Submitted:
05 February 2024
Posted:
06 February 2024
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Materials and Methods
2.1. Trichoderma in soil and endophytes
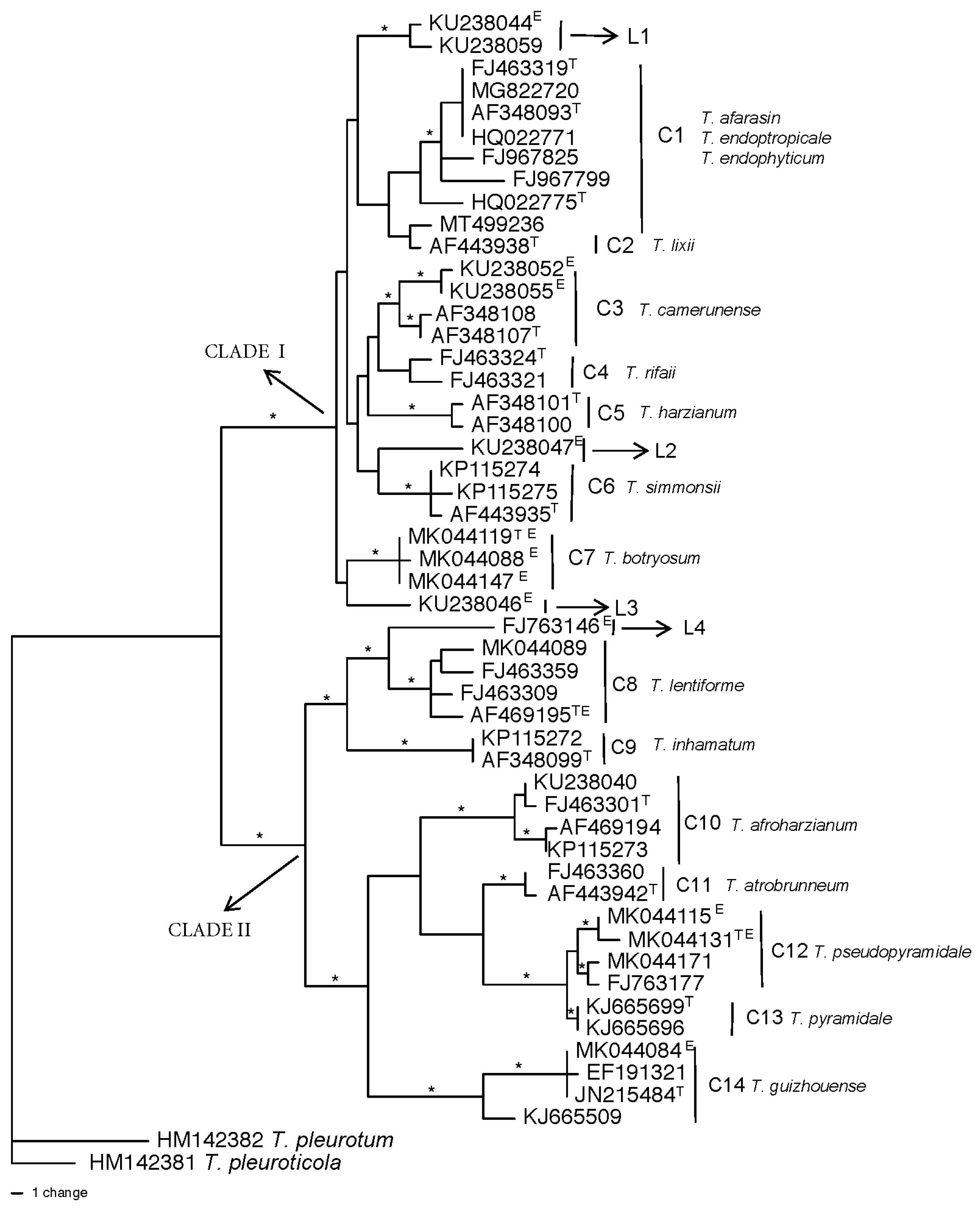
2.2. Harzianum Complex Clade species
2.3. Phylogenetic analysis
3. Results
3.1. Trichoderma soil and endophyte survey compilation
| Region or Country | Total isolates identifiedb | asperellum/ asperelloidesc | harzianumd | virens | atroviride | hamatum | Total dominant (% of all) isolatese | Referencef |
|---|---|---|---|---|---|---|---|---|
| Central and South America | ||||||||
| South America | 183 | 60/0 | 49 | 8 | 3 | 2 | 122 (67%) | [5] |
| Colombia | 21 | 10/0 | 3 | 0 | 4 | 0 | 17 (81%) | [11] |
| Brazil | 54 | 2/13 | 23 | 0 | 0 | 2 | 40 (74%) | [12] |
| Central and South America | 54 | 4/0 | 20 | 2 | 4 | 0 | 30 (56%) | [13] |
| Europe | ||||||||
| Poland | 170 | 0 | 43 | 6 | 20 | 9 | 78 (46%) | [14] |
| Island of Sardinia | 482 | 3/0 | 277 | 19 | 0 | 22 | 321 (67%) | [15] |
| Southern Italy | 16 | 0 | 6 | 0 | 4 | 0 | 10 (63%) | [16] |
| Africa | ||||||||
| Ethiopia | 164 | 64/32 | 8 | 0 | 0 | 6 | 110 (67%) | [17] |
| Tunisia | 53 | 0 | 15 | 0 | 7 | 14 | 36 (68%) | [18] |
| Algeria | 9 | 0 | 4 | 0 | 0 | 0 | 4 (44%) | [19] |
| Egypt | 20 | 0 | 14 | 0 | 0 | 0 | 14 (70%) | [20] |
| Asia | ||||||||
| Russia, Siberia, Himalaya | 75 | 2/0 | 31 | 5 | 14 | 15 | 67 (89%) | [21] |
| Iran | 159 | 0 | 87 | 19 | 0 | 0 | 106 (67%) | [22] |
| Nepal | 41 | 18/19 | 4 | 0 | 0 | 0 | 41 (100%) | [23] |
| Malaysia | 326 | 86/0 | 156 | 9 | 0 | 20 | 271 (83%) | [24] |
| South Korea | 26 | 2/4 | 3 | 6 | 1 | 1 | 17 (65%) | [25] |
| China (East agri. Fields) | 2078 | 425/0 | 429 | 340 | 73 | 397 | 1664 (80%) | [26] |
| China (Northwest) | 312 | 20/0 | 108 | 0 | 3 | 1 | 132 (42%) | [27] |
| China (four regions) | 64 | 4/0 | 26 | 2 | 13 | 0 | 45 (70%) | [28] |
| China (southeast sediments) | 254 | 32/0 | 63 | 1 | 134 | 1 | 231 (91%) | [29] |
| China | 13 | 12/0 | 1 | 0 | 0 | 0 | 13 (100) | [30] |
| China (Southwest) | 57 | 0 | 49 | 0 | 0 | 0 | 49 (86%) | [31] |
| Southeast Asia | 78 | 4/0 | 37 | 16 | 3 | 1 | 61 (78%) | [32] |
| Totalg | 4,709 | 816 | 1456 | 417 | 283 | 491 | 3479 (74%) | |
| Detection frequency among studiesh | N/Ai | 70%/17% | 100% | 52% | 57% | 57% | N/A |
| Country | Host | Total isolatesb | asp/aspoc | Harzianum Complexd | virens | atroviride | hamatum | Total and (%) | Referencee |
|---|---|---|---|---|---|---|---|---|---|
| North America | |||||||||
| Canada | Grapevines | 29 | 0/4 | 8 | 0 | 7 | 0 | 19 (66%) | [33] |
| South America | |||||||||
| Brazil | Rubber trees | 30 | 0 | 0 | 0 | 0 | 0 | 0 | [34] |
| Brazil | Cerrado-Caatinga ecotone | 19 | 0 | 0 | 0 | 0 | 0 | 0 | [35] |
| Peru* | Wild rubber tree | 39 | 0 | 31 | 0 | 0 | 0 | 31 (79%) | [36] |
| Europe | |||||||||
| United Kingdom | various garden trees | 40 | 0 | 15 | 1 | 0 | 4 | 20 (50%) | [37] |
| Hungary | Grapevines | 10 | 0 | 8 | 0 | 0 | 0 | 8 (80%) | [38] |
| Africa | |||||||||
| Ethiopia, Cameroon, Kenya | Coffee (cultivated and wild) | 76 | 0 | 46 | 1 | 1 | 3 | 51 (67%) | [39] |
| Ethiopia | Coffee | 48 | 0 | 14 | 0 | 0 | 20 | 14 (48%) | [9] |
| Asia | |||||||||
| Malaysia | 35 plant families | 93 | 13/22 | 27 | 22 | 0 | 0 | 84 (90%) | [10] |
| Indonesia | Theobroma cacao | 21 | 19 | 0 | 2 | 0 | 0 | 21 (100%) | [40] |
| Thailand | Rubber trees | 12 | 3/0 | 3 | 2 | 0 | 3 | 11 (92%) | [41] |
| Iran | Vinca sp. | 7 | 1/0 | 0 | 0 | 0 | 0 | 1 (14%) | [42] |
| Iran* | Cuppressaceae family plants | 5 | 0 | 0 | 0 | 4 | 0 | 4 (80%) | [43] |
| Totalg | N/Ai | 429 | 62 | 152 | 28 | 12 | 27 | 281 (66%) | |
| Detection frequency among studiesh | N/A | N/A | 38% | 61% | 38% | 23% | 30% |
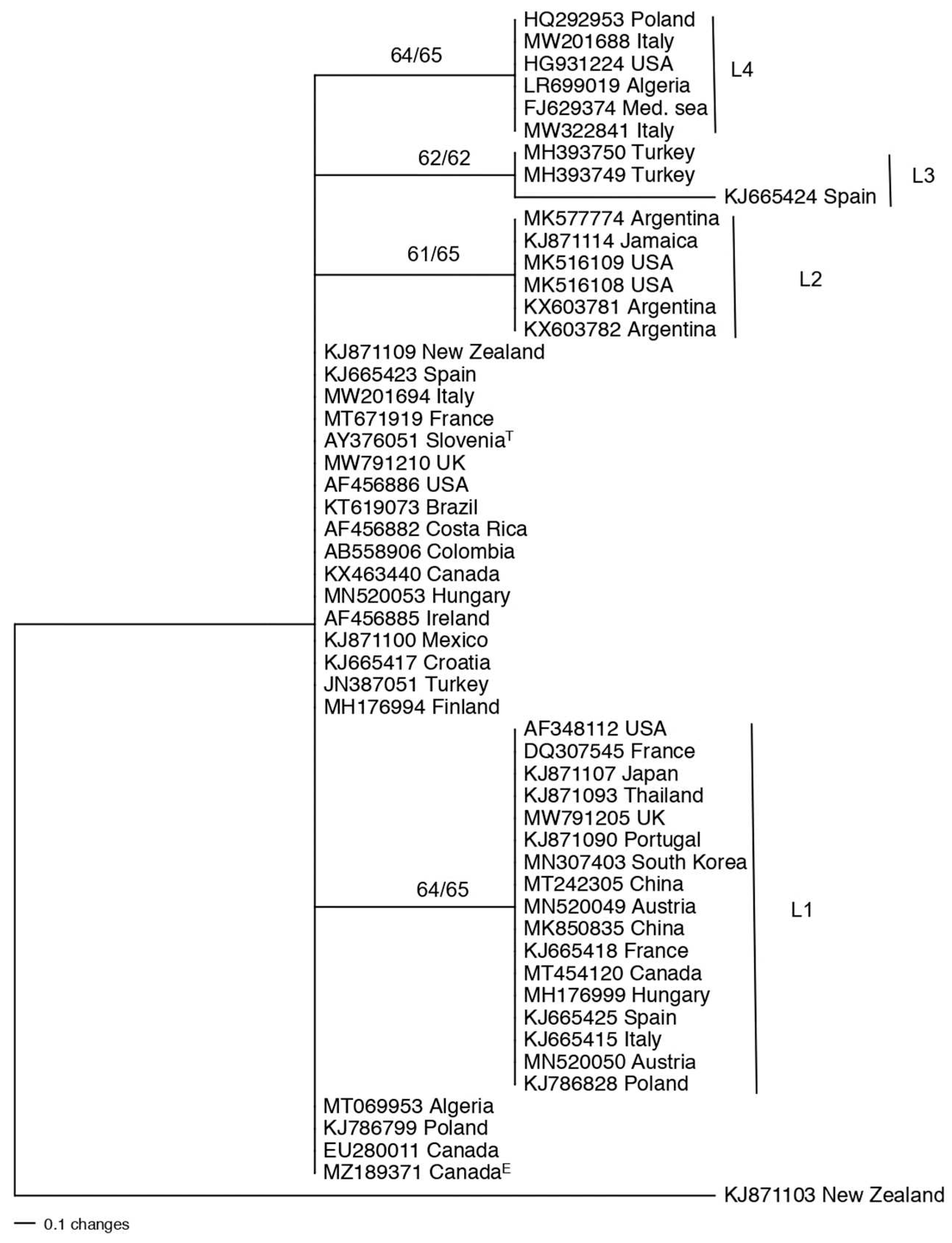
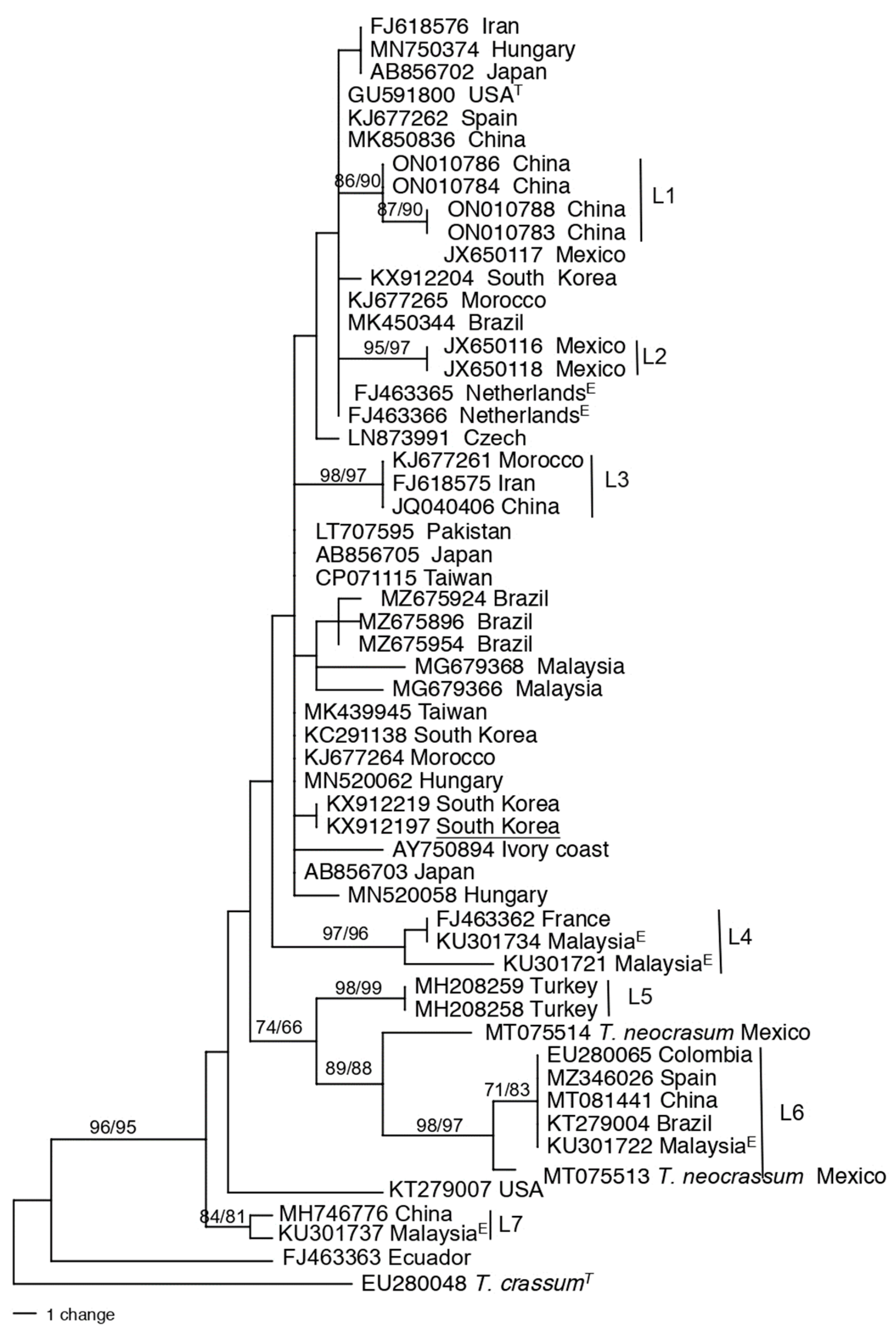
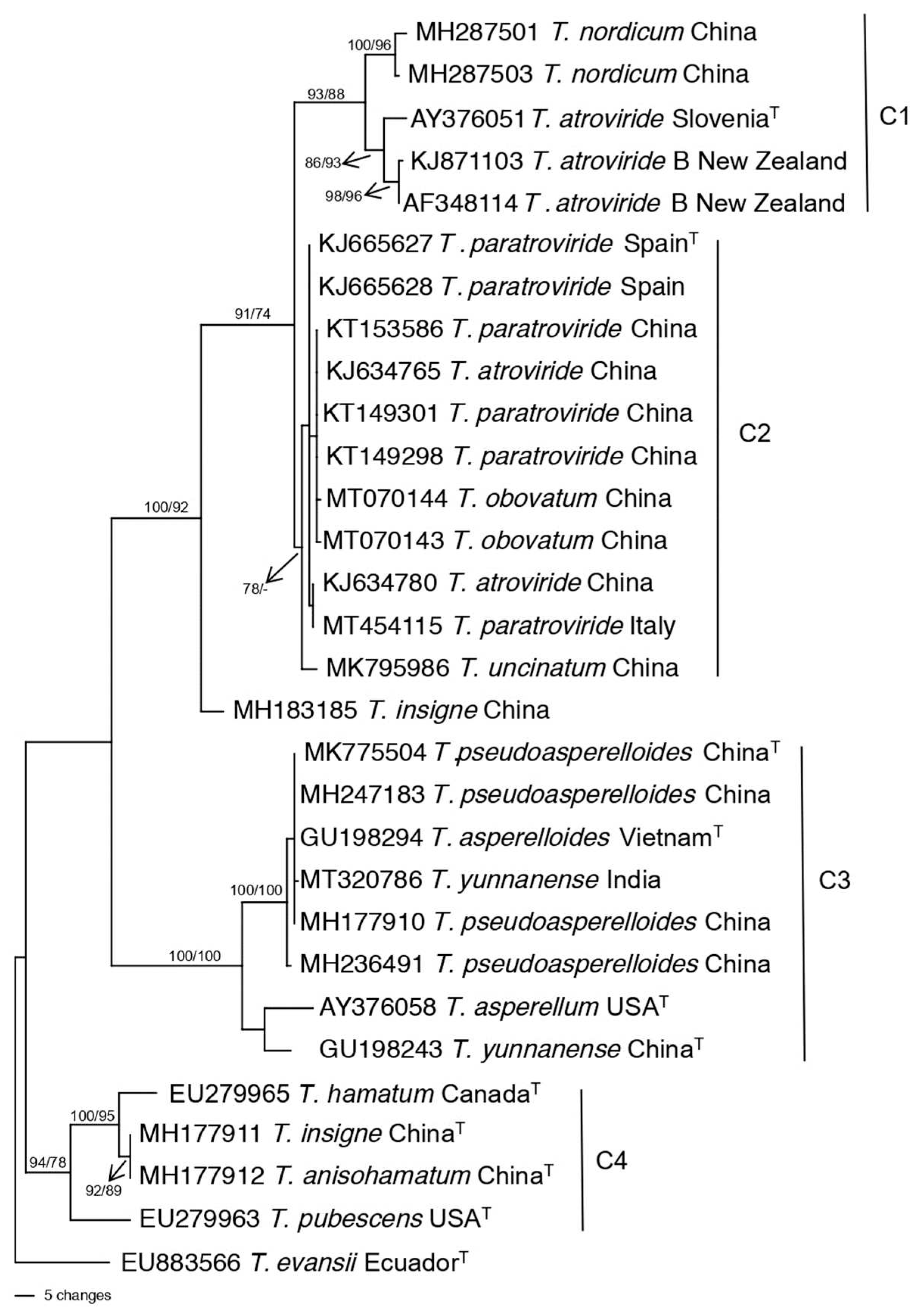
3.2. Population structure and genetic diversity of T. atroviride
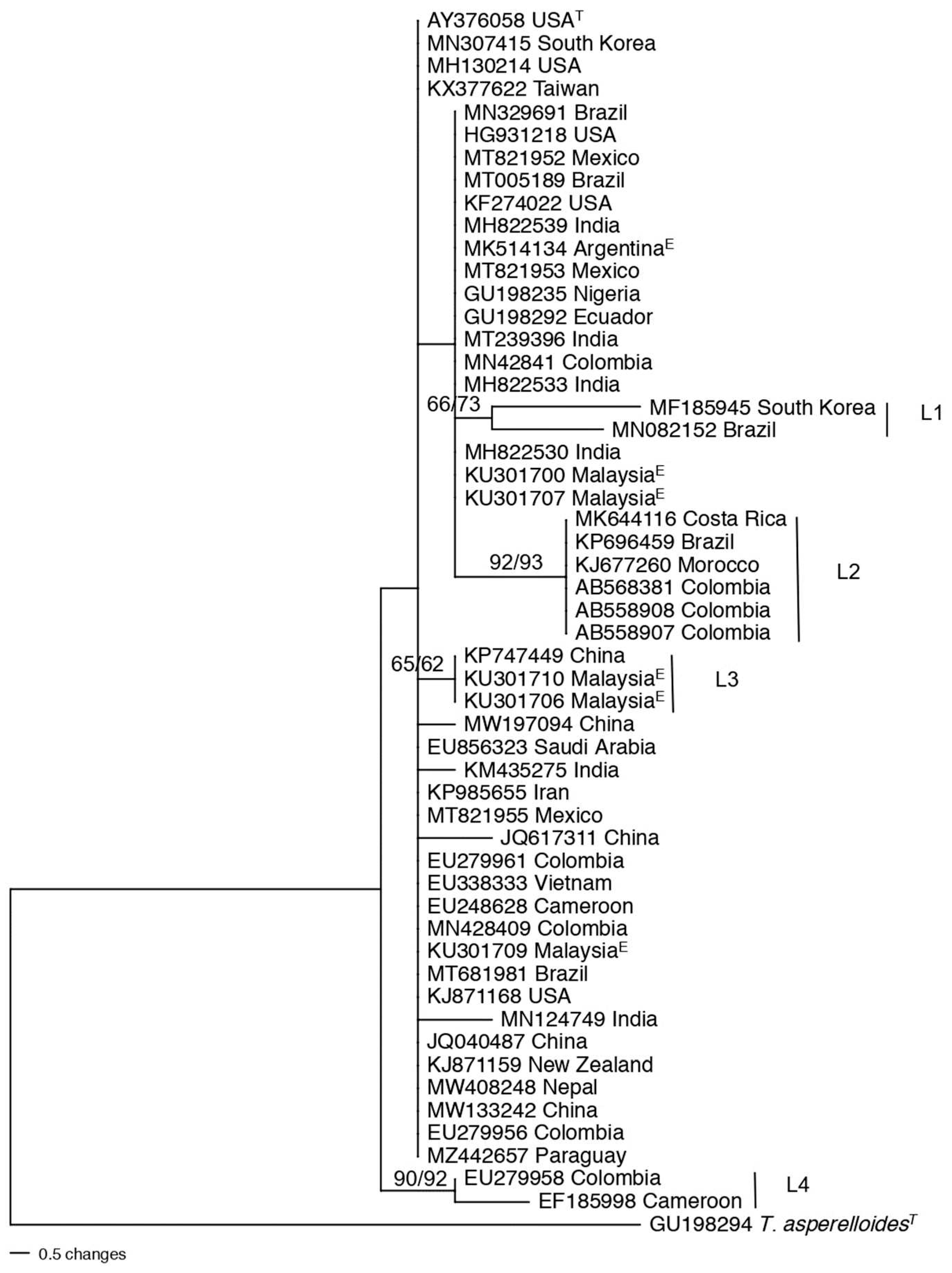
3.3. Population structure and genetic diversity of the T. asperellum/asperelloides species group
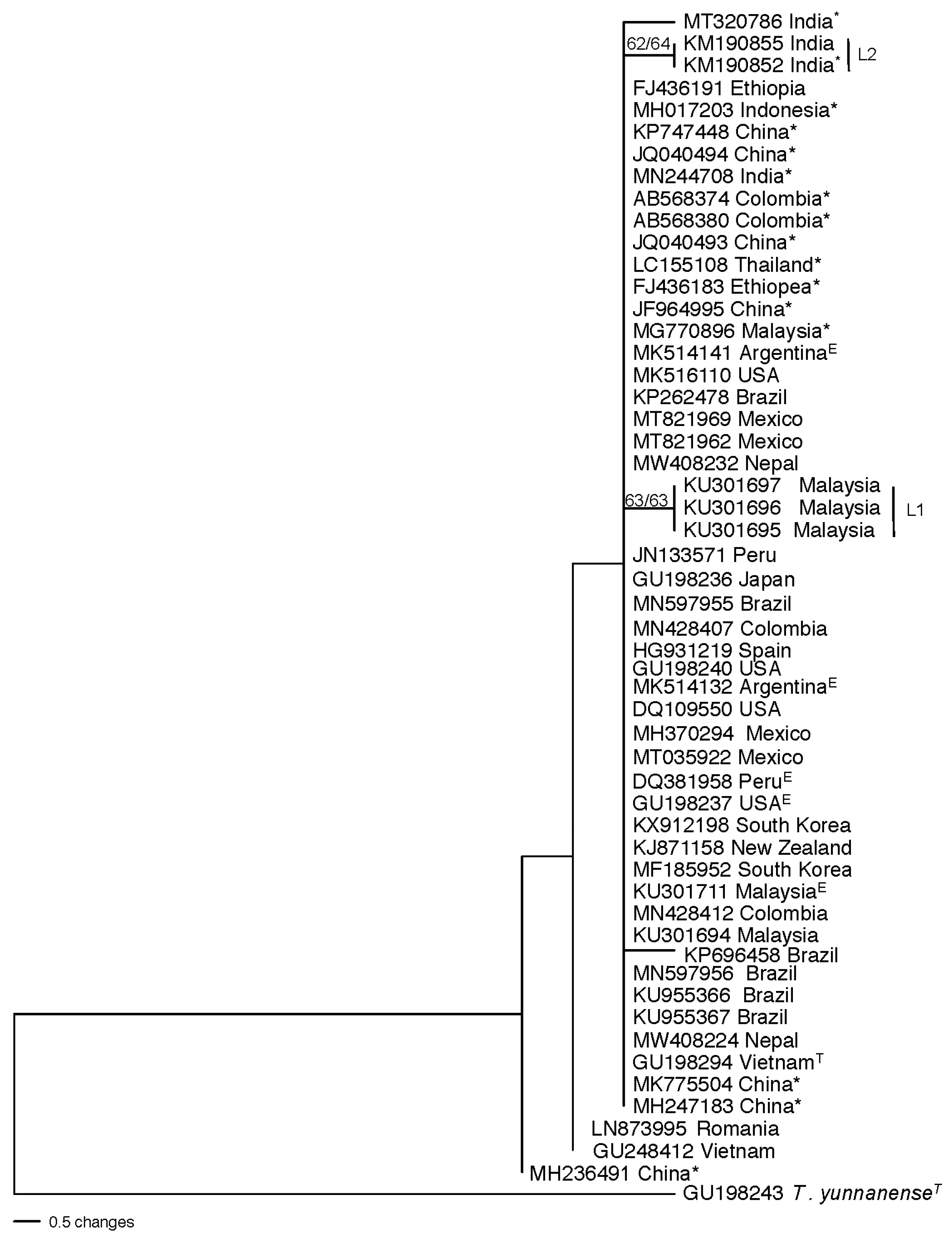
3.4. Population structure and genetic diversity of T. hamatum
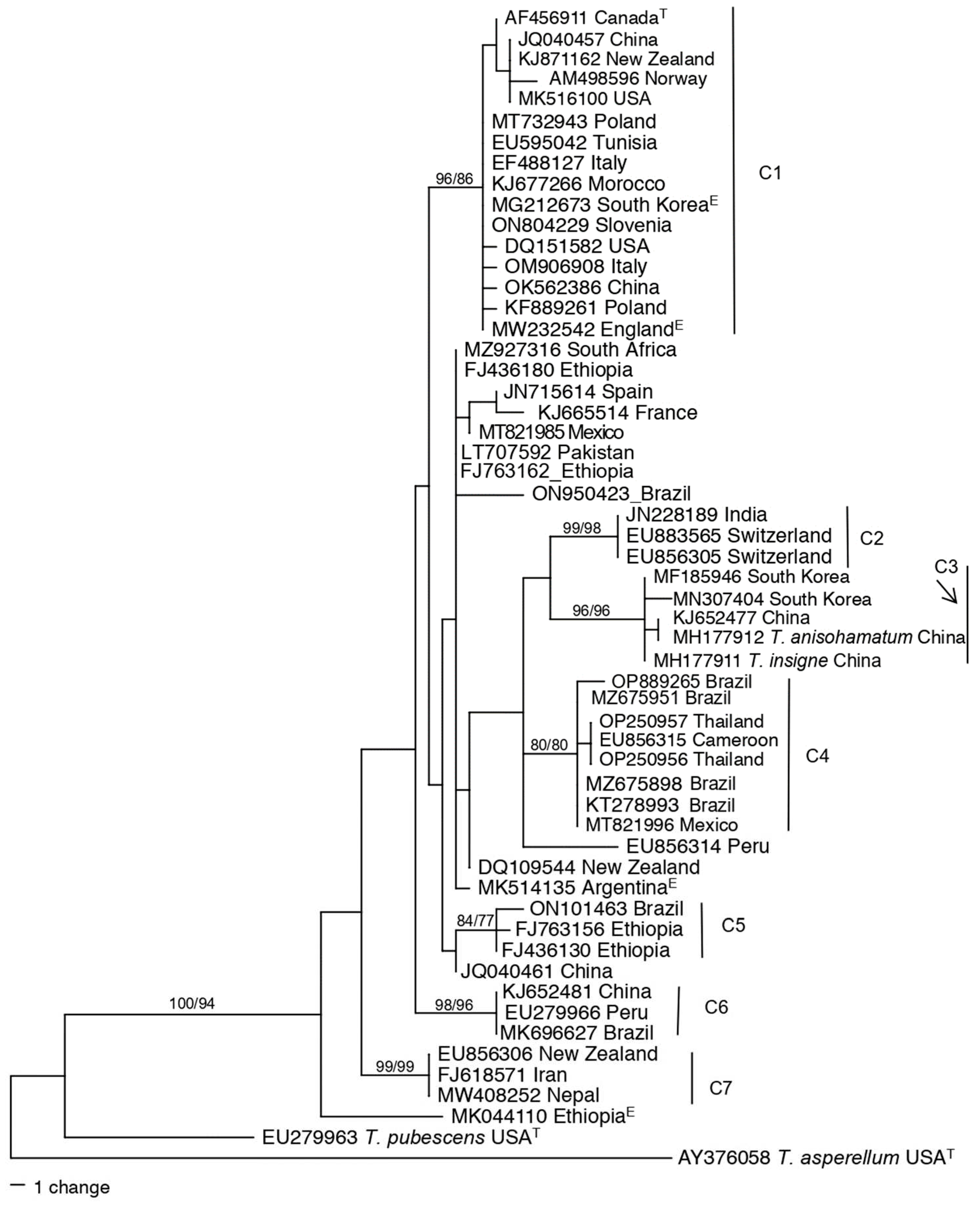
3.5. Genetic diversity of Harzianum Complex Clade species
| Speciesb | GenBank Hitsc | Habitat | Geographic Region | Referenced |
|---|---|---|---|---|
| T. lentiforme | 481 | endophytes; few from soil | South America | [7] |
| T. inhamatum | 117 | Soil | South America | Mycobank# 104673 |
| T. guizhouense | 204 | commonly in soil; endophytes in Africa | World-wide | [44] |
| T. afroharzianum | 644 | commonly in soil; few endophytic | World-wide | [7] |
| T. pyramidale | 26 | soil and decaying wood | Europe | [7] |
| T. atrobrunneum | 234 | commonly in soil | Europe | [7] |
| T. simmonsii | 176 | commonly in soil or decaying wood | North America, Europe | [7] |
| T. harzianum | N/Ae | soil, endophytic | North America, Europe | [7,45] |
| T. camerunense | 38 | commonly in soil | Africa | [7] |
| T. botryosum | 44 | endophytic in coffee | Africa | [39] |
| T. pseudopyramidale | 82 | endophytic in coffee and mycoparasite | Africa | [39] |
| T. afarasin | 27 | mostly endophytic | Africa | [7] |
| T. neotropicale | 37 | endophytes of tropical trees | South America | [7] |
| T. endophyticum | 72 | endophytes of tropical trees | South America | [7] |
| T. rifaii | 40 | endophytes of tropical trees | South America | [7] |
| T. lixii | 113 | soil or decaying wood | Southeast Asia | [7] |
3.6. Population structure and genetic diversity of T. virens
3.7. Nearest relative analysis
4. Discussion
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Harman, G.E.; Obregón, M.A.; Samuel, G.J.; Lorito, M. Changing models for commercialization and implementation of biocontrol in the developing and the developed world. Plant Dis. 2010, 8, 928–939. [Google Scholar] [CrossRef]
- Woo, S.; Hermosa, R.; Lorito, M.; Monte, E. Trichoderma: a multipurpose, plant-beneficial microorganism for eco-sustainable agriculture. Nature Rev. Microbiol. 2023, 21, 312–326. [Google Scholar] [CrossRef]
- Cai, F.; Druzhinina, I.S. In honor of John Bissett: authoritative guidelines on molecular identification of Trichoderma. Fungal Divers. 2021, 107, 1–69. [Google Scholar] [CrossRef]
- Rush, T.A.; Shrestha, H.K.; Gopalakrishnan Meena, M.; Spangler, M.K.; Ellis, J.C.; Labbé, J.L.; Abraham, P.E. Bioprospecting Trichoderma: A systematic roadmap to screen genomes and natural products for biocontrol applications. Front. Fungal Biol. 2021, 2, 716511. [Google Scholar] [CrossRef] [PubMed]
- Hoyos-Carvajal, L.; Orduz, S.; Bissett, J. Genetic and metabolic biodiversity of Trichoderma from Colombia and adjacent neotropic regions. Fungal Genet. Biol. 2009, 46, 615–631. [Google Scholar] [CrossRef] [PubMed]
- Samuels, G.J.; Ismaiel, A.; Bon, M.C.; De Respinis, S.; Petrini, O. Trichoderma asperellum sensu lato consists of two cryptic species. Mycologia. 2010, 102, 944–966. [Google Scholar] [CrossRef]
- Chaverri, P.; Branco-Rocha, F.; Jaklitsch, W.; Gazis, R.; Degenkolb, T.; Samuels, G. J. Systematics of the Trichoderma harzianum species complex and the re-identification of commercial biocontrol strains. Mycologia. 2015, 107, 558–590. [Google Scholar] [CrossRef] [PubMed]
- Tamura, K.; Stecher, G.; Kumar, S. MEGA11: Molecular evolutionary genetics analysis version 11. Mol. Biol. Evol. 2021, 38, 3022–3027. [Google Scholar] [CrossRef] [PubMed]
- Mulaw, T.B.; Druzhinina, I.S.; Kubicek, C.P.; Atanasova, L. Novel endophytic Trichoderma spp. isolated from healthy Coffea arabica roots are capable of controlling coffee tracheomycosis. Diversity. 2013, 5, 750–766. [Google Scholar] [CrossRef]
- Cummings, N.J.; Ambrose, A.; Braithwaite, M.; Bissett, J.; Roslan, H.A.; Abdullah, J.; Stewart, A.; Agbayani, F.V.; Steyaert, J.; Hill, R.A. Diversity of root-endophytic Trichoderma from Malaysian Borneo. Mycol. Progress. 2016, 15, 50. [Google Scholar] [CrossRef]
- Smith, A.; Beltrán, C.A.; Kusunoki, M.; Cotes, A.M.; Motohashi, K.; Kondo, T.; Deguchi, M. Diversity of soil-dwelling Trichoderma in Colombia and their potential as biocontrol agents against the phytopathogenic fungus Sclerotinia sclerotiorum (Lib.) de Bary. J. Gen. Plant Pathol. 2013, 79, 74–85. [Google Scholar] [CrossRef]
- Inglis, P.W.; Mello, S.C.M.; Martins, I.; Silva, J.B.T.; Macêdo, K.; Sifuentes, D.N.; Inglis, M.C.V. Trichoderma from Brazilian garlic and onion crop soils and description of two new species: Trichoderma azevedoi and Trichoderma peberdyi. PloS ONE. 2020, 15, e0228485. [Google Scholar] [CrossRef] [PubMed]
- Druzhinina, I.; Kopchinski, A.; Komoñ-Zelazowska, M.; Kubicek, C.P. An oligonucleotide barcode for species identification in Trichoderma and Hypocrea. Fungal Genet. Biol. 2005, 42, 813–828. [Google Scholar] [CrossRef] [PubMed]
- Błaszczyk, L.; Popiel, D.; Chełkowski, J.; Koczyk, G.; Samuels, G.J.; Sobieralski, K.; Siwulski, M. Species diversity of Trichoderma in Poland. J. Appl. Genet. 2011, 52, 233–243. [Google Scholar] [CrossRef] [PubMed]
- Migheli, Q.; Balmas, V.; Komoñ-Zelazowska, M.; Scherm, B.; Fiori, S.; Kopchinskiy, A.G.; Kubicek, C.P.; Druzhinina, I.S. Soils of a Mediterranean hot spot of biodiversity and endemism (Sardinia, Tyrrhenian Islands) are inhabited by pan-European, invasive species of Hypocrea/Trichoderma. Environ. Microbiol. 2009, 11, 35–46. [Google Scholar] [CrossRef] [PubMed]
- Úrbez-Torres, J.R.; Tomaselli, E.; Pollard-Flamand, J.; Boule, J.; Gerin, D.; Pollastro, S. Characterization of Trichoderma isolates from southern Italy, and their potential biocontrol activity against grapevine trunk disease fungi. Phytopathol.Mediterr. 2020, 59, 425–439. [Google Scholar] [CrossRef]
- Mulatu, A.; Megersa, N.; Abena, T.; Kanagarajan, S.; Liu, Q.; Tenkegna, T.A.; Vetukuri, R.R. Biodiversity of the genus Trichoderma in the rhizosphere of coffee (Coffea arabica) plants in Ethiopia and their potential use in biocontrol of coffee wilt Disease. Crops. 2022, 2, 120–141. [Google Scholar] [CrossRef]
- Sadfi-Zouaoui, N.; Hannachi, I.; Rouaissi, M.; Hajlaoui, M.R.; Rubio, M.B.; Monte, E.; Boudabous, A.; Hermosa, M.R. Biodiversity of Trichoderma strains in Tunisia. Can. J. Microbiol. 2009, 55, 154–162. [Google Scholar] [CrossRef]
- Haouhach, S.; Karkachi, N.; Oguiba, B.; Sidaoui, A.; Chamorro, I.; Kihal, M.; Monte, E. Three new reports of Trichoderma in Algeria: T. atrobrunneum, (South) T. longibrachiatum (South), and T. afroharzianum (Northwest). Microorganisms 2020, 8, 1455. [Google Scholar] [CrossRef]
- Gherbawy, Y.; Druzhinina, I.; Shaban, G.M.; Wuczkowsky, M.; Yasar, M.; El-Naghy, M.A.; Prillinger, H.J.; Kubicek, C.P. Trichoderma populations from alkaline agricultural soil in the Nile valley, Egypt, consist of only two species. Mycol. Prog. 2004, 3, 211–218. [Google Scholar] [CrossRef]
- Kullnig, C.; Szakacs, G.; Kubicek, C.P. Molecular identification of Trichoderma species from Russia, Siberia and the Himalaya. Mycol. Res. 2000, 104, 1117–1125. [Google Scholar] [CrossRef]
- Mirkhani, F.; Alaei, H. Species diversity of indigenous Trichoderma from alkaline pistachio soils in Iran. Mycol. Iran. 2015, 2, 22–37. [Google Scholar] [CrossRef]
- Khadka, R.B.; Miller, S.A. Synergy of anaerobic soil disinfestation and Trichoderma spp. in Rhizoctonia root rot suppression. Front. Sustain. Food Syst. 2021, 5, 76. [Google Scholar] [CrossRef]
- Rahman, S.S.M.S.A.; Zainudin, N.A.I.M.; Aziz, N.A.A. Evaluation of Trichoderma asperellum B1902 in controlling Fusarium wilt of Cavendish banana cultivar. Sains Malays. 2021, 50, 2549–2561. [Google Scholar] [CrossRef]
- Jang, S.; Jang, Y.; Kim, C.W.; Lee, H.; Hong, J.H.; Heo, Y.M.; Lee, Y.M.; Lee, D.W.; Lee, H.B.; Kim, J.J. Five new records of soil-derived Trichoderma in Korea: T. albolutescens, T. asperelloides, T. orientale, T. spirale, and T. tomentosum. Mycobiology. 2017, 45, 1–8. [Google Scholar] [CrossRef]
- Jiang, Y.; Wang, J.L.; Chen, J.; Mao, L.J.; Feng, X.X.; Zhang, C.L.; Lin, F.C. Trichoderma biodiversity of agricultural fields in east China reveals a gradient distribution of species. PloSONE. 2016, 11, e0160613. [Google Scholar] [CrossRef]
- Ma, J.; Tsegaye, E.; Li, M.; Wu, B.; Jiang, X. Biodiversity of Trichoderma from grassland and forest ecosystems in Northern Xinjiang, China. 3 Biotech 2020, 10, 362. [Google Scholar] [CrossRef]
- Zhang, C.L.; Druzhinina, I.S.; Kubicek, C.P.; Xu, T. Trichoderma biodiversity in China: evidence for a North to South distribution of species in East Asia. FEMS Microbiol. Lett. 2005, 251, 251–257. [Google Scholar] [CrossRef] [PubMed]
- Saravanakumar, K.; Yu, C.; Dou, K.; Wang, M.; Li, Y.; Chen, J. Biodiversity of Trichoderma community in the tidal flats and wetland of southeastern China. PloS ONE. 2016, 11(12), e0168020. [Google Scholar] [CrossRef]
- Xue, M.; Wang, R.; Zhang, C.; Wang, W.; Zhang, F.; Chen, D.; Ren, S.; Manman, Z.; Hou, J.; Liu, T. Screening and identification of Trichoderma strains isolated from natural habitats in China with potential agricultural applications. Biomed. Res. Int. 2021, 7913950. [Google Scholar] [CrossRef]
- Tang, G.T.; Li, Y.; Zhou, Y; Zhou, Y.; Zhu, Y.H.; Zheng, X.J.; Chang, X.L.; Zhang, S. R.; Gong, G.S. Diversity of Trichoderma species associated with soil in the Zoige alpine wetland of Southwest China. Sci. Rep. 2022, 12, 21709. [Google Scholar] [CrossRef]
- Kubicek, C.P.; Bissett, J.; Druzhinina, I.; Kullnig-Gradinger, C.; Szakacs, G. Genetic and metabolic diversity of Trichoderma: A case study on South-East Asian isolates. Fungal Genet. Biol. 2003, 38, 310–319. [Google Scholar] [CrossRef]
- Pollard-Flamand, J.; Boulé, J.; Hart, M.; Úrbez-Torres, J.R. Biocontrol activity of Trichoderma species isolated from grapevines in British Columbia against Botryosphaeria dieback fungal pathogens. J. Fungi. 2022, 8, 409. [Google Scholar] [CrossRef]
- Nascimento Brito, V.; Lana Alves, J.; Sírio Araújo, K.; de Souza Leite, T.; Borges de Queiroz, C.; Liparini Pereira, O.; de Queiroz, M.V. Endophytic Trichoderma species from rubber trees native to the Brazilian Amazon, including four new species. Front. Microbiol. 2023, 14, 1095199. [Google Scholar] [CrossRef]
- Morais, E.M.; Silva, A.A.R.; Sousa, F.W.A.; Azevedo, I.M.B.; Silva, H. F. Endophytic Trichoderma strains isolated from forest species of the Cerrado-Caatingaecotone are potential biocontrol agents against crop pathogenic fungi. PloSOne. 2022, 17(4), e0265824. [Google Scholar] [CrossRef]
- Gazis, R.; Chaverri, P. Diversity of fungal endophytes in leaves and stems of wild rubber trees (Hevea brasiliensis) in Peru. Fungal Ecol. 2010, 3, 240–254. [Google Scholar] [CrossRef]
- Rees, H.J.; Bashir, N.; Drakulic, J.; Cromey, M.G.; Bailey, A.M.; Foster, G.D. Identification of native endophytic Trichoderma spp. for investigation of in vitro antagonism towards Armillaria mellea using synthetic- and plant-based substrates. J. Appl. Microbiol. 2021, 131, 392–403. [Google Scholar] [CrossRef] [PubMed]
- Kovács, C.; Csótó, A.; Pál, K.; Nagy, A.; Fekete, E.; Karaffa, L.; Kubicek, C.P.; Sándor, E. The biocontrol potential of endophytic Trichoderma fungi isolated from Hungarian grapevines. Part I. Isolation, identification and in vitro studies. Pathogens. 2021, 10, 1612. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez, M.C.H.; Evans, H.C.; de Abreu, L.M.; Macedo, D.M.; Ndacnou, M.K.; Bekele, B.K.; Barreto, R.W. New species and records of Trichoderma isolated as mycoparasites and endophytes from cultivated and wild coffee in Africa. Sci. Rep. 2021, 11, 5671–5630. [Google Scholar] [CrossRef]
- Rosmana, A.; Samuels, G.J.; Ismaiel, A.; Ibrahim, E.S.; Chaverri, P.; Herawati, Y.; Asman, A. Trichoderma asperellum: a dominant endophyte species in cacao grown in Sulawesi with potential for controlling vascular streak dieback disease. Trop. Plant Pathol. 2015, 40, 19–25. [Google Scholar] [CrossRef]
- Sirikamonsathien, T.; Kenji, M.; Dethop, T. Potential of endophytic Trichoderma in controlling Phytophthora leaf fall disease in rubber (Hevea brasiliensis). Biol. Control, 2023, 179, 105175. [Google Scholar] [CrossRef]
- Leylaie, S.; Zafari, D. Antiproliferative and antimicrobial activities of secondary metabolites and phylogenetic study of endophytic Trichoderma species from Vinca plants. Front. Microbiol. 2018, 9, 1484. [Google Scholar] [CrossRef]
- Hosseyni-Moghaddam, M.S.; Soltani, J. Bioactivity of endophytic Trichoderma fungal species from the plant family Cupressaceae. Ann. Microbiol. 2014, 64, 753–761. [Google Scholar] [CrossRef]
- Li, Q.R.; Tan, P.; Jiang, Y.L.; Hyde, K.D.; Mckenzie, E.H.C.; Bahkali, A.H.; Kang, J.C.; Wang, Y. A novel Trichoderma species isolated from soil in Guizhou, T. guizhouense. Mycol Progress. 2013, 12, 167–172. [Google Scholar] [CrossRef]
- Rifai, M.A. A revision of the genus Trichoderma. Mycol. Pap. 1969, 116, 1–56. [Google Scholar]
- Dodd, S.L.; Lieckfeldt, E.; Samuels, G.J. Hypocrea atroviridis sp. Nov., the teleomorph of Trichoderma atroviride. Mycologia. 2003, 95, 27–40. [Google Scholar] [CrossRef]
- Samuels, G.J.; Hebbar, P.K. Trichoderma: Identification and agricultural application, 1st ed.; The American Phytopathological Society: St. Paul, Minnesota, USA, 2015; p. 204. [Google Scholar]
- Bissett, J. A revision of the genus Trichoderma. I. Sect. Longibrachiatum sect. nov. Can. J. Bot. 1984, 62, 924–931. [Google Scholar] [CrossRef]
- Bissett, J. A revision of the genus Trichoderma. II. Infrageneric classification. Can J Bot 1991, 69, 2357–2372. [Google Scholar] [CrossRef]
- Bissett, J. A revision of the genus Trichoderma. III. Sect. Pachybasium. Can. J. Bot. 1991, 69, 2373–2417. [Google Scholar] [CrossRef]
- Bissett, J. A revision of the genus Trichoderma. IV. Additional notes on section Longibrachiatum. Can. J. Bot. 69, 2418–2420. [CrossRef]
- Kindermann, J.; El-Ayouti, Y.; Samuels, G.J.; Kubicek, C.P. Phylogeny of the genus Trichoderma based on sequence analysis of the internal transcribed spacer region 1 of the rDNA clade. Fungal Genet. Biol. 1998, 24, 298–309. [Google Scholar] [CrossRef]
- Parnell, J.J.; Berka, R.; Young, H.A.; Sturino, J.M.; Kang, Y.; Barnhart, D.M.; DiLeo, M.V. From the lab to the farm: An industrial perspective of plant beneficial microorganisms. Front. Plant Sci. 2016, 7, 1100. [Google Scholar] [CrossRef]
- Zin, N.A.; Badaluddin, N.A. Biological functions of Trichoderma spp. for agriculture applications. Agric. Sci. 2020, 65, 68–178. [Google Scholar] [CrossRef]
- Lorito, M.; Woo, S.L.; Harman, G.E.; Monte, E. Translational research on Trichoderma: From omics to the field. Annu. Rev. Phytopathol. 2010, 48, 395–417. [Google Scholar] [CrossRef]
- Harman, G.E.; Howell, C.R.; Viterbo, A.; Chet, I.; Lorito, M. Trichoderma species – opportunistic, avirulent plant symbionts. Nature Rev. Microbiol. 2004, 2, 43–56. [Google Scholar] [CrossRef] [PubMed]
- Shoresh, M.; Harmen, G.E.; Mastouri, F. Induced systemic resistance and plant responses to fungal biocontrol agents. Annu. Rev. Phytopathol. 2010, 48, 21–43. [Google Scholar] [CrossRef] [PubMed]
- Marco, S.; Larendana, M.; Riccardo, V.; Raffaella, B.; Walter, C.; Luca, N. Microbe-assisted crop improvement: a sustainable weapon to restore holobiont functionality and resilience. Hort. Res. 2022, 9, uhac160. [Google Scholar] [CrossRef] [PubMed]
- Glare, T.; Caradus, J.; Gelernter, W.; Jackson, T.; Keyhani, N.; Köhl, J.; Marrone, P.; Morin, L.; Stewart, A. Have biopesticides come of age? Trends Biotechnol. 2012, 30, 250–258. [Google Scholar] [CrossRef] [PubMed]
- Kubheka, B.P.; Ziena, L.W. Trichoderma: A biofertilizer and a bio-fungicide for sustainable crop production. [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





