Submitted:
10 December 2025
Posted:
11 December 2025
You are already at the latest version
Abstract
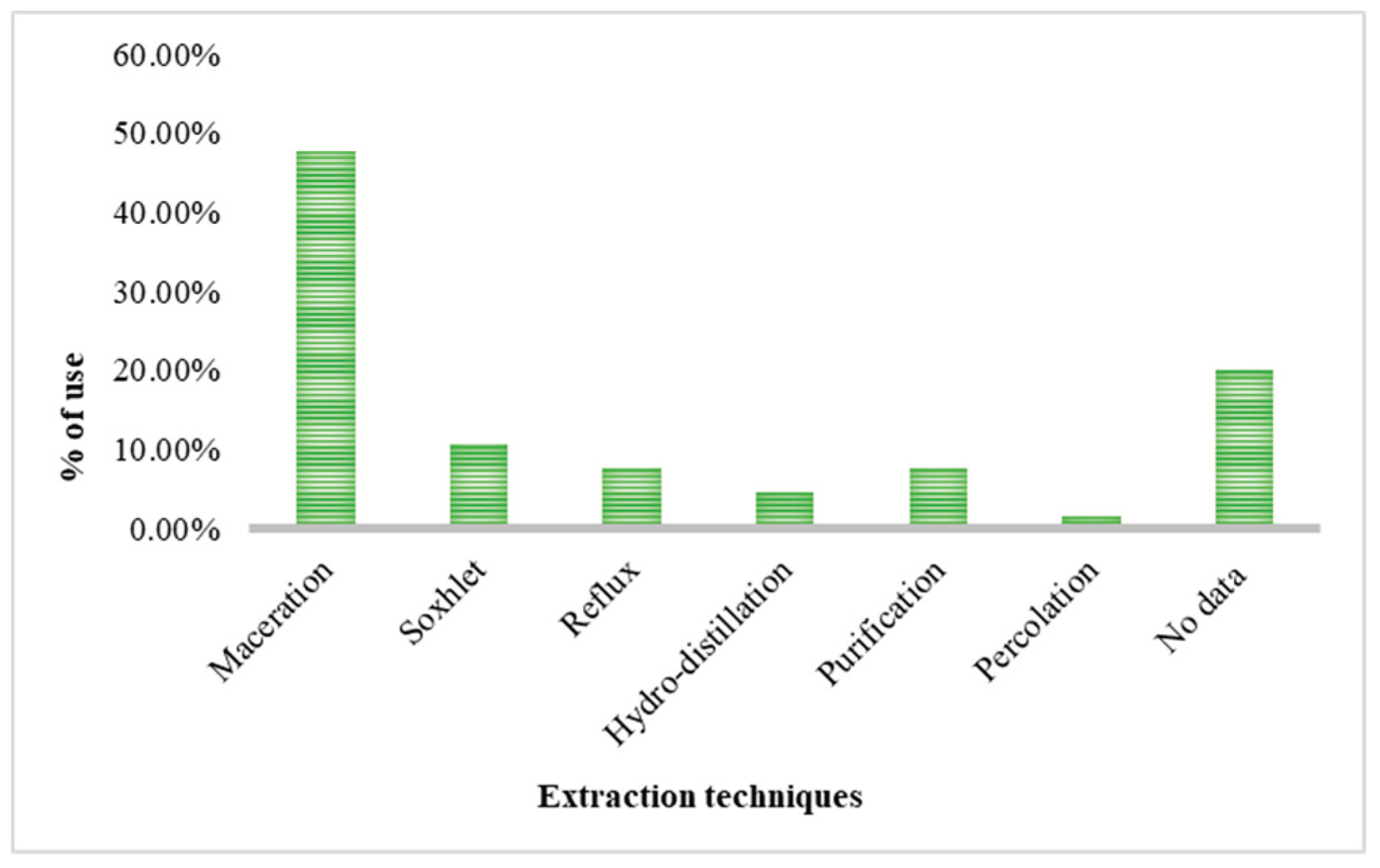
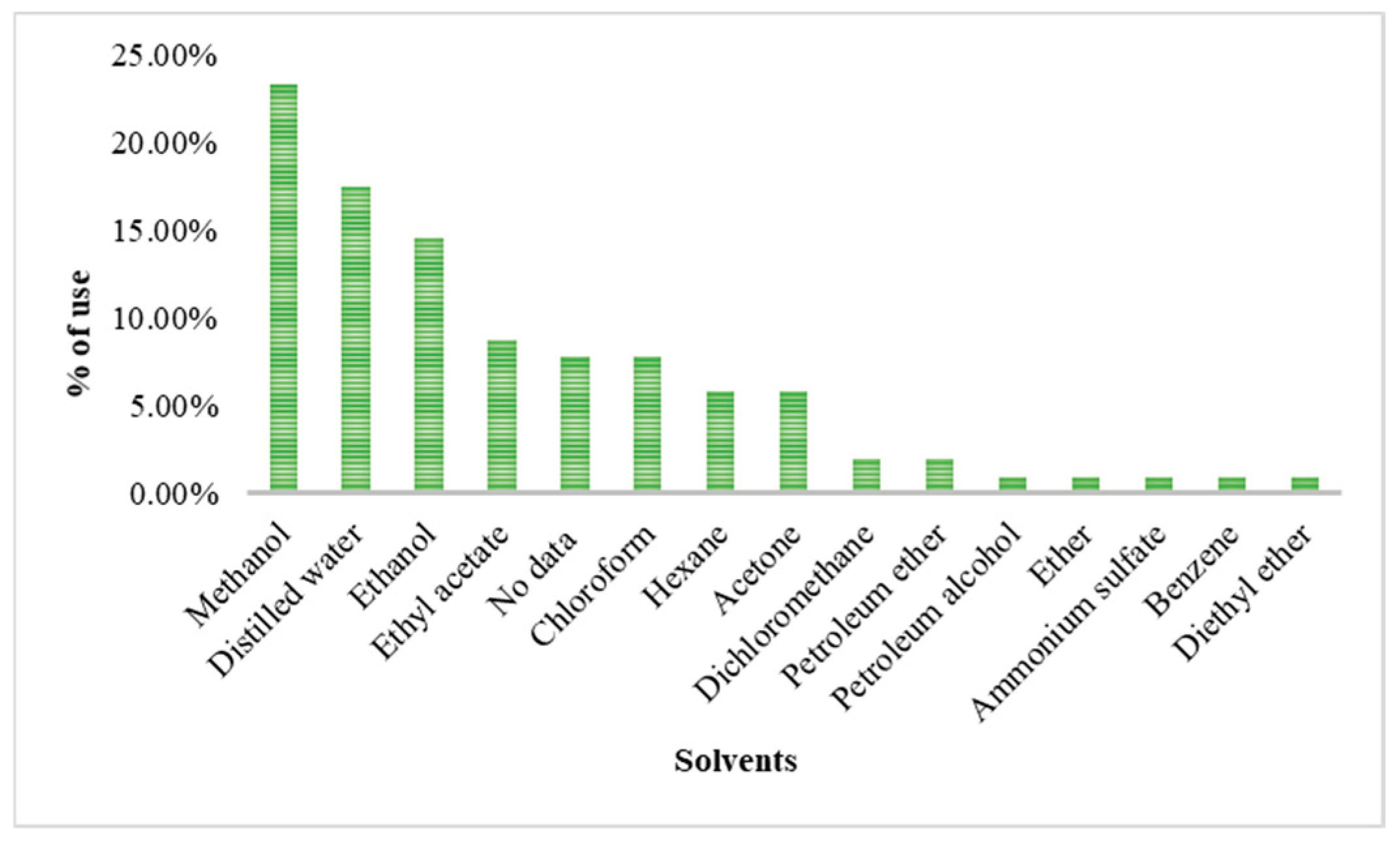
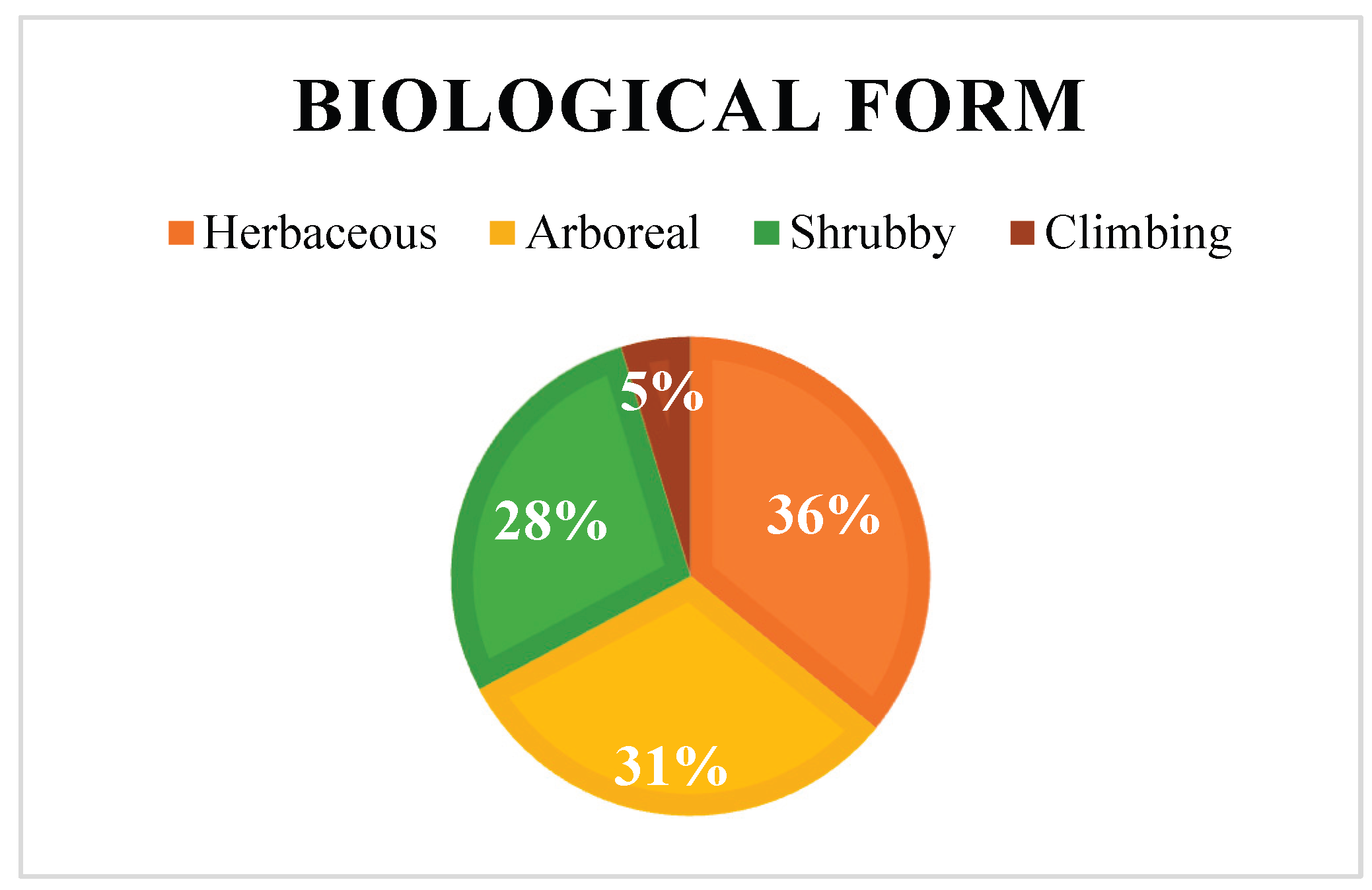
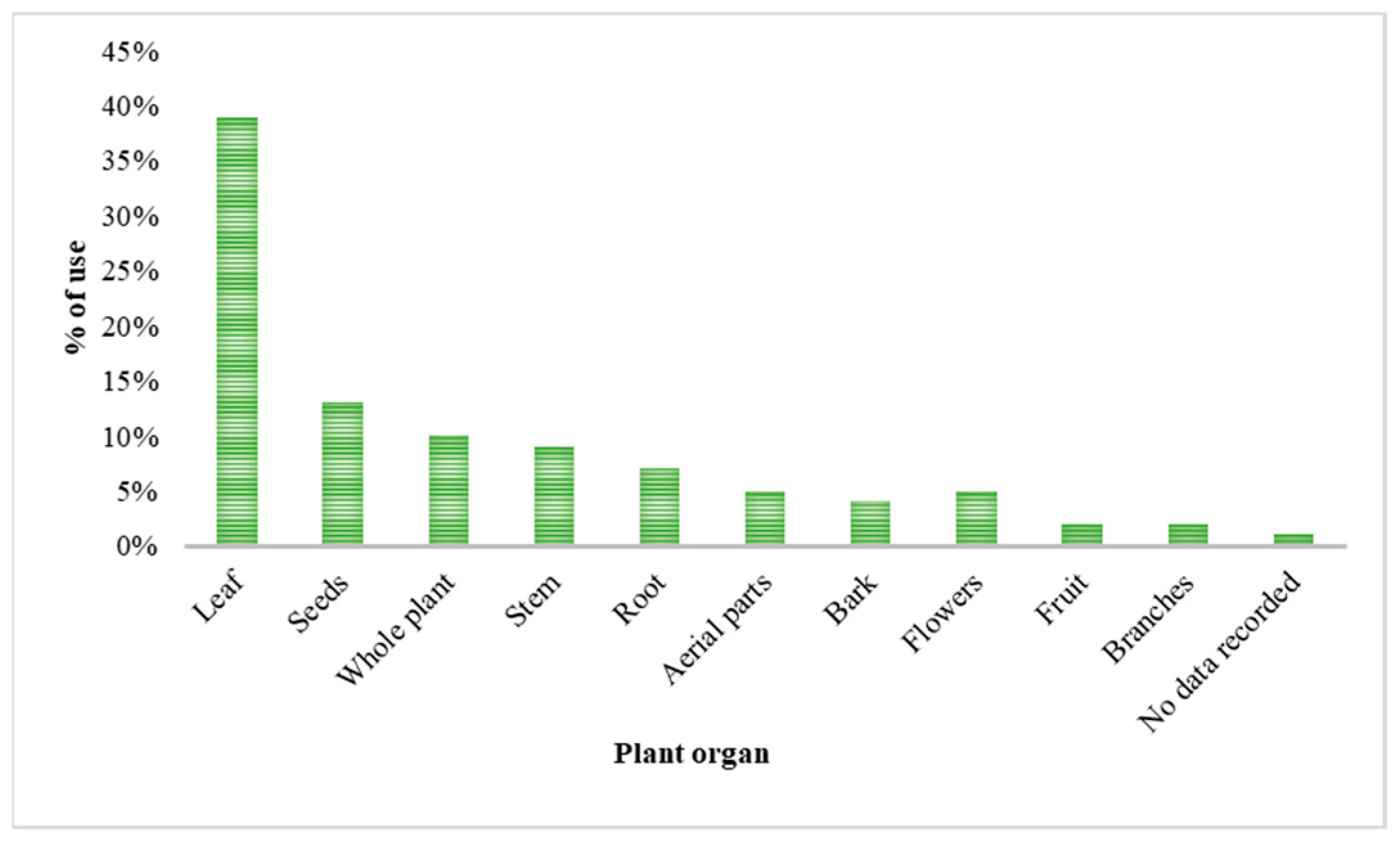
The increasing resistance to antibiotics resulting from their indiscriminate use in humans and animals is a serious public health concern recognized by the WHO and WOAH. In this context, phytotherapy based on medicinal plants represents a promising alternative, particularly due to the presence of bioactive compounds such as flavonoids and alkaloids with antimicrobial potential. The Fabaceae family stands out for its remarkable diversity and pharmacological relevance. This review integrates available information on the 347 species recorded in the state of Tamaulipas, Mexico. Only 64 species have been subjected to phytochemical studies, and 46 are traditionally used in medicine, mainly to treat digestive disorders (32%), dermatological conditions (18%), and parasitic infections (15%). The most frequently reported metabolites are tannins and flavonoids, which support their empirical use and therapeutic potential. The main extraction techniques identified were maceration (47.7%) and Soxhlet (10.8%), employing solvents such as methanol (21.5%), water, ethanol, ethyl acetate, and hexane. Herbaceous and arboreal plants were the most investigated. Phenols and flavonoids exhibited antioxidant properties with antibacterial and antifungal activity, whereas alkaloids showed antibacterial, antifungal, anticancer, and anti-inflammatory effects. The greatest metabolic diversity was found in leaves. Microbiological studies highlight notable activity against Staphylococcus aureus, Escherichia coli, and Candida albicans, mainly evaluated through the disk diffusion method.
Keywords:
1. Introduction
2. Results and Discussion
2.1. Applications in Traditional Medicine
2.2. Extraction Methods
2.2.1. Solvents
2.2.2. Biological Categories
2.3. Isolated Compounds
2.3.1. Properties of the Main Isolated Compounds
2.4. Evaluated Microorganisms
- A)
- B)
- Escherichia coli (Gram-negative bacterium), inhibited by tannins, terpenoids, and hydrolyzed proteins. Tannins modulate the bacterial cell membrane, while specific terpenoids produce significant inhibition zones (5.5–6 mm). Antimicrobial flavonoids act by inhibiting DNA gyrase, thereby affecting cell replication [14].
- C)
2.5. Antimicrobial Evaluation Techniques
3. Materials and Methods
4. Conclusions
Author Contributions
Acknowledgments
Conflicts of Interest
References
- Sakkas, H.; Papadopoulou, C. Antimicrobial Activity of Basil, Oregano, and Thyme Essential Oils. J Microbiol Biotechnol 2017, 27(3), 429–438. [Google Scholar] [CrossRef]
- Rodríguez-Flores, O.R. Plantas utilizadas para el tratamiento de enfermedades en los animales domésticos; Reserva Natural El Tisey, Estelí., 2005. [Google Scholar]
- Organizacion Mundial, d.l.s., OMS. Cuidados Innovadores para las condiciones crónicas. In Organización y prestación de atención de alta calidad a las enfermedades crónicas no transmisibles en las Américas; 2025.
- animal, O.m.d.l.s., OMSA. Riesgos sanitarios mundiales y desafíos del mañana. 2025.
- Rodríguez-Garza, N.E. In vitro biological activity and lymphoma cell growth inhibition by selected mexican medicinal plants. Life 2023, 13(4), 958. [Google Scholar] [CrossRef]
- Salehi, B. Antioxidants: positive or negative actors? Biomolecules 2018, 8(4), 124. [Google Scholar] [CrossRef]
- El-Saber Batiha, G. Chemical constituents and pharmacological activities of garlic (Allium sativum L.): A review. 2020, 12(3), 872.
- Camacho-Escobar, M.A. Las defensas físico-químicas de las plantas y su efecto en la alimentación de los rumiantes. 2020, 38(2), 443–453.
- Sabir, A. article Therapeutic Potential of Fabaceae Species: A Phytochemical and Bioactivity Investigation. Ars Pharmaceutica (Internet) 2025, 66(3), 301–313. [Google Scholar] [CrossRef]
- Rubio-Pequeño, L.G. Riqueza y distribución de leguminosas en un gradiente ambiental dentro del Área Natural Protegida Altas Cumbres, Tamaulipas, México. Botanical Sciences 2024, 102(4), 1284–1299. [Google Scholar] [CrossRef]
- Molares, S.; Ladio, A. The usefulness of edible and medicinal Fabaceae in Argentine and Chilean Patagonia: environmental availability and other sources of supply. Evidence-Based Complementary and Alternative Medicine 2012, 2012(1), 901918. [Google Scholar] [CrossRef] [PubMed]
- Llamas García, F.; Acedo Casado, M.C. Las leguminosas (Leguminosae o Fabaceae): una síntesis de las clasificaciones, taxonomía y filogenia de la familia a lo largo del tiempo. AmbioCiencias 2016. [Google Scholar]
- Medellín-Morales, S.G. Traditional knowledge and valuation of useful plants in el cielo biosphere reserve, tamaulipas, mexico. Agricultura, sociedad y desarrollo 2018, 15(3), 354–377. [Google Scholar]
- Gallegos-Flores, P.I. Antibacterial activity of five terpenoid compounds: carvacrol, limonene, linalool, α-terpinene and thymol. 2019.
- Villacrés-Vallejo, J. Conservación de la familia Fabácea en el Jardín Botánico del Instituto de Medicina Tradicional-EsSalud. 2021.
- Castañeda, R. Leguminosas (Fabaceae) silvestres de uso medicinal del distrito de Lircay, provincia de Angaraes (Huancavelica, Perú). Boletín latinoamericano y del Caribe de plantas medicinales y aromáticas 2017, 16(2), 136–149. [Google Scholar]
- Lozano, H.B. Investigation of the Antimicrobial Activity and Secondary Metabolites of Leaf Extracts from Vachellia Rigidula, Vachellia Farnesiana, Senegalia Berlandiery, and Senegalia Gregii. 2021.
- Nava-Solis, U. Antimicrobial activity of the methanolic leaf extract of Prosopis laevigata. Scientific reports 2022, 12(1), 20807. [Google Scholar] [CrossRef] [PubMed]
- Tajbakhsh, S. Invitro antibacterial activity of the Prosopis juliflora seed pods on some common pathogens. Journal of clinical and diagnostic research: JCDR 2015, 9(8), p. DC13. [Google Scholar] [CrossRef]
- Bezerra dos Santos, A.T. Organic extracts from Indigofera suffruticosa leaves have antimicrobial and synergic actions with erythromycin against Staphylococcus aureus. Frontiers in Microbiology 2015, 6, 13. [Google Scholar] [CrossRef]
- Feng, L. Chemical Composition and Antibacterial, Antioxidant, and Cytotoxic Activities of Essential Oils from Leaves and Stems of Aeschynomene indica L. Molecules 2024, 29(15), 3552. [Google Scholar] [CrossRef] [PubMed]
- Matela, K.S.; Mekbib, S.B.; Pillai, M.K. Antimicrobial activities of extracts from Gleditsia triacanthos L. and Schinus molle L. Pharmacol Online 2018, 2, 85–92. [Google Scholar]
- Baez, D.A.; Zepeda Vallejo, L.G.; Jimenez-estrada, M. Phytochemical studies on Senna skinneri and Senna wislizeni. Natural Product Letters 1999, 13(3), 223–228. [Google Scholar] [CrossRef]
- Borges-Argáez, R. Estudios citotóxicos y efectos in vitro de trans-3, 4, 4’, 5-tetrametoxiestilbeno, compuesto bioactivo aislado de Lonchocarpus punctatus Kunth. Polibotánica 2017, 43, 165–175. [Google Scholar]
- Vargas-Hernández, M. Bioactivity and gene expression studies of an arbustive Mexican specie Acaciella angustissima (Timbe). Industrial Crops and Products 2014, 52, 649–655. [Google Scholar] [CrossRef]
- Chan, E.W.L. Galloylated flavonol rhamnosides from the leaves of Calliandra tergemina with antibacterial activity against methicillin-resistant Staphylococcus aureus (MRSA). Phytochemistry 2014, 107, 148–154. [Google Scholar] [CrossRef]
- Fonseca, V.J.A. Lectins ConA and ConM extracted from Canavalia ensiformis (L.) DC and Canavalia rosea (Sw.) DC inhibit planktonic Candida albicans and Candida tropicalis. Archives of Microbiology 2022, 204(6), 346. [Google Scholar] [CrossRef]
- Lossio, C.F. Lectin from Canavalia villosa seeds: A glucose/mannose-specific protein and a new tool for inflammation studies. International Journal of Biological Macromolecules 2017, 105, 272–280. [Google Scholar] [CrossRef] [PubMed]
- Cortes-Morales, J.A. In vitro anthelmintic activity and colocalization analysis of hydroxycinnamic acids obtained from Chamaecrista nictitans against two Haemonchus contortus isolates. Veterinary Parasitology 2024, 331, 110282. [Google Scholar] [CrossRef] [PubMed]
- Belofsky, G. Activity of isoflavans of Dalea aurea (Fabaceae) against the opportunistic ameba Naegleria fowleri. Planta medica 2006, 72(04), 383–386. [Google Scholar] [CrossRef]
- Morales-Ubaldo, Y.A. Dalea bicolor: Una alternativa para el tratamiento de bacterias de importancia en salud pública. Revista de Investigaciones Veterinarias del Perú 2022, 33(6). [Google Scholar] [CrossRef]
- Villa-Ruano, N. Chemical profile, nutraceutical and anti-phytobacterial properties of the essential oil from Dalea foliolosa (Fabaceae). Emirates Journal of Food & Agriculture (EJFA) 2017, 29(9), 724–728. [Google Scholar]
- Belofsky, G. Antimicrobial isoflavans and other metabolites of Dalea nana. Phytochemistry 2024, 226, 114224. [Google Scholar] [CrossRef]
- Pitkin, F. A comparative study of the antimicrobial effects of the Desmodium incanum and the Moringa oleifera extracts on select microbes. Int J Public Health Health Syst 2019, 4, 27–35. [Google Scholar]
- Sarmiento-Pacurucu, J. DNA barcode analysis and antioxidant activity of Desmodium molliculum and Desmodium adscendens. 2024.
- Ndukwe, I. Phytochemical and antimicrobial studies on the leaves and stem ofDesmodium scorpiurus Der (sw). African Journal of Biotechnology 2006, 5(19). [Google Scholar]
- Rodríguez, J.-L. Chemical Characterization, Antioxidant Capacity and Anti-Oxidative Stress Potential of South American Fabaceae Desmodium tortuosum. nutrients 2023, 15(3), 746. [Google Scholar] [CrossRef] [PubMed]
- Gomez-Flores, R. In vitro antimicrobial activity and polyphenolics content of tender and mature Ebenopsis ebano seeds. Medicinal Plants-International Journal of Phytomedicines and Related Industries 2009, 1(1), 11–19. [Google Scholar] [CrossRef]
- Cagri-Mehmetoglu, A.; Sowemimo, A.; van de Venter, M. Evaluation of antibacterial activity and phenolic contents of four nigerian medicinal plants. International Journal 2017, 4(1), 13. [Google Scholar] [CrossRef]
- Tanaka, H. Antibacterial constituents from the roots of Erythrina herbacea against methicillin-resistant Staphylococcus aureus. Planta medica 2010, 76(09), 916–919. [Google Scholar] [CrossRef]
- Pablo-Pérez, S.S.; Estévez-Carmona, M.M.; Meléndez-Camargo, M.E. Diuretic activity of the bark of Eysenhardtia polystachya. Bangladesh Journal of Pharmacology 2016, 11(1), 212–217. [Google Scholar] [CrossRef]
- Ragab, E.A. Acylated triterpenoidal saponins and cytokinins from Gleditsia aquatica. J Pharmacog Phytother 2010, 2, 24–33. [Google Scholar]
- Kumar, N.S.; Simon, N. In vitro antibacterial activity and phytochemical analysis of Gliricidia sepium (L.) leaf extracts. Journal of Pharmacognosy and Phytochemistry 2016, 5(2), 131–133. [Google Scholar]
- Adeniyi, B.A. Antibacterial and antifungal activities of methanol extracts of Desmodium adscendens root and Bombax buonopozense leaves. International Journal of Biological and Chemical Sciences 2013, 7(1), 185–194. [Google Scholar] [CrossRef]
- Sharma, R.; Parashar, B.; Kabra, A. Efficacy of aqueous and methanolic extracts of plant Desmodium triflorum for potential antibacterial activity. International Journal of Pharmaceutical Sciences and Research 2013, 4(5), 1975. [Google Scholar]
- Rivero-Cruz, J.F. Antimicrobial compounds isolated from Haematoxylon brasiletto. Journal of Ethnopharmacology 2008, 119(1), 99–103. [Google Scholar] [CrossRef] [PubMed]
- Lozano, C.M. In vitro antimicrobial activity screening of tropical medicinal plants used in Santo Domingo, Dominican Republic. Part I. Pharmacognosy Communications 2013, 3(2), 64. [Google Scholar] [CrossRef]
- Aderibigbe, S. A.; Adetunji, O. A.; Odeniyi, M. A. Antimicrobial and Pharmaceutical Properties of The Seed Oil of Leucaena leucocephala (Lam.) De Wit (Leguminosae). Afr. J. Biomed. Res. 2011, 14, 63–68. [Google Scholar]
- Borges-Argáez, R. Cytotoxic studies and in vitro effects of trans-3, 4, 4’, 5-tetramethoxystilbene, a bioactive compound isolated from Lonchocarpus punctatus Kunth. Polibotánica 2017, 43, 165–175. [Google Scholar] [CrossRef]
- Garibo, D. Green synthesis of silver nanoparticles using Lysiloma acapulcensis exhibit high-antimicrobial activity. scientific reports 2020, 10(1), 12805. [Google Scholar] [CrossRef]
- Prabu, P.; Losetty, V. Green synthesis of copper oxide nanoparticles using Macroptilium Lathyroides (L) leaf extract and their spectroscopic characterization, biological activity and photocatalytic dye degradation study. Journal of Molecular Structure 2024, 1301, 137404. [Google Scholar] [CrossRef]
- Pérez Narváez, O.A. Actividad antimicrobiana y antioxidante de extractos etanólicos de hoja de Arbutus xalapensis Kunt, Mimosa malacophylla Gray y Teucrium cubense Jacquin. 2019.
- Salau, A. O. Odeleye, Antimicrobial activity of Mucuna pruriens on selected bacteria. African Journal of Biotechnology 2007, 6(18). [Google Scholar] [CrossRef]
- Rahman, A.A. Antiparasitic and antimicrobial indolizidines from the leaves of Prosopis glandulosa var. glandulosa. Planta medica 2011, 77(14), 1639–1643. [Google Scholar] [CrossRef] [PubMed]
- Zainuddin, I.D. Metabolites Profiling and Antimicrobial Activities in Roots and Leaves of Neptunia Oleracea. International Journal of Pharmaceutical Research (09752366) 2020, 12(1). [Google Scholar]
- Barrera- Necha, L.L. Antifungal activity of seed powders, extracts, and secondary metabolites of Pachyrhizus erosus (L.) urban (Fabaceae) against three postharvest fungi. Revista Mexicana de Fitopatología 2004, 22(3), 356–361. [Google Scholar]
- Parveen-Qureshi, S. Antibacterial activity and phytochemical screening of crude leaves extract of Parkinsonia aculeata Linn. International Journal of Researches in Biosciences, Agriculture and Technology 2017, 2, 667–670. [Google Scholar]
- López-Millán, A. Biosynthesis of gold and silver nanoparticles using Parkinsonia florida leaf extract and antimicrobial activity of silver nanoparticles. Materials Research Express 2019, 6(9), 095025. [Google Scholar] [CrossRef]
- Ordaz-Hernández, A. Parkinsonia praecox bark as a new source of antibacterial and anticancer compounds. European Journal of Integrative Medicine 2024, 71, 102401. [Google Scholar] [CrossRef]
- Chen, J. A novel sialic acid-specific lectin from Phaseolus coccineus seeds with potent antineoplastic and antifungal activities. Phytomedicine 2009, 16(4), 352–360. [Google Scholar] [CrossRef]
- Tamayo, J. Antimicrobial, Antioxidant and Anti-Inflammatory Activities of Proteins of Phaseoulus lunatus (Fabaceae) Baby Lima Beans Produced in Ecuador. bioRxiv 2018, 401323. [Google Scholar] [CrossRef]
- Hamed, E.-S.; Ibrahim, E.; Mounir, S. Antimicrobial activities of lectins extracted from some cultivars of phaseolus vulgaris seeds. J Microb Biochem Technol 2017, 9(3), 109–116. [Google Scholar]
- Kumar, M.; Nehra, K.; Duhan, J. Phytochemical analysis and antimicrobial efficacy of leaf extracts of Pithecellobium dulce. Asian journal of pharmaceutical and clinical research 2013, 6(1), 70–76. [Google Scholar]
- Gundidza, M. Phytochemical composition and biological activities of essential oil of Rhynchosia minima (L)(DC)(Fabaceae). African Journal of Biotechnology 2009, 8(5). [Google Scholar]
- Serrano-Vega, R. Phytochemical composition, anti-inflammatory and cytotoxic activities of chloroform extract of Senna crotalarioides Kunth. American Journal of Plant Sciences 2021, 12(6), 887–900. [Google Scholar] [CrossRef]
- Essien, E.E. Senna occidentalis (L.) Link and Senna hirsuta (L.) HS Irwin & Barneby: constituents of fruit essential oils and antimicrobial activity. Natural Product Research 2019, 33(11), 1637–1640. [Google Scholar] [PubMed]
- Doughari, J.; El-Mahmood, A.; Tyoyina, I. J Antimicrobial activity of leaf extracts of Senna obtusifolia (L). African Journal of Pharmacy and Pharmacology 2008, 2(1), 7–13. [Google Scholar]
- Tsado, N. Antioxidants and antimicrobial-activities of methanol leaf extract of senna occidentalis. Journal of Advances in Medical and Pharmaceutical Sciences 2016, 8(2), 1–7. [Google Scholar] [CrossRef]
- Monteiro, J. Bioactivity and toxicity of Senna cana and Senna pendula extracts. Biochemistry Research International 2018, 2018(1), 8074306. [Google Scholar] [CrossRef]
- Alonso-Castro, A.J. Diuretic activity and neuropharmacological effects of an ethanol extract from Senna septemtrionalis (Viv.) HS Irwin & Barneby (Fabaceae). Journal of Ethnopharmacology 2019, 239, 111923. [Google Scholar] [CrossRef]
- Hussiny, S. Phytochemical investigation using GC/MS analysis and evaluation of antimicrobial and cytotoxic activities of the lipoidal matter of leaves of Sophora secundiflora and Sophora tomentosa. Archives of Pharmaceutical Sciences Ain Shams University 2020, 4(2), 207–214. [Google Scholar] [CrossRef]
- Panduranga Murthy, G.; Mokshith, M.; Ravishankar, H. Isolation, partial purification of protein and detection of Antibacterial acivity in leaf extracts of Tephrosia cinerea (L.) Pers.-An Ethno-medicinal plant practiced by Tribal activity at Biligirirangana Hills of Karnataka, India. Internation Journal of Pharma & Biosciences 2011, 2(3), 513–519. [Google Scholar]
- Lam, S.-H. Chemical constituents of Vigna luteola and their anti-inflammatory bioactivity. Molecules 2019, 24(7), 1371. [Google Scholar] [CrossRef]
- Leu, Y.-L. Constituents from Vigna vexillata and their anti-inflammatory activity. International Journal of molecular sciences 2012, 13(8), 9754–9768. [Google Scholar] [CrossRef] [PubMed]
- Agbafor, K. Chemical and antimicrobial properties of leaf extracts of Zapoteca portoricensis. 2011.
- Arunkumar, R. The essential oil constituents of Zornia diphylla (L.) Pers, and anti-inflammatory and antimicrobial activities of the oil. Records of Natural Products 2014, 8(4), 385. [Google Scholar]
- Azmir, J. Techniques for extraction of bioactive compounds from plant materials: A review. Journal of food engineering 2013, 117(4), 426–436. [Google Scholar] [CrossRef]
- Azmir, J. Techniques for extraction of bioactive compounds from plant materials: A review. Journal of food engineering 2013, 117(4), 426–436. [Google Scholar] [CrossRef]
- Azwanida, N. A review on the extraction methods use in medicinal plants, principle, strength and limitation. Med aromat plants 2015, 4(196), 2167–0412. [Google Scholar]
- Abubakar, A.R.; Haque, M. Preparation of medicinal plants: Basic extraction and fractionation procedures for experimental purposes. Journal of Pharmacy and Bioallied Sciences 2020, 12(1), 1–10. [Google Scholar] [CrossRef]
- Altemimi, A. Phytochemicals: Extraction, isolation, and identification of bioactive compounds from plant extracts. Plants 2017, 6(4), 42. [Google Scholar] [CrossRef]
- Tongnuanchan, P.; Benjakul, S. Essential oils: extraction, bioactivities, and their uses for food preservation. Journal of food science 2014, 79(7), R1231–R1249. [Google Scholar] [CrossRef]
- Vankar, P.S. Essential oils and fragrances from natural sources. Resonance 2004, 9(4), 30–41. [Google Scholar] [CrossRef]
- Sauerschnig, C. Methanol generates numerous artifacts during sample extraction and storage of extracts in metabolomics research. Metabolites 2017, 8(1), 1. [Google Scholar] [CrossRef]
- Maticorena-Quevedo, J. Neuropatía óptica y necrosis putaminal bilateral: Reporte de un caso de intoxicación por metanol. Neurología Argentina 2022, 14(1), 61–66. [Google Scholar] [CrossRef]
- Memon, A.H. A comparative study of conventional and supercritical fluid extraction methods for the recovery of secondary metabolites from Syzygium campanulatum Korth. Journal of Zhejiang University-SCIENCE B 2016, 17(9), 683–691. [Google Scholar] [CrossRef]
- Zapata-Boada, S. Techno-economic and environmental analysis of algae biodiesel production via lipid extraction using alternative solvents. Industrial & Engineering Chemistry Research 2022, 61(49), 18030–18044. [Google Scholar] [CrossRef]
- Armas-Bardales, J.J.; Teco, R.M. Vigo. Estudio etnobotánico de plantas medicinales en las comunidades El Chino y Buena Vista. Tahuayo-Perú; 2011. [Google Scholar]
- Martínez-López, G. Local uses and tradition: ethnobotanical study of usefel plants in San Pablo Cuatro Venados (Valles Centrales, Oaxaca). 2021, 52, 193–212.
- Tungmunnithum, D. Flavonoids and other phenolic compounds from medicinal plants for pharmaceutical and medical aspects: An overview. Medicines 2018, 5(3), 93. [Google Scholar] [CrossRef] [PubMed]
- Cao, H. Extensive metabolic profiles of leaves and stems from the medicinal plant Dendrobium officinale Kimura et Migo. Metabolites 2019, 9(10), 215. [Google Scholar] [CrossRef]
- Sławińska, N.; Prochoń, K.; Olas, B. A review on berry seeds—A special Emphasis on their chemical content and health-promoting properties. Nutrients 2023, 15(6), 1422. [Google Scholar] [CrossRef] [PubMed]
- Boy Mendo, F.R. Compuestos bioactivos en extractos de plantas aromáticas y medicinales de la dehesa extremeña. 2023.
- Shamsudin, N.F. Flavonoids as antidiabetic and anti-inflammatory agents: A review on structural activity relationship-based studies and meta-analysis. International journal of molecular sciences 2022, 23(20), 12605. [Google Scholar]
- Rodríguez Pérez, B. Composición química, propiedades antioxidantes y actividad antimicrobiana de propóleos mexicanos. Acta universitaria 2020, 30. [Google Scholar] [CrossRef]
- Soto Vásquez, M.R. Actividad antinociceptiva y antibacteriana de los alcaloides totales de dos especies de la familia Solanaceae. Revista Cubana de Plantas Medicinales 2014, 19(4), 361–373. [Google Scholar]
- Santa Cruz-López, C.Y. Susceptibilidad in vitro de bacterias patógenas a los extractos de Rosmarinus officinalis y Caesalpinia spinosa. Revista Cubana de Medicina Militar 2023, 52(3), e02302933–e02302933. [Google Scholar]
- Sharma, K. Saponins: A concise review on food related aspects, applications and health implications. Food Chemistry Advances 2023, 100191. [Google Scholar] [CrossRef]
- Nazzaro, F. Essential oils and antifungal activity. Pharmaceuticals 2017, 10(4), 86. [Google Scholar] [CrossRef]
- Bermúdez-Vásquez, M.J.; Granados-Chinchilla, F.; Molina, A. Composición química y actividad antimicrobiana del aceite esencial de Psidium guajava y Cymbopogon citratus. Agron. Mesoam 2019, 147–163. [Google Scholar] [CrossRef]
- Cáceres-Huambo, A. Determinación de la estructura primaria de la lectina V-2 de semillas de arveja (Pisum sativum L.) y su efecto antibacteriano en Staphylococcus aureus y Escherichia coli. Idesia (Arica) 2017, 35(1), 11–18. [Google Scholar] [CrossRef]
- Cushnie, T.T.; Lamb, A.J. Recent advances in understanding the antibacterial properties of flavonoids. International journal of antimicrobial agents 2011, 38(2), 99–107. [Google Scholar] [CrossRef]
- Domingo, D.; López-Brea, M. Plantas con acción antimicrobiana. Rev Esp Quimioterap 2003, 16(4), 385–393. [Google Scholar]
- Kaczmarek, B. Tannic acid with antiviral and antibacterial activity as a promising component of biomaterials—A minireview. Materials 2020, 13(14), 3224. [Google Scholar] [CrossRef] [PubMed]
- Balouiri, M.; Sadiki, M.; Ibnsouda, S.K. Methods for in vitro evaluating antimicrobial activity: A review. Journal of pharmaceutical analysis 2016, 6(2), 71–79. [Google Scholar] [CrossRef] [PubMed]
- Bonev, B.; Hooper, J.; Parisot, J. Principles of assessing bacterial susceptibility to antibiotics using the agar diffusion method. Journal of antimicrobial chemotherapy 2008, 61(6), 1295–1301. [Google Scholar] [CrossRef] [PubMed]
- Andrews, J.M. Determination of minimum inhibitory concentrations. Journal of antimicrobial Chemotherapy 2001, 48 suppl_1, 5–16. [Google Scholar] [CrossRef] [PubMed]
- Villaseñor, J.L. Checklist of the native vascular plants of Mexico. Revista mexicana de biodiversidad 2016, 87(3), 559–902. [Google Scholar] [CrossRef]
- Bánki, O.; Roskov, Y.; Döring, M.; Ower, G.; Hernández Robles, DR; Plata Corredor, CA; Stjernegaard Jeppesen, T.; Örn, A.; Pape, T.; Hobern, D.; Garnett, S.; Little, H.; DeWalt, RE; Miller, J.; Orrell, T.; Aalbu, R.; Abbott, J.; Aedo, C.; Aescht, E. Catálogo de la Vida (Versión 2025-09-11).; Fundación Catálogo de Vida, 2025. [Google Scholar]
| Botanical name | Synonyms | Common name in México | Traditional use | References (Study location) |
|---|---|---|---|---|
| Acaciella angustissima (Mill.) Britton & Rose | - | Guajillo | No data recorded | [25] (Queretaro, Mexico) |
| Aeschynomene indica L. | - | No data recorded | Urticaria, furuncle, nyctalopia, hepatitis, enteritis, and diarrhea. | [21] (Quzhou, China) |
| Calliandra tergemina (L.) Benth. | - | No data recorded | No data recorded | [26] (Klang, Malaysia) |
| Canavalia rosea (Sw.) DC. | - | Frijol de playa | No data recorded | [27] (Crato, Brazil) |
| Canavalia villosa Benth. | - | Gallinitas | No data recorded | [28] (Brazil) |
| Chamaecrista nictitans (L.) Moench | - | Guajito | Fever and antiviral | [29] (Morelos, Mexico) |
| Dalea aurea Nutt. ex Pursh | - | No data recorded | Diarrhea, stomach pain, and cramps | [30] (Oklahoma, USA) |
| Dalea bicolor Humb. & Bonpl. ex Willd. | - | Escobilla | Gastrointestinal problems, vomiting, and diarrhea | [31] (Hidalgo, Mexico) |
| Dalea foliolosa (Aiton) Barneby | - | Almaraduz | Anti-inflammatory and hypoglycemic | [32] (Oaxaca, Mexico) |
| Dalea nana Torr. ex A.Gray | - | Trébol enano de pradera | No data recorded | [33] (Arizona, USA) |
| Dalea versicolor Zucc. | - | No data recorded | No data recorded | [33] (Arizona, USA) |
| Desmodium incanum (Sw.) DC. | - | Amor seco | Back pain, colds, and kidney Problems | [34] (Manchester, Jamaica) |
| Desmodium molliculum (Kunth) DC. | - | Hierba de los niños | Infections, body Pain, fever, cough, dyspnea | [35] (Santa Rosa, Ecuador) |
| Desmodium scorpiurus (Sw.) Poir. | - | No data recorded | Constipation, cough, convulsions, venereal infections, tinea | [36] (Kaduna, Niger) |
| Desmodium tortuosum (Sw.) DC. | - | Cadillo | Cardiovascular events | [37] (Ucayali, Peru) |
| Ebenopsis ebano (Berland.) Barneby & J.W.Grimes | - | Ébano | No data recorded | [38] (Nuevo Leon, Mexico) |
| Enterolobium cyclocarpum (Jacq.) Griseb. | - | Guanacaste | No data recorded | [39] (Oyo, Niger) |
| Erythrina herbacea L. | - | Hierba de colorín | No data recorded | [40] (Texas, USA) |
| Eysenhardtia platycarpa Pennell & Saff. | - | No data recorded | Kidney and gallbladder diseases | [38] (Nuevo Leon, Mexico) |
| Eysenhardtia polystachya (Ortega) Sarg. | - | Palito azul | Diuretic, kidney and bladder infections | [41] (Hidalgo, Mexico) |
| Gleditsia aquatica Marshall | - | No data recorded | No data recorded | [42] (Giza, Egypt) |
| Gleditsia triacanthos L. | - | Acacia de tres espinas | Pain, whooping cough, measles, smallpox, skin diseases, asthma | [22] (South Africa) |
| Gliricidia sepium (Jacq.) Kunth | - | Cacahuananche | Wounds, diarrhea, repelling mosquitoes, fumigating | [43] (Kerala, India) |
| Grona adscendens (Sw.) H.Ohashi & K.Ohashi | Desmodium adscendens (Sw.) DC. | Amor seco | Oral-dental and urogenital problems, and opportunistic infections | [44] (Ibadan, Niger) |
| Grona triflora (L.) H.Ohashi & K.Ohashi | Desmodium triflorum (L.) DC. | Hierba cuartillo | Diarrhea, convulsions, tonic, diuretic, and biliary conditions. | [45] (Lucknow, India) |
| Haematoxylum brasiletto H.Karst. | - | Madera de Brasil | Oral and kidney infections, hypertension, gastrointestinal disorders, and diabetes. | [46] (Sonora, Mexico) |
| Indigofera suffruticosa Mill. | - | Anileira | Healing | [20] (Pernambuco, Brazil) |
| Inga vera Willd. | - | No data recorded | Treatment of diseases | [47] (Santo Domingo, Dominican Republic) |
| Leucaena leucocephala (Lam.) de Wit | - | No data recorded | Gastrointestinal | [48] (Ibadan, Niger) |
| Lonchocarpus punctatus Kunth | - | Balché | Parasitic | [49] (Yucatan, Mexico) |
| Lysiloma acapulcense (Kunth) Benth. | - | No data recorded | Respiratory, gastrointestinal, urinary, and skin infections | [50] (Baja California, Mexico) |
| Macroptilium lathyroides (L.) Urb. | - | No data recorded | No data recorded | [51] (Chennai, India) |
| Mimosa malacophylla A.Gray | - | No data recorded | Diuretic and kidney stones | [52] (Nuevo Leon, Mexico) |
| Mucuna pruriens (L.) DC. | - | Mucuna | Purgative and diuretic | [53] (Osun, Niger) |
| Neltuma glandulosa (Torr.) Britton & Rose | Prosopis glandulosa Torr. | Mesquite dulce | Gastrointestinal, rashes, eye infections, hernias, skin conditions, sore throat | [54] (Nevada, USA) |
| Neltuma juliflora ( Sw. ) Raf. | Prosopis juliflora (Sw.) DC. | Mesquite | Colds, diarrhea, flu, hoarseness, inflammation, measles, sore throat, liver and eye problems | [19] (Bushehr, Iran) |
| Neltuma laevigata (Humb. & Bonpl. ex Willd.) Britton & Rose | Prosopis laevigata (Humb. & Bonpl. ex Willd.) M.C.Johnst. | Mesquite | Skin, gastrointestinal, and respiratory diseases | [18] (Zapotitlan Salinas, Mexico) |
| Neptunia oleracea Lour. | - | Mimosa de agua | Diabetes mellitus, inflammation, and fever | [55] (Selangor, Malaysia) |
| Pachyrhizus erosus (L.) Urb. | - | Jícama | Skin rashes | [56] (Morelos, Mexico) |
| Parkinsonia aculeata L. | - | Escoba | Skin and urinary tract infections | [57] (Maharashtra, India) |
| Parkinsonia florida (Benth. ex A.Gray) S.Watson | - | Palito azul verdoso | No data recorded | [58] (Sonora, Mexico) |
| Parkinsonia praecox (Ruiz & Pav.) Hawkins | - | Palo brea | Gastrointestinal, antitussive, wound healing, headaches, earaches, and scorpion stings | [59] (Oaxaca, Mexico) |
| Phaseolus coccineus L. | - | Ayocote | No data recorded | [60] (Dali, China) |
| Phaseolus lunatus L. | - | Habas | Food | [61] (Machala, Ecuador) |
| Phaseolus vulgaris L. | - | Frijoles | Food | [62] (Giza, Egypt) |
| Pithecellobium dulce (Roxb.) Benth. | - | Jungli Jalebi | Earache, leprosy, peptic ulcer, and toothache | [63] (Haryana, India) |
| Rhynchosia minima (L.) DC. | - | Frijolillo | Skin conditions and to relieve boils. | [64] (Harare, Zimbabwe) |
| Senegalia berlandieri (Benth.) Britton & Rose | - | Espino | No data recorded | [17] (Texas, USA) |
| Senegalia greggii (A.Gray) Britton & Rose | - | Tesota | No data recorded | [17] (Texas, USA) |
| Senna crotalarioides (Kunth) H.S.Irwin & Barneby | - | No data recorded | Inflammation | [65] (San Luis Potosi, Mexico) |
| Senna hirsuta (L.) H.S.Irwin & Barneby | - | Cuajillo | Hypertension, dropsy, diabetes, fevers, bile, rheumatism, tinea, and eczema | [66] (Uyo, Niger) |
| Senna obtusifolia (L.) H.S.Irwin & Barneby | - | Tasba | Eye infection and laxative | [67] (Yola, Niger) |
| Senna occidentalis (L.) Link | - | Candelilla pequeña | Malaria and trypanosomiasis | [68] (Minna, Niger) |
| Senna pendula (Humb. & Bonpl. ex Willd.) H.S.Irwin & Barneby | - | Pito canuto | Liver diseases and psoriasis | [69] (Ceará, Brazil) |
| Senna septemtrionalis (Viv.) H.S.Irwin & Barneby | - | Cafecillo | Diuretic, anti-inflammatory, laxative, expectorant, and fungicide, fever, burns, cholera, hemorrhoids, pain, gastroenteritis. | [70] (Guanajuato, Mexico) |
| Senna wislizeni (A.Gray) H.S.Irwin & Barneby | - | Carrozo | Laxative properties, skin and parasitic diseases | [23] (Morelos, Mexico) |
| Sophora tomentosa L. | - | No data recorded | Cholera, diarrhea, gastrointestinal antidote | [71] (Giza, Egypt) |
| Tephrosia cinerea (L.) Pers. | - | Bardana medicinal | Diarrhea, diuretic, bronchitis, asthma, inflammation | [72] (Chamrajanagar, India) |
| Vachellia farnesiana (L.) Wight & Arn. | - | Huizache | No data recorded | [17] (Texas, USA) |
| Vachellia rigidula (Benth.) Seigler & Ebinger | - | Chaparro prieto | No data recorded | [17] (Texas, USA) |
| Vigna luteola (Jacq.) Benth. | - | Porotillo | No data recorded | [73] (Nantou, Taiwan) |
| Vigna vexillata (L.) A.Rich. | - | Bejuco pato | No data recorded | [74] (Nantou, Taiwan) |
| Zapoteca portoricensis (Jacq.) H.M.Hern. | - | Palo blanco | Convulsions, constipation, skin infections | [75] (Abakaliki, Niger) |
| Zornia diphylla (L.) Pers. | - | Raíz de víbora | Diarrhea and venereal diseases | [76] (Kerala, India) |
| Botanical name | Biological form | Organ used | Extraction technique | Solvent | References |
|---|---|---|---|---|---|
| Acaciella angustissima (Mill.) Britton & Rose | Shrubby | Seeds | Soxhlet | No data recorded | [25] |
| Aeschynomene indica L. | Shrubby | Leaves and stems | Hydro-distillation | Distilled water | [21] |
| Calliandra tergemina (L.) Benth. | Shrubby | Leaves | Maceration | No data recorded | [26] |
| Canavalia rosea (Sw.) DC. | Herbaceous | Seeds | Purification | Distilled water | [27] |
| Canavalia villosa Benth. | Climbing | Seeds | Purification | Distilled water | [28] |
| Chamaecrista nictitans (L.) Moench | Herbaceous | Aerial parts | Maceration | Ethyl acetate | [29] |
| Dalea aurea Nutt. ex Pursh | Herbaceous | Whole plant | Maceration | Methanol | [30] |
| Dalea bicolor Humb. & Bonpl. ex Willd. | Shrubby | Whole plant | Maceration | Methanol | [31] |
| Dalea foliolosa (Aiton) Barneby | Herbaceous | Leaves | Hydro-distillation | Distilled water | [32] |
| Dalea nana Torr. ex A.Gray | Herbaceous | Roots and aerial parts | No data recorded | No data recorded | [33] |
| Dalea versicolor Zucc. | Herbaceous | Whole plant | No data recorded | Ethanol and methanol | [33] |
| Desmodium incanum (Sw.) DC. | Herbaceous | Leaves and flowers | Maceration | Methanol and distilled water | [34] |
| Desmodium molliculum (Kunth) DC. | Herbaceous | Aerial parts | Maceration | Methanol | [35] |
| Desmodium scorpiurus (Sw.) Poir. | Herbaceous | Aerial parts | Soxhlet | Petroleum alcohol, chloroform, and methanol. | [36] |
| Desmodium tortuosum (Sw.) DC. | Shrubby | Stems and leaves | Reflux | Distilled water | [37] |
| Ebenopsis ebano (Berland.) Barneby & J.W.Grimes | Arboreal | Seeds | No data recorded | No data recorded | [38] |
| Enterolobium cyclocarpum (Jacq.) Griseb. | Arboreal | Leaves | Reflux | Ethanol | [39] |
| Erythrina herbacea L. | Shrubby | Roots | Maceration | Ethyl acetate, n-hexane, acetone | [40] |
| Eysenhardtia platycarpa Pennell & Saff. | Arboreal | Branches and leaves | Maceration | Distilled water and methanol | [38] |
| Eysenhardtia polystachya (Ortega) Sarg. | Arboreal | Bark | Reflux | Distilled water | [41] |
| Gleditsia aquatica Marshall | Arboreal | Fruit | Maceration | Ethanol | [42] |
| Gleditsia triacanthos L. | Arboreal | Leaf, seeds, and stems | Maceration | Methanol | [22] |
| Gliricidia sepium (Jacq.) Kunth | Arboreal | Leaf | Maceration | Ethanol | [43] |
| Grona adscendens (Sw.) H.Ohashi & K.Ohashi | Herbaceous | Root | Maceration | Methanol | [44] |
| Grona triflora (L.) H.Ohashi & K.Ohashi | Herbaceous | Whole plant | Maceration | Distilled water and methanol | [45] |
| Haematoxylum brasiletto H.Karst. | Arboreal | Stems | Maceration | Methanol | [46] |
| Indigofera suffruticosa Mill. | Arboreal | Leaf | Maceration | Acetone, ether, and chloroform | [20] |
| Inga vera Willd. | Arboreal | Bark | Maceration | Ethanol | [47] |
| Leucaena leucocephala (Lam.) de Wit | Arboreal | Seeds | Maceration | Hexane | [48] |
| Lonchocarpus punctatus Kunth | Arboreal | Inflorescence | Maceration | Ethanol | [49] |
| Lysiloma acapulcense (Kunth) Benth. | Arboreal | Stems and root | No data recorded | Distilled water | [50] |
| Macroptilium lathyroides (L.) Urb. | Herbaceous | Leaf | Maceration | Distilled water | [51] |
| Mimosa malacophylla A.Gray | Shrubby | Leaf | No data recorded | Ethanol | [52] |
| Mucuna pruriens (L.) DC. | Climbing | Leaf | No data recorded | Methanol | [53] |
| Neltuma glandulosa (Torr.) Britton & Rose | Arboreal | Leaf | Percolation | Ethanol | [54] |
| Neltuma juliflora (Sw.) Raf. | Arboreal | Seeds | Maceration | Distilled water, methanol, and ethyl acetate | [19] |
| Neltuma laevigata (Humb. & Bonpl. ex Willd.) Britton & Rose | Arboreal | Leaf | No data recorded | Methanol | [18] |
| Neptunia oleracea Lour. | Herbaceous | Leaf and stem | Soxhlet | Methanol | [55] |
| Pachyrhizus erosus (L.) Urb. | Herbaceous | Seeds | No data recorded | Hexane, dichloromethane, and acetone | [56] |
| Parkinsonia aculeata L. | Shrubby | Leaf | Soxhlet | Ethanol, methanol | [57] |
| Parkinsonia florida (Benth. ex A.Gray) S.Watson | Arboreal | Leaf | Reflux | Distilled water | [58] |
| Parkinsonia praecox (Ruiz & Pav.) Hawkins | Arboreal | Bark | Maceration | Methanol | [59] |
| Phaseolus coccineus L. | Herbaceous | Seeds | Purification | No data recorded | [60] |
| Phaseolus lunatus L. | Herbaceous | Seeds | Purification | No data recorded | [61] |
| Phaseolus vulgaris L. | Herbaceous | Seeds | Purification | Ammonium sulfate | [62] |
| Pithecellobium dulce (Roxb.) Benth. | Arboreal | Leaf | Maceration | Chloroform, acetone, methanol, and distilled water | [63] |
| Rhynchosia minima (L.) DC. | Climbing | Leaf | Hydro-distillation | Distilled water | [64] |
| Senegalia berlandieri (Benth.) Britton & Rose | Shrubby | Leaf | Soxhlet | Ethanol, chloroform, ethyl acetate | [17] |
| Senegalia greggii (A.Gray) Britton & Rose | Shrubby | Leaf | Soxhlet | Ethanol, chloroform, ethyl acetate | [17] |
| Senna crotalarioides (Kunth) H.S.Irwin & Barneby | Shrubby | No data recorded | No data recorded | No data recorded | [65] |
| Senna hirsuta (L.) H.S.Irwin & Barneby | Shrubby | Fruit | No data recorded | No data recorded | [66] |
| Senna obtusifolia (L.) H.S.Irwin & Barneby | Herbaceous | Leaf | Reflux | Acetone, hexane, methanol | [67] |
| Senna occidentalis (L.) Link | Herbaceous | Leaf | Maceration | Methanol | [68] |
| Senna pendula (Humb. & Bonpl. ex Willd.) H.S.Irwin & Barneby | Shrubby | Leaf, flowers, and branches | Maceration | Hexane and ethanol | [69] |
| Senna septemtrionalis (Viv.) H.S.Irwin & Barneby | Shrubby | Aerial parts | Maceration | Ethanol | [70] |
| Senna wislizeni (A.Gray) H.S.Irwin & Barneby | Shrubby | Whole plant | Maceration | Methanol and hexane | [23] |
| Sophora tomentosa L. | Shrubby | Leaf | Maceration | Petroleum ether | [71] |
| Tephrosia cinerea (L.) Pers. | Herbaceous | Leaf | No data recorded | Ethyl acetate, acetone, petroleum ether | [72] |
| Vachellia farnesiana (L.) Wight & Arn. | Arboreal | Leaf | Soxhlet | Ethanol, chloroform, ethyl acetate | [17] |
| Vachellia rigidula (Benth.) Seigler & Ebinger | Shrubby | Leaf | Soxhlet | Ethanol, chloroform, ethyl acetate | [17] |
| Vigna luteola (Jacq.) Benth. | Herbaceous | Whole plant | Maceration | Methanol | [73] |
| Vigna vexillata (L.) A.Rich. | Herbaceous | Whole plant | Maceration | Methanol, chloroform, and distilled water | [74] |
| Zapoteca portoricensis (Jacq.) H.M.Hern. | Shrubby | Leaf | Maceration | Water, methanol, ethyl acetate, diethyl ether | [75] |
| Zornia diphylla (L.) Pers. | Herbaceous | Whole plant | Hydro-distillation | Distilled water | [76] |
| Botanical name | Isolated compounds | Bioactive properties | Effect on microorganisms | Study/dose used | References |
|---|---|---|---|---|---|
| Acaciella angustissima (Mill.) Britton & Rose | Phenols and flavonoids | Antioxidants, antimutagenic, antidiabetic, anticancer, and anti-inflammatory. | Rhizoctonia solani, Fusarium oxysporum y Phytophtora capsici | Dextrose potato agar culture (200 mg/mL) | [25] |
| Aeschynomene indica L. | Essential oils | Antibacterial, antioxidant, and cytotoxic | Staphylococcus aureus y Bacillus subtilis | Broth dilution (0.312–0.625 mg/mL) |
[21] |
| Calliandra tergemina (L.) Benth. | Flavonol | Antioxidant | Staphylococcus aureus | Broth dilution (0.02–1.00 mg/mL) |
[26] |
| Canavalia rosea (Sw.) DC. | Lectins | No data recorded | Candida albicans | Microdilution (512 to 0.5 µg/mL) |
[27] |
| Canavalia villosa Benth. | Lectins | Hemagglutination activity | No data recorded | No data recorded | [28] |
| Chamaecrista nictitans (L.) Moench | Flavonoids, ellagic acid, and proanthocyanidin oligomers | Anthelmintic, antioxidant, and prebiotic | Haemonchus contortus | Ovicidal activity (2134 and 601 µg/mL) |
[29] |
| Dalea aurea Nutt. ex Pursh | Isoflavones | Anti-amebic | N. fowleri | In vitro assay (10 µg/mL) | [30] |
| Dalea bicolor Humb. & Bonpl. ex Willd. | No data recorded | No data recorded |
Salmonella choleraesuis, E. coli, Staphylococcus aureus Bacillus subtilis Pseudomonas aeruginosa Salmonella Typhi |
Broth dilution (50 and 100 mg/mL) |
[31] |
| Dalea foliolosa (Aiton) Barneby | Monoterpenes, sesquiterpenes, and aliphatic hydrocarbons | Antioxidant, anti-a-glucosidase | Pseudomonas syringae | Microdilution (35–155 μg mL-1) | [32] |
| Dalea nana Torr. ex A.Gray | Flavonoids | Antimicrobial | Cryptococcus neoformans, Staphylococcus aureus, Candida albicans. | Microdilution (6.7–37.0 μM) | [33] |
| Dalea versicolor Zucc. | Flavonoids | Antimicrobial | Staphylococcus aureus y Bacillus cereus | Microdilution (10–30 µg/mL) | [33] |
| Desmodium incanum (Sw.) DC. | No data recorded. | Antimicrobial | Staphylococcus aureus, Streptococcus y Klebsiella Pneumoniae | Well diffusion (5–100 mg/dL) |
[34] |
| Desmodium molliculum (Kunth) DC. | Flavonoids, phenols, terpenes, essential oils, and alkaloids; hypocholesterolemic and hepatoprotective effects. | Antioxidant, antibacterial, anti-inflammatory | No data recorded | No data recorded | [35] |
| Desmodium scorpiurus (Sw.) Poir. | Alkaloids, saponins, glycosides, steroids, and flavonoids. | Antibacterial | Pseudomonas aeruginosa, Escherichia coli y Streptococcus pyrogenes | Broth dilution (200 mg/mL) |
[36] |
| Desmodium tortuosum (Sw.) DC. | Phenols, flavonoids, carotenoids. | Antioxidant | No data recorded | Microdilution (200 µg/mL) |
[37] |
| Ebenopsis ebano (Berland.) Barneby & J.W.Grimes | Phenols. | Antimicrobial | Escherichia coli, S. enterica y Candida albicans | Colorimetric assay (125–500 mg/mL) |
[38] |
| Enterolobium cyclocarpum (Jacq.) Griseb. | Phenols | Antimicrobial | Serratia liquefaciens y Staphylococcus warneri | Disc diffusion (10 μl) |
[39] |
| Erythrina herbacea L. | Alkaloids | No data recorded | Staphylococcus aureus | Microdilution (6.25–50 μg/mL) |
[40] |
| Eysenhardtia platycarpa Pennell & Saff. | No data recorded | Anti-inflammatory, antifungal | No data recorded | No data recorded | [38] |
| Eysenhardtia polystachya (Ortega) Sarg. | Anthraquinones, cardiac glycosides, coumarins, reducing sugars, saponins, and tannins | Blood purifier, antitussive, antispasmodic, antidiabetic, febrifuge, anti-inflammatory, antirheumatic, and analgesic | No data recorded | In vivo activity (500 and 750 mg/kg) |
[41] |
| Gleditsia aquatica Marshall | Saponins | Cytotoxic | No data recorded | No data recorded | [42] |
| Gleditsia triacanthos L. | No data recorded | Analgesic, anti-inflammatory, hepatoprotective, and antimicrobial activity | Proteus spp., Streptococcus spp., E. coli y Enterobacter spp. y one yeast species viz.C. albicans. | Well diffusion (1000, 500, 250, 125, 62.5 and 31. 25 μg/mL) |
[22] |
| Gliricidia sepium (Jacq.) Kunth | Glycosides, phytosterols, alkaloids, oils, saponins, phenols, and flavonoids | Antibacterial, antifungal, antiviral, and antioxidant | Escherichia coli y Pseudomonas aeroginosa | Disc diffusion (0.1g/1ml) |
[43] |
| Grona adscendens (Sw.) H.Ohashi & K.Ohashi | Tannins, saponins, alkaloids, and flavonoids | Antimicrobial | Staphylococcus aureus, Candida albicans | No data recorded (0.25 - 0.50 mg/ml) |
[44] |
| Grona triflora (L.) H.Ohashi & K.Ohashi | Alkaloids, steroids, tannins, saponins, and flavonoids | Antispasmodic, sympathomimetic, central nervous system stimulant, and diuretic | Staphylococcus aureus, Micrococcus luteus, Bacillus pumilus, Pseudomonas aeruginosa, Pseudomonas fluorescens, Escheria coli | Disc diffusion (50 and 100 μg/ml) |
[45] |
| Haematoxylum brasiletto H.Karst. | Flavonoids | Antimicrobial | Candida albicans | Disc diffusion (8.7 to 128 μg/mL) |
[46] |
| Indigofera suffruticosa Mill. | Alkaloids, flavonoids, phenylpropanoids, triterpenoids, volatile oils | Anti-inflammatory and anticonvulsant | Staphylococcus aureus | Disc diffusion (0.78 - 6.25 mg/mL) |
[20] |
| Inga vera Willd. | No data recorded | Antimicrobial | Escherichia coli, Klebsiella pneumoniae, Staphylococcus aureus, Pseudomona aeruginosa, y Candida albicans | Disc diffusion (35 μg/mL) |
[47] |
| Leucaena leucocephala (Lam.) de Wit | Essential oils | Central nervous system depressant, anthelmintic, and antidiabetic | Staphylococcus aureus, Esherichia coli, Bacillus subtilis y Pseudomonas aeruginosa, Aspergilus niger, Rhizopus stolon, Penicillum notatum y Candida albicans | Microdilution (100μg/ml, 50μg/ml, 25μg/ml, 12.5μg/ml) |
[48] |
| Lonchocarpus punctatus Kunth | Alkaloids, camptothecins, epipodophyllotoxins, and taxanes | Anticancer | No data recorded | Colorimetric assay | [49] |
| Lysiloma acapulcense (Kunth) Benth. | Tannins | Antimicrobial | E. coli, P. aeruginosa, S. aureus y C. albicans | Well diffusion (2.5 µg/mL to 5.0 µg/mL) |
[50] |
| Macroptilium lathyroides (L.) Urb. | Flavonoids, polyphenols, terpenoids, saponins, and alkaloids | Antioxidant, antibacterial, cytotoxic, anticancer, and antifungal. | Staphylococcus aureus and Escherichia coli | Disc diffusion (1000 µg/mL, 750 µg/mL, and 500 µg/mL) |
[51] |
| Mimosa malacophylla A.Gray | No data available | No data | Stenotrophomonas maltophilia | Well diffusion (2.9 ± 0.5 mg/mL-1) |
[52] |
| Mucuna pruriens (L.) DC. | No data available | Astringent, laxative, anthelmintic, alexipharmic, and tonic | Staphylococcus aureus, Escherichia coli, Bacillus subtilis, Pseudomonas aeruginosa | Well diffusion (240 mg/mL) |
[53] |
| Neltuma glandulosa (Torr.) Britton & Rose | Alkaloids | Antibacterial, antifungal, anti-infective, and antiparasitic activity | Leishmania donovani, Plasmodium falciparum, Cryptococcus neoformans, Mycobacterium intracellulare | Microdilution (0.66-20 μg/mL) |
[54] |
| Neltuma juliflora ( Sw. ) Raf. | Alkaloids | Antibacterial | Staphylococcus aureus, Staphylococcus epidermidis, Escherichia coli y Pseudomonas aeruginosa | Broth dilution (2.5 mg/mL) |
[19] |
| Neltuma laevigata (Humb. & Bonpl. ex Willd.) Britton & Rose | Phenols and alkaloids | Antimicrobial and antioxidant | Staphylococcus aureus , Escherichia coli , Candida tropicalis y Fusarium moniliforme | Broth dilution (0.08-4.62 mg/mL) |
[18] |
| Neptunia oleracea Lour. | Alkaloids, glycosides, flavonoids, proteins, terpenoids, phytosterols, and tannins | Antioxidants and anti-inflammatory | Staphylococcus aureus, Escherichia coli, Pseudomonas aeruginosa y Candida albicans | Disc diffusion (10-100 mg/mL) |
[55] |
| Pachyrhizus erosus (L.) Urb. | Isoflavones | Antifungal | C. gloeosporioides, F. oxysporum, y R. stolonifer | Disc diffusion (0.5–250 µg/mL) |
[56] |
| Parkinsonia aculeata L. | Alkaloids, glycosides, flavonoids, terpenoids, and tannins | Antibacterial | Staphylococcus aureus, Escherichia coli, y Pseudomonas aeruginosa | Disc diffusion (12.5–50 mg/mL) |
[57] |
| Parkinsonia florida (Benth. ex A.Gray) S.Watson | Alkaloids, carbohydrates, saponins, phenols, flavonoids, proteins, cardiac glycosides | Antibacterial | Staphylococcus aureus y Escherichia coli. | Disc diffusion (125–2000 µg/mL) |
[58] |
| Parkinsonia praecox (Ruiz & Pav.) Hawkins | Triterpenes | Anticancer, antibacterial | Listeria monocytogenes | Microdilution (2000 µg/mL) |
[59] |
| Phaseolus coccineus L. | Lectins | Antinoplastic and antifungal. | Candida albicans, Penicillium italicum, Helminthosporium maydis, Sclerotinia sclerotiorum, Gibberalla sanbinetti y Rhizoctonia solani | Disc diffusion (31.3–250 mg/mL) |
[60] |
| Phaseolus lunatus L. | Isolated and hydrolyzed proteins | Antibacterial, antioxidant, anti-inflammatory | Staphylococcus aureus, Escherichia coli, Bacillus cereus, Listeria monocytogenes y Pseudomonas aeruginosa | Well diffusion (500, 375, 250, 200, and 150 mg/mL) |
[61] |
| Phaseolus vulgaris L. | Lectins | Antibacterial and antifungal | Staphylococcus aureus ATCC 6538, and Streptococcus mutants ATCC 25175, Pseudomonas aeruginosa ATCC 10145 and Klebsiella pneumonia | Microdilution (0.24–1000 μg/mL) |
[62] |
| Pithecellobium dulce (Roxb.) Benth. | Alkaloids, anthraquinones, flavonoids, cardiac glycosides, proteins, tannins, sugars, and terpenoids. | Anti-inflammatory, antivenom, protease inhibitor, spermicide, antimicrobial, and antituberculosis activity | Bacillus subtilis, Enterococcus faecalis, Micrococcus luteus, Staphylococcus aureus and Staphylococcus epidermidis), Aeromonas hydrophila, Alcaligenes faecalis, Enterobacter aerogenes, Escherichia coli, Klebsiella pneumoniae, Pseudomonas aeruginosa y Salmonella typhimurium | Microdilution (200–1000 µg/mL) |
[63] |
| Rhynchosia minima (L.) DC. | Essential oils | Antimicrobianas y antioxidantes | Acenotobacter calcoacetilus, Bacillus subtilis, Citrobacter freundii, Escherichia coli, Proteus vulgaris, Pseudomonas aeruginosa, Salmonella typhii, Staphylococcus aureus y Yersinia enterocolitica. | Well diffusion (100 µg/mL) |
[64] |
| Senegalia berlandieri (Benth.) Britton & Rose | No data recorded | Antibacterial | No antibacterial effects observed | Disc diffusion (100 mg/mL) |
[17] |
| Senegalia greggii (A.Gray) Britton & Rose | No data recorded | Antibacterial | No antibacterial effects observed | Disc diffusion (100 mg/mL) |
[17] |
| Senna crotalarioides (Kunth) H.S.Irwin & Barneby | No data recorded | Anti-inflammatory | No data recorded | No data recorded | [65] |
| Senna hirsuta (L.) H.S.Irwin & Barneby | Essential oils | Antimicrobial | Escherichia coli, Staphylococcus aureus, Bacillus subtilis y Aspergillus niger | Microdilution (78-625 μg/mL) |
[66] |
| Senna obtusifolia (L.) H.S.Irwin & Barneby | Saponins, tannins, alkaloids, and flavonoids. | Antimicrobial | Neisseria gonorrheae, Salmonella sp., Pseudomonas aeruginosa, Proteus vulgari, Staphylococcus aureus y Streptococcus aerugenosa | Disc diffusion (200 - 1000 μg/mL) |
[67] |
| Senna occidentalis (L.) Link | Tannins, alkaloids, glycosides, flavonoids, steroids, saponins, anthraquinones, and flobanoids | Antimalarial, antitrypanosomal, immunosuppressive, anti-inflammatory, larvicidal, antidiabetic, anticancer, antiulcer, and hepatoprotective. | Escherichia coli, Klebsiella pneumoniae, Candida albicans, Staphylococcus aureus, Pseudimonas aeruginosa y Salmonella typhi | Well diffusion (80 and 120 mg/mL) |
[68] |
| Senna pendula (Humb. & Bonpl. ex Willd.) H.S.Irwin & Barneby | Anthraquinones, steroids, flavones, flavonols, saponins, tannins, triterpenoids, xanthones | Inflammatory, antimicrobial, antitumor, antimalarial, cardioprotective, and antioxidant. | No studies on M.O. are presented. | No data recorded | [69] |
| Senna septemtrionalis (Viv.) H.S.Irwin & Barneby | No data recorded | Diuretic activity and neuropharmacological effects | No studies on M.O. are presented. | No data recorded | [70] |
| Senna wislizeni (A.Gray) H.S.Irwin & Barneby | Flavonols | Laxative, antimicrobial, antiviral, antifungal, anti-inflammatory, antitumor, antioxidant | Escherichia coli y Salmonella thyphimurium | Agar overlay bioautography | [23] |
| Sophora tomentosa L. | Hydrocarbons, sterols, terpenes | Antioxidants, antimicrobials, anti-inflammatories, and anticancer agents | B. subtilis, S. aureus y E. coli | Well diffusion (50 mg/mL) |
[71] |
| Tephrosia cinerea (L.) Pers. | Phenols | Antimicrobial | Pseudomonas aeruginosa, E. coli | Broth dilution (10-90 mg/mL) |
[72] |
| Vachellia farnesiana (L.) Wight & Arn. | Phenols, tannins, diterpenes, sterols, triterpenes, and saponins | Antibacterial | M. roseus | Disc diffusion (100 mg/mL) |
[17] |
| Vachellia rigidula (Benth.) Seigler & Ebinger | Phenols, tannins, diterpenes, sterols, triterpenes, and saponins | Antibacterial | P. alcalifaciens | Disc diffusion (100 mg/mL) |
[17] |
| Vigna luteola (Jacq.) Benth. | Flavonoids and isoflavonoids | Antioxidant, antifungal, antitumor, antiparasitic, hypoglycemic, hepatoprotective, renal protection, antibacterial, hypotensive, and hypolipidemic | No studies on M.O. are presented. | No data recorded | [73] |
| Vigna vexillata (L.) A.Rich. | Sterols and isoflavones | Hypoglycemia, antihypertensive, cholesterol-lowering, antioxidant, antibacterial, anticancer | No studies on M.O. are presented. | No data recorded | [74] |
| Zapoteca portoricensis (Jacq.) H.M.Hern. | Alkaloids, saponins, tannins, terpenoids, flavonoids | Antimicrobial, antiviral, antioxidant | S. aureus, S.pyogenes, E. coli, K. pneumoniae, P. aeruginosa, C. albicans, M. audouini | Disc diffusion (5.0, 10.0, 20.0 mg/mL) |
[75] |
| Zornia diphylla (L.) Pers. | Essential oils | Antifungal, antimicrobial | Salmonella typh | Microdilution (50 µg/mL) |
[76] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).




