Submitted:
31 December 2024
Posted:
03 January 2025
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Materials and Methods
2.1. Cellulose Based Bioplastic Film from Sugarcane Bagasse
2.1.1. Sample Collection
2.1.2. Pre-Treatment of Sugarcane Bagasse
2.1.3. Extraction of Cellulose
2.1.4. Bioplastic Preparation from the Extracted Cellulose
2.1.5. Influence of Alkali
2.1.6. Influence of Acid
2.1.7. Influence of Salt
2.2. Keratin Based Bioplastic Film from Chicken Feathers
2.2.1. Sample Collection
2.2.2. Pre-Treatment of Feathers
2.2.3. Extraction of Keratin
2.2.4. Preparation of Bioplastic from the Extracted Keratin
2.2.5. Influence of Alkali
2.2.6. Influence of Acid
2.2.7. Influence of Salt
2.3. Short-Term Degradation Analysis of the Prepared Cellulose and Keratin Based Bioplastics and Low-Density Polyethylene in Soil
2.3.1. Short-Term Biodegradation Analysis of the Prepared Cellulose and Keratin Based Bioplastics and Low-Density Polyethylene in Soil
3. Results
3.1. Cellulose Based Bioplastic Film from Sugarcane Bagasse
3.1.1. Extraction of Cellulose
3.1.2. Cellulose Yield
3.1.3. Bioplastic Preparation
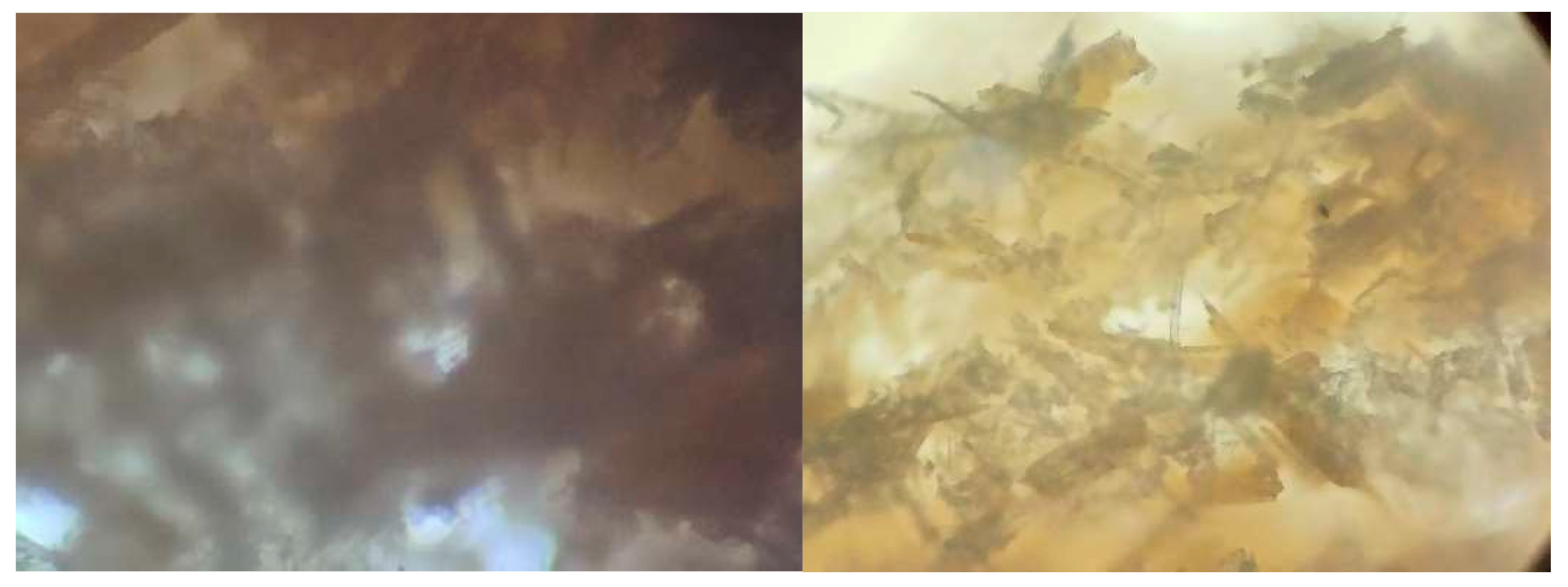
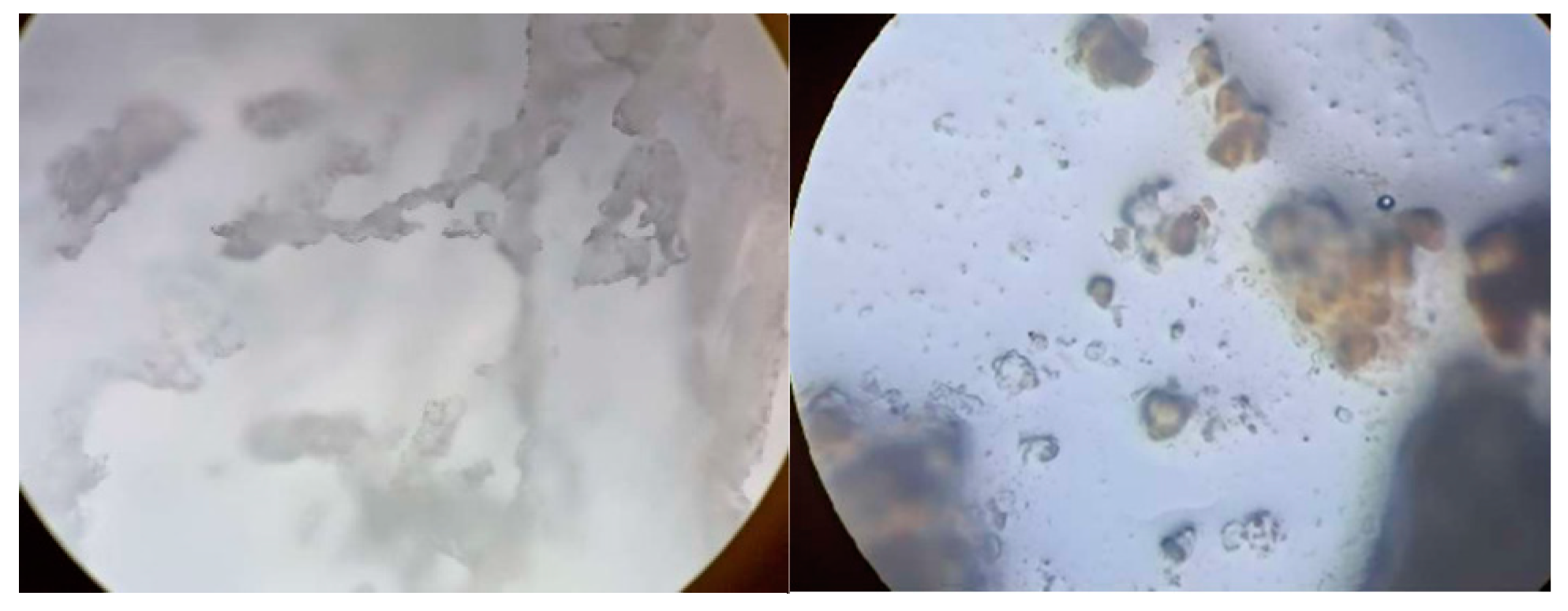
3.1.4. Light Microscopy Results of the Prepared Bioplastic
3.1.5. Influence of Alkali
3.1.6. Influence of Acid
3.1.7. Influence of Salt
3.2. Keratin Based Bioplastic Film from Chicken Feathers
3.2.1. Extraction of Keratin
3.2.2. Keratin Yield
3.2.3. Bioplastic Preparation
3.2.4. Light Microscopy Results of the Prepared Bioplastic
3.2.5. Influence of Alkali
3.2.6. Influence of Acid
3.2.7. Influence of Salt
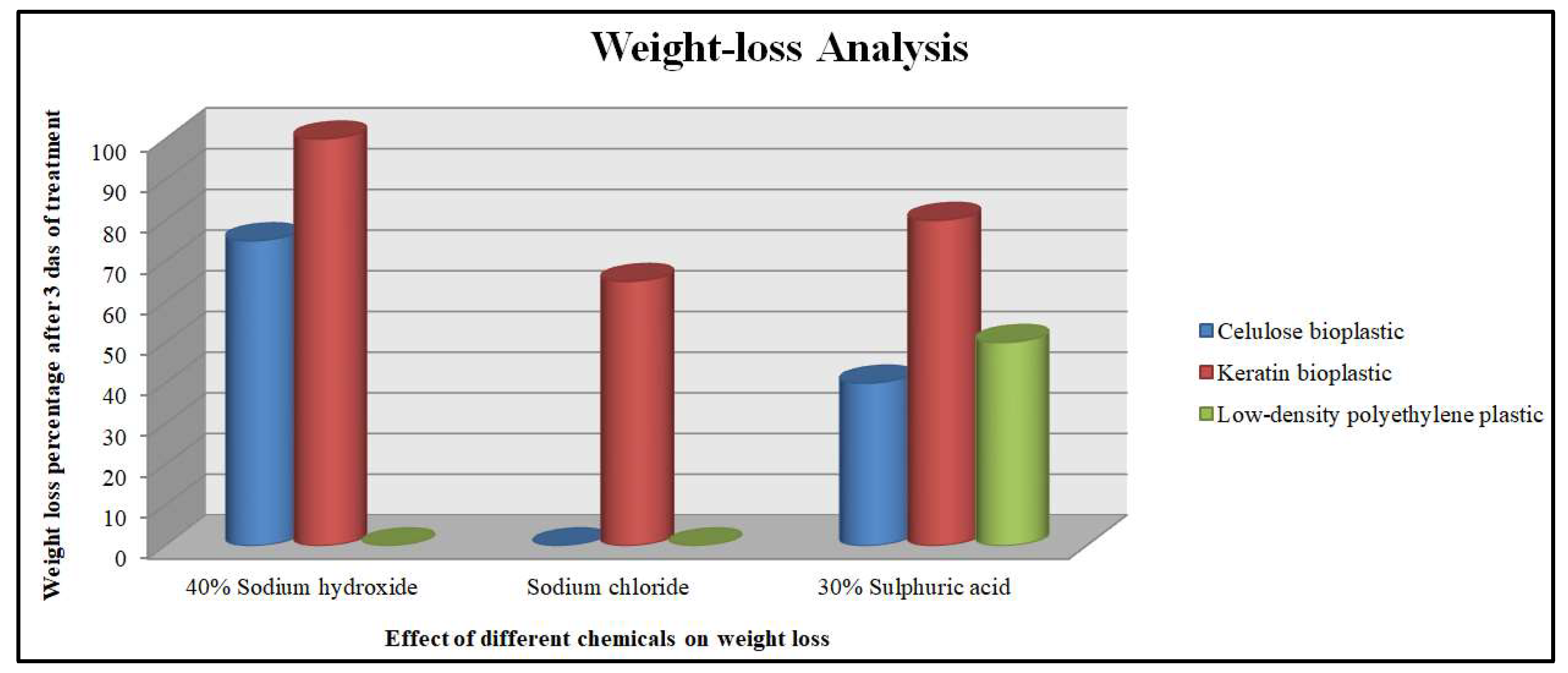
3.2.8. Comparitive Analysis of Weight-Loss Study
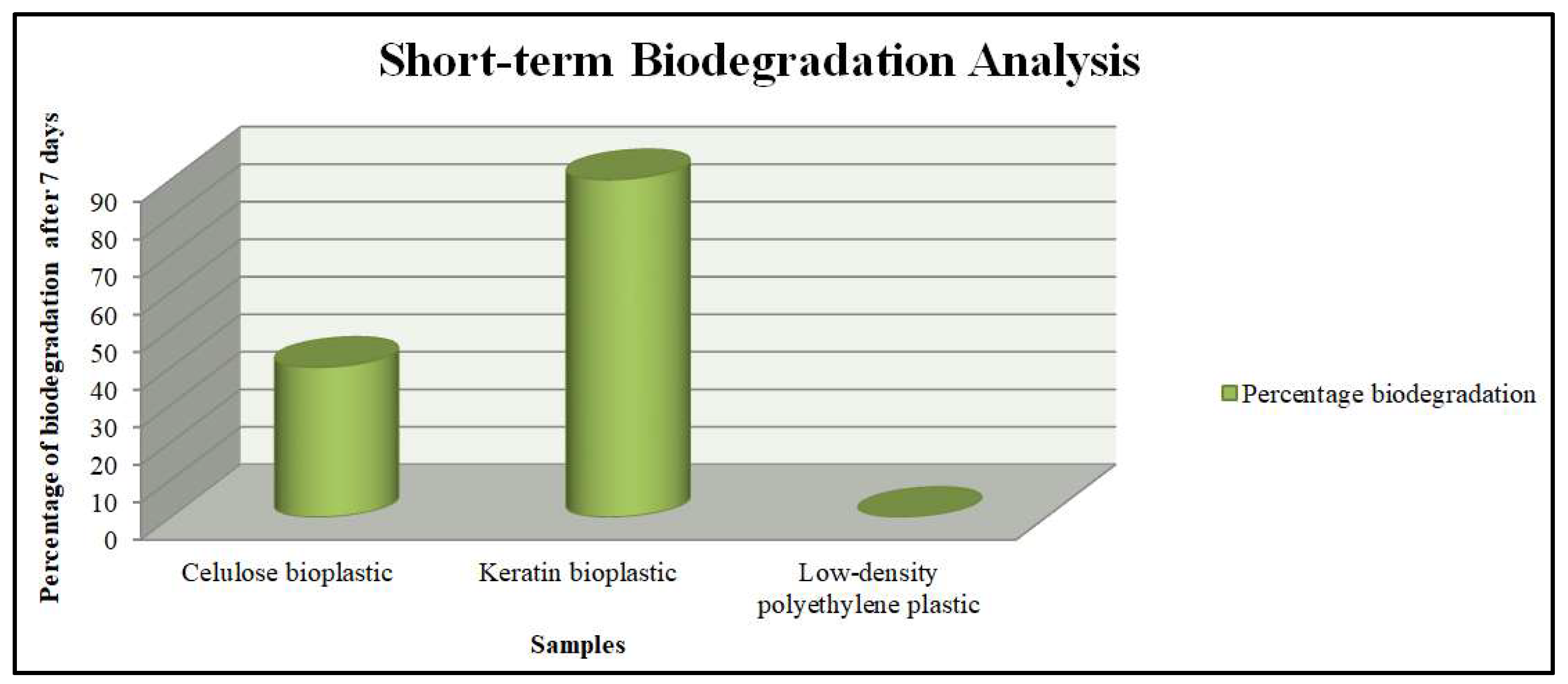
3.3. Short-Term Biodegradation Analysis of the Prepared Cellulose and Keratin Based Bioplastics and Low-Density Polyethylene in Soil
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Azmin, S. N. H. M., Hayat, N. A. binti M., & Nor, M. S. M. Development and characterization of food packaging bioplastic film from cocoa pod husk cellulose incorporated with sugarcane bagasse fibre. Journal of Bioresources and Bioproducts 2020,. [CrossRef]
- Ansink, E., Wijk, L., & Zuidmeer, F. No clue about bioplastics. Ecological Economics 2022, 191, 107245.
- 3. Guang-Yong Zhu; Xian Zhu; Xue-Liang Wan; Qi Fan; Yan-Hua Ma; Jing Qian; Xiao-Lei Liu; Yi-Jing Shen; Jun-Hui Jiang Hydrolysis technology and kinetics of poultry waste to produce amino acids in subcritical water. Journal of Analytical and Applied Pyrolysis 2010, 88(2), 187– 191. [CrossRef]
- E. Jin, N. Reddy, Z. Zhu, Y. Yang, Graft polymerization of native chicken feathers for thermoplastic applications. J. Agric. Food Chem. 2011, 59 (5), 1729–1738. [CrossRef]
- Mokrejs, P., Svoboda, P., Hrncirik, J., Janacova, D., & Vasek, V. Processing poultry feathers into keratin hydrolysate through alkaline-enzymatic hydrolysis. Waste management & research. The journal of the International Solid Wastes and Public Cleansing Association, ISWA 2011, 29(3), 260–267. [CrossRef]
- Fiorentino, G., Ripa, M., & Ulgiati, S. Chemicals from biomass: technological versus environmental feasibility. A review. Biofuels, Bioproducts and Biorefining 2017, 11(1), 195-214.
- Nigam, S., Das, A. K., & Patidar, M. K. Synthesis, characterization and biodegradation of bioplastic films produced from Parthenium hysterophorus by incorporating a plasticizer (PEG600). Environmental Challenges 2021, 5, 100280.
- Wayman, C.&Niemann, H. The fate of plastic in the ocean environment–a minireview. Environmental Science: Processes & Impacts 2021, 23(2), 198-212.
- Lunt, J. Large-Scale Production, Properties and Commercial Applications of Polylactic Acid Polymers. Polymers Degradation Stability 1998, 59, 145-152. https://dx.doi.org/10.1016/S0141-3910(97)00148-.
- Bonanomi, G., Chiurazzi, M., Caporaso, S., Del Sorbo, G., Moschetti, G. and Felice, S. Soil Solarization with Biodegradable Materials and Its Impact on Soil Microbial Communities. Soil Biology and Microbiology 2008, 40, 1989- 1998.
- Perez-Puyana, V. M., Jiménez-Rosado, M., Martínez, I., & Romero, A. Tuning the Mechanical and Functional Properties of Pea Protein-Based Bioplastics via Different Physical and Chemical Cross-Linking Methods. ACS Applied Polymer Materials 2024, 6(3), 1891-1899.
- Behera, L., Mohanta, M., & Thirugnanam, A. Intensification of yam-starch based biodegradable bioplastic film with bentonite for food packaging application. Environmental Technology & Innovation 2022, 25, 102180.
- Amin, M. R., Chowdhury, M. A., & Kowser, M. A. Characterization and performance analysis of composite bioplastics synthesized using titanium dioxide nanoparticles with corn starch. Heliyon 2019, 5(8), e02009. [CrossRef]
- Liliani, & Tjahjono, B. A conceptual framework for a dyadic supplier-customer co- innovation of bioplastic packaging. Procedia CIRP 2020, 90, 339- 343. [CrossRef]
- Filho, W. L., Salvia, A. L., Bonoli, A., Saari, U. A., Voronova, V., Klõga, M., … Barbir, J. An assessment of attitudes towards plastics and bioplastics in Europe. Science of The Total Environment 2021, 755, 142732. [CrossRef]
- MohammanAsaduzzaman Chowdhury, Nayem Hossain, Tauhidul Islam Noman, Ali Hasan, Alam Shafiul, Kashem Mohammod Abul. Biodegradable, physical and microbial analysis of tamarind seed starch infused eco-friendly bioplastics by different percentage of Arjuna powder, Results in Engineering 2022, 13, 100387, ISSN 2590-1230. [CrossRef]
- Mrithula Shanmathy, Monalisha Mohanta, A. Thirugnanam. Development of biodegradable bioplastic films from Taro starch reinforced with bentonite, Carbohydrate Polymer Technologies and Applications 2021, 2, 100173, ISSN 2666-8939. [CrossRef]
- M.K. Marichelvam, P. Manimaran, M.R. Sanjay, S. Siengchin, M. Geetha, K. Kandakodeeswaran, Pawinee Boonyasopon, Sergey Gorbatyuk. Extraction and development of starch-based bioplastics from Prosopis Juliflora Plant: Eco-friendly and sustainability aspects, Current Research in Green and Sustainable Chemistry 2022, 5, 100296, ISSN 2666-0865. [CrossRef]
- Alashwal, B. Y., Saad Bala, M., Gupta, A., Sharma, S., & Mishra, P. Improved properties of keratin-based bioplastic film blended with microcrystalline cellulose: A comparative analysis. Journal of King Saud University- Science 2019. [CrossRef]
- J.I. Moran, V.A. Alvarez, V.P. Cyras, A. Vazquez. Extraction of cellulose and preparation of nanocellulose from sisal fibers, Cellulose 15 2008, 149–159. [CrossRef]
- C.U. Maheswari, K.O. Reddy, M. Muzenda, B.R. Guduri, A.V. Rajulu. Extraction and characterization of cellulose microfibrils from agricultural residue - Cocos nucifera L, Biomass Bioenergy 2012, 46, 555–563. [CrossRef]
- K.O. Reddy, C. Uma Maheswari, E. Muzenda, M. Shukla, A.V. Rajulu. Extraction and characterization of cellulose from pretreated ficus (peepal tree) leaf fibers, J. Nat. Fibers 2016, 13, 1, 54–64. [CrossRef]
- R. Candido, G. Godoy, A.R. Gonçalves, Characterization and application of cellulose acetate synthesized from sugarcane bagasse, Carbohydr. Polym. 2017, 167, 280–289. [CrossRef]
- Sharma, S., Gupta, A., Chik, S. M. S. T., Gek, K. C., Podde, P. K., Thraisingam, J., & Subramaniam, M. Extraction and characterization of keratin from chicken feather waste biomass: a study. In Proceedings of the national conference for postgraduate research (NCON-PGR 2016), Universiti Malaysia Pahang (UMP), Pekan 2016, 693-699.
- S. Sharma, A. Gupta, S.M.S.B.T. Chik, C.Y.G. Kee, P.K. Poddar. Dissolution and characterization of biofunctional keratin particles extracted from chicken feathers, IOP Conference Series: Materials Science and Engineering, IOP Publishing 2017, p 012013. [CrossRef]
- Rahman, M. M., Hassan, A., Hossain, I., Jahangir, M. M. R., Chowdhury, E. H., & Parvin, R. Current state of poultry waste management practices in Bangladesh, environmental concerns, and future recommendations. Journal of Advanced Veterinary and Animal Research 2022, 9(3), 490.
- Leavitt, SW, Danzer, SR. Method for processing small wood samples to holocellulose for stable-carbon isotope analysis. Analytical Chemistry 1993, 65(1):87–9.
- Kaustubh C. Patankar, Saptarshi Maiti, Girendra Pal Singh, Mohammad Shahid, Sandeep More, Ravindra V. Adivarekar. Chemically modified wool waste keratin for flame retardant cotton finishing, Cleaner Engineering and Technology 2021, 5, 100319, ISSN 2666-7908. [CrossRef]
- Phuong VT, Verstichel S, Cinelli P, Anguillesi I, Coltelli M-B, Lazzeri A. Cellulose acetate blends – Effect of plasticizers on properties and biodegradability. J Renew Mater 2014, 2(1):35–41.
- Decroix, C., Chalamet, Y., Sudre, G., & Caroll, V. Thermo-mechanical properties and blend behaviour of cellulose acetate/lactates and acid systems: Natural-based plasticizers. Carbohydrate polymers 2020, 237, 116072.
- N. Mostafa, A.A. Farag, H.M. Abo-Dief, A.M. Tayeb. Production of biodegradable plastic from agricultural wastes, Arab. J. Chem. 2018, 11, 546–553. [CrossRef]
- Zimmermann, L., Dombrowski, A., Völker, C., & Wagner, M. Are bioplastics and plant-based materials safer than conventional plastics? In vitro toxicity and chemical composition. Environment International 2020, 106066. [CrossRef]
- K.O. Reddy, C. Uma Maheswari, E. Muzenda, M. Shukla, A.V. Rajulu. Extraction and characterization of cellulose from pretreated ficus (peepal tree) leaf fibers, J. Nat. Fibers 2016, 13, 1, 54–64. [CrossRef]
- Bian, J., Peng, P., Peng, F., Xiao, X., Xu, F., & Sun, R. C. Microwave-assisted acid hydrolysis to produce xylooligosaccharides from sugarcane bagasse hemicelluloses. Food chemistry 2014, 156, 7-13.
- B.S. Kaith, H. Mittal, R. Jindal, M. Maiti, S. Kalia. Environment benevolent biodegradable polymers: synthesis, biodegradability, and applications, in: Cellulose Fibers: Bio- and Nano-Polymer Composites 2011, 425–451. [CrossRef]




















Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).




