Submitted:
07 August 2024
Posted:
08 August 2024
You are already at the latest version
Abstract
Keywords:
1. Introduction
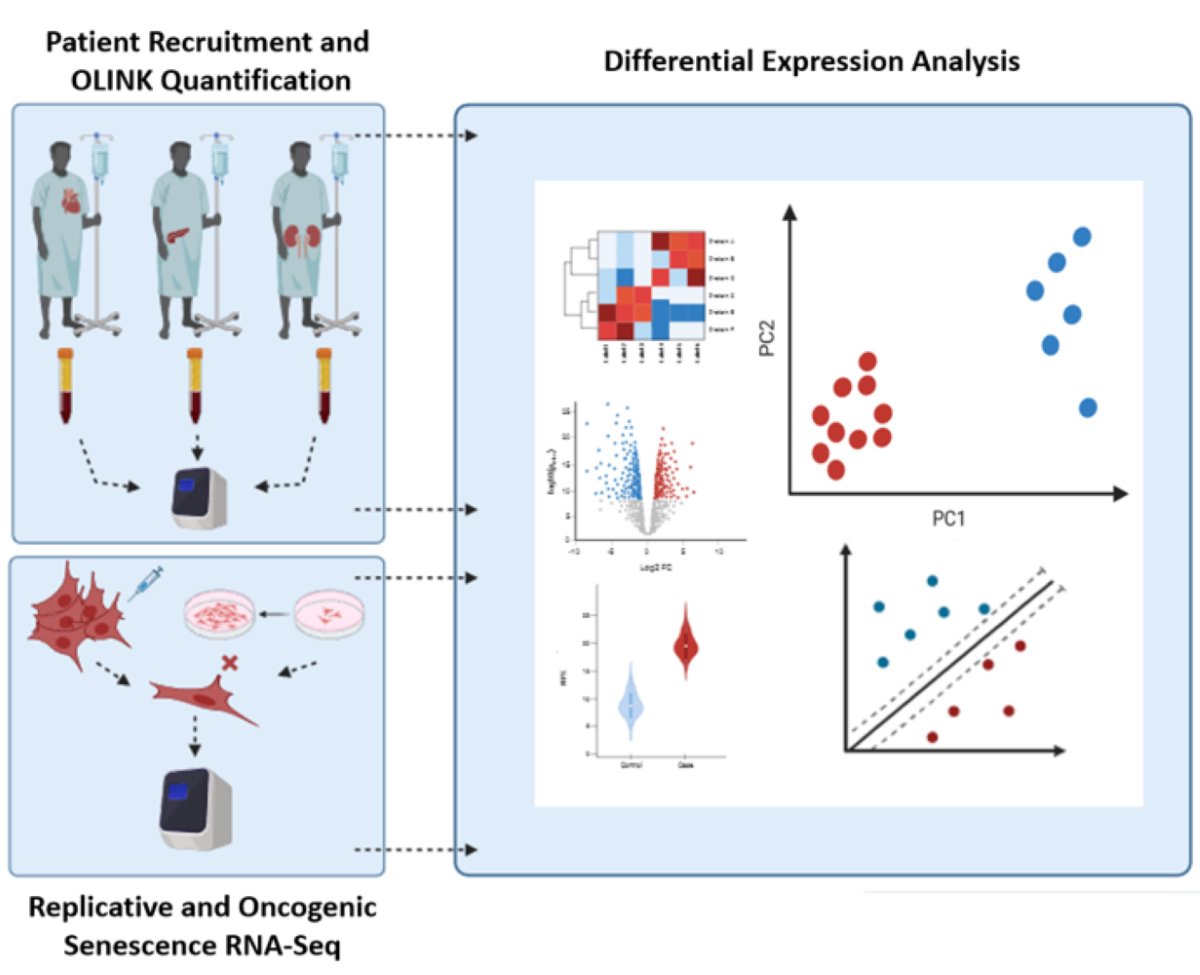
2. Materials and Methods
2.1. Participant Recruitment
2.2. Proteomic Analysis and Profiling Using Proximity Extension Assay
2.3. Statistical Analysis
2.4. Machine Learning Predictions
3. Results
3.1. Demographics for AKI Patients, CKD Patients, and Controls
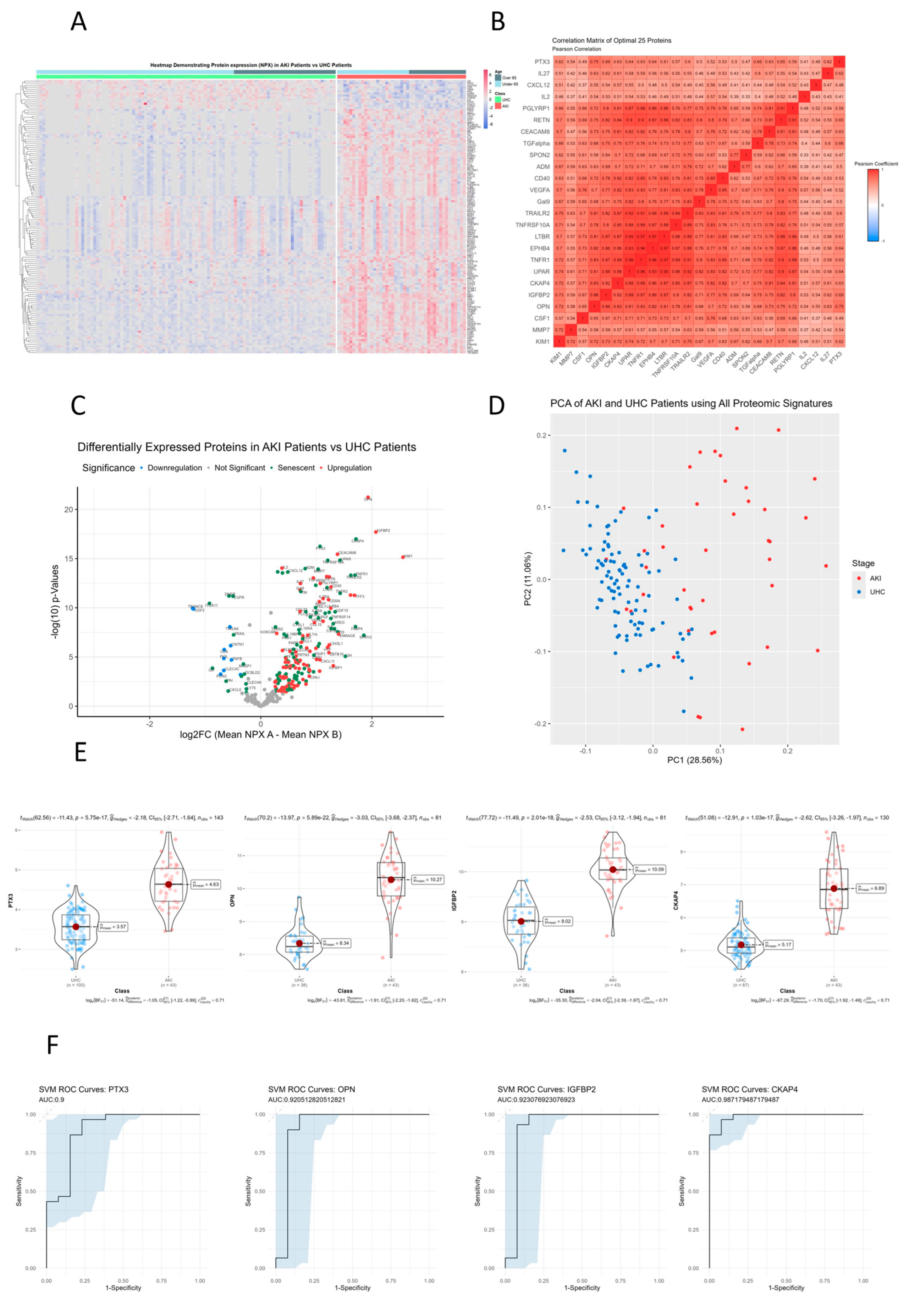
3.2. MMP3, IL16, TNFRSF10C, CCL23, GDF15, TNFR1 and UPAR that Are Significantly Different between AKI Patients and Controls on Hierarchical Clustered Heatmaps
3.3. TRAILR2, TNFRSF10A, LTBR, EPHB4, TNFR1, UPAR, CKAP4 and IGFBP2 Show Directly Proportional Correlation of Expression between AKI and Unhealthy Control Cohorts
3.4. Senescence Markers CKAP4, PTX3, UPAR, and TNFRSF10A Are Upregulated in AKI Patients Compared to Unhealthy Cohorts
3.5. Principle Component Analysis (PCA) of AKI Patients Compared to Controls
3.5. CKAP4, PTX3, OPN and IGFBP2 Are the Most Differentially Expressed Senescent Proteins between AKI and Unhealthy Control Cohorts
3.6. Receiver Operator Characteristic Curves for Individual Proteins for AKI vs. Controls
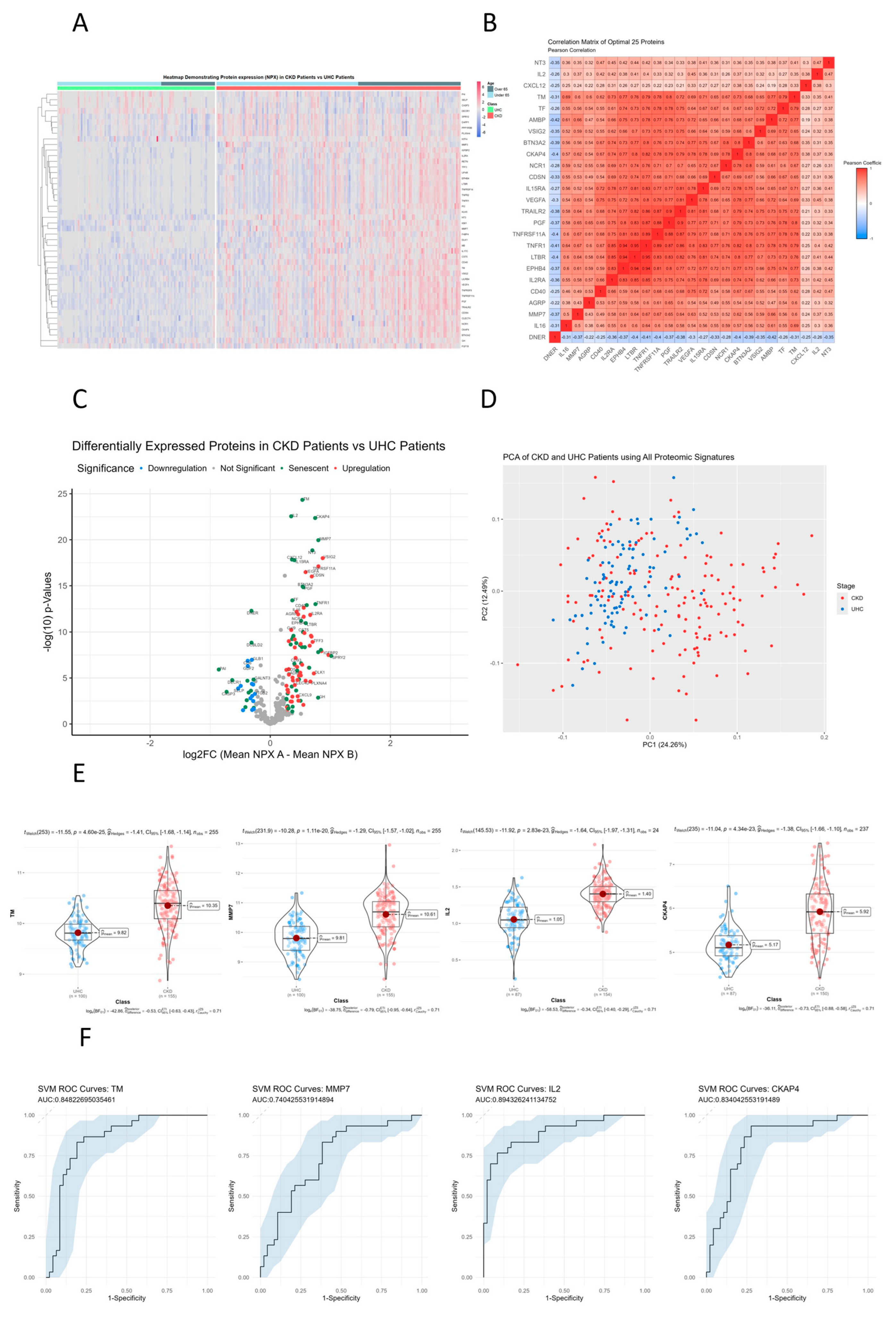
3.7. Senescent Proteins TM, CKAP4 and MMP7 Are Significantly Different between CKD Patients and Controls on Hierarchical Clustered Heatmaps
3.8. TNFR1, LTB3, EPHB4 and IL2RA Show Directly Proportional Correlation of Expression between CKD and Unhealthy Control Cohorts
3.9. Senescent Proteins TM, IL2, CKAP4 and MMP7 Are Significantly Differentially Expressed Senescent Proteins between CKD and Unhealthy Control Cohorts
3.10. Principle Component Analysis (PCA) of CKD Patients Compared to Controls
3.11. TM, CKAP4, IL2 and MMP7 Are the Most Differentially Expressed Senescent Proteins between CKD and Unhealthy Control Cohorts
3.12. Receiver Operator Characteristic Curves for Individual Proteins for CKD vs. Controls
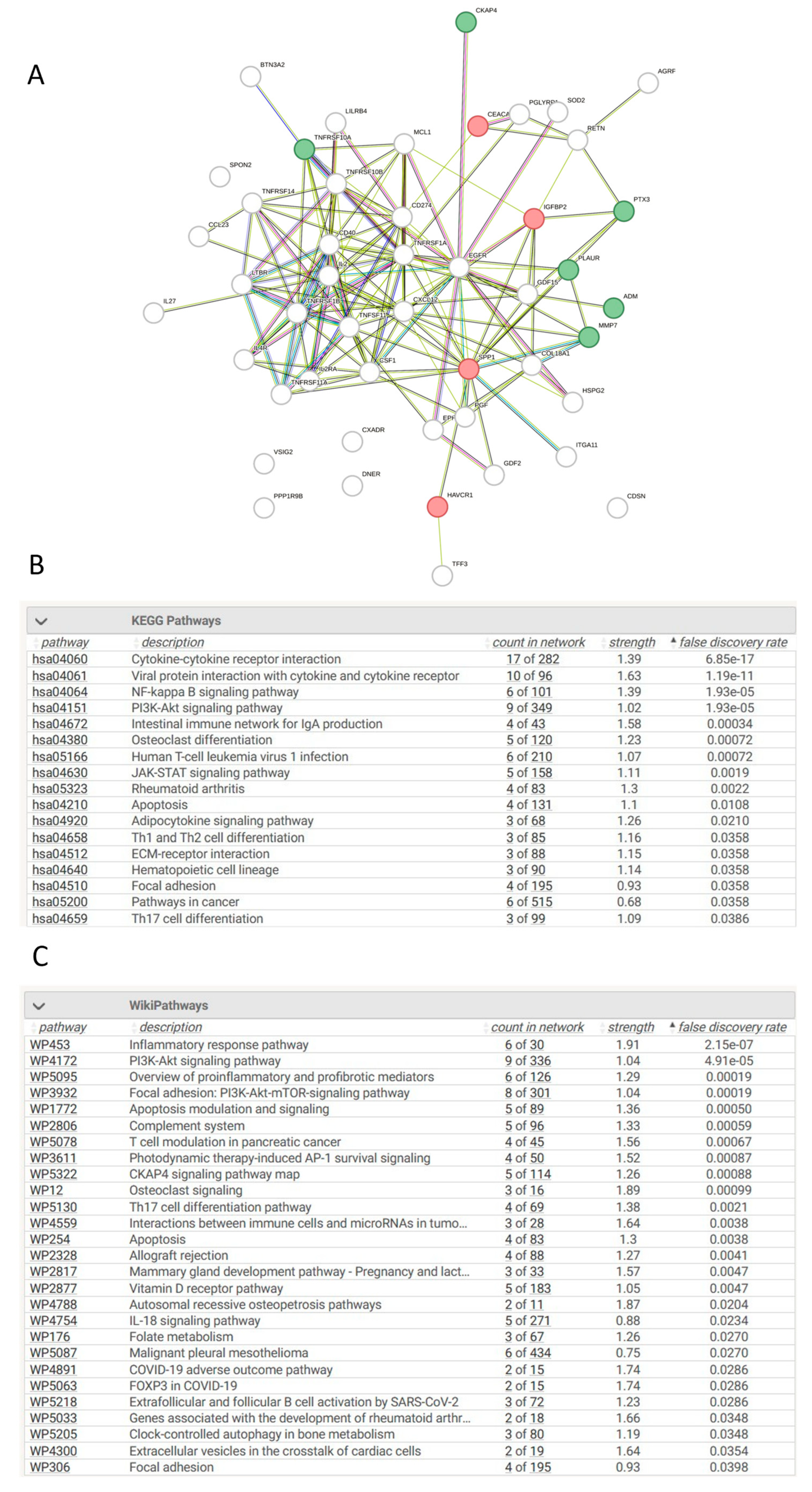
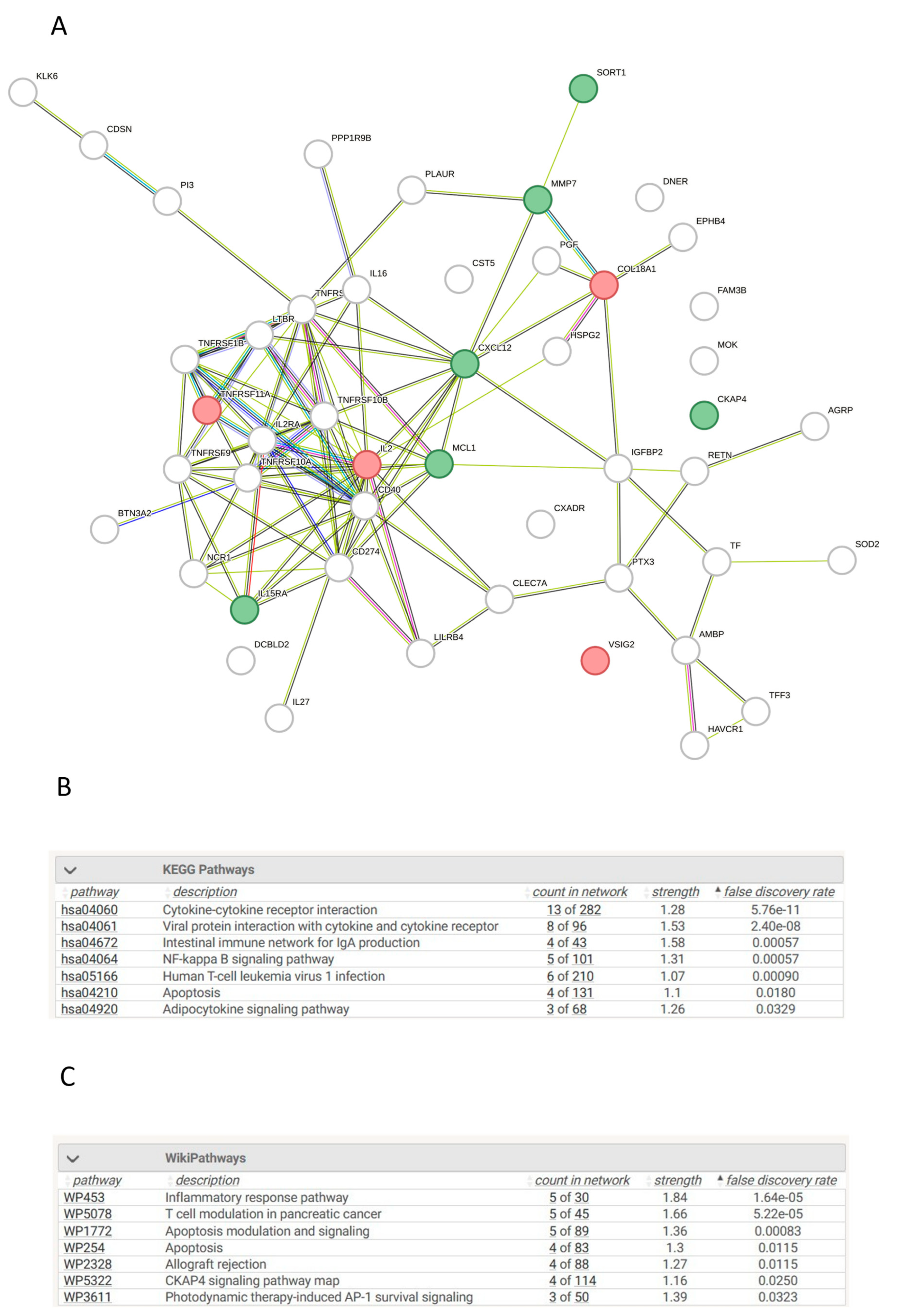
3.13. Network Analysis of Differentially Expressed Proteins in AKI Compared to Controls
3.14. Network Analysis of Differentially Expressed Proteins in CKD Compared to Controls
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Lankadeva, Y.R.; Kosaka, J.; Evans, R.G.; Bailey, S.R.; Bellomo, R.; May, C.N. Intrarenal and urinary oxygenation during norepinephrine resuscitation in ovine septic acute kidney injury. Kidney Int. 2016, 90, 100–108. [CrossRef]
- Levey, A.S. and J. Coresh, Chronic kidney disease. The Lancet, 2012. 379(9811): p. 165-180.
- Luyckx, V.A.; Cherney, D.Z.; Bello, A.K. Preventing CKD in Developed Countries. Kidney Int. Rep. 2019, 5, 263–277. [CrossRef]
- Sato, Y.; Takahashi, M.; Yanagita, M. Pathophysiology of AKI to CKD progression. Semin. Nephrol. 2020, 40, 206–215. [CrossRef]
- Webster, A.C., et al., Chronic Kidney Disease. The Lancet, 2017. 389(10075): p. 1238-1252.
- Coca, S.G.; Singanamala, S.; Parikh, C.R. Chronic kidney disease after acute kidney injury: A systematic review and meta-analysis. Kidney Int. 2012, 81, 442–448. [CrossRef]
- Noble, R.; Taal, M.W. Epidemiology and causes of chronic kidney disease. Medicine 2019, 47, 562–566. [CrossRef]
- Zhang, X.; Agborbesong, E.; Li, X. The Role of Mitochondria in Acute Kidney Injury and Chronic Kidney Disease and Its Therapeutic Potential. Int. J. Mol. Sci. 2021, 22, 11253. [CrossRef]
- Schanstra, J.P.; Zürbig, P.; Alkhalaf, A.; Argiles, A.; Bakker, S.J.; Beige, J.; Bilo, H.J.; Chatzikyrkou, C.; Dakna, M.; Dawson, J.; et al. Diagnosis and Prediction of CKD Progression by Assessment of Urinary Peptides. J. Am. Soc. Nephrol. 2015, 26, 1999–2010. [CrossRef]
- Semaan, V.; Noureddine, S.; Farhood, L. Prevalence of depression and anxiety in end-stage renal disease: A survey of patients undergoing hemodialysis. Appl. Nurs. Res. 2018, 43, 80–85. [CrossRef]
- Zhong, J.; Yang, H.-C.; Fogo, A.B. A perspective on chronic kidney disease progression. Am. J. Physiol. Physiol. 2017, 312, F375–F384. [CrossRef]
- Griffin, B.R.; Gist, K.M.; Faubel, S. Current Status of Novel Biomarkers for the Diagnosis of Acute Kidney Injury: A Historical Perspective. J. Intensiv. Care Med. 2020, 35, 415–424. [CrossRef]
- Levey, A.S.; Cattran, D.; Friedman, A.; Miller, W.G.; Sedor, J.; Tuttle, K.; Kasiske, B.; Hostetter, T. Proteinuria as a Surrogate Outcome in CKD: Report of a Scientific Workshop Sponsored by the National Kidney Foundation and the US Food and Drug Administration. Am. J. Kidney Dis. 2009, 54, 205–226. [CrossRef]
- Takahashi, S.; Nakasatomi, M.; Takei, Y.; Ikeuchi, H.; Sakairi, T.; Kaneko, Y.; Hiromura, K.; Nojima, Y.; Maeshima, A. Identification of Urinary Activin A as a Novel Biomarker Reflecting the Severity of Acute Kidney Injury. Sci. Rep. 2018, 8, 1–10. [CrossRef]
- Colombo, M., et al., Serum kidney injury molecule 1 and beta(2)-microglobulin perform as well as larger biomarker panels for prediction of rapid decline in renal function in type 2 diabetes. Diabetologia, 2019. 62(1): p. 156-168.
- Looker, H.C.; Colombo, M.; Hess, S.; Brosnan, M.J.; Farran, B.; Dalton, R.N.; Wong, M.C.; Turner, C.; Palmer, C.N.; Nogoceke, E.; et al. Biomarkers of rapid chronic kidney disease progression in type 2 diabetes. Kidney Int. 2015, 88, 888–896. [CrossRef]
- Schrauben, S.J.; Shou, H.; Zhang, X.; Anderson, A.H.; Bonventre, J.V.; Chen, J.; Coca, S.; Furth, S.L.; Greenberg, J.H.; Gutierrez, O.M.; et al. Association of Multiple Plasma Biomarker Concentrations with Progression of Prevalent Diabetic Kidney Disease: Findings from the Chronic Renal Insufficiency Cohort (CRIC) Study. J. Am. Soc. Nephrol. 2020, 32, 115–126. [CrossRef]
- Skupien, J.; Warram, J.H.; Niewczas, M.A.; Gohda, T.; Malecki, M.; Mychaleckyj, J.C.; Galecki, A.T.; Krolewski, A.S. Synergism Between Circulating Tumor Necrosis Factor Receptor 2 and HbA1c in Determining Renal Decline During 5–18 Years of Follow-up in Patients With Type 1 Diabetes and Proteinuria. Diabetes Care 2014, 37, 2601–2608. [CrossRef]
- Avelar, R.A.; Ortega, J.G.; Tacutu, R.; Tyler, E.J.; Bennett, D.; Binetti, P.; Budovsky, A.; Chatsirisupachai, K.; Johnson, E.; Murray, A.; et al. A multidimensional systems biology analysis of cellular senescence in aging and disease. Genome Biol. 2020, 21, 1–22. [CrossRef]
- Anderson, R.; Lagnado, A.; Maggiorani, D.; Walaszczyk, A.; Dookun, E.; Chapman, J.; Birch, J.; Salmonowicz, H.; Ogrodnik, M.; Jurk, D.; et al. Length-independent telomere damage drives post-mitotic cardiomyocyte senescence. EMBO J. 2019, 38. [CrossRef]
- Piechota, M., et al., Is senescence-associated β-galactosidase a marker of neuronal senescence? Oncotarget, 2016. 7(49): p. 81099-81099.
- Burton, D.G.; Stolzing, A. Cellular senescence: Immunosurveillance and future immunotherapy. Ageing Res. Rev. 2018, 43, 17–25. [CrossRef]
- Demaria, M.; Ohtani, N.; Youssef, S.A.; Rodier, F.; Toussaint, W.; Mitchell, J.R.; Laberge, R.-M.; Vijg, J.; Van Steeg, H.; Dollé, M.E.; et al. An Essential Role for Senescent Cells in Optimal Wound Healing through Secretion of PDGF-AA. Dev. Cell 2014, 31, 722–733. [CrossRef]
- Faget, D.V.; Ren, Q.; Stewart, S.A. Unmasking senescence: context-dependent effects of SASP in cancer. Nat. Rev. Cancer 2019, 19, 439–453. [CrossRef]
- Childs, B.G.; Baker, D.J.; Wijshake, T.; Conover, C.A.; Campisi, J.; Van Deursen, J.M. Senescent intimal foam cells are deleterious at all stages of atherosclerosis. Science 2016, 354, 472–477. [CrossRef]
- Campisi, J. The biology of replicative senescence. Eur. J. Cancer 1997, 33, 703–709. [CrossRef]
- En, A.; Takauji, Y.; Ayusawa, D.; Fujii, M. The role of lamin B receptor in the regulation of senescence-associated secretory phenotype (SASP). Exp. Cell Res. 2020, 390, 111927. [CrossRef]
- Van Deursen, J.M., The role of senescent cells in ageing. Nature 2014 509:7501, 2014. 509(7501): p. 439-446.
- Xue, W.; Zender, L.; Miething, C.; Dickins, R.A.; Hernando, E.; Krizhanovsky, V.; Cordon-Cardo, C.; Lowe, S.W. Senescence and tumour clearance is triggered by p53 restoration in murine liver carcinomas. Nature 2007, 445, 656–660. [CrossRef]
- Takasugi, M.; Yoshida, Y.; Hara, E.; Ohtani, N. The role of cellular senescence and SASP in tumour microenvironment. FEBS J. 2022, 290, 1348–1361. [CrossRef]
- Wang, Y., et al., Implication of cellular senescence in the progression of chronic kidney disease and the treatment potencies. Biomedicine & Pharmacotherapy, 2021. 135: p. 111191-111191.
- Jin, H.; Zhang, Y.; Ding, Q.; Wang, S.S.; Rastogi, P.; Dai, D.-F.; Lu, D.; Purvis, M.; Cao, C.; Wang, A.; et al. Epithelial innate immunity mediates tubular cell senescence after kidney injury. J. Clin. Investig. 2019, 4. [CrossRef]
- Andrade, L.; Rodrigues, C.E.; Gomes, S.A.; Noronha, I.L. Acute Kidney Injury as a Condition of Renal Senescence. Cell Transplant. 2018, 27, 739–753. [CrossRef]
- Basile, D.P., et al., Progression after AKI: Understanding Maladaptive Repair Processes to Predict and Identify Therapeutic Treatments. J Am Soc Nephrol, 2016. 27(3): p. 687-97.
- Dai, L.; Qureshi, A.R.; Witasp, A.; Lindholm, B.; Stenvinkel, P. Early Vascular Ageing and Cellular Senescence in Chronic Kidney Disease. Comput. Struct. Biotechnol. J. 2019, 17, 721–729. [CrossRef]
- Benz, K.; Hilgers, K.-F.; Daniel, C.; Amann, K. Vascular Calcification in Chronic Kidney Disease: The Role of Inflammation. Int. J. Nephrol. 2018, 2018, 1–7. [CrossRef]
- Figuer, A.; Bodega, G.; Tato, P.; Valera, G.; Serroukh, N.; Ceprian, N.; de Sequera, P.; Morales, E.; Carracedo, J.; Ramírez, R.; et al. Premature Aging in Chronic Kidney Disease: The Outcome of Persistent Inflammation beyond the Bounds. Int. J. Environ. Res. Public Heal. 2021, 18, 8044. [CrossRef]
- Wang, W.J., G.Y. Cai, and X.M. Chen, Cellular senescence, senescence-associated secretory phenotype, and chronic kidney disease. Oncotarget, 2017. 8(38): p. 64520-64520.
- Rai, T.S.; Cole, J.J.; Nelson, D.M.; Dikovskaya, D.; Faller, W.J.; Vizioli, M.G.; Hewitt, R.N.; Anannya, O.; McBryan, T.; Manoharan, I.; et al. HIRA orchestrates a dynamic chromatin landscape in senescence and is required for suppression of neoplasia. Genes Dev. 2014, 28, 2712–2725. [CrossRef]
- Shi Shanghai Jiao Tong, Y., et al., CKAP4 regulates the progression of vascular calcication in chronic kidney disease by modulating YAP phosphorylation and MMP2 expression. 2020.
- Tan, H., et al., CKAP4 participates in tryptase-induced phenotypic conversion in atrial fibroblasts through PAR2/p38/JNK pathway. American Journal of Translational Research, 2021. 13(4): p. 2270-2270.
- Summers, M.E.; Richmond, B.W.; Kropski, J.A.; Majka, S.A.; Bastarache, J.A.; Hatzopoulos, A.K.; Bylund, J.; Ghosh, M.; Petrache, I.; Foronjy, R.F.; et al. Balanced Wnt/Dickkopf1 signaling by mesenchymal vascular progenitor cells in the microvascular niche maintains distal lung structure and function. Am. J. Physiol. Physiol. 2020, 320, C119–C131. [CrossRef]
- Gladka, M.M.; Molenaar, B.; de Ruiter, H.; van der Elst, S.; Tsui, H.; Versteeg, D.; Lacraz, G.P.; Huibers, M.M.; van Oudenaarden, A.; van Rooij, E. Single-Cell Sequencing of the Healthy and Diseased Heart Reveals Cytoskeleton-Associated Protein 4 as a New Modulator of Fibroblasts Activation. Circulation 2018, 138, 166–180. [CrossRef]
- Osugi, Y.; Fumoto, K.; Kikuchi, A. CKAP4 Regulates Cell Migration via the Interaction with and Recycling of Integrin. Mol. Cell. Biol. 2019, 39. [CrossRef]
- Singbartl, K.; Miller, L.; Ruiz-Velasco, V.; Kellum, J.A. Reversal of Acute Kidney Injury–Induced Neutrophil Dysfunction: A Critical Role for Resistin. Crit. Care Med. 2016, 44, e492–e501. [CrossRef]
- Bottazzi, B.; Inforzato, A.; Messa, M.; Barbagallo, M.; Magrini, E.; Garlanda, C.; Mantovani, A. The pentraxins PTX3 and SAP in innate immunity, regulation of inflammation and tissue remodelling. J. Hepatol. 2016, 64, 1416–1427. [CrossRef]
- Cappuzzello, C.; Doni, A.; Dander, E.; Pasqualini, F.; Nebuloni, M.; Bottazzi, B.; Mantovani, A.; Biondi, A.; Garlanda, C.; D’amico, G. Mesenchymal Stromal Cell-Derived PTX3 Promotes Wound Healing via Fibrin Remodeling. J. Investig. Dermatol. 2016, 136, 293–300. [CrossRef]
- Jaillon, S.; Moalli, F.; Ragnarsdottir, B.; Bonavita, E.; Puthia, M.; Riva, F.; Barbati, E.; Nebuloni, M.; Krajinovic, L.C.; Markotic, A.; et al. The Humoral Pattern Recognition Molecule PTX3 Is a Key Component of Innate Immunity against Urinary Tract Infection. Immunity 2014, 40, 621–632. [CrossRef]
- Wu, X.-Y.; Li, K.-T.; Yang, H.-X.; Yang, B.; Lu, X.; Zhao, L.-D.; Fei, Y.-Y.; Chen, H.; Wang, L.; Li, J.; et al. Complement C1q synergizes with PTX3 in promoting NLRP3 inflammasome over-activation and pyroptosis in rheumatoid arthritis. J. Autoimmun. 2019, 106, 102336. [CrossRef]
- Rodriguez-Grande, B.; Swana, M.; Nguyen, L.; Englezou, P.; Maysami, S.; Allan, S.M.; Rothwell, N.J.; Garlanda, C.; Denes, A.; Pinteaux, E. The Acute-Phase Protein PTX3 is an Essential Mediator of Glial Scar Formation and Resolution of Brain Edema after Ischemic Injury. J. Cereb. Blood Flow Metab. 2013, 34, 480–488. [CrossRef]
- Bonavita, E.; Gentile, S.; Rubino, M.; Maina, V.; Papait, R.; Kunderfranco, P.; Greco, C.; Feruglio, F.; Molgora, M.; Laface, I.; et al. PTX3 Is an Extrinsic Oncosuppressor Regulating Complement-Dependent Inflammation in Cancer. Cell 2015, 160, 700–714. [CrossRef]
- Ristagno, G.; Fumagalli, F.; Bottazzi, B.; Mantovani, A.; Olivari, D.; Novelli, D.; Latini, R. Pentraxin 3 in Cardiovascular Disease. Front. Immunol. 2019, 10, 823. [CrossRef]
- Chen, J.; Matzuk, M.M.; Zhou, X.J.; Lu, C.Y. Endothelial pentraxin 3 contributes to murine ischemic acute kidney injury. Kidney Int. 2012, 82, 1195–1207. [CrossRef]
- de Oliveira, T.H.C., et al., Tissue Dependent Role of PTX3 During Ischemia-Reperfusion Injury. Front Immunol, 2019. 10: p. 1461.
- Lech, M.; Römmele, C.; Gröbmayr, R.; Susanti, H.E.; Kulkarni, O.P.; Wang, S.; Gröne, H.-J.; Uhl, B.; Reichel, C.; Krombach, F.; et al. Endogenous and exogenous pentraxin-3 limits postischemic acute and chronic kidney injury. Kidney Int. 2013, 83, 647–661. [CrossRef]
- Lee, H.H.; Kim, S.Y.; Na, J.C.; Yoon, Y.E.; Han, W.K. Exogenous pentraxin-3 inhibits the reactive oxygen species-mitochondrial and apoptosis pathway in acute kidney injury. PLOS ONE 2018, 13, e0195758. [CrossRef]
- Chu-bing, R., Serum and Urinary PTX-3 for Prediction of the Current Occurrence of Acute Kidney Injury in Critical Patients. JOURNAL OF CLINICAL TRANSFUSION AND LABORATORY MEDICINE, 2020. 22(1): p. 104-108.
- Sjöberg, B.; Qureshi, A.R.; Heimbürger, O.; Stenvinkel, P.; Lind, L.; Larsson, A.; Bárány, P.; Ärnlöv, J. Association between levels of pentraxin 3 and incidence of chronic kidney disease in the elderly. J. Intern. Med. 2015, 279, 173–179. [CrossRef]
- Yilmaz, M.I., et al., Soluble TWEAK and PTX3 in Nondialysis CKD Patients: Impact on Endothelial Dysfunction and Cardiovascular Outcomes. Clinical Journal of the American Society of Nephrology, 2011. 6(4): p. 785-792.
- El-Badawy, A.; Omar, R.; El-Sayed, M.; Maksoud, A.A.; El-Sayed, A. Study of the Relation between Plasma PTX3 Levels and Preclinical Atherosclerotic Cardiovascular Complications in Type 2 Diabetic Patients. Benha J. Appl. Sci. 2019, 4, 9–15. [CrossRef]
- Inoue, K.; Kodama, T.; Daida, H. Pentraxin 3: A Novel Biomarker for Inflammatory Cardiovascular Disease. Int. J. Vasc. Med. 2012, 2012, 1–6. [CrossRef]
- Hannan, M., et al., Risk Factors for CKD Progression: Overview of Findings from the CRIC Study. Clin J Am Soc Nephrol, 2021. 16(4): p. 648-659.
- Jiang, M.; Bai, M.; Lei, J.; Xie, Y.; Xu, S.; Jia, Z.; Zhang, A. Mitochondrial dysfunction and the AKI-to-CKD transition. Am. J. Physiol. Physiol. 2020, 319, F1105–F1116. [CrossRef]
- Gameiro, J.; Fonseca, J.A.; Outerelo, C.; Lopes, J.A. Acute Kidney Injury: From Diagnosis to Prevention and Treatment Strategies. J. Clin. Med. 2020, 9, 1704. [CrossRef]
- Rimes-Stigare, C.; Ravn, B.; Awad, A.; Torlén, K.; Martling, C.-R.; Bottai, M.; Mårtensson, J.; Bell, M. Creatinine- and Cystatin C-Based Incidence of Chronic Kidney Disease and Acute Kidney Disease in AKI Survivors. Crit. Care Res. Pr. 2018, 2018, 1–8. [CrossRef]
| Label | Levels | AKI | CKD | UHC | p-value |
| Gender | Female | 23 | 58 | 53 | |
| (%) | (53.5) | (37.4) | (53.0) | 0.024 | |
| Male | 20 | 97 | 47 | ||
| (%) | (46.5) | (62.6) | (47.0) | ||
| BMI | Mean | 30.1 | 29.3 | 30.7 | |
| (SD) | (10.1) | (5.5) | (7.7) | 0.306 | |
| Age | Mean | 60.5 | 58.7 | 59.5 | |
| (SD) | (14.8) | (17.3) | (12.9) | 0.788 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





