Submitted:
13 January 2025
Posted:
14 January 2025
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Materials and Methods
2.1. Waste Collection and Preparation
2.2. Larval Rearing and Experimental Design
2.3. Analytical Analysis
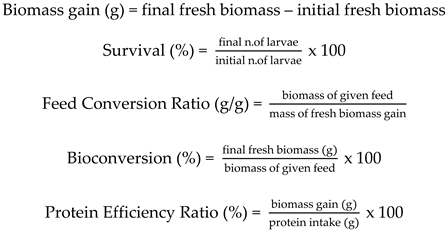
2.3.1. Biomass Gain, Survival, Feed Conversion Ratio, Bioconversion, and Protein Efficiency Ratio
2.3.2. Nutritional Composition
2.3.3. Total Polyphenol Concentration
2.4. Total Polyphenol Concentration
3. Results
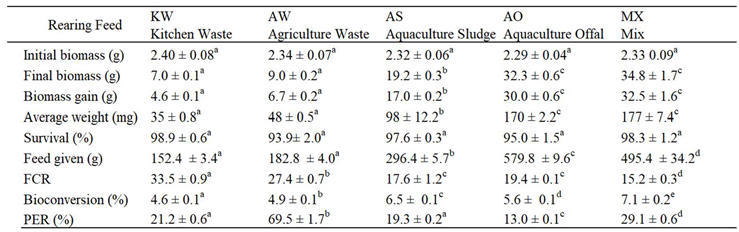
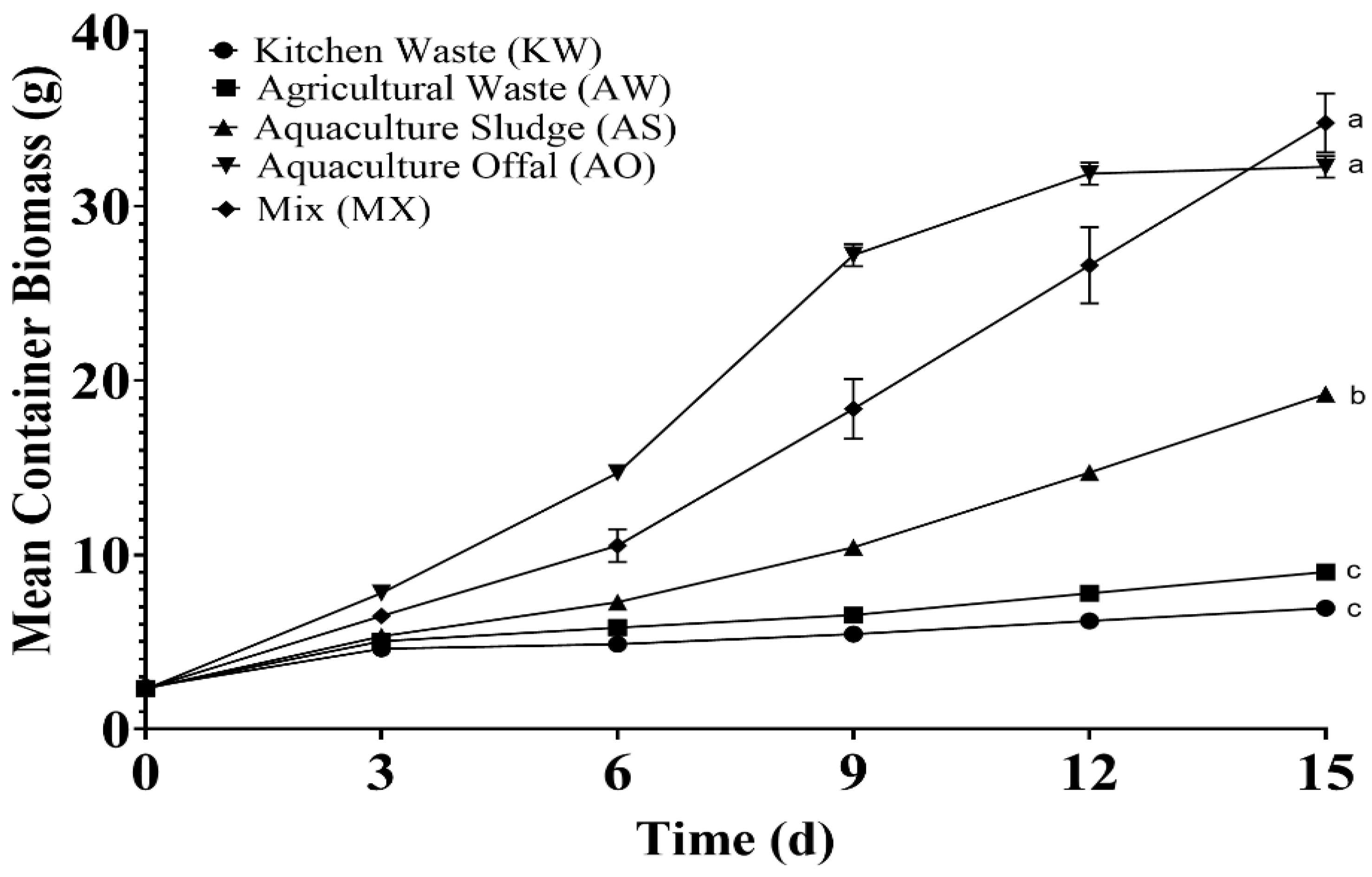
3.1. Growth and Survival
3.2. Feed Conversion Ratio, Bioconversion, and Protein Efficiency Ratio
3.3. Nutritional Composition
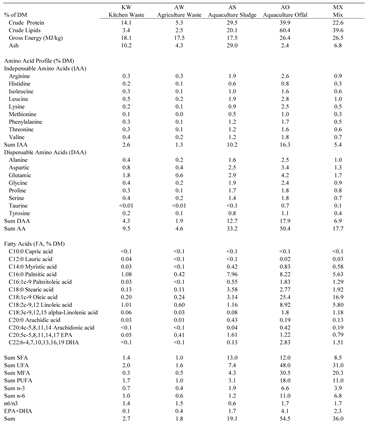
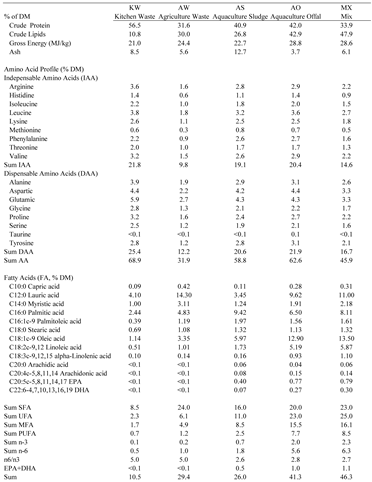
3.3.1. Feeds
3.3.2. Larvae
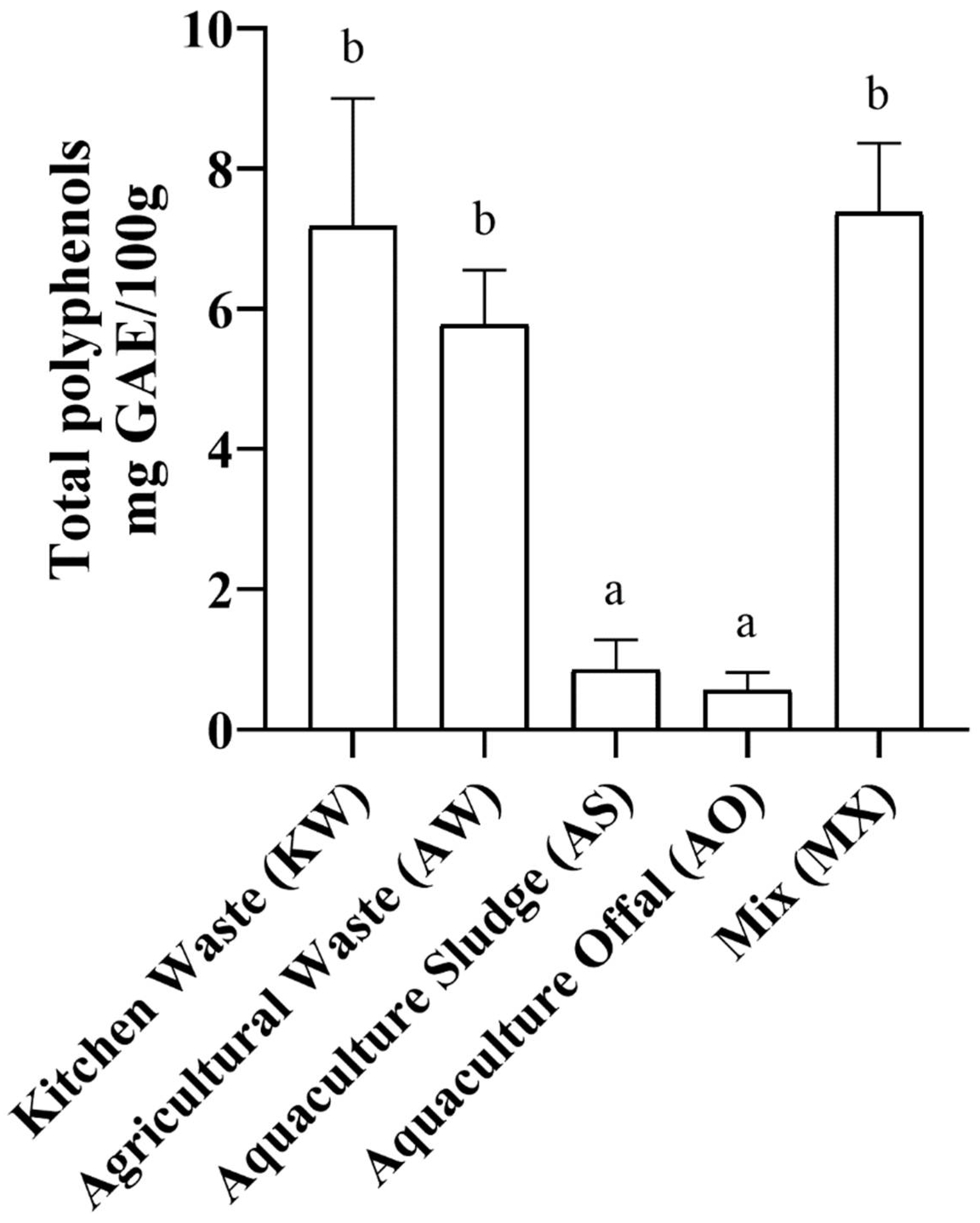
3.3.3. Total Polyphenol Concentration
4. Discussion
5. Conclusions
Author Contributions
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| BSFL | Black Soldier Fly Larvae |
| KW | Kitchen Waste |
| AW | Agriculture Waste |
| AS | Aquaculture Sludge |
| AO | Aquaculture Offal |
| MX | Mix |
| FCR | Feed Conversion Ratio |
| PER | Protein Efficiency Ratio |
| GAE | Gallic Acid Equivalent |
| BSFLM | Black Soldier Fly Larvae Meal |
| HCC | Hydrate Coconut Coir |
| TAA | Total Amino Acids |
| IAA | Indispensable Amino Acids |
| DAA | Dispensable Amino Acids |
| LC-PUFA | Long Chain – Polyunsaturated Fatty Acids |
References
- Naylor, R.L.; Hardy, R.W.; Buschmann, A.H.; Bush, S.R.; Cao, L.; Klinger, D.H.; Little, D.C.; Lubchenco, J.; Shumway, S.E.; Troell, M. A 20-year retrospective review of global aquaculture. Nature 2021, 591, 551–563. [Google Scholar] [CrossRef] [PubMed]
- Turchini, G.M.; Trushenski, J.T.; Glencross, B.D. Thoughts for the future of aquaculture nutrition: Realigning perspectives to reflect contemporary issues related to judicious use of marine resources in aquafeeds. North Am. J. Aquac. 2019, 81, 13–39. [Google Scholar] [CrossRef]
- Booman, M.; Forster, I.; Vederas, J.C.; Groman, D.B.; Jones, S.R.M. Soybean meal-induced enteritis in Atlantic salmon (Salmo salar) and Chinook salmon (Oncorhynchus tshawytscha) but not in pink salmon (O. gorbuscha). Aquaculture 2018, 483, 238–243. [Google Scholar] [CrossRef]
- van Huis, A. Insects as food and feed, a new emerging agricultural sector: A review. J. Insects Food Feed 2019, 6, 27–44. [Google Scholar] [CrossRef]
- Schiavone, A.; De Marco, M.; Martínez, S.; Dabbou, S.; Renna, M.; Madrid, J.; Gasco, L. Nutritional value of a partially defatted and a highly defatted black soldier fly larvae meal for broiler chickens: Apparent nutrient digestibility, apparent metabolizable energy, and apparent ileal amino acid digestibility. J. Anim. Sci. Biotechnol. 2017, 8, 1–10. [Google Scholar] [CrossRef]
- Makkar, H.P.S.; Tran, G.; Heuze, V.; Ankers, P. State-of-the-art on use of insects as animal feed. Anim. Feed Sci. Technol. 2014, 197, 1–33. [Google Scholar] [CrossRef]
- Gold, M.; Tomberlin, J.K.; Diener, S.; Zurbrügg, C.; Mathys, A. Decomposition of biowaste macronutrients, microbes, and chemical properties during the conversion process by black soldier fly larvae (Hermetia illucens). Sci. Total Environ. 2018, 613, 130–141. [Google Scholar] [CrossRef]
- Surai, P.F. Polyphenol compounds in the chicken/animal diet: From the past to the future. J. Anim. Physiol. Anim. Nutr. 2016, 100, 653–664. [Google Scholar] [CrossRef]
- Henry, M.; Gasco, L.; Piccolo, G.; Fountoulaki, E. Review on the use of insects in the diet of farmed fish: Past and future. Anim. Feed Sci. Technol. 2015, 203, 1–22. [Google Scholar] [CrossRef]
- Gasco, L.; Finke, M.; van Huis, A. Can diets containing insects promote animal health? J. Insects Food Feed 2018, 4, 1–4. [Google Scholar] [CrossRef]
- Parodi, A.; Leip, A.; De Boer, I.J.M.; Slegers, P.M.; Ziegler, F.; Temme, E.H.M.; van Zanten, H.H.E. The potential of future foods for sustainable and healthy diets. Nat. Sustain. 2018, 1(12), 782–789. [Google Scholar] [CrossRef]
- Lock, E.R.; Arsiwalla, T.; Waagbø, R. Insect larvae meal as an alternative source of nutrients in the diet of Atlantic salmon (Salmo salar) postsmolt. Aquac. Nutr. 2021, 27(2), 568–579. [Google Scholar] [CrossRef]
- Moutinho, S.; Oliva-Teles, A.; Fontinha, F.; Martins, N.; Monroig, Ó.; Peres, H. Black soldier fly larvae meal as a potential modulator of immune, inflammatory, and antioxidant status in gilthead seabream juveniles. Comp. Biochem. Physiol. B 2024, 271, 110951. [Google Scholar] [CrossRef] [PubMed]
- Weththasinghe, P.; Rocha, S.D.C.; Øyås, O.; et al. Modulation of Atlantic salmon (Salmo salar) gut microbiota composition and predicted metabolic capacity by feeding diets with processed black soldier fly (Hermetia illucens) larvae meals and fractions. Anim. Microbiome 2022, 4, 9. [Google Scholar] [CrossRef]
- AOAC Official Method 990.03. Protein (crude) in animal feed, combustion method. Official Methods of Analysis of AOAC International, 2006, 18th Ed.; AOAC International: Gaithersburg, MD, USA.
- Bligh, E.G.; Dyer, W.J. A rapid method for total lipid extraction and purification. Can. J. Biochem. Physiol. 1959, 37, 911–917. [Google Scholar] [CrossRef]
- International Organization of Standardization. Animal feeding stuffs, animal products, and faeces or urine — Determination of gross calorific value — Bomb calorimeter method, ISO Standard No. 9831.1998. ISO 1998.
- AOAC Official Method 969.33. Fatty acids in oils and fats. Official Methods of Analysis of AOAC International, 1969, AOAC International: Gaithersburg, MD, USA.
- AOAC Official Method 969.22. Methyl esters of fatty acids in oils and fats. Gas chromatographic method. Official Methods of Analysis of AOAC International, 1997, AOAC International: Gaithersburg, MD, USA.
- Olivares-Ferretti, P.; Chavez, V.; Hernandez, K.; Parodi, J. Polyphenols extracts from Didymosphenia geminata (Lyngbye) Schmidt altered the motility and viability of Daphnia magna. Res. Square 2021. [CrossRef]
- Belperio, S.; Cattaneo, A.; Nannoni, E.; Sardi, L.; Martelli, G.; Dabbou, S.; Meneguz, M. Assessing substrate utilization and bioconversion efficiency of black soldier fly (Hermetia illucens) larvae: Effect of diet composition on growth and development temperature. Animals 2024, 14(9). [CrossRef]
- Chia, S.Y.; Tanga, C.M.; Khamis, F.M.; Mohamed, S.A.; Salifu, D.; Sevgan, S.; Ekesi, S. Threshold temperatures and thermal requirements of black soldier fly Hermetia illucens: Implications for mass production. PLoS One 2020, 15(1), e0228541. [Google Scholar] [CrossRef]
- Nyakeri, E.M.; Ogola, H.J.O.; Ayieko, M.A.; Amimo, F.A. An open system for farming black soldier fly larvae as a source of proteins for small-scale poultry and fish production. J. Insects Food Feed 2017, 3(1), 51–56. [Google Scholar] [CrossRef]
- Lalander, C.; Diener, S.; Zurbrügg, C.; Vinnerås, B. Effects of feedstock on larval development and process efficiency in waste treatment with black soldier fly (Hermetia illucens). J. Clean. Prod. 2018, 233, 116–126. [Google Scholar] [CrossRef]
- Bekker, N.S.; Heidelbach, S.; Vastergaard, S.Z.; Nielsen, M.E.; Riisgaard-Jensen, M.; Zeuner, E.J.; Bahrndorff, S.; Eriksen, T. Impact of substrate moisture content on growth and metabolic performance of black soldier fly larvae. Waste Manag. 2021, 127, 73–79. [Google Scholar] [CrossRef]
- Barragan-Fonseca, K.B.; Dicke, M.; van Loon, J.J. Influence of larval density and dietary nutrient concentration on performance, body protein, and fat contents of black soldier fly larvae (Hermetia illucens). Entomol. Exp. Appl. 2018, 166(9), 761–770. [Google Scholar] [CrossRef] [PubMed]
- Diener, S.; Zurbrügg, C.; Gutiérrez, F.R.; Nguyen, D.H.; Morel, A.; Koottatep, T.; Tockner, K. Black soldier fly larvae for organic waste treatment—Prospects and constraints. Proceedings of WasteSafe 2011 – 2nd International Conference on Solid Waste Management in the Developing Countries, Khulna, Bangladesh, 13-15 February 2011. [Google Scholar]
- Li, R.; Lin, T.; Fan, X.; Dai, X.F.; Huang, J.H.; Zhang, Y.F.; Guo, R.B.; Fu, S.F. Effects of salinity in food waste on the growth of black soldier fly larvae and global warming potential analysis. Chem. Eng. J. 2024, 480. [Google Scholar] [CrossRef]
- Spranghers, T.; Ottoboni, M.; Klootwijk, C.; Ovyn, A.; Deboosere, S.; De Meulenaer, B.; Michiels, J.; Eeckhout, M.; De Clercq, P.; De Smet, S. Nutritional composition of black soldier fly (Hermetia illucens) prepupae reared on different organic waste substrates. J. Sci. Food Agric. 2017, 97(8), 2594–2600. [Google Scholar] [CrossRef] [PubMed]
- St-Hilaire, S.; Cranfill, K.; McGuire, M.A.; Mosley, E.E.; Tomberlin, J.K.; Newton, L.; Sealey, W.; Sheppard, C.; Irving, S. Fish offal recycling by the black soldier fly produces a foodstuff high in omega-3 fatty acids. J. World Aquac. Soc. 2007, 38(2). [CrossRef]
- Liu, Y.; Liu, J.; He, J.; Lu, H.; Sun, S.; Ji, F.; Dong, X.; Bao, Y.; Xu, J.; He, G.; Xu, W. Chronological and carbohydrate-dependent transformation of fatty acids in the larvae of black soldier fly following food waste treatment. Molecules 2023, 28, 41903. [Google Scholar] [CrossRef]
- Giannetto, A.; Oliva, S.; Riolo, K.; Savastano, D.; Parrino, V.; Cappello, T.; Maisano, M.; Fasulo, S.; Mauceri, A. Waste valorization via Hermetia illucens to produce protein-rich biomass for feed: Insight into the critical nutrient taurine. Animals 2020, 10(9), 1710. [Google Scholar] [CrossRef]
- Eggink, K.M.; Lund, I.; Pedersen, P.B.; Hansen, B.W.; Dalsgaard, J. Biowaste and by-products as rearing substrates for black soldier fly (Hermetia illucens) larvae: Effects on larval body composition and performance. PLoS One 2022, 17(9), e0275213. [Google Scholar] [CrossRef]
- Li, X.; Dong, Y.; Sun, Q.; Tan, X.; You, C.; Huang, Y.; Zhou, M. Growth and fatty acid composition of black soldier fly (Hermetia illucens) larvae are influenced by dietary fat sources and levels. Animals 2022, 12(4), 486. [Google Scholar] [CrossRef]
- Ewald, N.; Vidakovic, A.; Langeland, M.; Kiessling, A.; Sampels, S.; Lalander, C. Fatty acid composition of black soldier fly larvae (Hermetia illucens) – Possibilities and limitations for modification through diet. Waste Manag. 2020, 102, 40–47. [Google Scholar] [CrossRef]
- Barroso, F.G.; Sanchez-Muros, M.J.; Rincon, M.A.; Rodriguez-Rodriguez, M.; Fabrikov, D.; Morote, E.; Guil-Guerrero, J.S. Production of n-3-rich insects by bioaccumulation of fishery waste. J. Food Compos. Anal. 2019, 82, 103237. [Google Scholar] [CrossRef]
- Erbland, P.; Alyokhin, A.; Perkins, B.; Peterson, M. Dose-dependent retention of omega-3 fatty acids by black soldier fly larvae (Diptera: Stratiomyidae). J. Econ. Entomol. 2020, 113(3), 1221–1226. [Google Scholar] [CrossRef]
- Tylewicz, U.; Nowacka, M.; Marin-Garcia, B.; Wiktor, A. Target sources of polyphenols in different food products and their processing by-products. In: Polyphenols: Properties, Recovery, and Applications; Woodhead Publishing, 2018, pp. 135-175. [CrossRef]
- García-Valverde, V.; Inmaculada, N.G.; García-Alonso, J.; Periago, M.J. Antioxidant bioactive compounds in selected industrial processing and fresh consumption tomato cultivars. Food Bioprocess Technol. 2013, 6, 391–402. [Google Scholar] [CrossRef]
- Islam, M.S.; Yoshimoto, M.; Yahara, S.; Okuno, S.; Ishiguro, K.; Yamakawa, O. Identification and characterization of foliar polyphenolic composition in sweet potato (Ipomoea batatas L.) genotypes. J. Agric. Food Chem. 2002, 50(13), 3718-3722. [CrossRef]
- Sun, Y.; Pan, Z.; Yang, C.; Jia, Z.; Guo, X. Comparative assessment of phenolic profiles, cellular antioxidant and antiproliferative activities in ten varieties of sweet potato (Ipomoea batatas) storage roots. Molecules 2019, 24(24), 4476. [Google Scholar] [CrossRef] [PubMed]
- Vella, F.; Calandrelli, R.; Cautela, D.; Laratta, B. Screening of bioactivity in extracts from different varieties of lettuce. In Proceedings of the International Electronic Conference on Plant Science; 2020. [Google Scholar] [CrossRef]
- Brar, J.S.; Sharma, S.; Kaur, H.; Singh, H.; Naik, E.K.; Adhikary, T. Phytochemical properties, antioxidant potential and fatty acids profiling of three dragon fruit species grown under sub-tropical climate. Not. Bot. Horti Agrobot. Cluj-Napoca 2023, 51(3). [CrossRef]
- Ouédraogo, N.; Sombie, P.A.E.D.; Traore, R.E.; Sama, H.; Bationo/Kando, P.; Sawadogo, M.; Lebot, V. Nutritional and phytochemical characterization of taro (Colocasia esculenta (L.) Schott) germplasm from Burkina Faso. J. Plant Breed. Crop Sci. 2023, 15(1), 32-41. [CrossRef]
- Faezah, N.; Aishah, S.; Kalsom, U. Comparative evaluation of organic and inorganic fertilizers on total phenolic, total flavonoid, antioxidant activity, and cyanogenic glycosides in cassava (Manihot esculenta). Afr. J. Biotechnol. 2013, 12(18), 2414–2421. [Google Scholar] [CrossRef]
- Bureau, D.P.; Kaushik, S.J.; Cho, C.Y. Bioenergetics. In Fish Nutrition, 3rd ed.; Halver, J.E.; Hardy, R.W., Eds.; Academic Press, 2002, pp. 1-59.
- Manach, C.; Scalbert, A.; Morand, C.; Rémésy, C.; Jiménez, L. Polyphenols: Food sources and bioavailability. Am. J. Clin. Nutr. 2004, 79(5), 727–747. [Google Scholar] [CrossRef]
- Scalbert, A.; Johnson, I.T.; Saltmarsh, M. Polyphenols: Antioxidants and beyond. Am. J. Clin. Nutr. 2005, 81(1), 215S–217S. [Google Scholar] [CrossRef]
- Liu, Z.; Minor, M.; Morel, P.C.; Najar-Rodriguez, A.J. Bioconversion of three organic wastes by black soldier fly (Diptera: Stratiomyidae) larvae. Environ. Entomol. 2018, 47(6), 1609–1617. [Google Scholar] [CrossRef]
- Liu, X.; Chen, X.; Wang, H.; Yang, Q.; Rehman, K.; Li, W.; Cai, M.; Li, Q.; Mazza, L.; Zhang, J.; Yu, Z.; Zheng, L. Dynamic changes of nutrient composition throughout the entire life cycle of black soldier fly. PLoS One 2017, 12(8), e0182601. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





