consistent with existing literature, which reports excellent long-term cancer control with RAPN [
14,
24,
25]. For instance, a systematic review by Vartolomei
, et al. [
26] analysed multiple RAPN series and reported positive surgical margin rates ranging from 0% to 10.5%, with local recurrence occurring in up to 3.6% of patients. Our study achieved excellent results in this parameter, further reinforcing the oncological efficacy of RAPN in complex renal tumours. While no local recurrences or port-site metastases were observed during the follow-up period, it is important to note that our mean follow-up duration is relatively short. As such, the current data do not allow for definitive conclusions regarding long-term oncological control or renal function preservation. Recurrence and delayed functional decline may manifest beyond this period. Therefore, extended follow-up is essential to validate the durability of these outcomes.
The high rate of 'trifecta' achievement (88.6%), defined as the absence of severe perioperative complications, negative surgical margins, and preservation of renal function, underscores the efficacy and safety of RAPN in managing complex kidney tumours. The low rate of significant complications further supports its utility, with only 11.4% of patients experiencing Clavien-Dindo Grade ≥ 2 events. These outcomes emphasize the reliability of RAPN for treating complex renal tumours [
27]. Our results align with global studies that report similarly high "trifecta" achievement rates for RAPN. Furukawa
, et al. [
28] conducted a large multicentre study in Japan and reported a trifecta rate of 89%, demonstrating the consistency of RAPN outcomes across diverse populations [
28]. Notably, our trifecta achievement is comparable to this result, despite exclusively including complex kidney tumours.
This study is the first to report RAPN outcomes for complex renal tumours in Malaysia, contributing valuable data to the limited literature on Southeast Asian populations. While studies from broader Asian regions, such as China and South Korea, have demonstrated RAPN’s safety and efficacy for complex tumours, data specific to Southeast Asia have been sparse [
20,
28,
29,
30]. A study by Hinata and Fujisawa [
31] discussed RAPN techniques and outcomes, highlighting the advantages of the procedure in minimally invasive surgery and nephron-sparing approaches. This study provides valuable insights; it does not specifically address the Southeast Asian context. Similarly, Pandolfo, Wu, Campi, Bertolo, Amparore, Mari, Verze, Manfredi, Franco, Ditonno, Cerrato, Ferro, Lasorsa, Contieri, Napolitano, Tufano, Lucarelli, Cilio, Perdonà, Siracusano, Autorino and Aveta [
17] conducted a systematic review on RAPN for renal hilar masses, focusing on surgical techniques and outcomes. Although comprehensive, this review lacks data specific to Southeast Asian populations.
The scarcity of region-specific studies underscores the need for more research on RAPN outcomes in Southeast Asia. Factors such as genetic diversity, healthcare infrastructure, and surgical expertise may influence outcomes, making it essential to contextualize findings within the region. According to a study, experienced surgeons for RAPN were linked to lower operative time, WIT and major postoperative complications [
4]. Our findings align with these studies, showcasing RAPN’s ability to achieve favourable perioperative and oncological outcomes in a Southeast Asian context, further validating its potential benefits for this region's unique demographic and clinical settings.
This study has a number of limitations. Its retrospective nature and small sample size reduce the ability to generalize the findings. Additionally, the short follow-up period may not fully capture long-term oncological and functional outcomes, which are crucial for evaluating RAPN’s effectiveness in complex cases. Future studies should focus on larger cohorts with extended follow-up periods to provide a more comprehensive assessment of RAPN’s long-term benefits and limitations. Comparative studies between RAPN and alternative surgical approaches could also further delineate its advantages for complex renal tumours
5. Conclusions
This study demonstrates that RAPN is a safe and effective surgical approach for managing complex renal tumours (RENAL nephrometry score ≥ 9), achieving high rates of ‘trifecta’ outcomes. The procedure resulted in positive perioperative and oncological outcomes, such as reduced blood loss, maintained renal function, and lack of positive surgical margins, even in highly complex cases. The choice of approach, whether transperitoneal or retroperitoneal, should be tailored to the characteristics of the tumour to optimize surgical outcomes.
This study represents a significant contribution to the limited data on RAPN outcomes in Southeast Asia, specifically in Malaysia, and aligns with global findings that highlight RAPN as a reliable option for nephron-sparing surgery. Despite being a single-centre study with a modest sample size, this is the first Malaysian study evaluating RAPN for complex renal tumours and offers important preliminary data. Future larger-scale, multi-centre studies are warranted to validate and expand upon these findings.
Author Contributions
Conceptualization, X.I.F. and I.H.R..; methodology, X.I.F., M.H.M.H, and I.H.R; formal analysis, M.H.M.H., X.I.F., and I.H.R.; investigation, M.H.M.H., X.I.F., and I.H.R.; writing—original draft preparation, M.H.M.H., and X.I.F.; writing—review and editing, R.I.H., Z.M.Z., L.Y.L., H.C.K., S.S., M.H.A., and X.I.F.; supervision, X.I.F.
Funding
This research received no external funding.
Institutional Review Board Statement
This study was performed in line with the principles of the Declaration of Helsinki. This study was approved by the Research Ethics Committee of The National University of Malaysia (IRB no. JEP-2024-804).
Informed Consent Statement
Prior to the procedure, written informed consent was acquired from all patients.
Data Availability Statement
No datasets were generated or analysed during the current study.
Conflicts of Interest
The authors declare no conflicts of interest.
Abbreviations
The following abbreviations are used in this manuscript:
| RAPN |
Robotic-assisted partial nephrectomy |
| LAPN |
Laparoscopic partial nephrectomy |
| PN |
Partial nephrectomy |
| NSS |
Nephrometry scoring systems |
| PADUA |
Preoperative Aspects and Dimensions Used for Anatomical |
| RENAL |
Radius, Exophytic/Endophytic, Nearness, Anterior/Posterior, Location |
| DAP |
Diameter-axial-polar |
| C-index |
Centrality index |
| ABC |
Arterial-Based Complexity |
| eGFR |
Estimated glomerular filtration rate |
| WIT |
Warm ischemia time |
References
- Koh, D.H.; Jang, W.S.; Park, J.W.; Ham, W.S.; Han, W.K.; Rha, K.H.; Choi, Y.D. Efficacy and Safety of Robotic Procedures Performed Using the da Vinci Robotic Surgical System at a Single Institute in Korea: Experience with 10000 Cases. Yonsei Med J 2018, 59, 975–981. [Google Scholar] [CrossRef] [PubMed]
- Brenner, Z.R.; Salathiel, M.; Macey, B.A.; Krenzer, M. Postoperative care for the robotic surgery bowel resection patient. Gastroenterol Nurs 2011, 34, 271–275. [Google Scholar] [CrossRef] [PubMed]
- Abdalla, R.Z.; Averbach, M.; Ribeiro-Junior, U.; Machado, M.A.; Luca-Filho, C.R. Robotic abdominal surgery: a Brazilian initial experience. Arq Bras Cir Dig 2013, 26, 190–194. [Google Scholar] [CrossRef]
- Bray, G.; Bahadori, A.; Mao, D.; Ranasinghe, S.; Tracey, C. Benefits of Robotic Assisted vs. Traditional Laparoscopic Partial Nephrectomy: A Single Surgeon Comparative Study. J Clin Med 2022, 11. [Google Scholar] [CrossRef]
- Jeong, I.G.; Khandwala, Y.S.; Kim, J.H.; Han, D.H.; Li, S.; Wang, Y.; Chang, S.L.; Chung, B.I. Association of Robotic-Assisted vs Laparoscopic Radical Nephrectomy With Perioperative Outcomes and Health Care Costs, 2003 to 2015. Jama 2017, 318, 1561–1568. [Google Scholar] [CrossRef]
- Huang, Q.; Gu, L.; Zhu, J.; Peng, C.; Du, S.; Liu, Q.; Chen, J.; Wang, B.; Fan, Y.; Gao, Y.; et al. A three-dimensional, anatomy-based nephrometry score to guide nephron-sparing surgery for renal sinus tumors. Cancer 2020, 126 Suppl 9, 2062–2072. [Google Scholar] [CrossRef]
- Diana, P.; Lughezzani, G.; Uleri, A.; Casale, P.; Saita, A.; Hurle, R.; Lazzeri, M.; Mottrie, A.; De Naeyer, G.; De Groote, R.; et al. Multi-institutional Retrospective Validation and Comparison of the Simplified PADUA REnal Nephrometry System for the Prediction of Surgical Success of Robot-assisted Partial Nephrectomy. Eur Urol Focus 2021, 7, 1100–1106. [Google Scholar] [CrossRef]
- Khoo, H.C.; Lim, L.Y.; Shukor, S.; Zainal Adwin, Z.A.; Zulkifli, M.Z.; Fam, X.I. Initial experience of laparoscopic retroperitoneal partial nephrectomy in an academic hospital in Malaysia. Med J Malaysia 2022, 77, 764–767. [Google Scholar]
- Carbonara, U.; Crocerossa, F.; Campi, R.; Veccia, A.; Cacciamani, G.E.; Amparore, D.; Checcucci, E.; Loizzo, D.; Pecoraro, A.; Marchioni, M.; et al. Retroperitoneal Robot-assisted Partial Nephrectomy: A Systematic Review and Pooled Analysis of Comparative Outcomes. Eur Urol Open Sci 2022, 40, 27–37. [Google Scholar] [CrossRef]
- Shen, Z.; Sun, Z. Systematic review and meta-analysis of randomised trials of perioperative outcomes comparing robot-assisted versus open radical cystectomy. BMC Urol 2016, 16, 59. [Google Scholar] [CrossRef] [PubMed]
- Rogers, C.; Sukumar, S.; Gill, I.S. Robotic partial nephrectomy: the real benefit. Curr Opin Urol 2011, 21, 60–64. [Google Scholar] [CrossRef]
- Seo, I.Y.; Choi, H.; Boldbaatr, Y.; Lee, J.W.; Rim, J.S. Operative outcomes of robotic partial nephrectomy: a comparison with conventional laparoscopic partial nephrectomy. Korean J Urol 2011, 52, 279–283. [Google Scholar] [CrossRef] [PubMed]
- Benway, B.M.; Bhayani, S.B. Surgical outcomes of robot-assisted partial nephrectomy. BJU Int 2011, 108, 955–961. [Google Scholar] [CrossRef] [PubMed]
- Lee, C.U.; Kang, M.; Sung, H.H.; Jeon, H.G.; Han, D.H.; Jeong, B.C.; Jeon, S.S.; Lee, H.M.; Choi, H.Y.; Seo, S.I. Comparison of 5-Year Outcomes of Robot-Assisted Laparoscopic and Laparoscopic Partial Nephrectomy in Patients With Localized Renal Cell Carcinoma. Korean J Urol Oncol 2017, 15, 172–177. [Google Scholar] [CrossRef]
- Lv, Z.; Chen, G.; Chen, X.; Li, Y.; Bao, E.; Hu, K.; Yu, X. Open versus robot-assisted partial nephrectomy for highly complex renal masses: a meta-analysis of perioperitive and functional outcomes. J Robot Surg 2023, 17, 1955–1965. [Google Scholar] [CrossRef]
- Sharma, G.; Sharma, A.P.; Tyagi, S.; Bora, G.S.; Mavuduru, R.S.; Devana, S.K.; Singh, S.K. Robot-assisted partial nephrectomy for moderate to highly complex renal masses. A systematic review and meta-analysis. Indian J Urol 2022, 38, 174–183. [Google Scholar] [CrossRef]
- Pandolfo, S.D.; Wu, Z.; Campi, R.; Bertolo, R.; Amparore, D.; Mari, A.; Verze, P.; Manfredi, C.; Franco, A.; Ditonno, F.; et al. Outcomes and Techniques of Robotic-Assisted Partial Nephrectomy (RAPN) for Renal Hilar Masses: A Comprehensive Systematic Review. Cancers (Basel) 2024, 16. [Google Scholar] [CrossRef]
- Jabaji, R.B.; Fischer, H.; Kern, T.; Chien, G.W. Trend of Surgical Treatment of Localized Renal Cell Carcinoma. Perm J 2019, 23, 18–108. [Google Scholar] [CrossRef]
- Lyu, X.; Jia, Z.; Ao, L.; Ren, C.; Wu, Y.; Xu, Y.; Chen, K.; Gao, Y.; Wang, B.; Ma, X.; et al. Robot-assisted partial nephrectomy: Can retroperitoneal approach suit for renal tumors of all locations?-A large retrospective cohort study. BMC Urol 2022, 22, 202. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.K.; Lee, H.; Oh, J.J.; Lee, S.; Hong, S.K.; Lee, S.E.; Byun, S.S. Comparison of robotic and open partial nephrectomy for highly complex renal tumors (RENAL nephrometry score ≥10). PLoS One 2019, 14, e0210413. [Google Scholar] [CrossRef]
- Liu, X.; Jin, D.; Zhang, Y.; Zhang, S. Limited non-linear impact of warm ischemia time on renal functional decline after partial nephrectomy: a propensity score-matched study. Int Urol Nephrol 2023, 55, 1699–1708. [Google Scholar] [CrossRef]
- Numakura, K.; Kobayashi, M.; Koizumi, A.; Kashima, S.; Yamamoto, R.; Nara, T.; Saito, M.; Narita, S.; Inoue, T.; Habuchi, T. Factors influencing warm ischemia time in robot-assisted partial nephrectomy change depending on the surgeon's experience. World J Surg Oncol 2022, 20, 202. [Google Scholar] [CrossRef] [PubMed]
- Thompson, R.H.; Lane, B.R.; Lohse, C.M.; Leibovich, B.C.; Fergany, A.; Frank, I.; Gill, I.S.; Blute, M.L.; Campbell, S.C. Renal function after partial nephrectomy: effect of warm ischemia relative to quantity and quality of preserved kidney. Urology 2012, 79, 356–360. [Google Scholar] [CrossRef]
- Benway, B.M.; Bhayani, S.B.; Rogers, C.G.; Dulabon, L.M.; Patel, M.N.; Lipkin, M.; Wang, A.J.; Stifelman, M.D. Robot assisted partial nephrectomy versus laparoscopic partial nephrectomy for renal tumors: a multi-institutional analysis of perioperative outcomes. J Urol 2009, 182, 866–872. [Google Scholar] [CrossRef]
- Hennessey, D.B.; Wei, G.; Moon, D.; Kinnear, N.; Bolton, D.M.; Lawrentschuk, N.; Chan, Y.K. Strategies for success: a multi-institutional study on robot-assisted partial nephrectomy for complex renal lesions. BJU Int 2018, 121 Suppl 3, 40–47. [Google Scholar] [CrossRef]
- Vartolomei, M.D.; Remzi, M.; Fajkovic, H.; Shariat, S.F. Robot-Assisted Partial Nephrectomy Mid-Term Oncologic Outcomes: A Systematic Review. J Clin Med 2022, 11. [Google Scholar] [CrossRef]
- Tanabalan, C.; Raman, A.; Mumtaz, F. Robot-assisted partial nephrectomy: How to minimise renal ischaemia. Arab J Urol 2018, 16, 350–356. [Google Scholar] [CrossRef]
- Furukawa, J.; Kanayama, H.; Azuma, H.; Inoue, K.; Kobayashi, Y.; Kashiwagi, A.; Segawa, T.; Takahashi, Y.; Horie, S.; Ogawa, O.; et al. 'Trifecta' outcomes of robot-assisted partial nephrectomy: a large Japanese multicenter study. Int J Clin Oncol 2020, 25, 347–353. [Google Scholar] [CrossRef] [PubMed]
- Jiang, X.L.; OuYang, K.; Yang, R.; Yu, X.Y.; Yang, D.D.; Wu, J.T.; Zhao, H.W. The application of internal traction technique in retroperitoneal robot-assisted partial nephrectomy for renal ventral tumors. World J Surg Oncol 2022, 20, 213. [Google Scholar] [CrossRef]
- Wang, L.; Deng, J.Y.; Liang, C.; Zhu, P.Y. Perioperative, functional, and oncological outcomes of robotic vs. laparoscopic partial nephrectomy for complex renal tumors (RENAL score ≥7): an evidence-based analysis. Front Oncol 2023, 13, 1195910. [Google Scholar] [CrossRef] [PubMed]
- Hinata, N.; Fujisawa, M. Robot assisted partial nephrectomy: technique and outcomes. In Endourology Progress: Technique, technology and training; Springer: 2019; pp. 117–126.
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).