Submitted:
01 October 2024
Posted:
01 October 2024
You are already at the latest version
Abstract
Keywords:
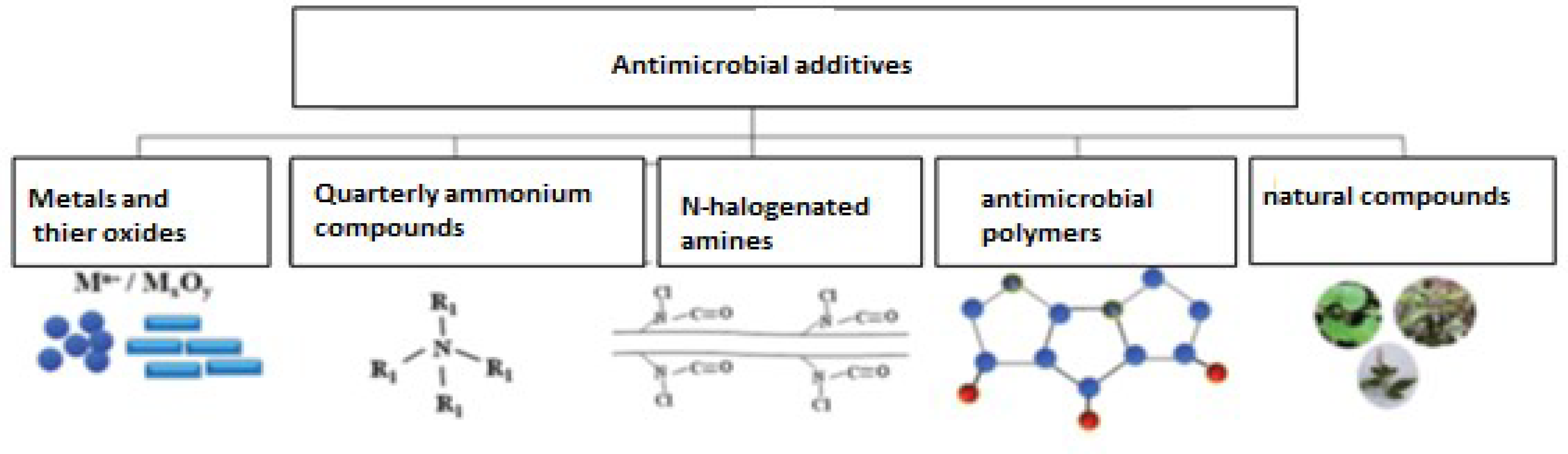
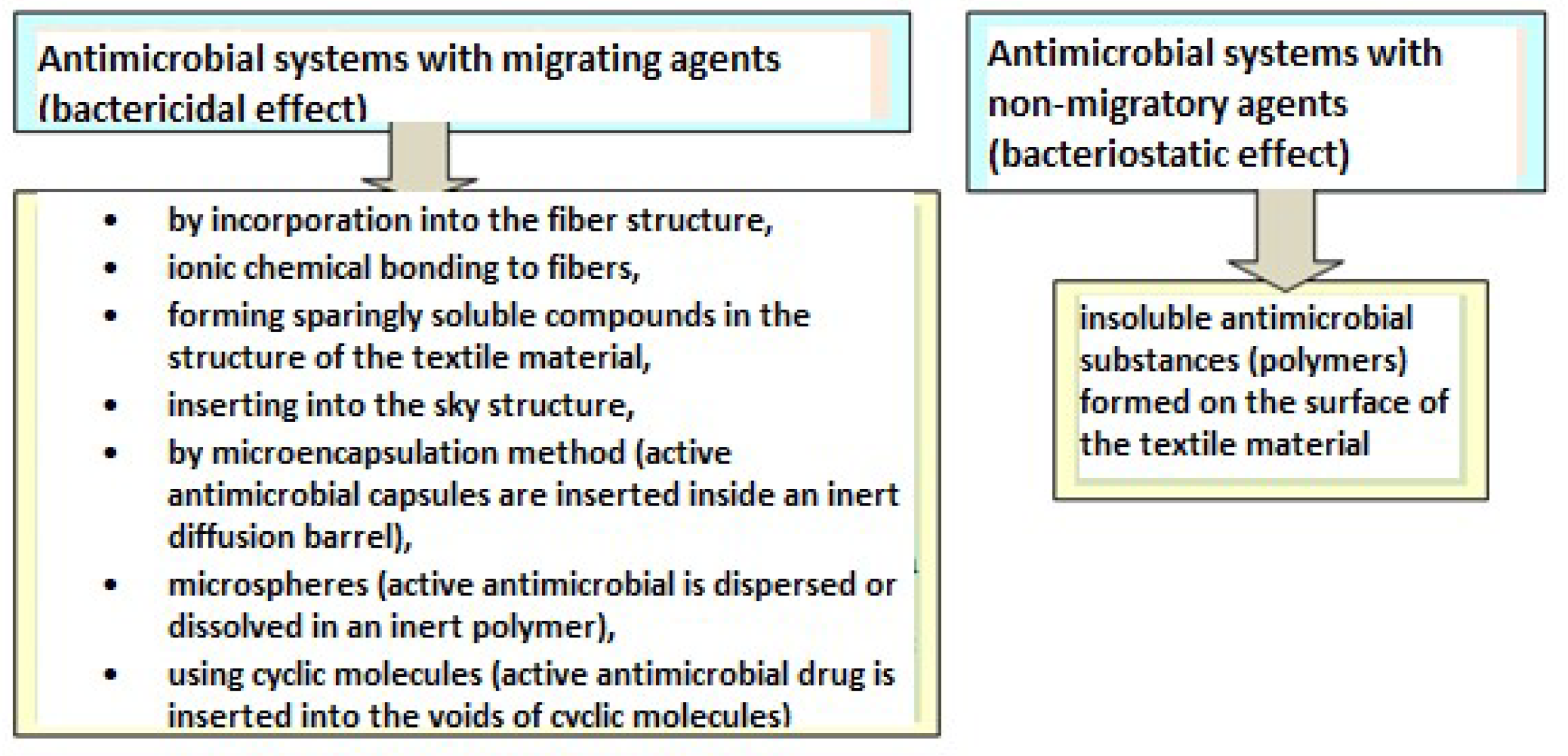
1. Introduction
2. Materials and Methods
2.1. Materials
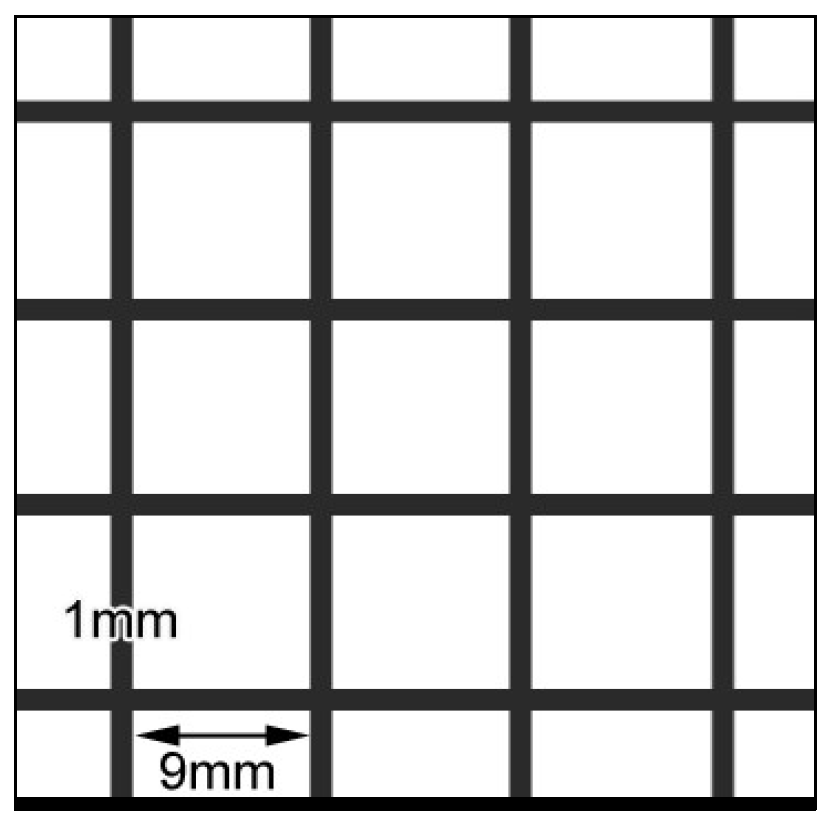
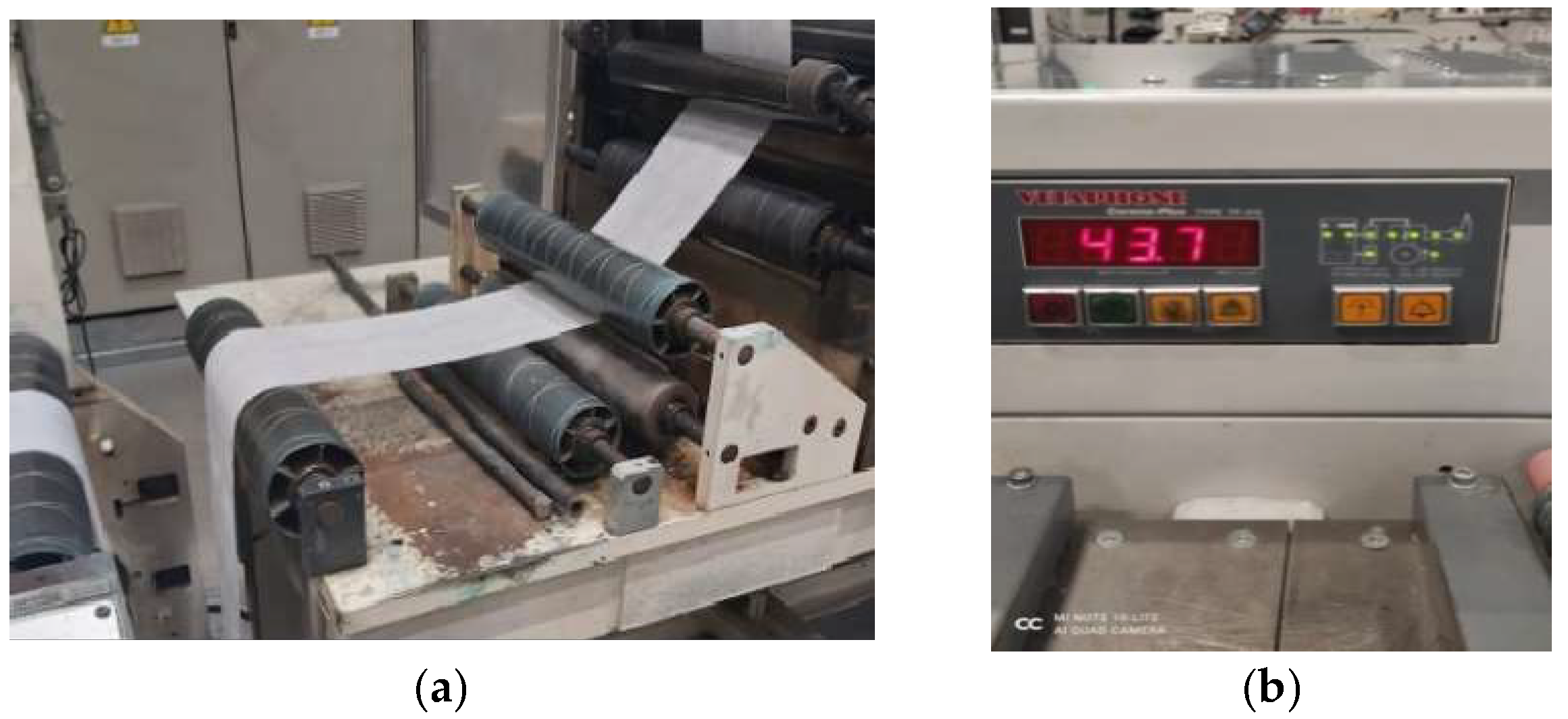
2.2. Coating of Nonwoven Fabric Samples with Prepared Compositions
2.3. Methods
2.3.1.“. Breathability” Test
- The differential pressure of 60 Pa/cm2 corresponds to an air permeability of about 93 mm/s at a pressure difference 100 Pa;
- The differential pressure of 70 Pa/cm2 corresponds to an air permeability of about 80 mm/s at a pressure difference 100 Pa.
2.3.2. Antibacterial Activity Test
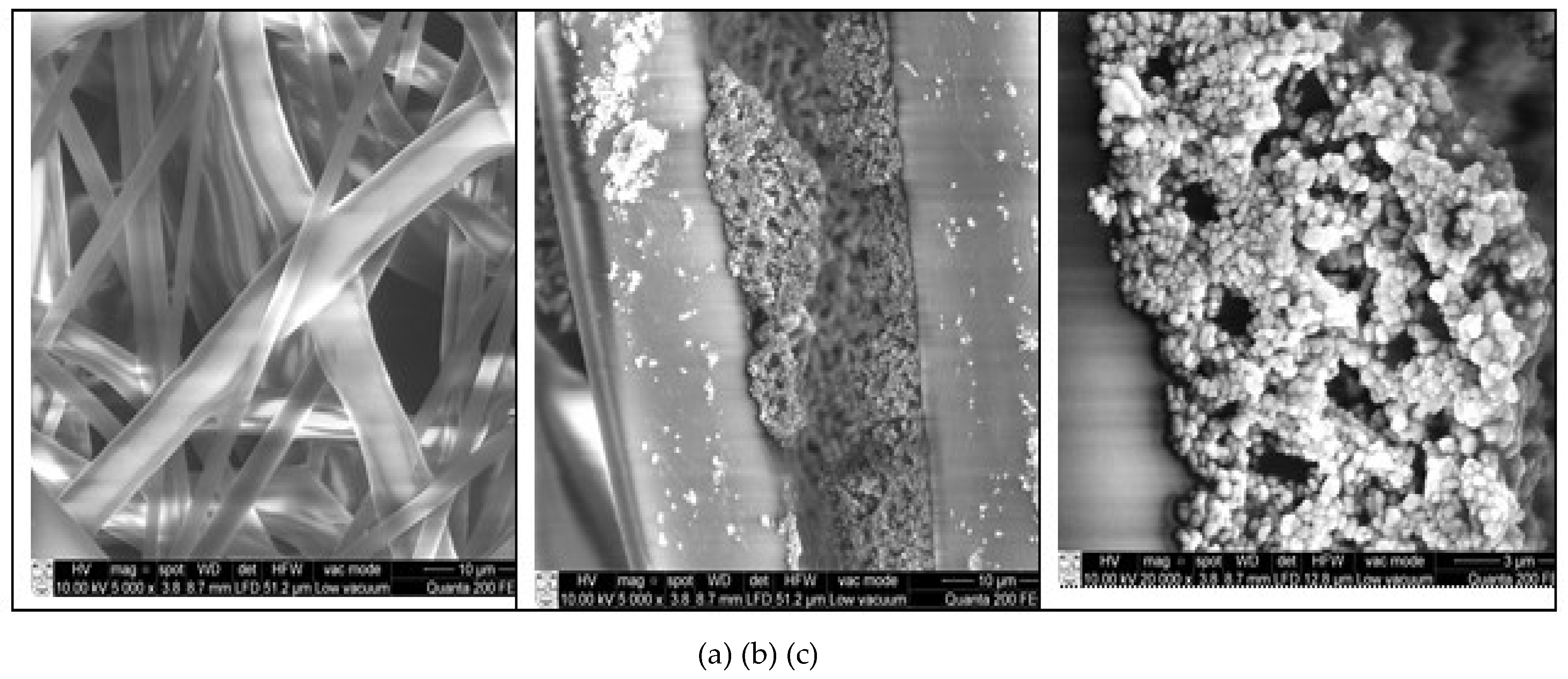
2.3.4. SEM
3. Results
| Marking of samples | Binder coating composition, g | |
| Full coating | Squared pattern coating (1cmx1cmx0,9cm) |
|
| DLP | LLP |
Tubicoat Thickener LP-3 Deionized water-97 |
| DSMT | LSMT |
CHT-Alginat SMT-4 Deionized water-96 |
| D126 | L126 |
Prematex RA 126-20 Deionized water-80 |
| DCM | LCM |
Prisulon CM70-6 Deionized water-80 |
| Antimicrobial additive | Formulation, g/ml | Marking of the sample with coating | ||
|---|---|---|---|---|
| Full coating | Cell coating | Dot coating | ||
| RUCO - BAC AGP |
Formulation B 1.RUCO-BACAGP-30 2.Tubicoat Thickener LP-3 3. Deionized water-67 Total: 100 |
- | MA21466 MA889 MA891 |
MA888 MA892 |
|
Formulation C 1.RUCO- BAC AGP - 80 2. Tubicoat Thickener LP-3 3. Deionized water -17 Total: 100 |
MA20849 | MA21466 MA890 |
- | |
| Marking of sample | Description of sample layers |
|---|---|
| MA888 | 1-st layer: non-woven PP fabric 2-nd layer: non-woven PP fabric with dot coating, Formulation B (Table 5) |
| MA888+1 layer | 1-st layer: non-woven PP fabric 2-nd layer: non-woven PP fabric with dot coating, Formulation B (Table 5) 3rd layer: non-woven PP fabric |
| MA889 | 1-st layer: non-woven PP fabric with squared pattern coating, Formulation B (Table 5) 2-nd layer: non-woven PP fabric |
| MA889+1 layer | 1-st layer: non-woven PP fabric 2-nd layer: non-woven PP fabric with squared pattern coating, Formulation B (Table 5) 3-rd layer: non-woven PP fabric |
| MA890 (mask layout) | 1-st layer: non-woven PP fabric 2-nd layer: non-woven PP fabric with squared pattern coating, Formulation C (Table 5) 3-rd layer: non-woven PP fabric |
| Description of the sample | Mass per unit area g/m2 |
Coverage deposit, g/m2 |
|---|---|---|
| Non-woven PP material coated with cell pattern by RUCO-BAC AGP composition (Formulation C) coating without Corona discharge in OMET device | 24,4 |
Very low (≤1) |
| Non-woven PP material coated with cell pattern by RUCO-BAC AGP composition (Formulation C) coating with Corona discharge in OMET device | 24,4 |
Very low (≤1) |
| Non-woven PP material (control, used for production test, KG) | 25,1 | - |
| Marking of sample | Antibacterial activity | |||
|
E.coli ATCC25922 |
S.aureus ATCC6338 |
K.pneumonia ATCC 13882 |
||
| MA20849 (1- layer) | Good | Good | Good | |
| MA21466 (1-layer) | Good | Good | Good | |
| MA13915 (1-layer) | Good | Limited | Limited | |
| MA888 (2- layers) | 1 | Good | Good | Good |
| 2 | Good | Limited | Limited | |
| MA889 (2-layers) | 1 | Limited | Good | Limited |
| 2 | Limited | Good | Limited | |
| MA890 (3- layers mask model) | 1 | Good | Good | Good |
| 3 | Good | Good | Good | |
| MA891 (1- layer) | Good | Good | Limited | |
| MA892 (1- layer) | Good | Good | Good | |
2.3.3. Production test
| No. | Marking of samples | Description of coating | Air permeability (R), mm/s |
|---|---|---|---|
| 1. | L126 | Squared pattern coating | 348±25 |
| 2. | D126 | Full coating | 84±9 |
| 3. | LLP | Squared pattern coating | 434±30 |
| 4. | DLP | Full coating | 369±27 |
| 5. | LSMT | Squared pattern coating | 376±28 |
| 6. | DSMT | Full coating | 116±15 |
| 7. | LCM | Squared pattern coating | 330±24 |
| 8. | DCM | Full coating | 20±3 |
| Description of the sample | Air permeability (R), mm/s |
|---|---|
| Nonwoven PP material, 1 layer | 451±22 |
| Nonwoven PP material, 2 layers | 223±16 |
| Nonwoven PP material, 3 layers | 150±11 |
4. Discussion
- Type I – minimum protection. This type of medical mask is intended to be worn by patients and others to reduce the risk of spreading infections, especially in the event of an epidemic or pandemic;
- Type II – This type of mask is primarily intended for use by healthcare professionals in an operating or other medical environment with similar requirements.
- Type IIR – used when the wearer seeks to protect himself from splashes of potentially contaminated liquids.
- BFE of type I medical masks - ≥ 95%,
- BFE of type II and IIR medical masks - ≥ 98%.
- The differential pressure of type I and type II medical masks must be <40 Pa/cm2,
- Type IIR - <60 Pa/cm2.
4. Conclusions
- The air permeability results presented in Table 9 show that this parameter was influenced not only by the surface area of the formed coating, but also by the chemical composition and concentration of the binder. LLP (squared pattern coating) and DLP (full coating) samples had the best air permeability properties of the 1- layer PP nonwoven fabric with binder coating. The air permeability of the samples of 1-layer non-woven PP material with different chemical binder coatings (Table 9) showed that the composition of the polyacrylic acid binder Tubicoat Thickener LP had the least effect on their “breathability” (samples LLP and DLP).
- Summarizing the air permeability of the 1- layer samples (Table 9) and the antibacterial efficiency results (Table 10), it can be stated that good antibacterial activity was observed for all three pathogens (Escherichia coli, Klebsiella pneumoniae and Staphylococcus aureus) and air permeability (341 mm / s) of sample MA21466 with squared pattern coating of RUCO-BAC AGP composition (Formulation C). Based on the obtained research data, a 3-layer medical mask model MA 890 was prepared, in the 1st and 3rd layers of raw non-woven PP material (Table 3), and in the 2nd layer - sample MA 21466 (Table 9).
- Microbiological studies have shown that the non-woven PP material (Bermed) of 3-layer medical mask model MA 890 with the 5.6 g/m2 coverage deposit coating of the antimicrobial RUCO-BAC AGP composition in the middle layer (Formulation C) in both sides of the model has antibacterial efficiency against three pathogens (E. coli, K. pneumoniae, and S. aureus).
- The performance of the mask model MA 890 has been found to meet the requirements for type I medical masks according to the EN 14683 standard [26].
- Study have shown that the microbial purity of the M 890 model is CFU/g <3. It can be stated that the microbial cleanliness of the mask model meets the requirements of all three types of medical masks.
- After the production test in OMET device preparation and application on non-woven PP material for masks composition RUCO-BAC AGP (Formulation C), it was determined that in order to achieve good antimicrobial efficiency it is necessary to modify the existing PTO drawing, increasing the surface area of the textile coating and a coverage deposit.
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- https://www.who.int/news/item/03-03-2020-shortage-of-personal-protective-equipment-endangering-health-workers-worldwide (revised in 2021.12.11).
- Prata, J. C., Silva, A. L., Walker, T. R., Duarte, A. C., & Rocha-Santos, T. COVID-19 pandemic repercussions on the use and management of plastics. Environmental Science & Technology 2020, 54 (13), pp. 7760-7765.
- https://www.globenewswire.com/news-release/2020/08/04/2072167/0/en/Global-face-mask-market-to-register-12-8-CAGR-through-202.html (revised 2021.12.05).
- Chu, D. K., Akl, E. A., Duda, S., Solo, K., Yaacoub, S., Schünemann, H. J. Physical distancing, face masks, and eye protection to prevent person-to person transmission of SARS-CoV-2 and COVID-19: a systematic review and meta-analysis. The Lancet 2020, 395, 1973–1956. [Google Scholar] [CrossRef] [PubMed]
- Aboubakr, H. A., Sharafeldin, T. A., & Goyal, S. M. Stability of SARS-CoV-2 and other coronaviruses in the environment and on common touch surfaces and the influence of climatic conditions: A review. Transboundary and Emerging Diseases 2020; 00, pp.1–17.
- J. W. Tang, T. J. Liebner, B. A. Craven, and G. S. Settles. A schlieren optical study of the human cough with and without wearing masks for aerosol infection control. J. R. Soc Interface 2009, 6 (Suppl 6), pp. S727–S736.
- C. Leung, T. H. Lam and K. K. Cheng. Mass masking in the COVID-19 epidemic: people need guidance. The Lancet 2020, 395, 945. [Google Scholar] [CrossRef] [PubMed]
- N. L. Belkin.The evolution of the surgical mask: Filtering efficiency versus effectiveness, Infect. Control Hosp. Epidemiol 1997, 18, 49–57.
- B. Hogan and L. P. Samaranayake. The surgical mask unmasked: A review. Oral Surg. Oral Med. Oral Pathol. 1990, 70, pp. 34–36.
- Shama, S., Kamal, H., Ayesha, S., Zeeshan, A. Production of antimicrobial, natural, and reusable material for stitching eco-friendly, extra-protective face masks. International Journal of Clothing Science and Technology 2022. [CrossRef]
- Omar, B.A.; Turki, A. Evaluation of the antibacterial activities of face masks coated with titanium dioxide nanoparticles. Scientific Reports volume 2022, 12, : 18739. [Google Scholar]
- Li, Z., Lihua, J., Huiyong, L., Cheng, H., Jianxun, S., Lei, L., Fanrong, M., Zhihong, D., Changchun, Z. Synthesis and characterization of silver-incorporated calcium phosphate antibacterial nanocomposites for mask filtration material. Composites Part B 153 2018, p.p. 387–392.
- Chaitanya B. H., Anuraj S. K., Vividha V. D., Tanaya K., Prasad J., Priyesh V. M. Enhanced anti-microbial response of commercial face mask using colloidal silver nanoparticles. Vacuum 2018, 14, 156, p.p. 475-482.
- Pinhong, C., Zhi, Y., Zhuoxian, M., Ziyun, H., Yongshuang, B., Shangjing, W., Xianming, D., Xianjun, F., Frank, K., Shiying, Z., Wenxu, Z., Shengsen, Z., Wuyi, Z. Electrospun nanofibrous membrane with antibacterial and antiviral properties decorated with Myoporum bontioides extract and silver-doped carbon nitride nanoparticles for medical masks application. Separation and Purification Technology 2022, Volume 298, Article number: 121565.
- Fangfei, Z., Junzhu, L., Mingwan, Y., Yun, W., Zhicheng, Y., Jiajun, H., Jie, S., Xuechang, Z., Zhiguang, G., Yabin, Z., Ben, W. High-breathable, antimicrobial and water-repellent face mask for breath monitoring. Chemical Engineering Journal 2023, Volume 466, 15, Article number: 143150.
- Natsathaporn, P., Herwig, G., ·Altenried, S., Ren, Q., Rossi, R. M., ·Crespy, D.· F. Itel. Functional Fiber Membranes with Antibacterial Properties for Face Masks. Advanced Fiber Materials. [CrossRef]
- Calais, G. B., Neto, J. B. M. R., Bataglioli, R. A., Chevalier, P., Tsukamoto, J., Weis Arn, C., Mantovani, D., Masumi Beppu, M. Bioactive textile coatings for improved viral protection: A study of polypropylene masks coated with copper salt and organic antimicrobial agents. Applied Surface Science 2023, Volume 638, 30, Article number: 158112.
- Song, X., Liu, P., Yu, L., and Zille, A. Fiber-Based Masks and Respirators: Using Decontamination Methods and Antimicrobial Treatment to Improve Its Reusability during Pandemic. Textiles 2022, 2, p.p. 318–335. [CrossRef]
- Krisciunaite, J., Kalendraite, B., Ragelienė, L., Merkelyte, E., Mikucioniene, D. Durable wash-resistant antimicrobial treatment of knitted fabrics. AUTEX Research Journal. [CrossRef]
- Wu, H., Huang, J., Zhang, C. J. P., He, Z. and Ming, W.-K.. Facemask shortage and the novel coronavirus disease (COVID-19) outbreak: Reflections on public health measures. Clinical Medicine 2020, 21, Article number: 100329.
- Brandelli, A. C. Ritter, F. F. Veras, Antimicrobial Activities of Metal Nanoparticles, Metal Nanoparticles in Pharma 2017, pp. 337–363.
- https://heiq.com/products/functional-textile-technologies/heiq-/Viroblock (revised 2021.11.25).
- https://www.rudolf.de/en/support/labels/detail/rucor-bac-agp/ (revised 2021.11.25).
- https://www.textileworld.com/textile-world/2018/02/devan-introduces-bi-ome-brand-develops-r-vital/(revised 2021.11.25).
- EN ISO 9237:1997.
- EN 14683:2019+AC.
- CWA 17553, June 2020.
- EN ISO 20645: 2005.
- Tsutsumi-Arai, C.; Iwamiya, Y.; Hoshino, R.; Terada-Ito, C.; Sejima, S.; Akutsu-Suyama, K.; Shibayama, M.; Hiroi, Z.; Tokuyama-Toda, R.; Iwamiya, R.; et al. Surface Functionalization of Non-Woven Fabrics Using a Novel Silica-Resin Coating Technology: Antiviral Treatment of Non-Woven Fabric Filters in Surgical Masks. Int. J. Environ. Res. Public Health 2022, 19, 3639. [Google Scholar] [CrossRef] [PubMed]
- ZoeA. Pollard, Madeline Karod & Jillian L. Goldfarb. Metal leaching from antimicrobial cloth face masks intended to slow the spread of COVID-19. www.nature.com/scientificreports.
- Omar B.Ahmed, T.Alamro. Evaluation of the antibacterial activities of face masks coated with titanium dioxide. www.nature.com/scientificreports.



| Name | Producer | Parameters |
|---|---|---|
| RUCO- BAC AGP | Rudolf Group | Chemical content (AgCl ≥1-<2,5%) etc., non-ionic, pH7 |
| BI-OME AM10 | Devan Chemicals | Chemical content – organic silane (6-10 %), etc., cationic, pH4. |
| Dispersion of elemental nano silver (Ag) | NANOIRON | Chemical content - Ag, water, organic stabilizers; Ag concentration - 100 ppm; Particle shape – spherical; Average particle size – 20-40 nm; pH 8-9, at a concentration of 10 ppm |
| Name of binder | Producer | Parameters | Content of the binder composition used for coating, % |
|---|---|---|---|
| Tubicoat Thickener LP | CHT R. Beitlich GmbH | Chemical content – polyacrylic acid, anionic, pH 7,5-8,2 (2%). | Binder, 3 Deionized water, 97 |
| CHT-Alginat SMT | CHT R. Beitlich GmbH | Chemical content – sodium alginate, anionic, 6,5-8,5 (4%) | Binder, 4 Deionized water-96 |
| Prematex RA 126 | CHT R. Beitlich GmbH | Chemical content – acrylic polymer ( ≥1 - < 2,5), etc. anionic, pH-7-9. |
Binder, 20 Deionized water-80 |
| Prisulon CM70 | CHT R. Beitlich GmbH | Chemical content - cellulose ether, non-ionic, pH-6,5 -8,5 (6%). | Binder, 20 Deionized water-80 |
| Parameters of fabric | |
|---|---|
 |
Colour-white; Mass per unit area- 24,12 g/cm2; Fiber content -100% polypropylene (PP); Produced using Meltblow method |
| Marking of sample | Description of the antimicrobial coating | Air permeability (R), mm/s |
|---|---|---|
| Control sample, K | Uncoated non-woven PP material | 451±30 |
| MA20849 | Full coating, Formulation C | 80±7 |
| MA21466 | Squared pattern coating, Formulation C | 369±25 |
| MA 891 | Squared pattern coating, Formulation B | 362±25 |
| MA 892 | Dot coating, Formulation B | 246±26 |
| Marking of sample | Description of sample layers | Air permeability (R), mm/s |
|---|---|---|
| MA888 |
1-st layer: non-woven PP fabric 2-nd layer: non-woven PP fabric with dot coating, Formulation B (Table 5) |
185±19 |
| MA888+1 layer |
1-st layer: non-woven PP fabric 2-nd layer: non-woven PP fabric with dot coating, Formulation B (Table 5) 3rd layer: non-woven PP fabric |
119±15 |
| MA889 |
1-st layer: non-woven PP fabric with squared pattern coating, Formulation B (Table 5) 2-nd layer: non-woven PP fabric |
203±21 |
| MA889+1 layer |
1-st layer: non-woven PP fabric 2-nd layer: non-woven PP fabric with squared pattern coating, Formulation B (Table 5) 3-rd layer: non-woven PP fabric |
144±16 |
|
MA890 (mask model) |
1-st layer: non-woven PP fabric 2-nd layer: non-woven PP fabric with squared pattern coating, Formulation C (Table 5) 3-rd layer: non-woven PP fabric |
140±16 |
| Description of the sample | Mass per unit area g/m2 |
Coverage deposit, g/m2 | Air permeability R, mm/s |
|---|---|---|---|
| Non-woven PP material (control used for laboratory tests, K) | 24,13 | - | 451±22 |
| Non-woven PP material coated with cell pattern by RUCO-BAC AGP composition (Formulation C) coating without Corona discharge in OMET device | 24,4 |
Very low (≤1) |
419±28 |
| Non-woven PP material coated with cell pattern by RUCO-BAC AGP composition (Formulation C) coating with Corona discharge in OMET device | 24,4 |
Very low (≤1) |
452±22 |
| Non-woven PP material (control, used for production test, KG) | 25,1 | - | 445±28 |
| Non-woven PP material coated with sieve template by RUCO-BAC AGP composition (Formulation C) with cell pattern coating |
29,73 |
5,6 |
369±18 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





