Submitted:
11 December 2025
Posted:
12 December 2025
You are already at the latest version
Abstract
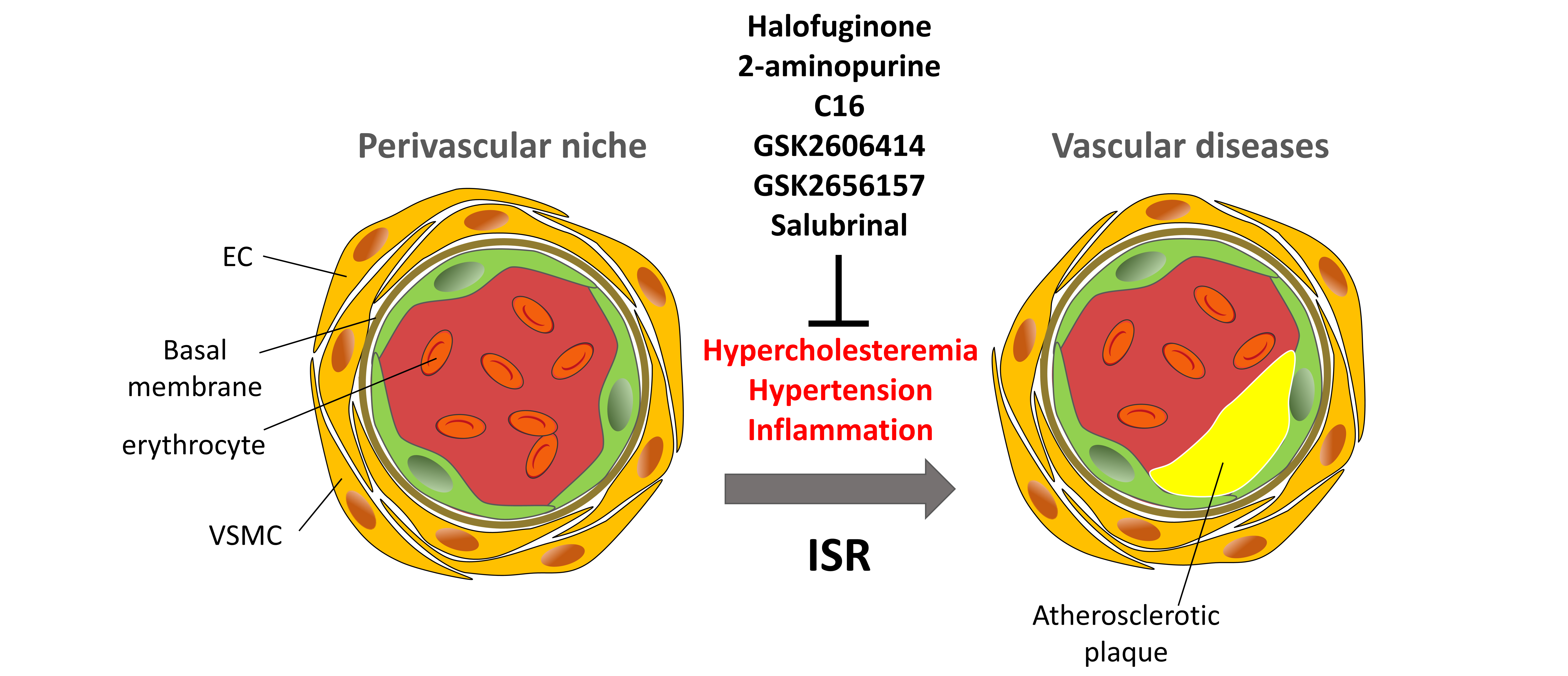
Keywords:
1. Introduction
2. The Molecular Mechanisms of the Integrated Stress Response (ISR)
3. The ISR Signaling and Its Modulators in Vascular Normal and Pathological Physiology
3.1. PERK
3.1.1. PERK Kinase as a Therapeutic Target in Atherosclerosis
3.1.1.1. CNPY2
3.1.1.2. LOX-1/NOX4
3.1.1.3. (cGAS)-STING Pathway
3.1.2. Role of PERK Kinase Signaling in Restenosis and Thrombosis
3.2. GCN2
3.2.1. Metabolic Roles of GCN2
3.2.2. Halofuginone
3.3. PKR
3.3.1. PKR as Regulator of Cellular Senescence and Proliferation
3.3.2. Role PKR in Vascular Disorders
3.3.3. PKR and Inflammasome Response
3.4. HRI
| ISR component | Modulator | Effect on the ISR pathway | Side effects | Off targets | Pathology | Pharmacological effect |
| PERK | GSK2606414 | Inhibition | Pancreas toxicity [176,177,178,179] Body weight loss [180,181,182] Hyperglycemia [181] |
At a concentration of less than 1μM: RIPK1 [182] c-kit [183,184] Aurora B kinase [184] BRK [184] MLK2/MAP3K10 [184] c-MER [184] DDR2 [184] MLCK2/MYLK2 [184] IKKe/IKBKE [184] Concentration of 1μ<: IKKe/IKBKE [184] TRKC [184] MLK3/MAP3K11 [184] RET [184] LCK [184] NEK4 [184] KHS/MAP4K5 [184] MLK1/MAP3K9 [184] TRKA [184] AXL [184] TRKB [184] YES/YES1 [184] WNK2 [184] |
Atherosclerosis | Beneficial [69,73] |
| Hypertension | Beneficial [74] | |||||
| Restenosis | Beneficial [82,83] | |||||
| Inflammation | Beneficial [83] | |||||
| Tumor growth | Beneficial [63,64] | |||||
| Thrombosis | Beneficial [82] | |||||
| GSK2656157 | Inhibition | No toxic effects on heart, liver, kidney and lung tissues [185] | RIPK1 [182] HRI [186] PKR [186] GCN2 [186,187] |
Diabetic cardiomyopathy | Detrimental [62] | |
| Tumor growth | Beneficial [66] | |||||
| CCT020312 | Activation | No toxic effects on liver and kidney [188] | no data | Hypertension | Detrimental [74] | |
| Tumor growth | Detrimental [63,64] | |||||
| GCN2 | Halofuginone | Activation | Skin and eyes irritation, acute toxicity at high doses [189] | TGF-β1/Smad 3 signalling [127,190,191,192,193] |
Inflammation | Beneficial [124,125,127] |
| PH | Beneficial [126,127] | |||||
| Restenosis | Beneficial [128] | |||||
| Tumor growth | Beneficial [129] | |||||
| PKR | C16 | Inhibition | no data | CDK2/CDK5 [194] | Hypertension | Beneficial [142] |
| Inflammation | Beneficial [142,143,148,151] | |||||
| PVOD | Beneficial [145,146] | |||||
| Atherosclerosis | Beneficial [152] | |||||
| 2-aminopurine | Inhibition | Mutagenic effects, Irritation to eyes, skin, and respiratory tract [195] | p53 signalling [196] DNA synthesis [197] |
Senescence | Beneficial [135] | |
| Inflammation | Beneficial [147,150] | |||||
| PH | Beneficial [150] | |||||
| Phosphorylation of eIF2α | ISRIB | Inhibition | No overt signs of toxicity [176,198] | no data | PVOD | Beneficial [145,146] |
| Salubrinal | Activation | No overt signs of toxicity [199,200,201] | Bcl-2 [202] | Atherosclerosis | Beneficial [71,75,84] | |
| PVOD | Beneficial [90] |
4. Conclusions
5. Future Directions
- Precision Therapeutics and Targeted Delivery
- Overcoming these limitations will require two complementary strategies. First, the rational design of next-generation ISR modulators—including improved ISRIB analogues and novel allosteric compounds—should prioritize enhanced selectivity for specific ISR branches (e.g., PERK- vs. GCN2-specific) and optimized pharmacokinetic and safety pro-files. Second, advances in delivery technologies, such as lipid nanoparticles, liposomes, and other nanocarriers, offer promising platforms for transient, non-genomic, and cell-specific modulation of ISR pathways. These clinically validated systems—already applied in mRNA vaccines and siRNA therapies—can be adapted to deliver ISR modulators (e.g., mRNA-encoded dominant-negative constructs or siRNAs targeting ISR effectors) to vascular smooth muscle cells, endothelial cells, or lesion macrophages, thereby minimizing systemic exposure and reducing off-target effects.
- Deciphering Cell-Type-Specific ISR Dynamics In Vivo. The functional outcome of ISR activation is highly context-dependent, varying by cell type (endothelial cells, VSMCs, macrophages), disease stage, and metabolic milieu. Leveraging single-cell RNA seq and other multi-omics technologies (transcriptomics, proteomics) on human vascular tissues and advanced animal models will be essential to map these heterogeneous responses and identify the most therapeutically vulnerable cellular targets.
- Elucidating the Switch from Adaptive to Maladaptive ISR. A fundamental unanswered question is what molecular mechanisms determine whether ISR signaling promotes cell survival or initiates apoptosis in vascular cells. Systematic studies combining genetic screening with temporal phospho-proteomics and metabolomics are needed to identify the critical checkpoints that dictate this fate decision. A paramount challenge is to map the "therapeutic windows" for ISR modulation across different vascular diseases. Computational modeling of ISR network dynamics in specific vascular cell types could further predict the tipping point between adaptive and maladaptive outcomes, guiding the timing and choice of intervention.
- Bridging the Translational Gap. Despite promising preclinical findings, in vivo data on the pharmacokinetics, long-term safety, and therapeutic efficacy of ISR modulators in chronic vascular disease contexts remain limited. Moving beyond proof-of-concept will re-quire robust, investigational new drug (IND)-enabling studies, including dose-response modeling, toxicological profiling, and biomarker-guided efficacy assessment. Expanding ISR modulation to conditions such as age-related vascular dysfunction is especially compelling, given the well-established links between ISR, senescence, and vascular aging.
- Integrating ISR with Vascular Immunology. The cross-talk between ISR and inflammasome activation, particularly via PKR, in endothelial cells and macrophages is a potent driver of pathology. A more integrated understanding of this axis could reveal novel combinatorial strategies to simultaneously dampen inflammatory and proteotoxic stress in diseases like atherosclerosis and pulmonary hypertension.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Derisbourg MJ, Hartman MD, Denzel MS (2021) Modulating the integrated stress response to slow aging and ameliorate age-related pathology. Nat Aging 1:760–768. [CrossRef]
- Peng Z, Shu B, Zhang Y, Wang M (2019) Endothelial Response to Pathophysiological Stress. Arterioscler Thromb Vasc Biol 39:. [CrossRef]
- Roth GA, Mensah GA, Johnson CO, et al (2020) Global Burden of Cardiovascular Diseases and Risk Factors, 1990–2019. J Am Coll Cardiol 76:2982–3021. [CrossRef]
- Druey KM, Arnaud L, Parikh SM (2024) Systemic capillary leak syndrome. Nat Rev Dis Prim 10:86. [CrossRef]
- Gül A, Aksentijevich I, Brogan P, et al (2025) The pathogenesis, clinical presentations and treatment of monogenic systemic vasculitis. Nat Rev Rheumatol 21:414–425. [CrossRef]
- Song P, Rudan D, Zhu Y, et al (2019) Global, regional, and national prevalence and risk factors for peripheral artery disease in 2015: an updated systematic review and analysis. Lancet Glob Heal 7:e1020–e1030. [CrossRef]
- Kolls BJ, Sapp S, Rockhold FW, et al (2019) Stroke in Patients With Peripheral Artery Disease. Stroke 50:1356–1363. [CrossRef]
- Jurenne D Hooi HES (1998) Risk factors and cardiovascular diseases associated with asymptomatic peripheral arterial occlusive disease: The Limburg PAOD Study. Scand J Prim Health Care 16:177–182. [CrossRef]
- Athavale A, Fukaya E, Leeper NJ (2024) Peripheral Artery Disease: Molecular Mechanisms and Novel Therapies. Arterioscler Thromb Vasc Biol 44:1165–1170. [CrossRef]
- Libby P, Buring JE, Badimon L, et al (2019) Atherosclerosis. Nat Rev Dis Prim 5:56. [CrossRef]
- Porsch F, Binder CJ (2024) Autoimmune diseases and atherosclerotic cardiovascular disease. Nat Rev Cardiol 21:780–807. [CrossRef]
- Libby P (2021) The changing landscape of atherosclerosis. Nature 592:524–533. [CrossRef]
- Björkegren JLM, Lusis AJ (2022) Atherosclerosis: Recent developments. Cell 185:1630–1645. [CrossRef]
- Di Nisio M, van Es N, Büller HR (2016) Deep vein thrombosis and pulmonary embolism. Lancet 388:3060–3073. [CrossRef]
- Yamashita A, Asada Y (2023) Underlying mechanisms of thrombus formation/growth in atherothrombosis and deep vein thrombosis. Pathol Int 73:65–80. [CrossRef]
- Wattanakit K, Lutsey PL, Bell EJ, et al (2012) Association between cardiovascular disease risk factors and occurrence of venous thromboembolism. Thromb Haemost 108:508–515. [CrossRef]
- Ortel TL, Neumann I, Ageno W, et al (2020) American Society of Hematology 2020 Guidelines for Management of Venous Thromboembolism: Treatment of Deep Vein Thrombosis and Pulmonary Embolism. Blood Adv 4:4693–4738. [CrossRef]
- Kearon C, Akl EA, Ornelas J, et al (2016) Antithrombotic Therapy for VTE Disease. Chest 149:315–352. [CrossRef]
- Boucly A, Gerges C, Savale L, et al (2023) Pulmonary arterial hypertension. Presse Med 52:104168. [CrossRef]
- Balko R, Edriss H, Nugent K, Test V (2017) Pulmonary veno-occlusive disease: An important consideration in patients with pulmonary hypertension. Respir Med 132:203–209. [CrossRef]
- Dorweiler B, Grechowa I, Wallrath A, et al (2014) Activation of the proapoptotic unfolded protein response in plaques of the human carotid artery. Eur J Vasc Endovasc Surg 48:248–57. [CrossRef]
- Zhou J, Lhoták S, Hilditch BA, Austin RC (2005) Activation of the Unfolded Protein Response Occurs at All Stages of Atherosclerotic Lesion Development in Apolipoprotein E–Deficient Mice. Circulation 111:1814–1821. [CrossRef]
- Thorp E, Li G, Seimon TA, et al (2009) Reduced Apoptosis and Plaque Necrosis in Advanced Atherosclerotic Lesions of Apoe−/− and Ldlr−/− Mice Lacking CHOP. Cell Metab 9:474–481. [CrossRef]
- Onat UI, Yildirim AD, Tufanli Ö, et al (2019) Intercepting the Lipid-Induced Integrated Stress Response Reduces Atherosclerosis. J Am Coll Cardiol 73:1149–1169. [CrossRef]
- Zhang G, Wang X, Li C, et al (2021) Integrated Stress Response Couples Mitochondrial Protein Translation With Oxidative Stress Control. Circulation 144:1500–1515. [CrossRef]
- Silva RA, Sarigol F, Karagöz GE, et al (2025) The unfolded protein response in progeria arteries originates from non-endothelial cell types. Life Sci Alliance 9:e202503485. [CrossRef]
- Hamczyk MR, Villa-Bellosta R, Quesada V, et al (2019) Progerin accelerates atherosclerosis by inducing endoplasmic reticulum stress in vascular smooth muscle cells. EMBO Mol Med 11:. [CrossRef]
- Rothenburg S, Georgiadis MM, Wek RC (2016) Evolution of eIF2α Kinases: Adapting Translational Control to Diverse Stresses. In: Evolution of the Protein Synthesis Machinery and Its Regulation. Springer International Publishing, Cham, pp 235–260.
- Taniuchi S, Miyake M, Tsugawa K, et al (2016) Integrated stress response of vertebrates is regulated by four eIF2α kinases. Sci Rep 6:32886. [CrossRef]
- Hinnebusch AG (2005) TRANSLATIONAL REGULATION OF GCN4 AND THE GENERAL AMINO ACID CONTROL OF YEAST. Annu Rev Microbiol 59:407–450. [CrossRef]
- Pakos-Zebrucka K, Koryga I, Mnich K, et al (2016) The integrated stress response. EMBO Rep 17:1374–1395. [CrossRef]
- Han A-P (2001) Heme-regulated eIF2alpha kinase (HRI) is required for translational regulation and survival of erythroid precursors in iron deficiency. EMBO J 20:6909–6918. [CrossRef]
- Wu C, Zhang Y, Hu C (2020) PKZ, a Fish-Unique eIF2α Kinase Involved in Innate Immune Response. Front Immunol 11:. [CrossRef]
- Lavoie H, Li JJ, Thevakumaran N, et al (2014) Dimerization-induced allostery in protein kinase regulation. Trends Biochem Sci 39:475–486. [CrossRef]
- Lu Y-N, Kavianpour S, Zhang T, et al (2021) MARK2 phosphorylates eIF2α in response to proteotoxic stress. PLOS Biol 19:e3001096. [CrossRef]
- Wu Z, Mei F, Gan Y, et al (2023) FAM69C functions as a kinase for eIF2α and promotes stress granule assembly. EMBO Rep 24:. [CrossRef]
- Melber A, Haynes CM (2018) UPRmt regulation and output: a stress response mediated by mitochondrial-nuclear communication. Cell Res 28:281–295. [CrossRef]
- Hewes RS, Schaefer AM, Taghert PH (2000) The cryptocephal Gene (ATF4) Encodes Multiple Basic-Leucine Zipper Proteins Controlling Molting and Metamorphosis in Drosophila. Genetics 155:1711–1723. [CrossRef]
- Brown B, Mitra S, Roach FD, et al (2021) The transcription factor Xrp1 is required for PERK-mediated antioxidant gene induction in Drosophila. Elife 10:. [CrossRef]
- Quirós PM, Prado MA, Zamboni N, et al (2017) Multi-omics analysis identifies ATF4 as a key regulator of the mitochondrial stress response in mammals. J Cell Biol 216:2027–2045. [CrossRef]
- Tran HC, Van Aken O (2020) Mitochondrial unfolded protein-related responses across kingdoms: similar problems, different regulators. Mitochondrion 53:166–177. [CrossRef]
- Steinberger J, Chu J, Maïga RI, et al (2017) Developing anti-neoplastic biotherapeutics against eIF4F. Cell Mol Life Sci 74:1681–1692. [CrossRef]
- Jennings MD, Pavitt GD (2010) eIF5 has GDI activity necessary for translational control by eIF2 phosphorylation. Nature 465:378–381. [CrossRef]
- Sidrauski C, Tsai JC, Kampmann M, et al (2015) Pharmacological dimerization and activation of the exchange factor eIF2B antagonizes the integrated stress response. Elife 4:. [CrossRef]
- Wek RC, Anthony TG, Staschke KA (2023) Surviving and Adapting to Stress: Translational Control and the Integrated Stress Response. Antioxid Redox Signal 39:351–373. [CrossRef]
- Ye J, Kumanova M, Hart LS, et al (2010) The GCN2-ATF4 pathway is critical for tumour cell survival and proliferation in response to nutrient deprivation. EMBO J 29:2082–2096. [CrossRef]
- Jousse C, Oyadomari S, Novoa I, et al (2003) Inhibition of a constitutive translation initiation factor 2α phosphatase, CReP, promotes survival of stressed cells. J Cell Biol 163:767–775. [CrossRef]
- Skabkin MA, Skabkina O V., Dhote V, et al (2010) Activities of Ligatin and MCT-1/DENR in eukaryotic translation initiation and ribosomal recycling. Genes Dev 24:1787–1801. [CrossRef]
- Vasudevan D, Neuman SD, Yang A, et al (2020) Translational induction of ATF4 during integrated stress response requires noncanonical initiation factors eIF2D and DENR. Nat Commun 11:4677. [CrossRef]
- Bohlen J, Harbrecht L, Blanco S, et al (2020) DENR promotes translation reinitiation via ribosome recycling to drive expression of oncogenes including ATF4. Nat Commun 11:4676. [CrossRef]
- Mukhopadhyay S, Amodeo ME, Lee ASY (2023) eIF3d controls the persistent integrated stress response. Mol Cell 83:3303-3313.e6. [CrossRef]
- Davies PF, Civelek M, Fang Y, Fleming I (2013) The atherosusceptible endothelium: endothelial phenotypes in complex haemodynamic shear stress regions in vivo. Cardiovasc Res 99:315–327. [CrossRef]
- Milusev A, Rieben R, Sorvillo N (2022) The Endothelial Glycocalyx: A Possible Therapeutic Target in Cardiovascular Disorders. Front Cardiovasc Med 9:. [CrossRef]
- Shih Y-T, Wei S-Y, Chen J-H, et al (2023) Vinculin phosphorylation impairs vascular endothelial junctions promoting atherosclerosis. Eur Heart J 44:304–318. [CrossRef]
- Hu X-Q, Zhang L (2021) Hypoxia and the integrated stress response promote pulmonary hypertension and preeclampsia: Implications in drug development. Drug Discov Today 26:2754–2773. [CrossRef]
- Lenna S, Han R, Trojanowska M (2014) Endoplasmic reticulum stress and endothelial dysfunction. IUBMB Life 66:530–537. [CrossRef]
- Liu Z, Lv Y, Zhao N, et al (2015) Protein kinase R-like ER kinase and its role in endoplasmic reticulum stress-decided cell fate. Cell Death Dis 6:e1822–e1822. [CrossRef]
- Julier C, Nicolino M (2010) Wolcott-Rallison syndrome. Orphanet J Rare Dis 5:29. [CrossRef]
- Shimizu T, Maruyama K, Kawamura T, et al (2020) PERK participates in cardiac valve development via fatty acid oxidation and endocardial-mesenchymal transformation. Sci Rep 10:20094. [CrossRef]
- Karali E, Bellou S, Stellas D, et al (2014) VEGF Signals through ATF6 and PERK to Promote Endothelial Cell Survival and Angiogenesis in the Absence of ER Stress. Mol Cell 54:559–572. [CrossRef]
- Liu C, Liu Y, He J, et al (2019) Liraglutide Increases VEGF Expression via CNPY2-PERK Pathway Induced by Hypoxia/Reoxygenation Injury. Front Pharmacol 10:. [CrossRef]
- Zhang S, Tian W, Duan X, et al (2024) Melatonin attenuates diabetic cardiomyopathy by increasing autophagy of cardiomyocytes via regulation of VEGF-B/GRP78/PERK signaling pathway. Cardiovasc Diabetol 23:19. [CrossRef]
- Cai W, Sun X, Jin F, et al (2021) PERK-eIF2α-ERK1/2 axis drives mesenchymal-endothelial transition of cancer-associated fibroblasts in pancreatic cancer. Cancer Lett 515:86–95. [CrossRef]
- Soni H, Bode J, Nguyen CDL, et al (2020) PERK-mediated expression of peptidylglycine α-amidating monooxygenase supports angiogenesis in glioblastoma. Oncogenesis 9:18. [CrossRef]
- Luxmi R, Mains RE, King SM, Eipper BA (2021) Amino Acids | Peptidylglycine α-Amidating Monooxygenase (PAM). In: Encyclopedia of Biological Chemistry III. Elsevier, pp 88–104.
- Atkins C, Liu Q, Minthorn E, et al (2013) Characterization of a Novel PERK Kinase Inhibitor with Antitumor and Antiangiogenic Activity. Cancer Res 73:1993–2002. [CrossRef]
- Blais JD, Addison CL, Edge R, et al (2006) Perk-Dependent Translational Regulation Promotes Tumor Cell Adaptation and Angiogenesis in Response to Hypoxic Stress. Mol Cell Biol 26:9517–9532. [CrossRef]
- Verginadis II, Avgousti H, Monslow J, et al (2022) A stromal Integrated Stress Response activates perivascular cancer-associated fibroblasts to drive angiogenesis and tumour progression. Nat Cell Biol 24:940–953. [CrossRef]
- Bingyu W, Jun Q, Bingyang L, et al (2024) Trimethylamine N-oxide promotes PERK-mediated endothelial-mesenchymal transition and apoptosis thereby aggravates atherosclerosis. Int Immunopharmacol 142:113209. [CrossRef]
- Saaoud F, Liu L, Xu K, et al (2023) Aorta- and liver-generated TMAO enhances trained immunity for increased inflammation via ER stress/mitochondrial ROS/glycolysis pathways. JCI Insight 8:. [CrossRef]
- Tao YK, Yu PL, Bai YP, et al (2016) Role of PERK/eIF2α/CHOP Endoplasmic Reticulum Stress Pathway in Oxidized Low-density Lipoprotein Mediated Induction of Endothelial Apoptosis. Biomed Environ Sci 29:868–876. [CrossRef]
- Hong D, Tang W, Li F, et al (2024) The short-chain fatty acid propionate prevents ox-LDL-induced coronary microvascular dysfunction by alleviating endoplasmic reticulum stress in HCMECs. PLoS One 19:e0304551. [CrossRef]
- Huang H, Tang N, Li Y, et al (2023) The role of CNPY2 in endothelial injury and inflammation during the progress of atherosclerosis. J Mol Histol 54:195–205. [CrossRef]
- Guo C, Liu H, Li B, Lu Z (2023) Angiotensin-(1–9) prevents angiotensin II-induced endothelial apoptosis through CNPY2/PERK pathway. Apoptosis 28:379–396. [CrossRef]
- Hong D, Bai Y-P, Gao H-C, et al (2014) Ox-LDL induces endothelial cell apoptosis via the LOX-1-dependent endoplasmic reticulum stress pathway. Atherosclerosis 235:310–317. [CrossRef]
- Rousset F, Zhang L, Lardy B, et al (2020) Transmembrane Nox4 topology revealed by topological determination by Ubiquitin Fusion Assay, a novel method to uncover membrane protein topology. Biochem Biophys Res Commun 521:383–388. [CrossRef]
- Hu H, Wang C, Jin Y, et al (2019) Catalpol Inhibits Homocysteine-induced Oxidation and Inflammation via Inhibiting Nox4/NF-κB and GRP78/PERK Pathways in Human Aorta Endothelial Cells. Inflammation 42:64–80. [CrossRef]
- Yu W, Li S, Wu H, et al (2021) Endothelial Nox4 dysfunction aggravates atherosclerosis by inducing endoplasmic reticulum stress and soluble epoxide hydrolase. Free Radic Biol Med 164:44–57. [CrossRef]
- Li X, Chen X, Zheng L, et al (2023) Non-canonical STING–PERK pathway dependent epigenetic regulation of vascular endothelial dysfunction via integrating IRF3 and NF-κB in inflammatory response. Acta Pharm Sin B 13:4765–4784. [CrossRef]
- Li P, Xie C, Zhong J, et al (2021) Melatonin Attenuates ox-LDL-Induced Endothelial Dysfunction by Reducing ER Stress and Inhibiting JNK/Mff Signaling. Oxid Med Cell Longev 2021:. [CrossRef]
- Galán M, Kassan M, Kadowitz PJ, et al (2014) Mechanism of endoplasmic reticulum stress-induced vascular endothelial dysfunction. Biochim Biophys Acta - Mol Cell Res 1843:1063–1075. [CrossRef]
- Wang B, Zhang M, Urabe G, et al (2020) PERK Inhibition Mitigates Restenosis and Thrombosis. JACC Basic to Transl Sci 5:245–263. [CrossRef]
- Wang B, Zhang M, Urabe G, et al (2021) PERK Inhibition Promotes Post-angioplasty Re-endothelialization via Modulating SMC Phenotype Changes. J Surg Res 257:294–305. [CrossRef]
- Huang Q, Yang Z, Zhou J-P, Luo Y (2017) HMGB1 induces endothelial progenitor cells apoptosis via RAGE-dependent PERK/eIF2α pathway. Mol Cell Biochem 431:67–74. [CrossRef]
- Misra J, Carlson KR, Spandau DF, Wek RC (2024) Multiple mechanisms activate GCN2 eIF2 kinase in response to diverse stress conditions. Nucleic Acids Res 52:1830–1846. [CrossRef]
- Castilho BA, Shanmugam R, Silva RC, et al (2014) Keeping the eIF2 alpha kinase Gcn2 in check. Biochim Biophys Acta - Mol Cell Res 1843:1948–1968. [CrossRef]
- Ma B, Li T, Li W, et al (2022) Patient-specific and gene-corrected induced pluripotent stem cell-derived endothelial cells elucidate single-cell phenotype of pulmonary veno-occlusive disease. Stem Cell Reports 17:2674–2689. [CrossRef]
- Nossent EJ, Antigny F, Montani D, et al (2018) Pulmonary vascular remodeling patterns and expression of general control nonderepressible 2 (GCN2) in pulmonary veno-occlusive disease. J Hear Lung Transplant 37:647–655. [CrossRef]
- Manaud G, Nossent EJ, Lambert M, et al (2020) Comparison of Human and Experimental Pulmonary Veno-Occlusive Disease. Am J Respir Cell Mol Biol 63:118–131. [CrossRef]
- Chen Z, Zhang J, Wei D, et al (2021) GCN2 Regulates ATF3-p38 MAPK Signaling Transduction in Pulmonary Veno-Occlusive Disease. J Cardiovasc Pharmacol Ther 26:677–689. [CrossRef]
- DuBrock HM, Kradin RL, Rodriguez-Lopez JM, Channick RN (2015) Pulmonary Capillary Hemangiomatosis: The Role of Invasive Cardiopulmonary Exercise Testing. Pulm Circ 5:580–586. [CrossRef]
- Best DH, Sumner KL, Austin ED, et al (2014) EIF2AK4 Mutations in Pulmonary Capillary Hemangiomatosis. Chest 145:231–236. [CrossRef]
- Longchamp A, Mirabella T, Arduini A, et al (2018) Amino Acid Restriction Triggers Angiogenesis via GCN2/ATF4 Regulation of VEGF and H2S Production. Cell 173:117-129.e14. [CrossRef]
- Eleftheriadis T, Tsogka K, Pissas G, et al (2016) Activation of general control nonderepressible 2 kinase protects human glomerular endothelial cells from harmful high-glucose-induced molecular pathways. Int Urol Nephrol 48:1731–1739. [CrossRef]
- Wang Y, Ning Y, Alam GN, et al (2013) Amino Acid Deprivation Promotes Tumor Angiogenesis through the GCN2/ATF4 Pathway. Neoplasia 15:989–997. [CrossRef]
- Yang R, Wek SA, Wek RC (2000) Glucose Limitation Induces GCN4 Translation by Activation of Gcn2 Protein Kinase. Mol Cell Biol 20:2706–2717. [CrossRef]
- Rolfes RJ, Hinnebusch AG (1993) Translation of the Yeast Transcriptional Activator GCN4 Is Stimulated by Purine Limitation: Implications for Activation of the Protein Kinase GCN2. Mol Cell Biol 13:5099–5111. [CrossRef]
- Deng J, Harding HP, Raught B, et al (2002) Activation of GCN2 in UV-Irradiated Cells Inhibits Translation. Curr Biol 12:1279–1286. [CrossRef]
- Zhan K, Narasimhan J, Wek RC (2004) Differential Activation of eIF2 Kinases in Response to Cellular Stresses in Schizosaccharomyces pombe. Genetics 168:1867–1875. [CrossRef]
- Liu Y, László C, Liu Y, et al (2010) Regulation of G1 Arrest and Apoptosis in Hypoxia by PERK and GCN2-Mediated eIF2α Phosphorylation. Neoplasia 12:61-IN6. [CrossRef]
- Jiménez-Díaz A, Remacha M, Ballesta JPG, Berlanga JJ (2013) Phosphorylation of Initiation Factor eIF2 in Response to Stress Conditions Is Mediated by Acidic Ribosomal P1/P2 Proteins in Saccharomyces cerevisiae. PLoS One 8:e84219. [CrossRef]
- Piecyk M, Ferraro-Peyret C, Laville D, et al (2024) Novel insights into the GCN2 pathway and its targeting. Therapeutic value in cancer and lessons from lung fibrosis development. FEBS J 291:4867–4889. [CrossRef]
- Missiaen R, Anderson NM, Kim LC, et al (2022) GCN2 inhibition sensitizes arginine-deprived hepatocellular carcinoma cells to senolytic treatment. Cell Metab 34:1151-1167.e7. [CrossRef]
- Cordova RA, Misra J, Amin PH, et al (2022) GCN2 eIF2 kinase promotes prostate cancer by maintaining amino acid homeostasis. Elife 11:1–31. [CrossRef]
- Bröer A, Rahimi F, Bröer S (2016) Deletion of Amino Acid Transporter ASCT2 (SLC1A5) Reveals an Essential Role for Transporters SNAT1 (SLC38A1) and SNAT2 (SLC38A2) to Sustain Glutaminolysis in Cancer Cells. J Biol Chem 291:13194–13205. [CrossRef]
- Fiore A, Zeitler L, Russier M, et al (2022) Kynurenine importation by SLC7A11 propagates anti-ferroptotic signaling. Mol Cell 82:920-932.e7. [CrossRef]
- Sannino S, Yates ME, Schurdak ME, et al (2021) Unique integrated stress response sensors regulate cancer cell susceptibility when Hsp70 activity is compromised. Elife 10:. [CrossRef]
- Parzych K, Saavedra-García P, Valbuena GN, et al (2019) The coordinated action of VCP/p97 and GCN2 regulates cancer cell metabolism and proteostasis during nutrient limitation. Oncogene 38:3216–3231. [CrossRef]
- Ge M-K, Zhang C, Zhang N, et al (2023) The tRNA-GCN2-FBXO22-axis-mediated mTOR ubiquitination senses amino acid insufficiency. Cell Metab 35:2216-2230.e8. [CrossRef]
- Ye J, Palm W, Peng M, et al (2015) GCN2 sustains mTORC1 suppression upon amino acid deprivation by inducing Sestrin2. Genes Dev 29:2331–2336. [CrossRef]
- Saxton RA, Sabatini DM (2017) mTOR Signaling in Growth, Metabolism, and Disease. Cell 168:960–976. [CrossRef]
- Kim J, Kundu M, Viollet B, Guan K-L (2011) AMPK and mTOR regulate autophagy through direct phosphorylation of Ulk1. Nat Cell Biol 13:132–141. [CrossRef]
- Gesualdi NM, Chirico G, Pirozzi G, et al (2007) Tumor necrosis factor-associated protein 1 (TRAP-1) protects cells from oxidative stress and apoptosis. Stress 10:342–350. [CrossRef]
- Matassa DS, Amoroso MR, Agliarulo I, et al (2013) Translational control in the stress adaptive response of cancer cells: a novel role for the heat shock protein TRAP1. Cell Death Dis 4:e851–e851. [CrossRef]
- Chen C, Xie Y, Qian S (2024) Multifaceted role of GCN2 in tumor adaptation and therapeutic targeting. Transl Oncol 49:102096. [CrossRef]
- Stonyte V, Mastrangelopoulou M, Timmer R, et al (2023) The GCN2/eIF2αK stress kinase regulates PP1 to ensure mitotic fidelity. EMBO Rep 24:. [CrossRef]
- Jung JG, Yi S-A, Choi S-E, et al (2015) TM-25659-Induced Activation of FGF21 Level Decreases Insulin Resistance and Inflammation in Skeletal Muscle via GCN2 Pathways. Mol Cells 38:1037–1043. [CrossRef]
- Santos-Ribeiro D, Lecocq M, de Beukelaer M, et al (2023) Disruption of GCN2 Pathway Aggravates Vascular and Parenchymal Remodeling during Pulmonary Fibrosis. Am J Respir Cell Mol Biol 68:326–338. [CrossRef]
- Zhu MM, Dai J, Dai Z, et al (2024) GCN2 kinase activation mediates pulmonary vascular remodeling and pulmonary arterial hypertension. JCI Insight 9:. [CrossRef]
- Giaid A, Yanagisawa M, Langleben D, et al (1993) Expression of Endothelin-1 in the Lungs of Patients with Pulmonary Hypertension. N Engl J Med 328:1732–1739. [CrossRef]
- Eleftheriadis T, Pissas G, Crespo M, et al (2021) The effect of anti-HLA class I antibodies on the immunological properties of human glomerular endothelial cells and their modification by mTOR inhibition or GCN2 kinase activation. Mol Med Rep 23:355. [CrossRef]
- Dushpanova A, Agostini S, Ciofini E, et al (2016) Gene silencing of endothelial von Willebrand Factor attenuates angiotensin II-induced endothelin-1 expression in porcine aortic endothelial cells. Sci Rep 6:30048. [CrossRef]
- Rai S, Szaruga M, Pitera AP, Bertolotti A (2024) Integrated stress response activator halofuginone protects mice from diabetes-like phenotypes. J Cell Biol 223:. [CrossRef]
- Zhong M, Zhang X, Shi X, Zheng C (2020) Halofuginone inhibits LPS-induced attachment of monocytes to HUVECs. Int Immunopharmacol 87:106753. [CrossRef]
- He B, Fu G-H, Du X-F, Chu H-M (2019) Halofuginone protects HUVECs from H2O2-induced injury by modulating VEGF/JNK signaling pathway. J Chinese Med Assoc 82:92–98. [CrossRef]
- Jain PP, Zhao T, Xiong M, et al (2021) Halofuginone, a promising drug for treatment of pulmonary hypertension. Br J Pharmacol 178:3373–3394. [CrossRef]
- Wang J, Guan L, Yu J, et al (2025) Halofuginone prevents inflammation and proliferation of high-altitude pulmonary hypertension by inhibiting the TGF-β1/Smad signaling pathway. Sci Rep 15:3619. [CrossRef]
- Guo L-W, Wang B, Goel SA, et al (2014) Halofuginone Stimulates Adaptive Remodeling and Preserves Re-Endothelialization in Balloon-Injured Rat Carotid Arteries. Circ Cardiovasc Interv 7:594–601. [CrossRef]
- Mi L, Zhang Y, Su A, et al (2022) Halofuginone for cancer treatment: A systematic review of efficacy and molecular mechanisms. J Funct Foods 98:105237. [CrossRef]
- Abramovitch R, Itzik A, Harel H, et al (2004) Halofuginone Inhibits Angiogenesis and Growth in Implanted Metastatic Rat Brain Tumor Model-an MRI Study. Neoplasia 6:480–489. [CrossRef]
- Gal-Ben-Ari S, Barrera I, Ehrlich M, Rosenblum K (2019) PKR: A kinase to remember. Front Mol Neurosci 11:1–20. [CrossRef]
- Zhu Z, Zhong H, Zhou Q, et al (2015) Inhibition of PKR impairs angiogenesis through a VEGF pathway. Am J Physiol Metab 308:E518–E524. [CrossRef]
- Zhu M, Liu X, Wang S, et al (2016) PKR promotes choroidal neovascularization via upregulating the PI3K/Akt signaling pathway in VEGF expression. Mol Vis 22:1361–1374.
- Kalinin A, Zubkova E, Menshikov M (2023) Integrated Stress Response (ISR) Pathway: Unraveling Its Role in Cellular Senescence. Int J Mol Sci 24:17423. [CrossRef]
- Li Y, Peng Z, Wang C, et al (2018) Novel role of PKR in palmitate-induced Sirt1 inactivation and endothelial cell senescence. Am J Physiol Circ Physiol 315:H571–H580. [CrossRef]
- Peng Z, Tan X, Xie L, et al (2023) PKR deficiency delays vascular aging via inhibiting GSDMD-mediated endothelial cell hyperactivation. iScience 26:105909. [CrossRef]
- Fasciano S, Hutchins B, Handy I, Patel RC (2005) Identification of the heparin-binding domains of the interferon-induced protein kinase, PKR. FEBS J 272:1425–1439. [CrossRef]
- Fasciano S, Patel RC, Handy I, Patel C V. (2005) Regulation of Vascular Smooth Muscle Proliferation by Heparin. J Biol Chem 280:15682–15689. [CrossRef]
- Handy I, Patel RC (2013) STAT1 requirement for PKR-induced cell cycle arrest in vascular smooth muscle cells in response to heparin. Gene 524:15–21. [CrossRef]
- Patel RC, Handy I, Patel C V. (2002) Contribution of Double-Stranded RNA-Activated Protein Kinase Toward Antiproliferative Actions of Heparin on Vascular Smooth Muscle Cells. Arterioscler Thromb Vasc Biol 22:1439–1444. [CrossRef]
- Ding Y, Sun Y, Wang H, et al (2024) Atherosis-associated lnc_000048 activates PKR to enhance STAT1-mediated polarization of THP-1 macrophages to M1 phenotype. Neural Regen Res 19:2488–2498. [CrossRef]
- Kalra J, Dasari D, Bhat A, et al (2020) PKR inhibitor imoxin prevents hypertension, endothelial dysfunction and cardiac and vascular remodelling in L-NAME-treated rats. Life Sci 262:118436. [CrossRef]
- Kalra J, Mangali S, Bhat A, et al (2020) Selective inhibition of PKR improves vascular inflammation and remodelling in high fructose treated primary vascular smooth muscle cells. Biochim Biophys Acta - Mol Basis Dis 1866:165606. [CrossRef]
- Wang H, Xu X, Fassett J, et al (2014) Double-Stranded RNA–Dependent Protein Kinase Deficiency Protects the Heart From Systolic Overload-Induced Congestive Heart Failure. Circulation 129:1397–1406. [CrossRef]
- Prabhakar A, Kumar R, Wadhwa M, et al (2024) Reversal of pulmonary veno-occlusive disease phenotypes by inhibition of the integrated stress response. Nat Cardiovasc Res 3:799–818. [CrossRef]
- Prabhakar A, Wadhwa M, Kumar R, et al (2024) Mechanisms underlying age-associated exacerbation of pulmonary veno-occlusive disease. JCI Insight 9:. [CrossRef]
- Lu B, Nakamura T, Inouye K, et al (2012) Novel role of PKR in inflammasome activation and HMGB1 release. Nature 488:670–674. [CrossRef]
- Karnam K, Sedmaki K, Sharma P, et al (2023) Selective inhibition of PKR by C16 accelerates diabetic wound healing by inhibiting NALP3 expression in mice. Inflamm Res 72:221–236. [CrossRef]
- PENG K, LIU L, WEI D, et al (2015) P2X7R is involved in the progression of atherosclerosis by promoting NLRP3 inflammasome activation. Int J Mol Med 35:1179–1188. [CrossRef]
- Li Y, Li Y, Li L, et al (2021) PKR deficiency alleviates pulmonary hypertension via inducing inflammasome adaptor ASC inactivation. Pulm Circ 11:1–13. [CrossRef]
- Jiang Y, Steinle JJ (2019) Epac1 inhibits PKR to reduce NLRP3 inflammasome proteins in retinal endothelial cells. J Inflamm Res Volume 12:153–159. [CrossRef]
- Zhu J, Chen H, Le Y, et al (2022) Salvianolic acid A regulates pyroptosis of endothelial cells via directly targeting PKM2 and ameliorates diabetic atherosclerosis. Front Pharmacol 13:. [CrossRef]
- Huang C, Sheu WH, Chiang A (2015) Docosahexaenoic acid and eicosapentaenoic acid suppress adhesion molecule expression in human aortic endothelial cells via differential mechanisms. Mol Nutr Food Res 59:751–762. [CrossRef]
- Koromilas AE, Cantin C, Craig AWB, et al (1995) The Interferon-inducible Protein Kinase PKR Modulates the Transcriptional Activation of Immunoglobulin κ Gene. J Biol Chem 270:25426–25434. [CrossRef]
- Bonnet MC, Weil R, Dam E, et al (2000) PKR Stimulates NF-κB Irrespective of Its Kinase Function by Interacting with the IκB Kinase Complex. Mol Cell Biol 20:4532–4542. [CrossRef]
- Demarchi F, Gutierrez MI, Giacca M (1999) Human Immunodeficiency Virus Type 1 Tat Protein Activates Transcription Factor NF-κB through the Cellular Interferon-Inducible, Double-Stranded RNA-Dependent Protein Kinase, PKR. J Virol 73:7080–7086. [CrossRef]
- Ge L, Zhang Y, Zhao X, et al (2021) EIF2AK2 selectively regulates the gene transcription in immune response and histones associated with systemic lupus erythematosus. Mol Immunol 132:132–141. [CrossRef]
- Jammi N V., Whitby LR, Beal PA (2003) Small molecule inhibitors of the RNA-dependent protein kinase. Biochem Biophys Res Commun 308:50–57. [CrossRef]
- Hu W, Hofstetter W, Wei X, et al (2009) Double-Stranded RNA-Dependent Protein Kinase-Dependent Apoptosis Induction by a Novel Small Compound. J Pharmacol Exp Ther 328:866–872. [CrossRef]
- HU Y, CONWAY TW (1993) 2-Aminopurine Inhibits the Double-Stranded RNA-Dependent Protein Kinase Both In Vitro and In Vivo. J Interferon Res 13:323–328. [CrossRef]
- Papadakis AI, Paraskeva E, Peidis P, et al (2010) eIF2α Kinase PKR Modulates the Hypoxic Response by Stat3-Dependent Transcriptional Suppression of HIF-1α. Cancer Res 70:7820–7829. [CrossRef]
- Watanabe T, Ninomiya H, Saitou T, et al (2020) Therapeutic effects of the PKR inhibitor C16 suppressing tumor proliferation and angiogenesis in hepatocellular carcinoma in vitro and in vivo. Sci Rep 10:5133. [CrossRef]
- Chen J-J (2025) HRI protein kinase in cytoplasmic heme sensing and mitochondrial stress response: Relevance to hematological and mitochondrial diseases. J Biol Chem 301:108494. [CrossRef]
- Girardin SE, Cuziol C, Philpott DJ, Arnoult D (2021) The eIF2α kinase HRI in innate immunity, proteostasis, and mitochondrial stress. FEBS J 288:3094–3107. [CrossRef]
- Sekine Y, Houston R, Eckl E-M, et al (2023) A mitochondrial iron-responsive pathway regulated by DELE1. Mol Cell 83:2059-2076.e6. [CrossRef]
- Chakrabarty Y, Yang Z, Chen H, Chan DC (2024) The HRI branch of the integrated stress response selectively triggers mitophagy. Mol Cell 84:1090-1100.e6. [CrossRef]
- Fu Y, Sacco O, DeBitetto E, et al (2023) Mitochondrial DNA breaks activate an integrated stress response to reestablish homeostasis. Mol Cell 83:3740-3753.e9. [CrossRef]
- Koncha RR, Ramachandran G, Sepuri NB V., Ramaiah KVA (2021) CCCP-induced mitochondrial dysfunction – characterization and analysis of integrated stress response to cellular signaling and homeostasis. FEBS J 288:5737–5754. [CrossRef]
- Perea V, Baron KR, Dolina V, et al (2023) Pharmacologic activation of a compensatory integrated stress response kinase promotes mitochondrial remodeling in PERK-deficient cells. Cell Chem Biol 30:1571-1584.e5. [CrossRef]
- Guo X, Aviles G, Liu Y, et al (2020) Mitochondrial stress is relayed to the cytosol by an OMA1–DELE1–HRI pathway. Nature 579:427–432. [CrossRef]
- Baron KR, Oviedo S, Krasny S, et al (2025) Pharmacologic Activation of Integrated Stress Response Kinases Inhibits Pathologic Mitochondrial Fragmentation.
- Bora P, Zaman M, Oviedo S, et al (2025) Drug repurposing screen identifies an HRI activating compound that promotes adaptive mitochondrial remodeling in MFN2-deficient cells. Proc Natl Acad Sci 122:. [CrossRef]
- Rosen MD, Woods CR, Goldberg SD, et al (2009) Discovery of the first known small-molecule inhibitors of heme-regulated eukaryotic initiation factor 2α (HRI) kinase. Bioorg Med Chem Lett 19:6548–6551. [CrossRef]
- Qin D, Hu J, Yang Y, et al (2025) Endothelial GTPBP3 directs developmental angiogenesis and neovascularization after limb ischemia via the mtROS/HRl/ATF4/mTORC1 axis. Angiogenesis 28:36. [CrossRef]
- Upcin B, Szi-Marton D, Henke E, et al (2020) Abstract 688: Targeting tumor associated host cells by modifiers of integrated endoplasmic reticulum stress response. Cancer Res 80:688–688. [CrossRef]
- Halliday M, Radford H, Sekine Y, et al (2015) Partial restoration of protein synthesis rates by the small molecule ISRIB prevents neurodegeneration without pancreatic toxicity. Cell Death Dis 6:e1672–e1672. [CrossRef]
- Hughes DT, Halliday M, Smith HL, et al (2020) Targeting the kinase insert loop of PERK selectively modulates PERK signaling without systemic toxicity in mice. Sci Signal 13:. [CrossRef]
- Yu Q, Zhao B, Gui J, et al (2015) Type I interferons mediate pancreatic toxicities of PERK inhibition. Proc Natl Acad Sci 112:15420–15425. [CrossRef]
- Mercado G, Castillo V, Soto P, et al (2018) Targeting PERK signaling with the small molecule GSK2606414 prevents neurodegeneration in a model of Parkinson’s disease. Neurobiol Dis 112:136–148. [CrossRef]
- Vieira FG, Tassinari VR, Kidd JD, et al (2024) PERK modulation, with GSK2606414, Sephin1 or salubrinal, failed to produce therapeutic benefits in the SOD1G93A mouse model of ALS. PLoS One 19:e0292190. [CrossRef]
- Moreno JA, Halliday M, Molloy C, et al (2013) Oral Treatment Targeting the Unfolded Protein Response Prevents Neurodegeneration and Clinical Disease in Prion-Infected Mice. Sci Transl Med 5:. [CrossRef]
- Rojas-Rivera D, Delvaeye T, Roelandt R, et al (2017) When PERK inhibitors turn out to be new potent RIPK1 inhibitors: critical issues on the specificity and use of GSK2606414 and GSK2656157. Cell Death Differ 24:1100–1110. [CrossRef]
- McLaughlin M, Pedersen M, Roulstone V, et al (2020) The PERK Inhibitor GSK2606414 Enhances Reovirus Infection in Head and Neck Squamous Cell Carcinoma via an ATF4-Dependent Mechanism. Mol Ther oncolytics 16:238–249. [CrossRef]
- Axten JM, Medina JR, Feng Y, et al (2012) Discovery of 7-Methyl-5-(1-{[3-(trifluoromethyl)phenyl]acetyl}-2,3-dihydro-1 H -indol-5-yl)-7 H -pyrrolo[2,3- d ]pyrimidin-4-amine (GSK2606414), a Potent and Selective First-in-Class Inhibitor of Protein Kinase R (PKR)-like Endoplasmic Reticulum Kinase (P. J Med Chem 55:7193–7207. [CrossRef]
- Pandey J, Larson-Casey JL, Pinthong N, Carter AB (2025) PERK Inhibitor: GSK2656157 Reverses Established Pulmonary Fibrosis. Am J Respir Crit Care Med 211:A3315–A3315. [CrossRef]
- Axten JM, Romeril SP, Shu A, et al (2013) Discovery of GSK2656157: An Optimized PERK Inhibitor Selected for Preclinical Development. ACS Med Chem Lett 4:964–968. [CrossRef]
- Szaruga M, Janssen DA, de Miguel C, et al (2023) Activation of the integrated stress response by inhibitors of its kinases. Nat Commun 14:. [CrossRef]
- Bruch J, Xu H, Rösler TW, et al (2017) PERK activation mitigates tau pathology in vitro and in vivo. EMBO Mol Med 9:371–384. [CrossRef]
- Halofuginone Safety Data Sheet. https://www.sigmaaldrich.com/US/en/sds/mm/5.05763.
- Roffe S, Hagai Y, Pines M, Halevy O (2010) Halofuginone inhibits Smad3 phosphorylation via the PI3K/Akt and MAPK/ERK pathways in muscle cells: Effect on myotube fusion. Exp Cell Res 316:1061–1069. [CrossRef]
- Nelson EF, Huang CW, Ewel JM, et al (2012) Halofuginone down-regulates Smad3 expression and inhibits the TGFbeta-induced expression of fibrotic markers in human corneal fibroblasts. Mol Vis 18:479–87. https://doi.org/22393274.
- Pines M (2008) Targeting TGFβ signaling to inhibit fibroblast activation as a therapy for fibrosis and cancer: effect of halofuginone. Expert Opin Drug Discov 3:11–20. [CrossRef]
- Juárez P, Fournier PGJ, Mohammad KS, et al (2017) Halofuginone inhibits TGF-β/BMP signaling and in combination with zoledronic acid enhances inhibition of breast cancer bone metastasis. Oncotarget 8:86447–86462. [CrossRef]
- Chen H, Wang L, D’Mello SR (2008) A chemical compound commonly used to inhibit PKR, {8-(imidazol-4-ylmethylene)-6H-azolidino[5,4-g] benzothiazol-7-one}, protects neurons by inhibiting cyclin-dependent kinase. Eur J Neurosci 28:2003–2016. [CrossRef]
- -Aminopurine Safety Data Sheet. www.sigmaaldrich.com/US/en/sds/sigma/a3509.
- Huang S, Qu L-K, Cuddihy AR, et al (2003) Protein kinase inhibitor 2-aminopurine overrides multiple genotoxic stress-induced cellular pathways to promote cell survival. Oncogene 22:3721–3733. [CrossRef]
- Sowers LC, Boulard Y, Fazakerley GV (2000) Multiple Structures for the 2-Aminopurine−Cytosine Mispair. Biochemistry 39:7613–7620. [CrossRef]
- Rabouw HH, Langereis MA, Anand AA, et al (2019) Small molecule ISRIB suppresses the integrated stress response within a defined window of activation. Proc Natl Acad Sci 116:2097–2102. [CrossRef]
- Alsterda A, Asha K, Powrozek O, et al (2021) Salubrinal Exposes Anticancer Properties in Inflammatory Breast Cancer Cells by Manipulating the Endoplasmic Reticulum Stress Pathway. Front Oncol 11:. [CrossRef]
- Matsuoka M, Komoike Y (2015) Experimental Evidence Shows Salubrinal, an eIF2α Dephosphorylation Inhibitor, Reduces Xenotoxicant-Induced Cellular Damage. Int J Mol Sci 16:16275–87. [CrossRef]
- Boyce M, Bryant KF, Jousse C, et al (2005) A Selective Inhibitor of eIF2α Dephosphorylation Protects Cells from ER Stress. Science (80- ) 307:935–939. [CrossRef]
- Kessel D (2006) Protection of Bcl-2 by salubrinal. Biochem Biophys Res Commun 346:1320–1323. [CrossRef]
- Koohestani F, Qiang W, MacNeill AL, et al (2016) Halofuginone suppresses growth of human uterine leiomyoma cells in a mouse xenograft model. Hum Reprod 31:1540–1551. [CrossRef]
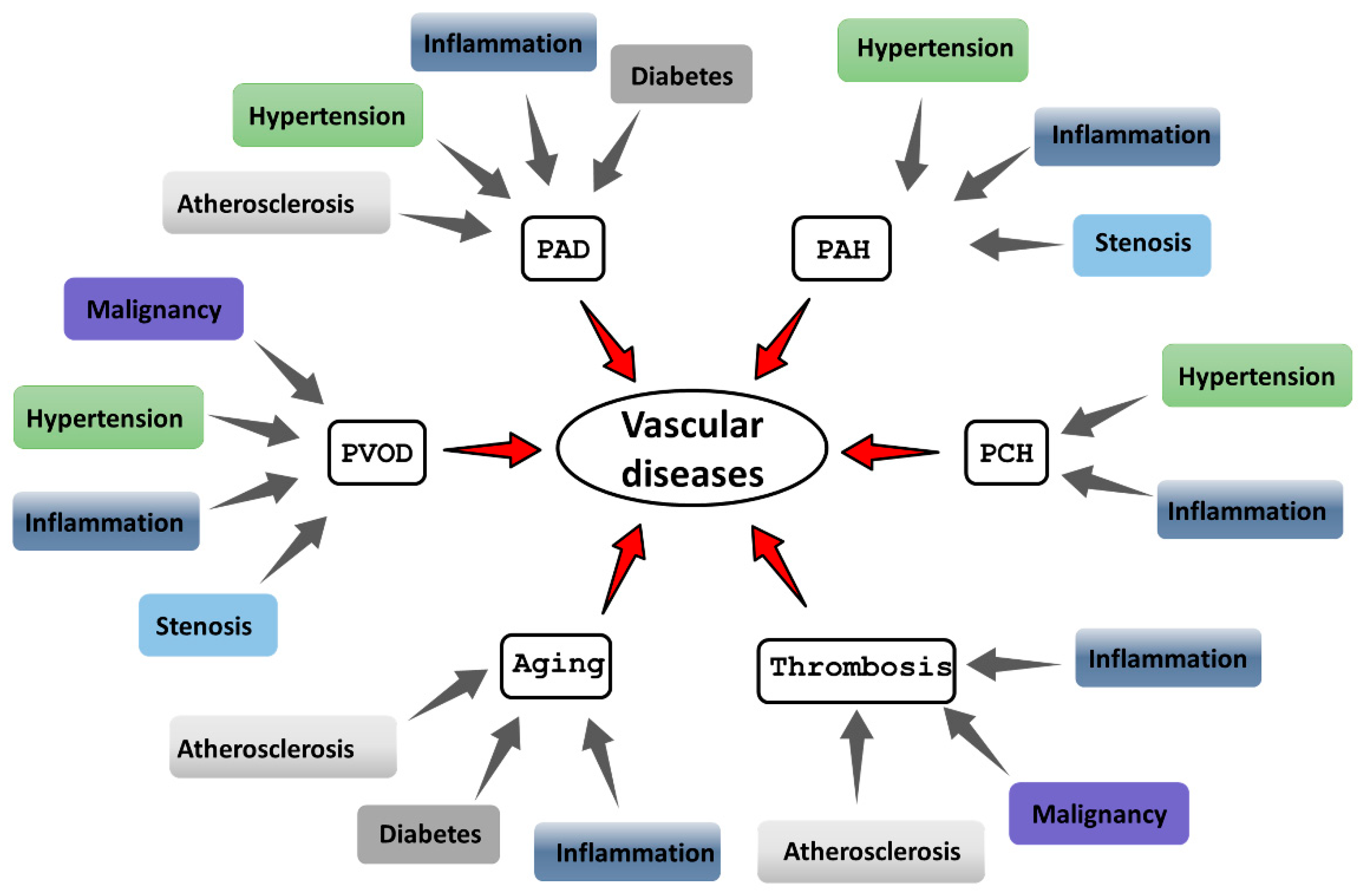
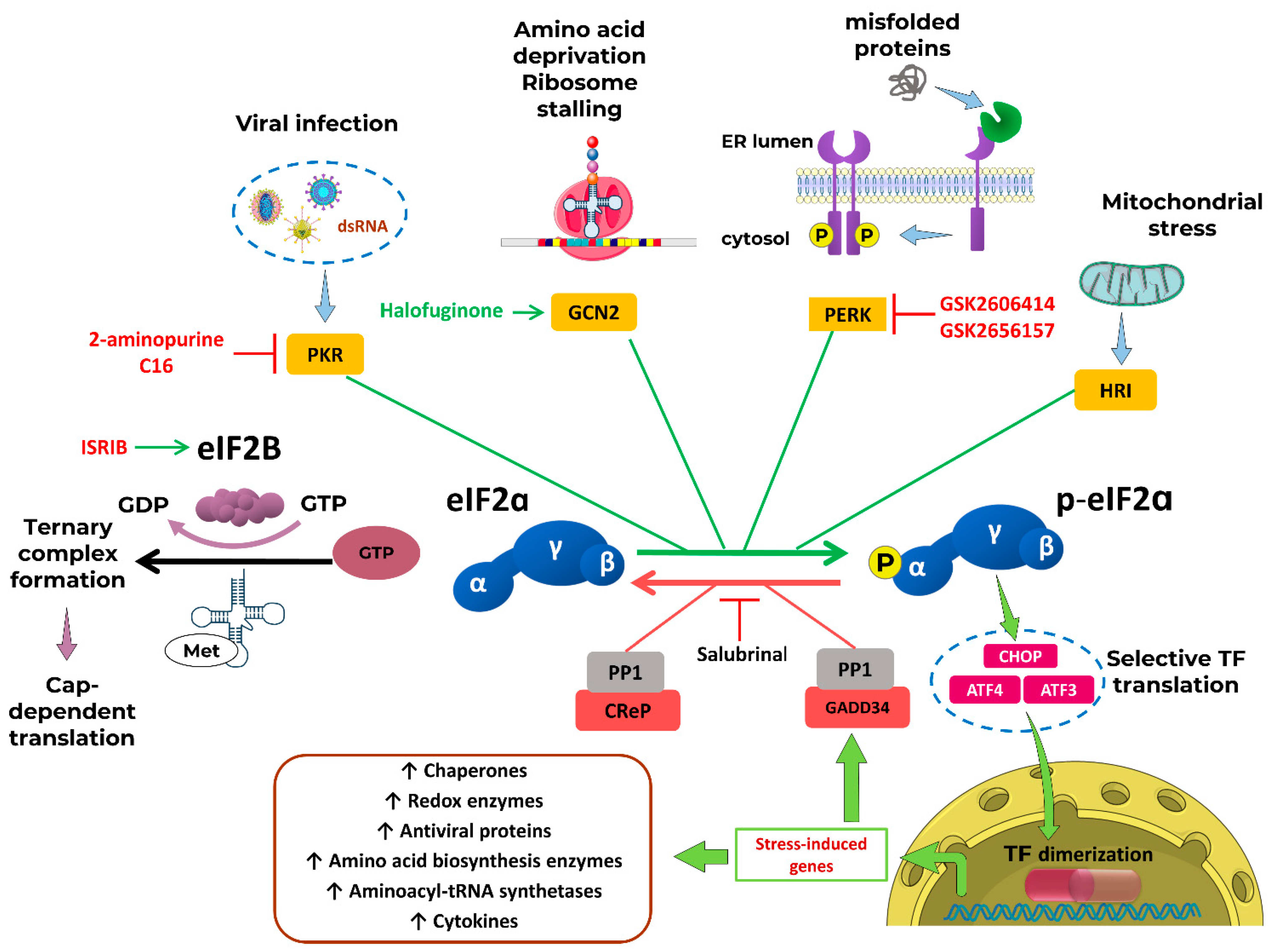
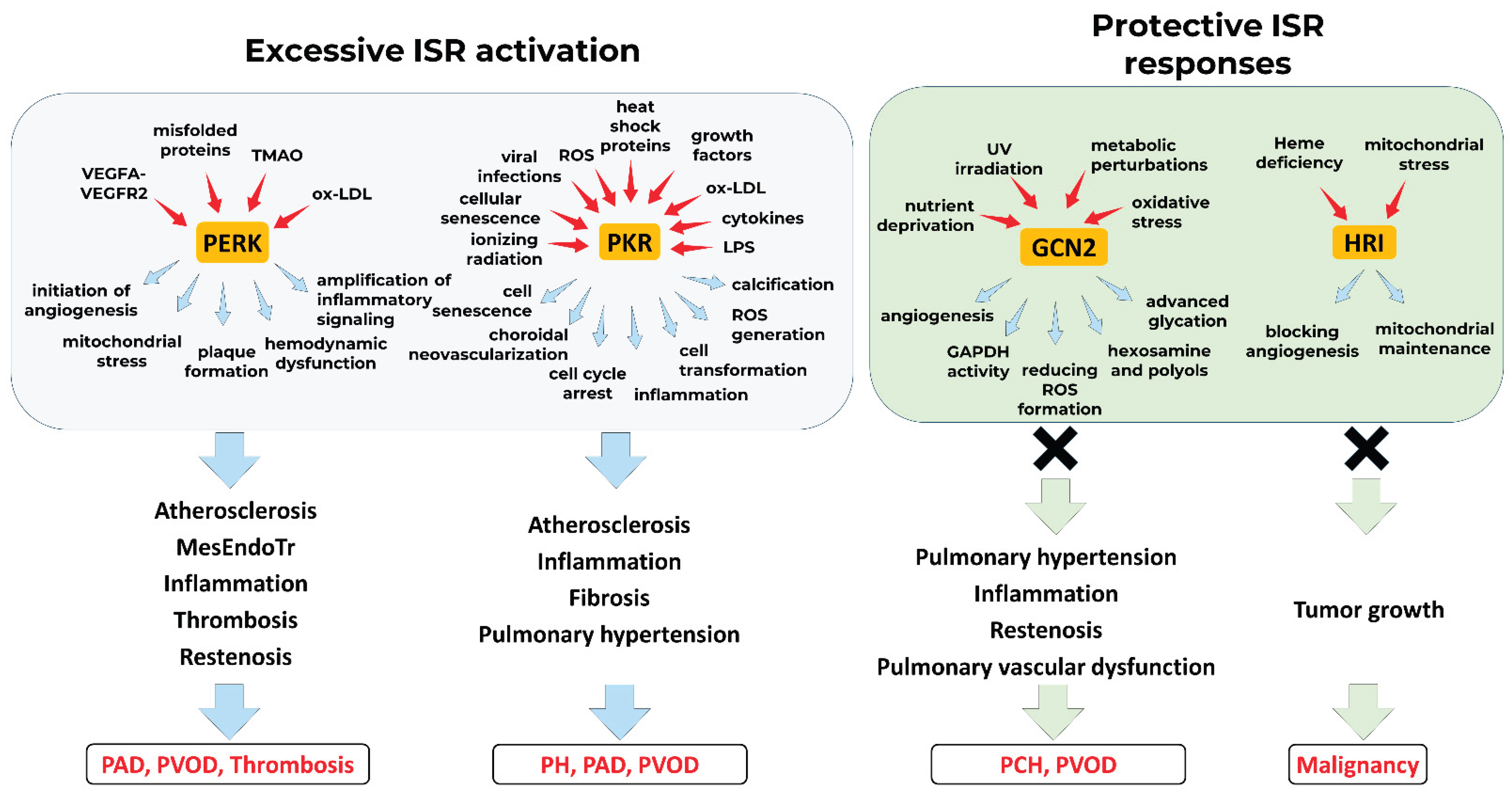
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).




