1. Introduction
To attain sustainable food production, it is necessary that nutrient depletion and soil degradation must be prevented by replacing the nutrients removed after crops were harvested. One of the different nutrients important for crops is Phosphorus (P), which is required in the largest quantities (Heuer et al., 2017). In all living cells, P is important and its functions cannot be replaced by other elements; life on Earth is not possible without it (Johnston et al., 2014). Plants absorb large quantities of P from the soil solute in the form of phosphate, primarily dihydrogen phosphate (H2PO4) (Roberts & Johnston, 2015). But the amount of soluble P in the soil is usually (400–1260 mg kg−1) which is very low (V. Fernández et al., 2014). P activators encompass a variety of techniques designed to enhance and expedite the conversion of soil P into forms that are soluble in plants within the soil solution. One method involves is utilizing the bacteria that solubilize phosphate (Zhu et al., 2018). To transform the insoluble phosphate to soluble phosphates in the soil, the PSB produce enzymes and organic acids and also excrete siderophores that can form complexes and chelate the metal ions (Rawat et al., 2021; T. Nikitha et al., 2017).
There are various common genera of PSB being isolated in the soil such including Acinetobacter, Pseudomonas, Erwinia, Bacillus, Enterobacter, Burkholderia, Flavobacterium, Agrobacterium, Arthrobacter, and Micrococcus (L. A. Fernández et al., 2007). According (Alaylar et al., 2019), Acinetobacter spp. with Enterobacter cloacae have been recognized as active phosphate-solubilizing strains. In the research of (Ogut et al., 2010), result revealed that the most effective solubilizers of tricalcium phosphate containing agar were the Acinetobacter strains. In the 5 days incubation, Acinetobacter strains dissolved P in liquid cultures with the mean ranged from 167 to 888 μg/ml P or 167 and 1022 ppm.
There were many studies that phosphate solubilizing bacteria were utilized as biofertilizer in root crops production. In the experiment of (Mazzuco et al., 2023) using the mixture of PSB in garlic production, improved the size of bulbs, dry mass, concentration of P in the leaf, and the crop’s yield. The germination, diameter of bulb, and growth of onion crops were positively affected by PSB inoculation (Colo, 2014). Using biofertilizer containing phosphate solubilizing bacteria as foliar spray, the bulb weight, bulb diameter, yield, and total soluble solids of onion increased by 6.7%, 4.10%, 19.14%, and 91.50% respectively (Elshazly et al., 2023).
The onion, scientifically known as
Allium cepa L., is a globally important part of daily cuisine, especially in the Philippines. The total area dedicated for onion production is 19,824.02 hectares in the country and according to the Onion Production Guide (
https://www.da.gov.ph/wp-content/uploads/2021/04/), a 1-ha onion production requires at least 45kg of available phosphate. From the total area devoted to onion, Nueva Ecija has the largest coverage with 9,495.16 hectares, followed by Mindoro Occidental with 4,023.15 hectares, and Ilocos Sur with 1,689.84 hectares (
https://psa.gov.ph, 2022). Onion farmers in the country particularly in Bongabon, Nueva Ecija experience the problem in the expensive cost of chemical fertilizers (Domingo, 2023) resulting to high cost of production.
To reduce dependence on synthetic P sources, harnessing the ability of PSB as potential bioinoculant for onion production may provide cheaper cost of production, environment-friendly, and sustainable P management approach. In addition, exploring the potentials of locally-isolated soil microorganisms for the development of bioinoculants and/or biofertilizers that are adapted to local agro-environment gradients is a more ecological manner to protect the local biodiversity than using introduced microorganisms.
2. Materials and Methods
2.1. Soil Collection and Analysis
A total of 3 samples were collected from top three onion producing regions in the Philippines namely: I, III, and IVB. Region I soil sample was collected at Brgy. Tay-ak, Bantay, Ilocos Sur (17°35'55"N 120°28'7"E); Region III soil sample was collected at Brgy. Vega, Bongabon, Nueva Ecija (15°39'12"N 121°8'8"E); while Region IVB soil sample was collected at Brgy. La Curva, San Jose, Mindoro Occidental (12° 24' 17" N · 121° 2' 45" E). Soil sampling methods followed the standard protocol according to the procedure stated at the leaflet of the Department of Agriculture (DA) - Bureau of Soils and Water Management (
https://www.bswm.da.gov.ph/download/how-to-collect-soil-samples-for-analysis/). In summary, a total of 10 subsamples (15 – 25cm depth) per location were obtained and were mixed thoroughly until a 1-kg of composite sample was taken. The 100 grams was put in an ice box and used for microbial analysis; the 900 grams of each sample were brought to the DA-Regional Field Office Number III-Regional Soils Laboratory (
https://rfo3.da.gov.ph/index.php/regional-soils-laboratory/, n.d.) in City of San Fernando, Pampanga, Philippines, for chemical and physical properties analyses.
Different methods were used to determine the parameters in Cation Exchange Capacity (CEC), pH, Electrical Conductivity (EC). The measurement of pH and EC were done thru Potentiometric; CEC was determined thru Cation Displacement Kjeldahl Distillation; organic matter was measured thru Walkley - Black-Colorimetric; Total Nitrogen was measured thru Kjeldahl; Phosphorus and Sodium were measure thru Olsen; Potassium was determined thru Leaching Flame AES; Calcium and Magnesium were measured thru Leaching-Flame AAS; Iron, Zinc, Manganese were tested thru DTPA Extraction Flame AAS; Lead was measured thru Acid Digestion ICP OES; Sulfate was tested thru Turbidimetric; and textural class was tested thru Bouyoucos Hydrometer (
https://rfo3.da.gov.ph/index.php/regional-soils-laboratory/, n.d.).
2.2. Isolation of Phosphate Solubilizing Bacteria from the Soil Samples
The soil samples were mixed in sterilized distilled water with a ratio of 99 ml sterile distilled water: 1 gram of soil and conducted the serial dilution to 10¯1 up to 10¯9 in the 9ml conical plastic tube, thereafter. The 10¯1 up to 10¯9 soil samples that were diluted serially were plated (0.1 ml) on the PVK agar having TCP as the source of phosphate (RI, P., 1948). PVK agar medium contained the following ingredients for 1liter capacity: Yeast extract 0.5g, Dextrose 10g, Tricalcium phosphate (Ca3(PO4)2) 5g, Ammonium sulfate (NH4)2SO4) 0.5g, Potassium chloride (KCl) 0.2g, Magnesium sulfate (MgSO4) 0.1g, Manganese sulfate (MNSO4) 0.0001g, Ferrous sulfate (FeSO4) 0.0001g, Bacteriological Agar/Agar 15g, purchased at (RTC Laboratory Services and Supply House, Quezon City, Phil.) and Distilled water 1 liter. The solution was mixed in Erlenmeyer Flask (EF) thoroughly until all the chemicals were dissolved, then autoclaved (TRIUP, Model TRS-50L) at 15 lbs pressure, 121oC for 15 minutes. In an inverted position, all petri dishes were incubated at 27-30oC for the duration of 7 days. After incubation, the colonies having clear halo zone were screened and selected. They were streaked in the freshly made PVK agar plates to get the pure colonies. Repeated streaking was done out of the same medium to validate the result. Isolated bacteria on petri plates were kept at 4oC for further study.
2.3. Screening and Measurement of Solubilizing Index (SI) of Isolated PSB
On the PVK agar plates, the isolated bacteria were screened for their capability of solubilizing tricalcium phosphate. Aseptically, the isolated bacteria were spot inoculated on the middle of petri plates with PVK agar. At 28oC ±2oC, all petri plates were incubated for the duration of 7 days. A clear halo zone which surrounds the growing colony is an indicator of the microorganisms in solubilizing phosphate. Measuring the solubilizing index (SI) was done and computed as (colony diameter + halo zone diameter) divided to the colony diameter (Edi Premono, 1997).
PSI = colony diameter + holozone diameter PSI
colony diameter
2.4. Selection of Representative Isolate for Sequence Analysis
The isolated bacteria were spot inoculated quarterly on the petri plate containing PVK agar. The colony with biggest halo zone around was selected, sliced from the agar plate with the use of aseptic surgical blade and put in the micro centrifuge tube then, covered with parafilm tightly. The samples were sent to MACROGEN Laboratory, Seoul, South Korea thru Kinovett Scientific Solutions Corporation, Quezon City, Phil. The study used a universal primer EU49f (5’-TTAACACATGCAAGTCGAACGG-3’) and EU1070r (5’-GGACTTAACCCAACATCTCACGA-3’) and run in the Polymerase Chain Reaction (PCR) condition of: initial denaturation for 5 minutes at 94oC, followed by 30 cycles (94oC for 60 seconds, 55 oC for 60 seconds and 74oC for 60 seconds) and final extension at 74oC for 5 minutes (Amri et al., 2023).
2.5. Construction of Phylogenetic Tree
The Basic Local Alignment Search Tool (BLAST) tool was used to align the sequences to the bacterial lineages that deposited in the GenBank of the National Center for Biotechnology Information (NCBI) (
http://www.ncbi.nlm.nih.gov/, n.d.). The alignment was done using ClustalW and Neighbor-Joining method was utilized to build the phylogenetic trees. The distances of the genetic were computed using Molecular Evolutionary Genetic Analysis (MEGA v11) software. Consequently, the phylogenetic trees were bootstrapped with 1000 replications.
3. Results and Discussions
3.1. Physical and Chemical Properties of the Soils
The characteristics of physical and chemical properties of the soils are shown in
Table 1. The study collected 3 soil samples from three sites intended for onion production. The soil pH in Ilocos Sur, Mindoro Occidental, and Nueva Ecija were 6.95, 6.97, and 6.92 respectively which are considered neutral. The pH of soil is directly linked to the populations of soil microbial communities and is commonly regarded as a general indicator of the structure characteristics of the communities of bacteria (Zheng et al., 2019). The estimated bacterial population of the higher soil pH of 6.8 is 60% more than that of the soil having the pH 5.1. The estimated bacterial abundance in soil with pH of 5.5 was 26% which was in larger quantity compare to more acidic soil of having a 4.1 pH. Bacterial population in soil pH 2.5-2.7 is relatively in low level (Fierer & Jackson, 2006). The CEC is an essential gauge in the determination of soil quality and it indicates the capacity of the soil to retain the positively charge ions (Khaledian et al., 2017). The CECs in soils from Ilocos Sur and Nueva Ecija were found to be 35.19cmol/kg and 15.44cmol/kg, respectively, both falling within the normal range while Mindoro Occidental which is clay loam in texture and has a CEC of 13.42cmol/kg, is considered low. The values of EC in three sites are considered non-saline.
The three study sites possess a very low organic matter. Ilocos Sur has 1.40%; 1.43% belonged to Mindoro Occidental, and 0.87% to Nueva Ecija. Farm management techniques, such as the massive and rampant application of chemical fertilizers and pesticides in crops, have direct correlation with the low concentration of organic matter in the soils. This statement is supported by (Pahalvi, 2021), that the reduction of quality of soil used in agricultural production and the decline of the organic matter in the soil is caused by the continuous application of chemical fertilizers.
In Ilocos Sur, total Nitrogen was 0.07%, 0.04% in Mindoro Occidental, and 0.05% in Nueva Ecija. In case of Potassium, Ilocos Sur has 0.61cmol/kg, Mindoro Occidental has 0.28cmol/kg, and Nueva Ecija has 0.24cmol/kg. The macronutrients such as Nitrogen and Potassium are very low in three sites. However, the Phosphorus was in normal ranged at 30.62ppm to 49.44ppm. P content in the soil was mainly because of the application of fertilizer containing P over a long period of time. According to (Shen et al., 2011), the P retention is dominated by precipitation reactions in neutral-to-calcareous soils. Exchangeable bases such as Calcium, Magnesium, and Sodium are high in three study sites. Furthermore, the textural class for Ilocos Sur was Sandy Clay Loam, for Mindoro Occidental was Clay Loam, and for Nueva Ecija was Sandy Loam.
3.2. Morphological Characterization and P-Solubilization Ability of the Isolated Bacteria
All bacterial isolates were assessed for their morphological characteristics. The bacterial colonies exhibited circular shapes with raised elevations, smooth, convex, entire, and whitish pigmentation on the agar plates, which is the same to the results of the study of (Patre, 2021; Rawat et al., 2021). As described by (Farokh et al., 2011; Gulati et al., 2009), Acinetobacter isolates were recognized in different characteristics such as negatively oxidase, positively catalase, gram-negative, non-motile, and short rods or coccobacilli. The capacity of the isolated bacteria to solubilize phosphate using tricalcium phosphate as the P source was assessed. The ability of the bacterial isolates in the three research sites to solubilize tricalcium phosphate was demonstrated by the distinct halo zones surrounding the colonies. Effective PSB in soil has been reported from bacterial strains, including Acinetobacter (ADI NUGROHO et al., 2020).
For clarity, the representative bacterial isolates were assigned with codes as follows: isolate from Nueva Ecija – NE PSB-RGL-2023; from Ilocos Sur – IS PSB-RGL-2023; from Mindoro Occidental – MOc PSB-RGL-2023.
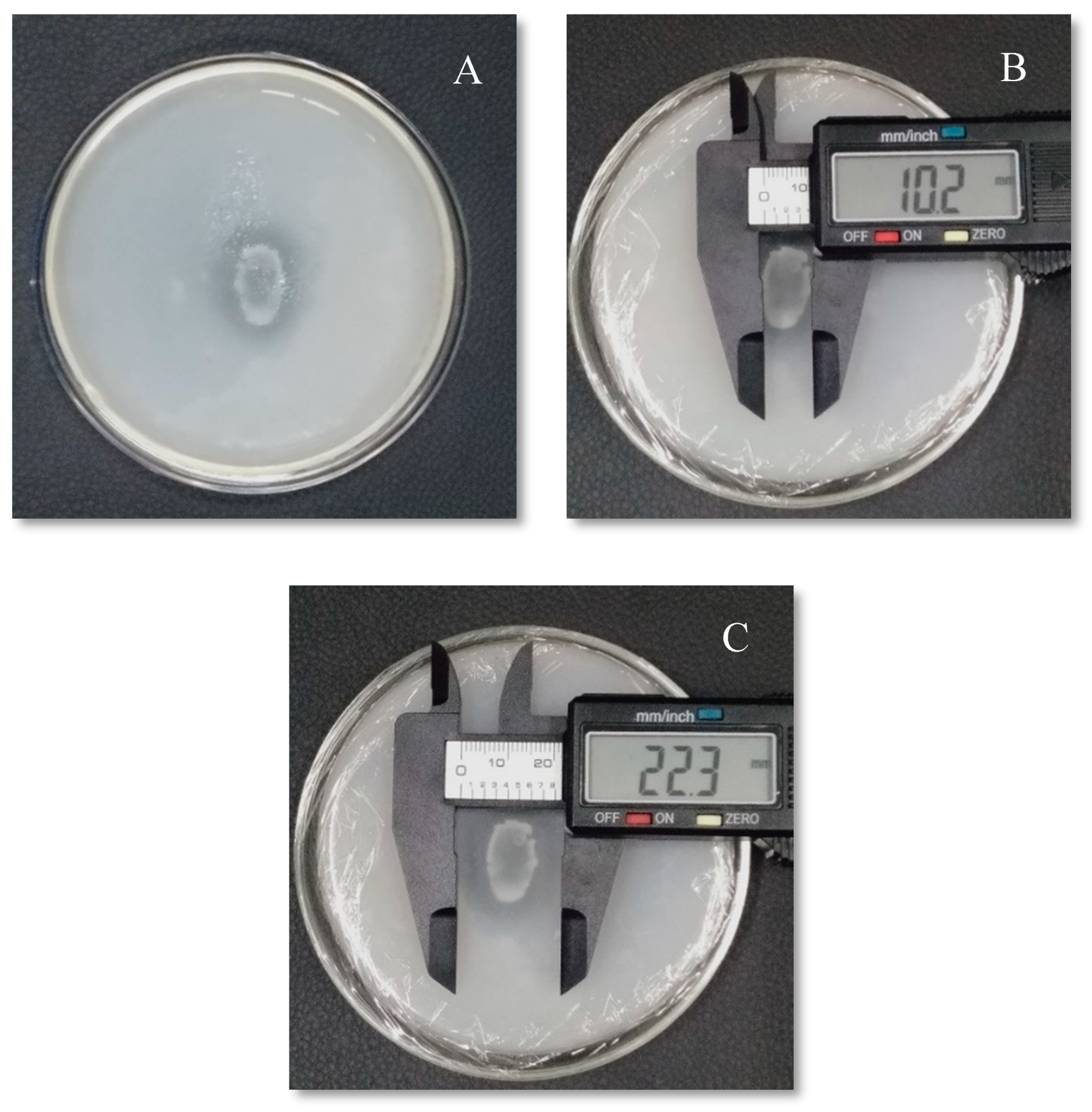
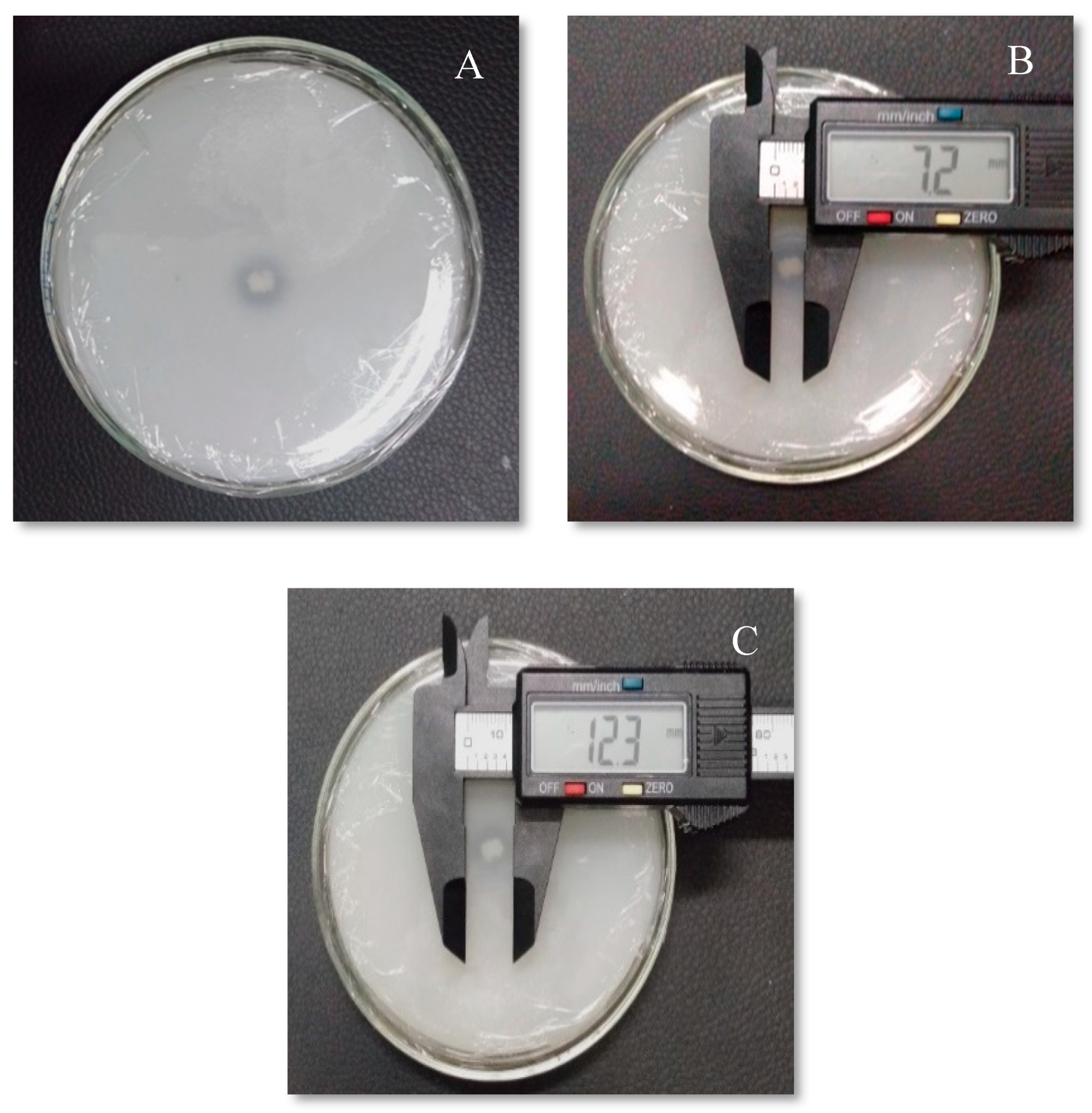
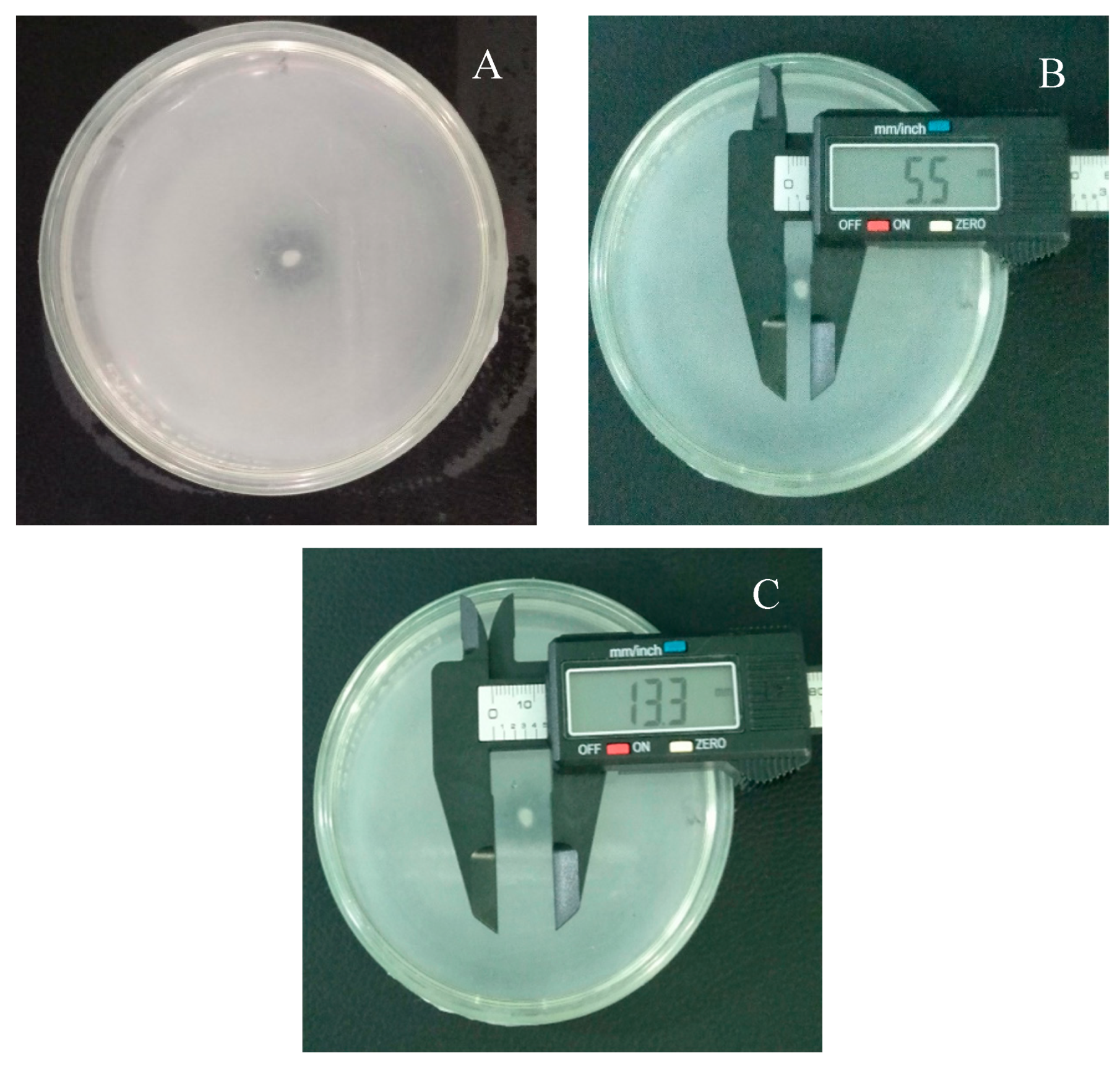
The solubilization index was used to quantify the capacity of isolated PSB to solubilize TCP on the PVK agar media (Paul and Sinha, 2017). The results of solubilizing indices were measured on the basis of clear halo zone and the SI was calculated in millimeter (mm). The clear halo zone formed around the colony of bacteria could be the polysaccharide, organic acid, enzyme phosphatase, and phytase productions (Kumar et al., 2024). It was discovered that the phosphate solubilizing capability of the isolates obtained in the soils from the three study sites are varied. The three bacterial isolates showed varying P solubilizing activity, as evidenced by the size differences between their colonies and P solubilizing halos. The isolates' halo zone to colony zone ratios varied from 2.70 mm to 3.42 mm (
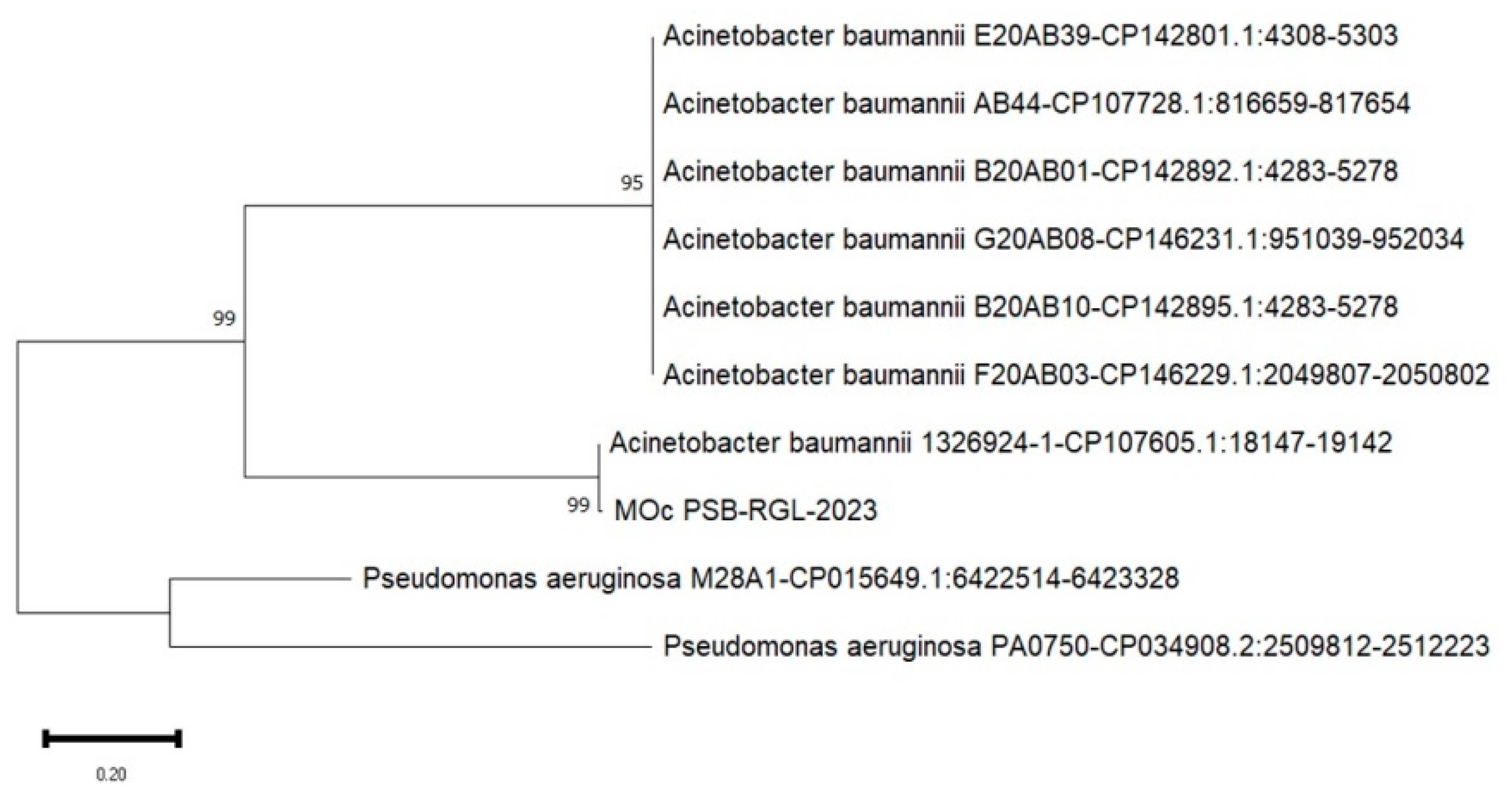
Table 2). The highest proficient bacterial isolates solubilizing tricalcium phosphate was Mindoro Occidental (MOc PSB-RGL-2023) isolate of 3.42 mm (
Figure 3), followed by Ilocos Sur isolate (IS PSB-RGL-2023) of 3.20 mm (
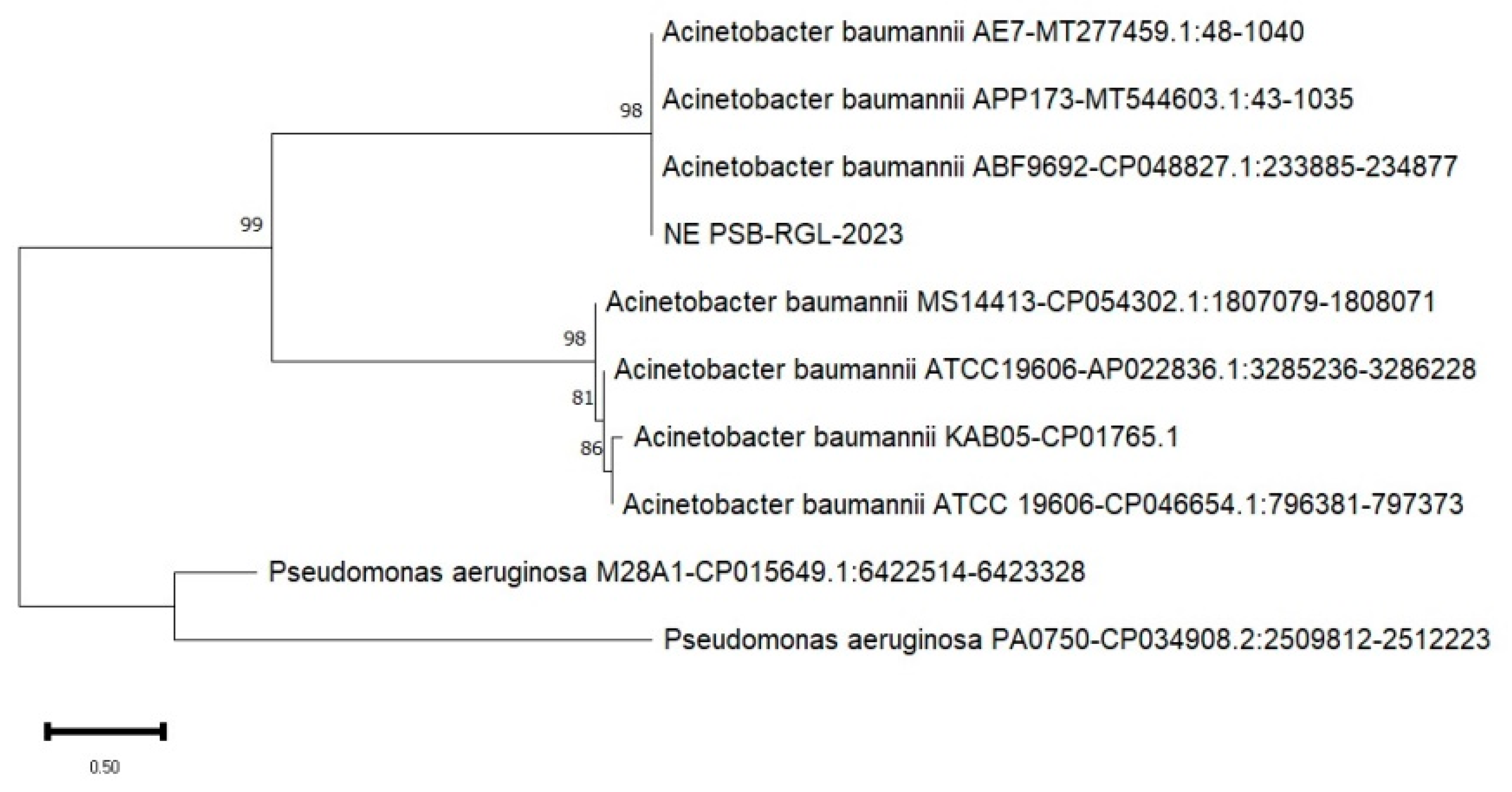
Figure 1), and the last, Nueva Ecija (NE PSB-RGL-2023) isolate of 2.70 mm (
Figure 2). Based on the result, the three locally-isolated bacteria showed efficient phosphate solubilization that can be utilized to increase the availability of P for plant’s uptake.
In a report by Adi Nughoro et al. (2020), it was found that Acinetobacter baumannii, after the 7 and 14 days incubation period, has a mean P dissolved in liquid cultures of 10.8 mg l-1 and 39.3 mg l-1, respectively and with the solubilization index of 1.83 mm; which are smaller SI than the isolates in this present report. Another study by Das et al. (2021) indicated that after 7 days of incubation, Acinetobacter strain SuKIC24 garnered a SI of 4.0 cm and produced 387 µg/ml of soluble phosphate in PVK media thru the process of phosphomolybdate method and the result demonstrates the strong capability of phosphate solubilization. Fatimah et al. (2023) reported that the highest solubilization index from 12 isolates from mangrove soil of East Java, Indonesia was only 2.82 and the authors earlier report (Fatimah et al., 2021) from 19 isolates had a highest solubilization index of 2.36. Meanwhile, researchers from Egypt evaluated 40 isolates for phosphate solubilizing property and obtained the highest SI of 2.3 wherein a combination of some of these isolates (with SI of 1.3, 1.8, and 2.0) significantly increased the P content of wheat plants by 76% and 12% over the full fertilized plants, suggesting that these SI values are highly efficient in solubilizing high amount of P from the soil for plant’s uptake. In this present report, the lowest SI is 2.70 while the highest is 3.42 which is a positive indication that these local isolates have the ability to solubilize a high amount of P as proven by several studies.
Figure 1.
The isolate (IS PSB-RGL-2023) from Bantay, Ilocos Sur, Philippines indicating the (A) colony morphology; (B) colony size; and (C) halo zone size with 16S rRNA gene similar to Acinetobacter baumannii.
Figure 1.
The isolate (IS PSB-RGL-2023) from Bantay, Ilocos Sur, Philippines indicating the (A) colony morphology; (B) colony size; and (C) halo zone size with 16S rRNA gene similar to Acinetobacter baumannii.
Figure 2.
The isolate (NE PSB-RGL-2023) from Bongabon, Nueva Ecija, Philippines indicating the (A) colony morphology; (B) colony size; and (C) halo zone size with 16S rRNA gene similar to Acinetobacter baumannii.
Figure 2.
The isolate (NE PSB-RGL-2023) from Bongabon, Nueva Ecija, Philippines indicating the (A) colony morphology; (B) colony size; and (C) halo zone size with 16S rRNA gene similar to Acinetobacter baumannii.
Figure 3.
The isolate (MOc PSB-RGL-2023) from San Jose, Occidental Mindoro, Philippines indicating the (A) colony morphology; (B) colony size; and (C) halo zone size with 16S rRNA gene similar to Acinetobacter baumannii.
Figure 3.
The isolate (MOc PSB-RGL-2023) from San Jose, Occidental Mindoro, Philippines indicating the (A) colony morphology; (B) colony size; and (C) halo zone size with 16S rRNA gene similar to Acinetobacter baumannii.
3.3. Genetic Characterizations of Isolated PSB from Soil Samples
Utilizing
16S rRNA sequences and matching them to the database stored in NCBI GenBank,
A. baumannii were isolated from the rhizospheric soils in onion fields at Bantay, Ilocos Sur, Bongabon, Nueva Ecija, and San Jose, Mindoro Occidental.
Acinetobacter is a genus of gram-negative, oxidase-negative, strictly aerobic bacteria, belong to
γ-Proteobacteria, and order
Pseudomonadales (Jung and Park, 2015).
Acinetobacter species exist in natural ecosystems such as soils, aquatic, marine, sediments, the polar region, and even in site with hydrocarbon contamination (Kotska, 2011; Mahjoubi, 2013). According to Peix et al. (2009),
Acinetobacter is an abundant bacteria comprehensively distributed in water and soil ecosystems and contains 17 validly well-explained species and 14 unidentified genomic species. Baumann (1968) reported that the estimate population of
Acinetobacter cells in soil and water was 10
5/mg. Dijkshoorn et al. (2007) cited that there existed 924 genomes of
Acinetobacter in the Integrated Microbial Genome database (IMG;
www.img.jgi.doe.gov) as of September 2014, of which 728 genomes or equivalent to 81% belong to
A. baumannii.
Acinetobacter are chemoheterotrophs and nutritionally diverse, the different substrates they utilized as derivatives of lone carbon and energy are the same of the aerobic Pseudomonad (Barbe et al., 2004). Due to soil complexity,
A. baumannii has been found to coexist with closely related
Acinetobacter species such as
A. bohemicus (Krizova et al., 2014).
Acinetobacter is an important Plant Growth Promoting Bacteria (PGPR) because it is known to solubilize phosphate, potassium, and zinc, produce antibiotics, siderophores, gibberellin, and Indole Acetic Acid (IAA). Acinetobacter has a wide range of uses in the removal of phosphate from wastewater environments due to its ability to sequester high amounts of inorganic phosphate (Mujumdar, 2023). Meanwhile, it was reported that A. baumannii solubilized 10.8 mgl-1 and 39.3 mgl-1 of P after the incubation of 7 and 14 days, respectively (ADI NUGROHO et al., 2020). In addition, a study reported that A. baumannii PUCM1029 strain solubilized phosphate of 64 mg/ml, produced IAA of 10 µg/ml, and produced siderophore of 74.20SU in a research conducted by Farokh et al. (2011). Moreover, A. baumannii was discovered to produce siderophores quantitatively, at a rate of 65.54SU (Singh et al., 2020). By making iron available to plants and creating a shortage of iron for pathogenic fungi, siderophores promote plant growth (Ahmad et al., 2008). A certain Acinetobacter sp. SK2 solubilized 682 μg ml−1 of TCP and 86 μg ml−1 of Rock Phosphate (RP), resulting in a pH drop of up to 4 owing to gluconate formation. The produced gluconate was mediated by enzymes membrane-bound bound GDH (mGDH) and soluble GDH (sGDH) and this is the biochemical basis of the P solubilization (Bharwad and Rajkumar, 2020). According to (An and Moe, 2016), the Gluconobacter, Pseudomonas, and Acinetobacter species which are gram-negative have the membrane-bound mGDH, however the sGDH is less particular. In a research of Sachdev et al. (2010), it was found that A. baumannii LRFN53 produced ethylene, thus, has the capability in the nitrogen fixation. In the case of iron-limiting situations, and after 48 hours of incubation, A. baumannii HIRFP40 secreted siderophore of 94.77SU, and A. baumannii LRFP52 strain solubilized Zinc most efficiently. According to Sidat (1999), Acinetobacter spp. is capable of accumulating amount of phosphate larger than what is needed for cell synthesis. The process is called luxury phosphate absorption. This supports the claim that Acinetobacter spp. is the primary microorganism responsible in improved absorption of Phosphorus. According to Islam et al. (2007), isolates of Acinetobacter spp. grow best in neutral media and are less resistant to extremely acidic environments. Therefore, aside from its ability as plant growth promoter and P-solubilizer, the species of Acinetobacter can be explored to be inoculated on acidic soil conditions to increase P availability that are being rendered unavailability by low soil pH.
Although in this report, the isolated bacteria were only sequenced based on 16S rRNA gene and its genetic identity has to be further verified through sequencing of other chromosomal, metabolic, and functional genes. Yet, this is the first report in the Philippines about A. baumanii strains that can be explored as a microbial inoculant or biofertilizer for crop production in the country. The first report about Acinetobacter in the Philippines was about the 117 A. baumanii isolates collected from the Philippines’ hospitals which were carbapenem-resistant was on December 2021. However, this report was not related to crop production.
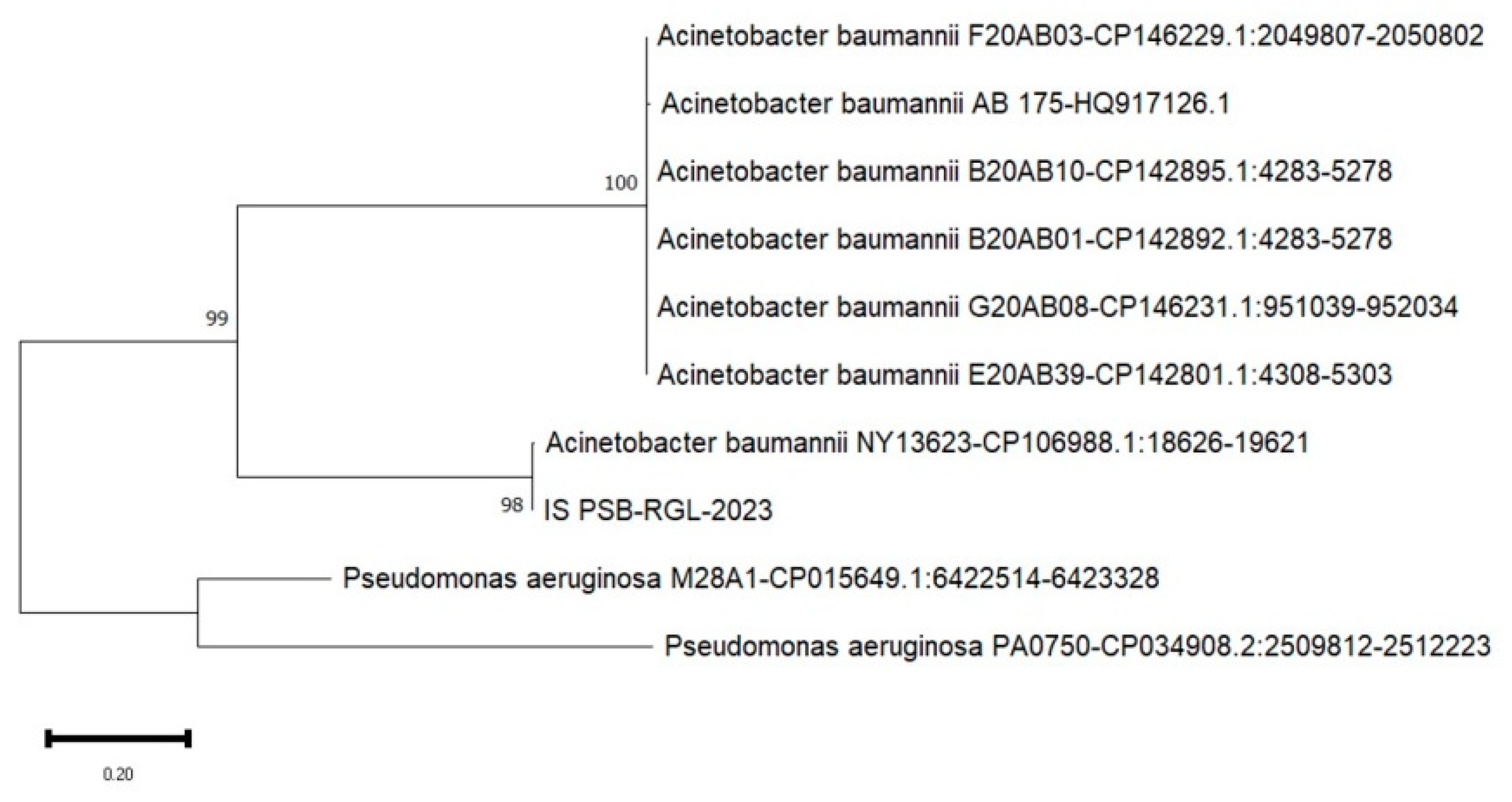
Shown on
Figure 4,
Figure 5 and
Figure 6 are the phylogenetic trees constructed to indicate the 16S rRNA gene nucleotide similarity obtained from the DNA database through BLAST between the known strains and the locally-isolated bacterial isolates of this study. It is evident that the phosphate solubilizing bacteria in the three study sites are categorized under the genus of
A. baumannii with IS PSB-RGL-2023 obtaining a 98% similarity to
A. baumannii NY13623 strain; NE PSB-RGL-2023 with 98% similarity to three strains such as
A. baumannii APP 173,
A. baumannii AE7,
A. baumannii ABF9692; and MOc-PSB RGL-2023 with 99% similarity to
A. baumannii 1326924-1.
4. Conclusion
PSB help to the increased availability of soluble phosphates in the soils for easy absorption of plants, thus reducing the usage of chemical fertilizers that contribute to lessen the environmental degradation. This study discovered the PSB present in the soils of onion fields in Ilocos Sur, Nueva Ecija, and Mindoro Occidental, Philippines which is dominated by genus Acinetobacter. This genus has the effective capacity of solubilizing phosphate, possess different mechanisms such as organic acid, IAA, siderophores productions which contribute to soil fertility and plant growth and development. With these, A. baumanni can be a potential biofertilizer that enhances crop productivity while also protecting or maintaining environmental conditions.
In the Philippines, this is the first report as of this time on A. baumannii strain as a phosphate solubilizer for onion production. This study serves an initiative in the formulation of biofertilizer using A. baumannii for increasing P availability for crops that require a high amount of P application, such as onion.
However, the study utilized only the 16S rRNA gene, so further research should focus on the identification of specific functional genes of phosphate solubilization and/or quantification of siderophore and gluconic acid, phytase enzyme productions, and other plant growth promoting hormones that are potentially released by these beneficial microorganisms. In addition, future research will include the molecular analysis of other genes related to its functions, evolution, and ecological niche to infer on its possible endemism.
Author Contributions
Rosalee G. Leander: performed the experiments; analyzed and interpreted the data; wrote and edited the paper. Dr. Maria Luisa T. Mason: conceived and designed the experiments; performed the experiments; contributed reagents, analyzed and interpreted the data; wrote and edited the paper. Dr. Ariel G. Mactal: conceived and designed the experiments; provided the materials for the research; edited the paper. Dr. Fernan T. Fiegalan: conceived and designed the experiments, edited and suggested for the improvement of the paper. Dr. Marilou M. Sarong: conceived and designed the experiments, edited the paper. Dr. Elaida R. Fiegalan: conceived and designed the experiments, edited the paper.
Funding
The funds for this study was provided by the DOST-ASTHRD, Republic of the Philippines.
Acknowledgments
This research study is a success because of the tremendous contributions of different entities. And with a grateful heart, the authors want to acknowledge the Department of Science and Technology (DOST), Republic of the Philippines for giving the funds, Ramon Magsaysay – Center for Agricultural Research and Environmental Study - Central Luzon State University (RM-CARES-CLSU) for providing the laboratory facility, and the Municipal Agricultural Offices (MAO) of Bantay, Ilocos Sur, Vega, Bongabon, Nueva Ecija, and San Jose, Mindoro Occidental, Philippines for providing the areas.
Conflicts of Interest
The authors declare no conflict of interest.
Additional information
No additional information is available for this paper.
References
- ADI NUGROHO, R., IRENE MEITINIARTI, V., & DAMAYANTI, C. (2020). Antagonistic Effect of Two Indigenous Phosphate Solubilizing Bacteria, Burkholderia contaminans PSB3 and Acinetobacter baumannii PSB11 Isolated from Different Crop Soils. Microbiology Indonesia, 14(2), 45–51. [CrossRef]
- Ahmad, F., Ahmad, I., & Khan, M. S. (2008). Screening of free-living rhizospheric bacteria for their multiple plant growth promoting activities. Microbiological Research, 163(2), 173–181. [CrossRef]
- Alaylar, B., Güllüce, M., Karadayi, M., & Isaoglu, M. (2019). Rapid Detection of Phosphate-Solubilizing Bacteria from Agricultural Areas in Erzurum. Current Microbiology, 76(7), 804–809. [CrossRef]
- Amri, M., Rjeibi, M. R., Gatrouni, M., Mateus, D. M. R., Asses, N., Pinho, H. J. O., & Abbes, C. (2023). Isolation, Identification, and Characterization of Phosphate-Solubilizing Bacteria from Tunisian Soils. Microorganisms, 11(3). [CrossRef]
- An, R., & Moe, L. A. (2016). Regulation of pyrroloquinoline quinone-dependent glucose dehydrogenase activity in the model rhizosphere-dwelling bacterium Pseudomonas putida KT2440. Applied and Environmental Microbiology, 82(16), 4955–4964. [CrossRef]
- Barbe, V., Vallenet, D., Fonknechten, N., Kreimeyer, A., Oztas, S., Labarre, L., Cruveiller, S., Robert, C., Duprat, S., Wincker, P., Ornston, L. N., Weissenbach, J., Marlière, P., Cohen, G. N., & Médigue, C. (2004). Unique features revealed by the genome sequence of Acinetobacter sp. ADP1, a versatile and naturally transformation competent bacterium. Nucleic Acids Research, 32(19), 5766–5779. [CrossRef]
- Baumann’, P. (1968). Isolation of Acinetobacter from Soil and Water. In JOURNAL OF BACTERIOLOGY (Vol. 96, Issue 1). https://journals.asm.org/journal/jb.
- Bharwad, K., & Rajkumar, S. (2020). Modulation of PQQ-dependent glucose dehydrogenase (mGDH and sGDH) activity by succinate in phosphate solubilizing plant growth promoting Acinetobacter sp. SK2. 3 Biotech, 10(1). [CrossRef]
- Colo, J. O. S. I. P. , H.-J. T. , D. S. , S. D. , & H. S. A. U. D. (2014). Plant growth promotion rhizobacteria in onion production. PolishJournalofMicrobiology, 63(1), 83. https://pdf.semanticscholar.org/1bb6/f03f8fc73e303c097f1ac0bbca9b6bd9f.pdf.
- Das, S., Sultana, K. W., & Chandra, I. (2021). Isolation and Characterization of a Plant Growth-Promoting Bacterium Acinetobacter sp. SuKIC24 From in vitro-Grown Basilicum polystachyon (L.) Moench. Current Microbiology, 78(8), 2961–2969. [CrossRef]
- Dijkshoorn, L., Nemec, A., & Seifert, H. (2007). An increasing threat in hospitals: Multidrug-resistant Acinetobacter baumannii. In Nature Reviews Microbiology (Vol. 5, Issue 12, pp. 939–951). [CrossRef]
- Edgar, R. C., & Batzoglou, S. (2006). Multiple sequence alignment. In Current Opinion in Structural Biology (Vol. 16, Issue 3, pp. 368–373). [CrossRef]
- Edi Premono, M. , M. A. M. & V. P. L. G. (1997). Effect of phosphate-solubilizing Pseudomonas putida on the growth of maize and its survival in the rhizosphere. https://doi.org/https://www.cabidigitallibrary.org/doi/full/10.5555/19970700762.
- Elshazly, M., Ahmed, A., Ibrahim, H., & Ismail, S. (2023). Using of Biofertilizers and Some Micronutrients in Improving Onion Productivity under Siwa Oasis Conditions. Journal of Soil Sciences and Agricultural Engineering, 0(0), 325–331. [CrossRef]
- Farokh, R. Z., Sachdev, D., Pour, N. K., Engineer, A., Pardesi, K. R., Zinjarde, S., Dhakephalkar, P. K., & Chopade, B. A. (2011). Characterization of plant-growth-promoting traits of Acinetobacter species isolated from rhizosphere of Pennisetum glaucum. Journal of Microbiology and Biotechnology, 21(6), 556–566. [CrossRef]
- Fernández, L. A., Zalba, P., Gómez, M. A., & Sagardoy, M. A. (2007). Phosphate-solubilization activity of bacterial strains in soil and their effect on soybean growth under greenhouse conditions. Biology and Fertility of Soils, 43(6), 805–809. [CrossRef]
- Fernández, V., Guzmán, P., Peirce, C. A. E., McBeath, T. M., Khayet, M., & McLaughlin, M. J. (2014). Effect of wheat phosphorus status on leaf surface properties and permeability to foliar-applied phosphorus. Plant and Soil, 384(1–2), 7–20. [CrossRef]
- Fierer, N., & Jackson, R. B. (2006). The diversity and biogeography of soil bacterial communities. www.pnas.orgcgidoi10.1073pnas.0507535103.
- Gulati, A., Vyas, P., Rahi, P., & Kasana, R. C. (2009). Plant growth-promoting and rhizosphere-competent Acinetobacter rhizosphaerae strain BIHB 723 from the cold deserts of the himalayas. Current Microbiology, 58(4), 371–377. https://doi.org/10.1007/s00284-008-9339-x Hanci, F. (2018). A Comprehensive Overview of Onion Production: Worldwide and Turkey. 11(9), 17–27.
- Heuer, S., Gaxiola, R., Schilling, R., Herrera-Estrella, L., López-Arredondo, D., Wissuwa, M., Delhaize, E., & Rouached, H. (2017). Improving phosphorus use efficiency: a complex trait with emerging opportunities. Plant Journal, 90(5), 868–885. [CrossRef]
- https://agrictech.tnau.ac.in/agriculture/agri_soil_sampling.html. (n.d.).
- https://psa.gov.ph. (2022).
- https://rfo3.da.gov.ph/index.php/regional-soils-laboratory/. (n.d.).
- http://www.ncbi.nlm.nih.gov/. (n.d.).
- Islam, M. T., Deora, A., Hashidoko, Y., Rahman, A., Ito, T., & Tahara, S. (2007). Isolation and Identification of Potential Phosphate Solubilizing Bacteria from the Rhizoplane of Oryza sativa L. cv. BR29 of Bangladesh. In Z. Naturforsch (Vol. 62). http://www.znaturforsch.com.
- Johnston, A. E., Poulton, P. R., Fixen, P. E., & Curtin, D. (2014). Phosphorus. Its Efficient Use in Agriculture. In Advances in Agronomy (Vol. 123, pp. 177–228). Academic Press Inc. [CrossRef]
- Jung, J., & Park, W. (2015). Acinetobacter species as model microorganisms in environmental microbiology: current state and perspectives. In Applied Microbiology and Biotechnology (Vol. 99, Issue 6, pp. 2533–2548). Springer Verlag. [CrossRef]
- Khaledian, Y., Brevik, E. C., Pereira, P., Cerdà, A., Fattah, M. A., & Tazikeh, H. (2017). Modeling soil cation exchange capacity in multiple countries. Catena, 158, 194–200. [CrossRef]
- Kotska, J. E. , P. O. , O. W. A. G. S. J. , F. G. , C. A. , & H. M. (2011). Hydrocarbon-degrading bcteria and the bacterial community response in Gulf of Mexico beach sands impacted by the Deepwater Horizon oil spill. Appliedandenvironmentalmicrobiology, 77(22), 7962–7974. [CrossRef]
- Krizova, L., Maixnerova, M., Sedo, O., & Nemec, A. (2014). Acinetobacter bohemicus sp. nov. widespread in natural soil and water ecosystems in the Czech Republic. Systematic and Applied Microbiology, 37(7), 467–473. [CrossRef]
- Kumar, V., Chourasia, H. K., Rajani, K., & Kumar, R. R. (2024). Exploration and Characterization of High-Efficiency Phosphate-Solubilizing Bacteria Isolates from Chickpea Rhizospheric Soil. International Journal of Bio-Resource and Stress Management, 15(Jan, 1), 01–09. [CrossRef]
- Mahjoubi, M. , J. A. , G. A. , A. S. B. , J. A. , C. H. , & C. A. (2013). Hydrocarbonoclastic bacteria isolated from petroleum contaminated sites in Tunisia: isolation, identification and characterization of the biotechnological potential. Newbiotechnology, 3(6), 723–733. [CrossRef]
- Mazzuco, V. R., Júnior, C. da C. T., & Botelho, G. R. (2023). Fluorescent Pseudomonas spp. and Bacillus spp. for phosphate solubilization and growth promotion of garlic. Pesquisa Agropecuaria Tropical, 53. [CrossRef]
- Mujumdar, S. , B. J. , A. A. , H. S. , A. N. , J. P. , & B. S. (2023). Acinetobacter: A versatile plant growth-promoting rhizobacteria (PGPR). In Plant-Microbe Interaction Recent Advances in Molecular and Biochemical Approaches . AcademicPress, 327–362. [CrossRef]
- Nikitha, T., M. S., B. Sadhana, E. U. B. R., & Vani, S. S. (2017). Phosphorous and Phosphate Solubilising Bacteria and their Role in Plant Nutrition. International Journal of Current Microbiology and Applied Sciences, 6(4), 2133–2144. [CrossRef]
- Ogut, M., Er, F., & Kandemir, N. (2010). Phosphate solubilization potentials of soil Acinetobacter strains. Biology and Fertility of Soils, 46(7), 707–715. [CrossRef]
- Pahalvi, H. N. , R. L. , R. S. , N. B. , & K. A. N. (2021). Chemical fertilizers and their impact on soil health. MicobiotaandBiofertilizersEcofriendlyToolsforReclamationofDegaradedSoilEnvirons, 2, 1–20. https://doi.org/https://link.springer.com/chapter/110.1007/978-3-030-61010-4. [CrossRef]
- Patre, A. S. , & P. J. K. (2021). Assessment of physicochemical parameters and characterization of multiple plant growth promotion traits of Pseudomonas aeruginosa L. PharmaInnov, 10, 18–27. https://www.thepharmajournal.com/archives/2021/vol10issue7/PartA/10-8-169-361.pdf.
- Paul, D., & Sinha, S. N. (2017). Isolation and characterization of phosphate solubilizing bacterium Pseudomonas aeruginosa KUPSB12 with antibacterial potential from river Ganga, India. Annals of Agrarian Science, 15(1), 130–136. [CrossRef]
- Peix, A., Ramírez-Bahena, M. H., & Velázquez, E. (2009). Historical evolution and current status of the taxonomy of genus Pseudomonas. In Infection, Genetics and Evolution (Vol. 9, Issue 6, pp. 1132–1147). [CrossRef]
- Rawat, P., Das, S., Shankhdhar, D., & Shankhdhar, S. C. (2021). Phosphate-Solubilizing Microorganisms: Mechanism and Their Role in Phosphate Solubilization and Uptake. In Journal of Soil Science and Plant Nutrition (Vol. 21, Issue 1, pp. 49–68). Springer Science and Business Media Deutschland GmbH. [CrossRef]
- Roberts, T. L., & Johnston, A. E. (2015). Phosphorus use efficiency and management in agriculture. Resources, Conservation and Recycling, 105, 275–281. [CrossRef]
- Sachdev, D., Nema, P., Dhakephalkar, P., Zinjarde, S., & Chopade, B. (2010). Assessment of 16S rRNA gene-based phylogenetic diversity and promising plant growth-promoting traits of Acinetobacter community from the rhizosphere of wheat. Microbiological Research, 165(8), 627–638. [CrossRef]
- Shen, J., Yuan, L., Zhang, J., Li, H., Bai, Z., Chen, X., Zhang, W., & Zhang, F. (2011). Phosphorus dynamics: From soil to plant. Plant Physiology, 156(3), 997–1005. [CrossRef]
- Sidat, M. , B. F. , & K. H. C. (1999). Polyphosphate accumulation by bacteria isolated from activated sludge . WaterSa, 25(2), 175–179. https://www.wrc.org.za/wp-content/uploads/mdocs/WaterSA_1999_02_apr99_p175.pdf.
- Singh, T. B., Sahai, V., Goyal, D., Prasad, M., Yadav, A., Shrivastav, P., Ali, A., & Dantu, P. K. (2020). Identification, Characterization and Evaluation of Multifaceted Traits of Plant Growth Promoting Rhizobacteria from Soil for Sustainable Approach to Agriculture. Current Microbiology, 77(11), 3633–3642. [CrossRef]
- Zheng, B. X., Zhang, D. P., Wang, Y., Hao, X. L., Wadaan, M. A. M., Hozzein, W. N., Peñuelas, J., Zhu, Y. G., & Yang, X. R. (2019). Responses to soil pH gradients of inorganic phosphate solubilizing bacteria community. Scientific Reports, 9(1). [CrossRef]
- Zhu, J., Li, M., & Whelan, M. (2018). Phosphorus activators contribute to legacy phosphorus availability in agricultural soils: A review. Science of the Total Environment, 612, 522–537. [CrossRef]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).