1. Introduction
Global temperature rise and climate instability are direct consequences of human-driven greenhouse gas emissions. The intensive consumption of fossil fuels not only threatens energy security but also causes irreversible ecological damage. The increasing atmospheric concentrations of CO₂, CH₄, and N₂O accelerate global warming, leading to sea-level rise, extreme weather events, biodiversity loss, and declining agricultural productivity. These challenges underscore the limitations of fossil fuel–based energy systems and highlight the urgent need for renewable and sustainable energy technologies [
1,
2,
3]. As renewable energy sources such as solar, wind, biomass, and hydrogen become more widely deployed, the development of advanced energy-storage systems, including batteries and supercapacitors, has become essential for safe and efficient energy utilization. Consequently, environmentally friendly, cost-effective, and high-performance energy technologies have emerged as a scientific and technological priority to enhance energy security, reduce carbon emissions, and achieve global sustainability targets [
4,
5,
6].
Supercapacitors are particularly attractive due to their high-power density, rapid charge–discharge rates, long cycle life, and excellent electrochemical stability [
7,
8]. They are classified into three main categories based on their charge-storage mechanisms: electrical double-layer capacitors (EDLCs), pseudocapacitors, and hybrid capacitors [
9,
10]. EDLCs store energy through electrostatic charge separation at the electrode–electrolyte interface, whereas pseudocapacitors rely on fast, reversible surface or bulk redox reactions [
11,
12,
13]. Hybrid supercapacitors integrate both mechanisms to achieve higher energy and power densities. In all cases, optimizing the morphological, chemical, and conductive properties of electrode materials is a key determinant of overall performance [
14,
15,
16].
In recent years, metal nanoparticles produced via green synthesis have gained attention for energy-storage and biosensing applications due to their sustainability, low cost, and environmental compatibility. These methods employ plant extracts as natural reducing and capping agents, eliminating toxic reagents and enabling the fabrication of biocompatible nanomaterials [
17,
18].
Rhus coriaria L. (sumac) is particularly notable for its high content of phenolics, flavonoids, and tannins, which effectively reduce metal ions and stabilize nanoparticle formation. Gold nanoparticles synthesized using
R. coriaria extract (Rc@AuNPs) exhibit high electrical conductivity and chemical stability, making them promising candidates for both sustainable energy-storage applications and biosensors [
19,
20].
Gold nanoparticles are widely utilized due to their strong surface plasmon resonance (SPR), high conductivity, and excellent affinity toward biomolecules, which enable highly sensitive biosensing. Their large electrochemically active surface area also promotes efficient ion transport, contributing to improved specific capacitance in supercapacitor systems [
21,
22,
23]. Thus, Rc@AuNPs produced via green synthesis represent multifunctional nanomaterials with strong potential in both high-performance energy storage and biosensing. This environmentally friendly approach advances the integration of bio-derived nanomaterials into future sustainable energy and biomedical technologies.
2. Materials and Methods
2.1. Preparation of Rhus coriaria L. Plant Extract
Seeds of Rhus coriaria L., commercially obtained from the Hasankeyf district of Batman, were washed several times with tap water and subsequently rinsed with distilled water. After drying at room temperature, the seeds were ground into a fine powder. A total of 40 g of this powder was mixed with 600 mL of distilled water and boiled. The resulting extract was cooled to room temperature and filtered through Whatman No. 1 filter paper.
2.2. Gold Nanoparticles (Rc@AuNPs) Synthesis
For nanoparticle synthesis, a 100 mg/L solution of Sigma-Aldrich tetrachloroauric acid (HAuCl₄·3H₂O) was prepared. Then, 200 mL of the R. coriaria extract was combined with the prepared 100 ppm gold solution. The reaction mixture was stirred at 60 °C for 2 hours. Following the reaction, the dark-colored solution was centrifuged, and the obtained solid was dried in an oven at 110 °C for 48 hours. The resulting gold nanomaterials (Rc@AuNPs) were collected and stored for subsequent electrochemical analyses.
2.3. Electrodes Preparation
The gold nanoparticle-coated electrode material (Rc@AuNPs) was prepared using a sustainable green synthesis method, taking advantage of the reducing properties of bioactive compounds in
R. coriaria plant extract. The resulting Rc@AuNPs nanocomposite was used as the active component of the electrode. A measured amount of graphite powder was added to increase the electrical conductivity of the composite, and mineral oil was used as the binder phase. The mixture for the electrode material was blended until a homogeneous paste was obtained, using a ratio of 75% active component (Rc@AuNPs), 5% graphite, and 20% binder (7.5:0.5:2, w/w) [
24].
The prepared nanocomposite paste was carefully packed into the cavity of a commercial carbon paste electrode (CPE; MF-2010, BASi), and its surface was smoothed and compacted to make it suitable for electrochemical analysis.
2.4. Electrochemical Measurements
All electrochemical measurements were carried out using an AUTOLAB PGSTAT128N potentiostat/galvanostat system. A three-electrode cell configuration manufactured by BASi was used for the experiments. In this electrochemical setup, a platinum wire (Pt; MF-1032, BASi) was used as the auxiliary electrode, and an Ag/AgCl (3 M KCl) electrode (MF-1063, BASi) served as the reference electrode.
The energy storage properties of the designed supercapacitor were investigated in various supporting electrolyte environments (1 M H₂SO₄, Na₂SO₄, and NaOH). The electrochemical properties of the capacitor were comprehensively analyzed using cyclic voltammetry (CV), galvanostatic charge–discharge (GCD), and electrochemical impedance spectroscopy (EIS) methods.
2.5. Calculation of Specific Capacitance
The energy storage performance of the Rc@ZnONPs electrode was assessed by calculating the specific capacitance (Cₛₚ) using three complementary electrochemical techniques: CV, GCD, and EIS. The corresponding calculations were performed based on well-established equations commonly reported in the literature [
25].
1) From CV measurements:
2) From GCD curves:
3) From EIS data:
Where A is the integrated area under the CV curve (A·V), r is the potential scan rate (V/s), ΔV is the potential window (V), I is the discharge current (A), tc and td are the charge and discharge times (s), respectively, m represents the mass of the active electrode material (g), ƒ is the frequency (Hz), and Z” is the imaginary component of impedance (Ω).
In addition to the specific capacitance, the energy density (Eₛ, Wh/kg) and power density (Pₛ, W/kg) of the electrode were calculated as follows:
4)
5)
3. Results and Discussion
3.1. Cyclic Voltammetry Results of the Rc@AuNPs Electrode
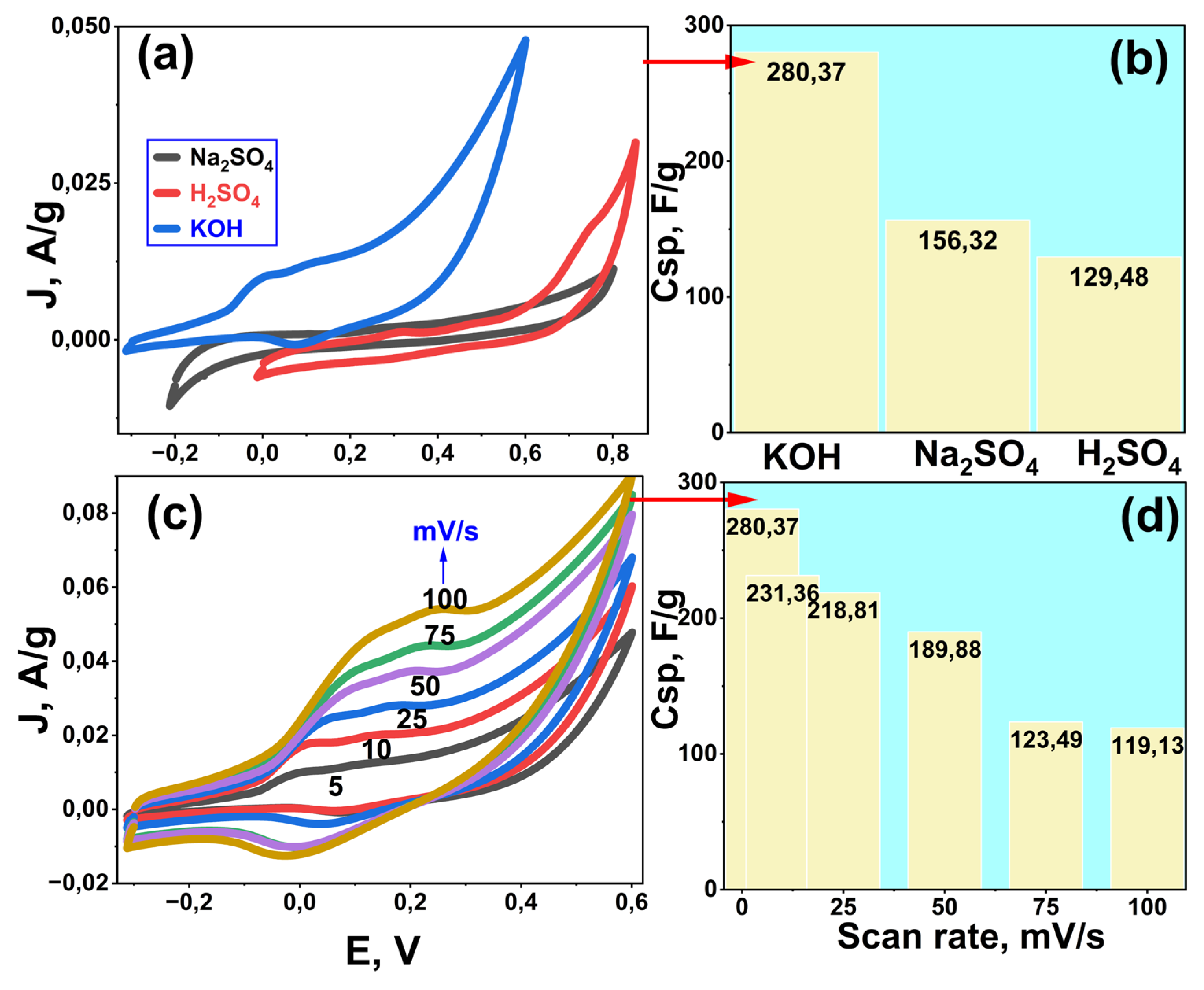
The electrochemical behavior of the Rc@AuNPs electrode material was investigated in different electrolyte solutions (H₂SO₄ – red curve, Na₂SO₄ – black curve, and KOH – blue curve) at a scan rate of 5 mV/s (
Figure 1a). The overall profile of the CV curves exhibits a rectangular shape typical of supercapacitors, with the blue curve in KOH showing a larger current area. This suggests that the Rc@AuNPs electrode exhibits a more pronounced pseudocapacitive behavior in KOH solution. The high specific capacitance value of 280.37 F/g in KOH is attributed not only to electrochemical double-layer capacitance (EDLC) but also to the pseudocapacitance effect resulting from surface redox processes. Pseudocapacitance is characterized by reversible surface redox reactions or electrode/ion interactions (for example, the literature reports an increase in pseudocapacitance when Au NPs are applied to Ni(OH)₂ electrodes) [
26].
The basis for this contribution is the electroactive behavior of Au nanoparticles (AuNPs) in alkaline media. In alkaline solutions, a redox transformation can occur on the Au surface as follows:
This process leads to the formation of Au–OH species on the surface, particularly in more positive potential regions. The resulting Au–OH and its derivatives facilitate rapid and reversible charge transfer across the electrode surface. Therefore, the high capacitance values observed in KOH can be interpreted as hybrid capacitance behavior, contributed by double-layer capacitance (EDLC) and surface redox reactions (Au/Au–OH transition).
In contrast, due to the lower concentration of OH⁻ ions and limited interaction with the Au surface in neutral (Na₂SO₄) and acidic (H₂SO₄) environments, AuNPs are less able to exhibit the same electroactive behavior. In this context, the influence of the electrolyte environment on AuNP–electrode interactions has been discussed in the literature [
27,
28]. This is consistent with the specific capacitance order KOH > Na₂SO₄ > H₂SO₄ shown in
Figure 1b.
CV curves (
Figure 1c), obtained at different scan rates (5–100 mV/s), provide additional information about ion transport and electrode kinetics. At lower scan rates, ions reach the active sites more easily, allowing both EDLC and pseudocapacitive contributions to participate [
29]. However, due to limited ion diffusion time at high scan rates, pseudocapacitive reactions cannot fully occur, and therefore the Cₛₚ value (
Figure 1d) decreases from 280.37 F/g to 119.13 F/g. This decrease indicates that the Rc@AuNPs material exhibits hybrid-capacitor behavior controlled by ion diffusion-dependent redox processes. Furthermore, the literature supports that AuNP doping increases electrode conductivity and charge-transfer rate; for instance, AuNPs have been shown to increase conductivity in carbon/metal-oxide electrode systems [
30]. This may promote pseudocapacitive activity by accelerating electrochemical kinetics.
Thus, the highly electroactive behavior of the Rc@AuNPs supercapacitor in KOH medium is associated with the efficient adsorption of OH⁻ ions on the Au surface and the rapid redox conversion of Au–OH species [
26,
31]. This feature demonstrates that the material exhibits a pseudocapacitive supercapacitor behavior that goes beyond the classical double-layer storage mechanism and provides enhanced energy density and strong cycling stability.
3.2. Charge–Discharge Behavior of the Rc@AuNPs Electrode
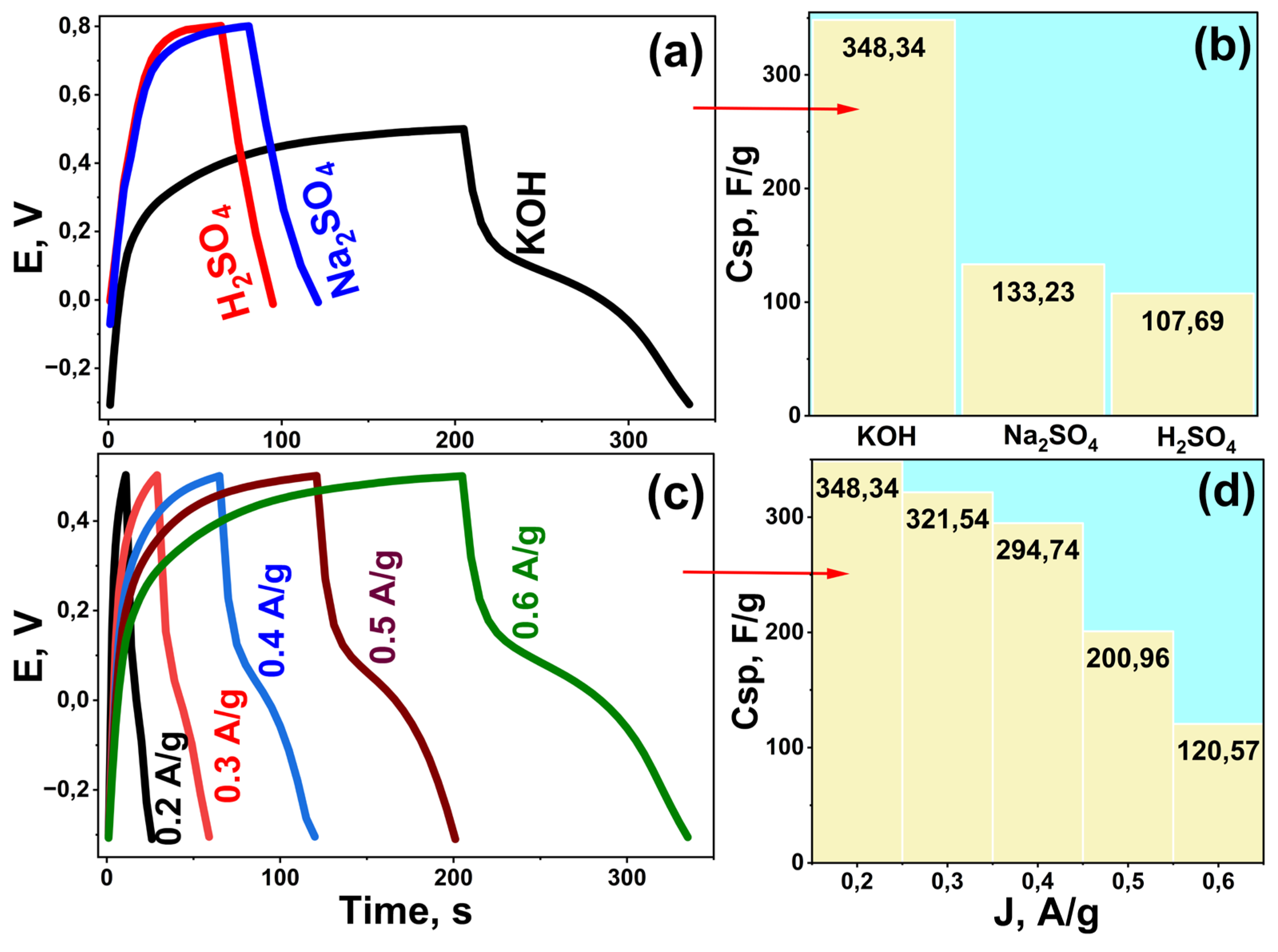
The supercapacitor performance of the Rc@AuNPs hybrid material was investigated in acidic, neutral, and basic electrolytes using the GCD technique. Measurements were performed in the potential range of –0.3 V to +0.5 V in KOH and 0.0 V to +0.8 V in Na₂SO₄ and H₂SO₄ at a constant current density of 0.6 A/g. Specific capacitance (Cₛₚ) values were calculated from the corresponding GCD curves (
Figure 2).
GCD analyses revealed distinct capacitive behaviors depending on the electrolyte type (
Figure 2a). The Cₛₚ values were 348.34 F/g in KOH, 133.23 F/g in Na₂SO₄, and 107.69 F/g in H₂SO₄, demonstrating that Rc@AuNPs achieved the highest energy storage capacity and superior electrochemical performance in a basic environment. This enhanced performance in KOH can be attributed to stronger interactions between AuNPs and plant-extract-derived functional groups, improved ion transport, and reduced internal resistance (as confirmed later by EIS, Figure 5). The slight curvature in the KOH GCD curves indicates pseudocapacitive contributions arising not only from double-layer capacitance but also from rapid surface-controlled redox reactions between AuNPs and functional groups [
28,
32]. These faradic contributions occur simultaneously with non-faradaic processes, enhancing total capacitance and the synergistic energy-storage effect of the hybrid electrode.
The effect of current density on capacitive behavior was further evaluated in KOH at 0.2–0.6 A/g (
Figure 2c and
Figure 2d). Specific capacitance decreased with increasing current density, from 348.34 F/g at 0.2 A/g to 120.57 F/g at 0.6 A/g. This decrease results from insufficient time for electrolyte ions to diffuse throughout the electrode’s porous structure at higher currents, preventing full utilization of active sites. At lower current densities, ions have sufficient time to access all active sites, enabling effective charge storage and higher Cₛₚ values.
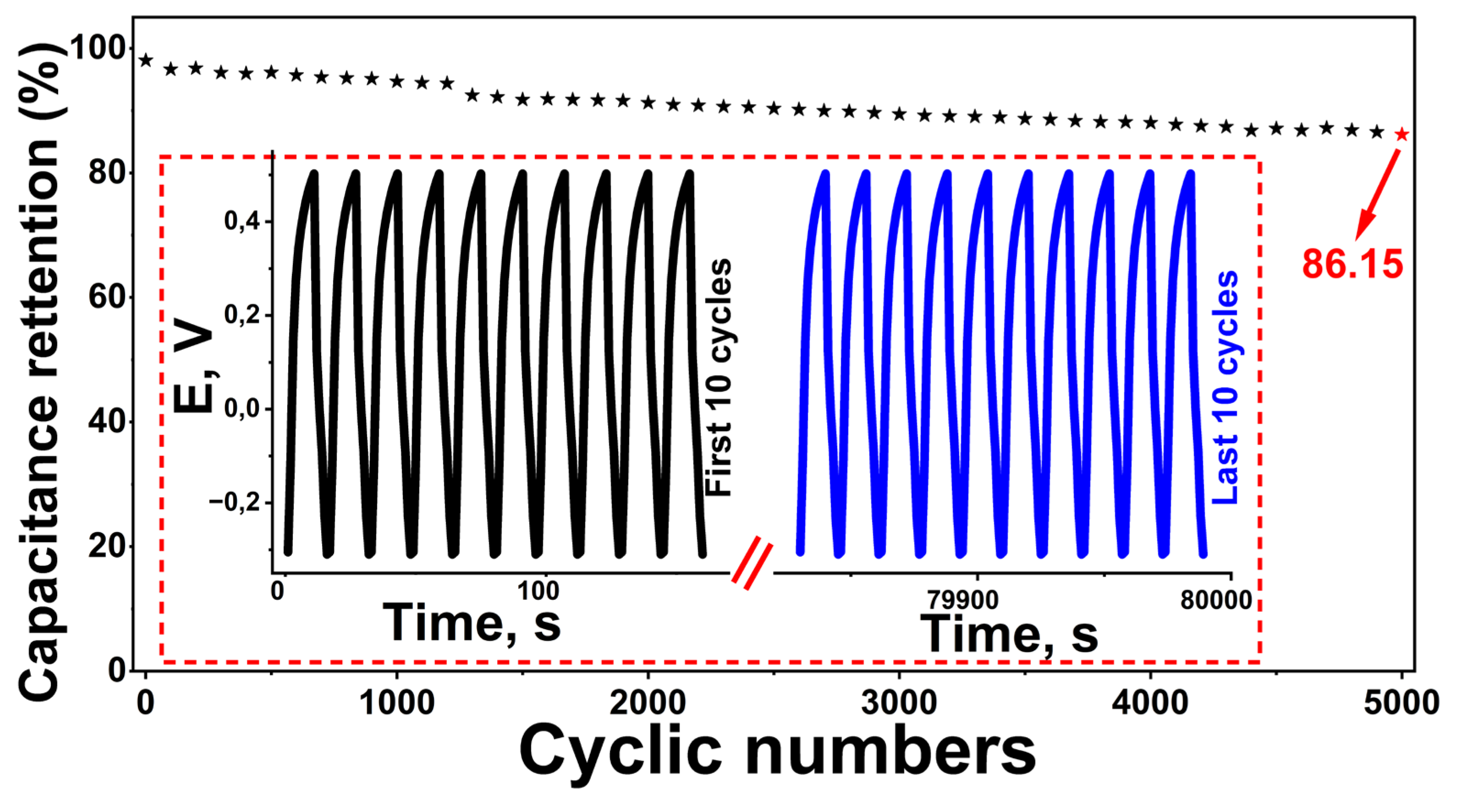
The practical applicability of energy storage devices depends largely on long cycle life and electrochemical stability. In this context, the cyclic performance of the supercapacitor designed using the Rc@AuNPs hybrid material was investigated for 5000 charge–discharge cycles at a current density of 0.6 A g⁻¹ in an aqueous KOH electrolyte (
Figure 3). The results revealed that the electrode material exhibited exceptional stability, retaining 86.15% of its initial C
sp value. This value is significantly higher than the capacity retention rates reported for AuNPs-based carbon hybrids in the literature and confirms the long-term structural integrity of the hybrid system [
28,
32,
33].
The high morphological consistency between the GCD curves for the first 10 and last 10 cycles, presented in
Figure 3, is consistent with low polarization and minimal capacity fading. This behavior is attributed to the synergistic effect of the electrochemical contributions from the redox-active functional groups of the
R. coriaria plant extract and the high conductivity and mechanical stability provided by AuNPs. Similarly, the stability-enhancing role of AuNPs in conductive polymers or carbon frameworks has been previously reported [
31,
34,
35]. These results demonstrate that the robust network structure formed by AuNPs effectively absorbs mechanical stresses during cycling, preventing structural deformation and maintaining continuous ion diffusion pathways. Furthermore, the use of
R. coriaria plant extract as a reducing and capping agent in the green synthesis process provides an environmentally sustainable production approach. In recent years, the applicability of similar green synthesis protocols in energy storage systems has increased, and the advantages of plant-extract-based metal nanoparticles, such as high electrochemical stability and environmentally friendly production, have been highlighted [
30,
31,
36].
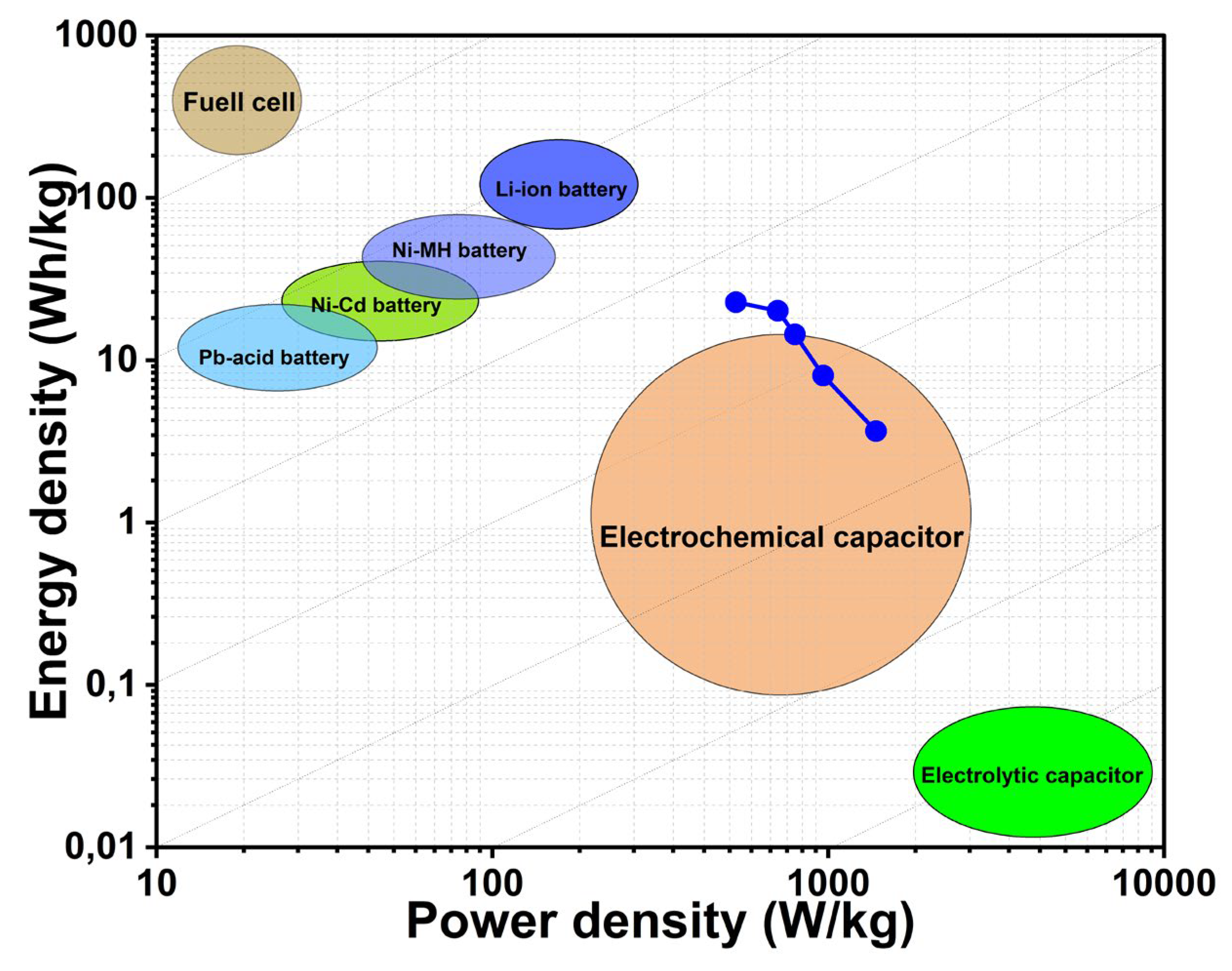
The Ragone diagram, commonly used to illustrate the performance limits of energy storage systems, highlights the fundamental trade-off between a device’s energy density and power density [
25,
37]. In this study, the performance of a supercapacitor based on Rc@AuNPs hybrid material, measured in KOH electrolyte, was evaluated using Ragone curves (
Figure 4).
It was observed that the calculated power density values increased from 530.77 Wh/kg to 1388 Wh/kg, while the energy density decreased from 22.78 Wh/kg to 3.67 Wh/kg. This inverse relationship is characteristic of supercapacitors, enabling rapid energy delivery at high power demands while partially reducing the stored energy. The highest measured energy density (22.78 Wh/kg) exceeds the typical range of conventional electrochemical capacitors and approaches the lower limits of lithium-ion batteries [
32,
38]. Simultaneously, power densities of up to 1388 Wh/kg are consistent with the material’s high conductivity and rapid ion adsorption/desorption kinetics. This balanced performance is enabled by the synergistic combination of redox-active functional groups from the
R. coriaria plant extract and efficient charge transfer with low internal resistance provided by the AuNPs [
31,
39].
Consequently, the Rc@AuNPs-based supercapacitor exhibits a “bridge material” property, combining the advantages of both systems by offering higher energy density than conventional capacitors and higher power density than batteries. This well-balanced performance profile is particularly promising for hybrid energy systems and electric vehicle applications, where high power output and mesoscale energy storage are critical.
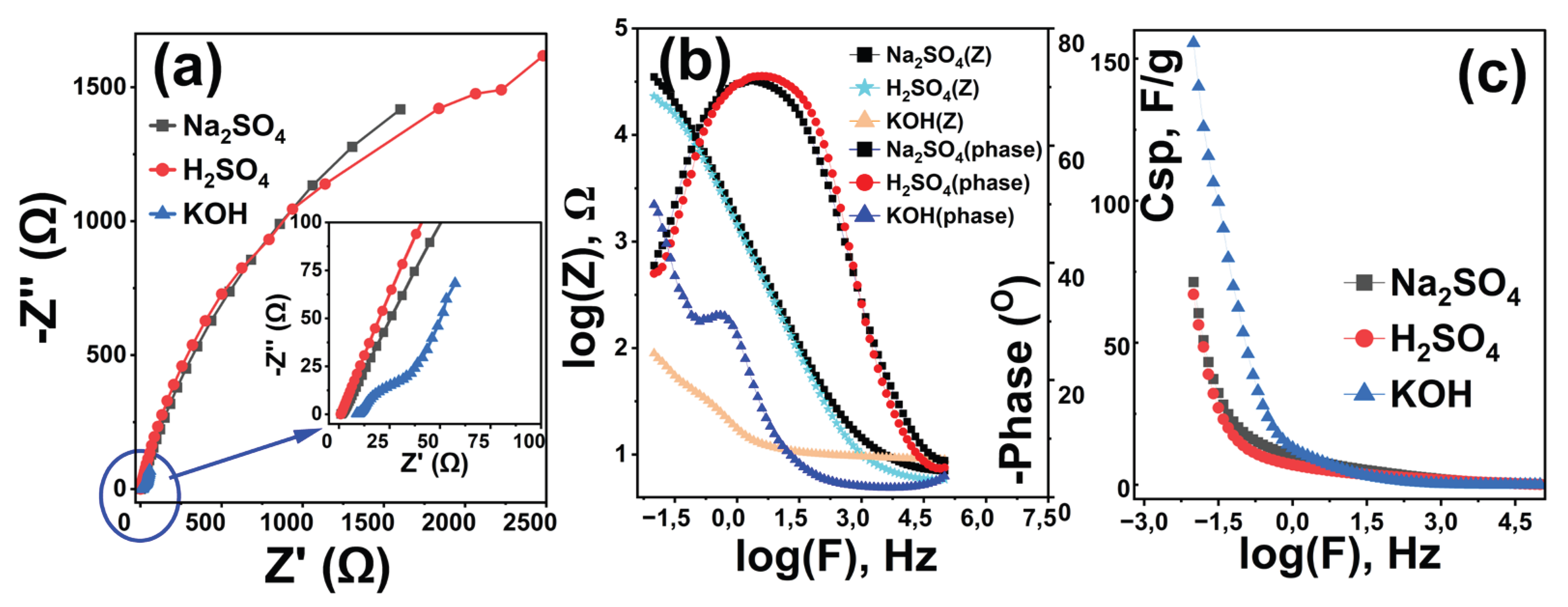
3.3. Electrochemical Impedance Spectroscopy Analysis
To evaluate the electrochemical behavior of the Rc@AuNPs hybrid material and the effect of electrolyte selection on its performance, EIS measurements were performed in three different electrolyte environments (KOH: blue, H₂SO₄: red, and Na₂SO₄: black) (
Figure 5). All measurements were obtained at open-circuit potential, with a signal amplitude of 10 mV and a frequency sweep from 0.01 Hz to 100 kHz [
40].
The findings clearly reveal that electrolyte chemistry plays a critical role in determining the ESR, capacitive behavior, and energy-storage capacity of the Rc@AuNPs hybrid system [
11,
28,
41]. ESR values calculated from Nyquist diagrams (
Figure 5a) showed that the lowest internal resistance was obtained in KOH medium, at 24.43 Ω. This finding is consistent with the high ionic conductivity of the KOH solution, indicating efficient charge transfer at the electrode/electrolyte interface. In contrast, the high ESR values observed in Na₂SO₄ (64.42 Ω) and H₂SO₄ (67.96 Ω) media indicate that ion transport in the system is limited and charge-transfer kinetics are slowed. To further evaluate the influence of electrolyte type, the effect on capacitive properties was investigated using Bode phase diagrams (
Figure 5b). The phase angle closest to the ideal capacitor behavior (90°) was obtained in the KOH electrolyte at 52.42°, indicating a strong capacitive nature of the system [
11,
31,
42]. In contrast, the low phase angles measured in Na₂SO₄ (39.65°) and H₂SO₄ (38.05°) environments suggest that capacitive behavior is partially impaired in these systems. Furthermore, resistance values derived from Bode diagrams (KOH: 1.95 Ω; Na₂SO₄: 4.36 Ω; H₂SO₄: 4.59 Ω) were found to be consistent with the ESR results and further supported the superior conductivity properties of the KOH electrolyte.
To evaluate the energy-storage capacity, C
sp values were calculated (
Figure 5c) [
32,
40]. The results show that the choice of electrolyte has a significant impact on energy-storage performance. At the lowest frequency, the highest C
sp value of 155.56 F/g was obtained in KOH medium, while this value was approximately halved in H₂SO₄ (71.62 F/g) and Na₂SO₄ (67.14 F/g) electrolytes. These differences are thought to be due to the hydration properties of the electrolyte ions, their ion sizes, and their interaction with the pore morphology of the hybrid electrode [
11,
32,
41,
43].
Overall, EIS analyses clearly demonstrated that the KOH electrolyte is the most suitable operating environment for the Rc@AuNPs hybrid material. The low ESR, high phase angle, and superior specific capacitance values obtained in the KOH environment confirm that this system offers high efficiency for supercapacitor applications. Furthermore, the gold nanoparticles incorporated into the hybrid structure are believed to significantly enhance the observed performance by improving the electrode’s overall conductivity [
44,
45,
46]. In addition, the high C
sp value obtained by the EIS technique in KOH medium is in full compliance with the electrochemical performance results determined by the CV and GCD techniques, which collectively confirms the superior capacitive behavior of the Rc@AuNPs electrode.
4. Conclusions
In this study, gold nanoparticles (Rc@AuNPs) were successfully synthesized using bioactive components derived from the Rhus coriaria (sumac) plant via an environmentally friendly, sustainable, and low-cost method. The polyphenolic compounds and phytochemical reducing agents in R. coriaria extract played an active role in the reduction of Au³⁺ ions and surface stabilization of the nanoparticles, achieving a green synthesis approach without any toxic chemical reducers.
Structural and electrochemical characterizations demonstrated that Rc@AuNPs nanoparticles are a promising electrode material with high energy storage performance. The electrochemical behavior of Rc@AuNPs in different electrolytes was evaluated comprehensively using cyclic voltammetry (CV), galvanostatic charge–discharge (GCD), and electrochemical impedance spectroscopy (EIS). The specific capacitance (Cₛₚ) values were 129.48 F/g, 156.32 F/g, and 280.37 F/g in H₂SO₄, Na₂SO₄, and KOH, respectively, while GCD measurements yielded Cₛₚ values of 107.69 F/g, 133.23 F/g, and 348.34 F/g in acidic (H₂SO₄), neutral (Na₂SO₄), and basic (KOH) environments, respectively. These results indicate that Rc@AuNPs achieve highest capacitive performance in basic media, with KOH providing the most suitable electrolyte due to its high ionic conductivity.
Equivalent series resistance (ESR) values obtained by EIS were 24.43 Ω, 67.96 Ω, and 64.42 Ω for KOH, H₂SO₄, and Na₂SO₄, respectively. The low ESR in KOH indicates efficient charge transfer at the electrode/electrolyte interface and low internal resistance, which correlates with the high capacitance observed.
Overall, the high specific capacitance, low internal resistance, and efficient charge transfer of Rc@AuNPs in KOH demonstrate that this material is a promising candidate for high-performance supercapacitors and energy storage systems. Furthermore, the environmental sustainability, green chemistry compatibility, and high electrochemical stability of biosynthesized Rc@AuNPs suggest that they could play a significant role in future renewable energy technologies and environmentally friendly energy storage applications.
Author Contributions
M.F.B.: conceptualization, investigation, writing – original draft; E.H.: investigation, methodology, writing – original draft; A.E.: data curation, investigation, project administration, writing – original draft; A.L.: formal analysis, investigation, supervision, writing – original draft; E.E.: formal analysis, investigation, writing – original draft; T.K.: data curation, investigation, writing – review and editing; O.Š.: investigation, methodology, validation; E.K.: funding acquisition, project administration; O.S.: supervision, methodology, writing – review and editing.
Acknowledgments
This work was supported in part by the Ministry of Education and Science of Ukraine (projects Nos. 0125U002005 and 0125U002033), National Research Foundation of Ukraine (project No. 2020.02/0100), Slovak Grant Agency VEGA (projects Nos. 2/0166/22 and 2/0131/25), and Slovak Research and Development Agency (project No. APVV-21-0335). T.K. also acknowledges the SAIA (Slovak Academic Information Agency) for scholarship in the Institute of Physics of the Slovak Academy of Sciences in the framework of the National Scholarship Program of the Slovak Republic. This work has also received funding through the MSCA4Ukraine project (grant No. 1128327), which is funded by the European Union. E.K. and O.S. acknowledge US National Science Foundation (NSF) grant CBET-2235349, including IMPRESS-U supplement, and NSF-BSF grant CBET-2422672.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Sarfraz, M. Global Warming Cause and Impact on Climate Change. Int. J. Emerg. Knowl. Stud. 2024, 3, 198–204. [Google Scholar]
- Mostafa, A.R.; Owes, A.M.; Ghoniem, S.A. Interconnected impacts of climate change on biodiversity, agriculture and human health. Adv. Nat. Appl. Sci. 2025, 4, 43–63. [Google Scholar]
- Amin, M.R.; Islam, M.R.; Shaili, S.J.; Khatun, M.R.; Mahedi, M. Climate change and its impact: A review of global strategies for adaptation and mitigation. J. Agric. Ecol. Res. Int. 2025, 26, 205–221. [Google Scholar] [CrossRef]
- Zhang, J.; Gu, M.; Chen, X. Supercapacitors for renewable energy applications: A review. Micro Nano Eng. 2023, 21, 100229. [Google Scholar] [CrossRef]
- Liu, X.; Li, W.; Guo, X.; Su, B.; Guo, S.; Jing, Y.; Zhang, X. Advancements in Energy-Storage Technologies: A Review of Current Developments and Applications. Sustainability 2025, 17, 8316. [Google Scholar] [CrossRef]
- Conde, H.J.C.; Demition, C.M.; Honra, J. Storage Is the New Black: A Review of Energy Storage System Applications to Resolve Intermittency in Renewable Energy Systems. Energies 2025, 18, 354. [Google Scholar] [CrossRef]
- Mansi, A.; Al Kiey, S.A.; Abedin, S.Z.E.; Bassyouni, M.; Wassel, A.R.; Yousif, A.M.; Hasanin, M.S. Recent Advances in Sustainable and Green Chemistry for Polyurethane-Based High-Performance Supercapacitor Electrodes. Trans. Tianjin Univ. 2025, 1, 1–26. [Google Scholar] [CrossRef]
- Kasprzak, D.; Mayorga-Martinez, C.C.; Pumera, M. Sustainable and flexible energy storage devices: a review. Energ. Fuel. 2022, 37, 74–97. [Google Scholar] [CrossRef]
- Bejjanki, D.; Puttapati, S.K. Supercapacitor basics (EDLCs, pseudo, and hybrid). In Multidimensional Nanomaterials for Supercapacitors: Next Generation Energy Storage; Bentham Science Publishers, 2024; pp. 29–48. [Google Scholar]
- Volfkovich, Y.M. Electrochemical supercapacitors (a review). Russ. J. Electrochem. 2021, 57, 311–347. [Google Scholar] [CrossRef]
- Patel, A.; Patel, S.K.; Singh, R.S.; Patel, R.P. Review on recent advancements in the role of electrolytes and electrode materials on supercapacitor performances. Discov. Nano 2024, 19, 188. [Google Scholar] [CrossRef]
- Kumar, N.; Kim, S.B.; Lee, S.Y.; Park, S.J. Recent advanced supercapacitor: a review of storage mechanisms, electrode materials, modification, and perspectives. Nanomater. 2022, 12, 3708. [Google Scholar] [CrossRef]
- Karthikeyan, S.; Narenthiran, B.; Sivanantham, A.; Bhatlu, L.D.; Maridurai, T.J.M.T.P. Supercapacitor: Evolution and review. Mater. Today Proc. 2021, 46, 3984–3988. [Google Scholar] [CrossRef]
- Gupta, R. A review of functionalized nanomaterials for supercapacitor and hybrid capacitor Technologies. Discov. Electron. 2024, 1, 24. [Google Scholar] [CrossRef]
- Javed, M.S.; Asim, S.; Najam, T.; Khalid, M.; Hussain, I.; Ahmad, A.; Han, W. Recent progress in flexible Zn-ion hybrid supercapacitors: Fundamentals, fabrication designs, and applications. Carbon Energy 2023, 5, e271. [Google Scholar] [CrossRef]
- Lobato-Peralta, D.R.; Lázaro, M.J.; Alegre, C. Carbon-based materials in metal-ion hybrid supercapacitors: Advances, challenges, and future directions. J. Energy Storage 2025, 131, 117568. [Google Scholar] [CrossRef]
- Radulescu, D.-M.; Surdu, V.-A.; Ficai, A.; Ficai, D.; Grumezescu, A.-M.; Andronescu, E. Green Synthesis of Metal and Metal Oxide Nanoparticles: A Review of the Principles and Biomedical Applications. Int. J. Mol. Sci. 2023, 24, 15397. [Google Scholar] [CrossRef]
- Shafey, A.M.E. Green synthesis of metal and metal oxide nanoparticles from plant leaf extracts and their applications: A review. Green Process. Synth. 2020, 9, 304–339. [Google Scholar] [CrossRef]
- Batiha, G.E.-S.; Ogunyemi, O.M.; Shaheen, H.M.; Kutu, F.R.; Olaiya, C.O.; Sabatier, J.-M.; De Waard, M. Rhus coriaria L. (Sumac), a Versatile and Resourceful Food Spice with Cornucopia of Polyphenols. Molecules 2022, 27, 5179. [Google Scholar] [CrossRef]
- Mazzara, E.; Caprodossi, A.; Mustafa, A.M.; Maggi, F.; Caprioli, G. Phytochemical investigation of Sumac (Rhus coriaria L.) fruits from different Sicilian accessions. Foods 2023, 12, 4359. [Google Scholar] [CrossRef] [PubMed]
- Gurung, B.; Lama, A.; Pokhrel, T.; Sinjali, B.B.; Thapa, S.; Bhusal, M.; Adhikari, A. Optical detection of the viruses by gold nanoparticles (AuNPs). J. Nanomater. 2023, 1, 8091118. [Google Scholar] [CrossRef]
- Karnwal, A.; Kumar Sachan, R.S.; Devgon, I.; Devgon, J.; Pant, G.; Panchpuri, M.; Kumar, G. Gold nanoparticles in nanobiotechnology: from synthesis to biosensing applications. ACS Omega 2024, 9, 29966–29982. [Google Scholar] [CrossRef]
- Lee, J.-H.; Cho, H.-Y.; Choi, H.K.; Lee, J.-Y.; Choi, J.-W. Application of Gold Nanoparticle to Plasmonic Biosensors. Int. J. Mol. Sci. 2018, 19, 2021. [Google Scholar] [CrossRef]
- Saka, C.; Levent, A. Fabrication of nitrogen and ZnO doped on carbon particles obtained from waste biomass and their use as supercapacitor electrodes for energy storage. Int. J. Hydrog. Energy 2024, 90, 1070–1083. [Google Scholar] [CrossRef]
- Ratha, S.; Samantara, A.K. Supercapacitor: instrumentation, measurement and performance evaluation techniques; Springer, 2018. [Google Scholar]
- Kim, S.I.; Thiyagarajan, P.; Jang, J.H. Great improvement in pseudocapacitor properties of nickel hydroxide via simple gold deposition. Nanoscale 2014, 6, 11646–11652. [Google Scholar] [CrossRef]
- Zakaria, N.D.; Omar, M.H.; Ahmad Kamal, N.N.; Abdul Razak, K.; Sönmez, T.; Balakrishnan, V.; Hamzah, H.H. Effect of supporting background electrolytes on the nanostructure morphologies and electrochemical behaviors of electrodeposited gold nanoparticles on glassy carbon electrode surfaces. ACS Omega 2021, 6, 24419–24431. [Google Scholar] [CrossRef]
- Ma, H.; Chen, Z.; Gao, X.; Liu, W.; Zhu, H. 3D hierarchically gold-nanoparticle-decorated porous carbon for high-performance supercapacitors. Sci. Rep. 2019, 9, 17065. [Google Scholar] [CrossRef]
- Levent, A.; Saka, C. Tunable energy storage in acidic and alkaline electrolytes using a NiO-embedded N, P-Doped biomass-derived electrode. Biomass and Bioenergy 2025, 202, 108225. [Google Scholar] [CrossRef]
- Li, J.; Li, Y.; Liu, H.; Ran, F. Nano gold for supercapacitors and batteries. Nano Energy 2024, 128, 109839. [Google Scholar] [CrossRef]
- Vivas, L.; Singh, D.P. A highly efficient graphene gold based green supercapacitor coin cell device for energy storage. Front. Energy Res. 2022, 9, 794604. [Google Scholar] [CrossRef]
- Wang, Y.; Song, Y.; Xia, Y. Electrochemical capacitors: mechanism, materials, systems, characterization and applications. Chem. Soc. Rev. 2016, 45, 5925–5950. [Google Scholar] [CrossRef]
- Bujewska, P.; Gorska, B.; Fic, K. Gold nanoparticles for power retention in electrochemical capacitors with KSCN-based aqueous electrolyte. J. Power Sources Adv. 2022, 14, 100087. [Google Scholar] [CrossRef]
- Hryniewicz, B.M.; Gil, I.C.; Vidotti, M. Enhancement of polypyrrole nanotubes stability by gold nanoparticles for the construction of flexible solid-state supercapacitors. J. Electroanal. Chem. 2022, 911, 116212. [Google Scholar] [CrossRef]
- Smiljanić, M.; Petek, U.; Bele, M.; Ruiz-Zepeda, F.; Šala, M.; Jovanovič, P.; Hodnik, N. Electrochemical stability and degradation mechanisms of commercial carbon-supported gold nanoparticles in acidic media. J. Phys. Chem. C 2021, 125, 635–647. [Google Scholar] [CrossRef]
- Meena, J.; Sivasubramaniam, S.; David, E. Green supercapacitors: review and perspectives on sustainable template-free synthesis of metal and metal oxide nanoparticles. RSC Sustain. 2024, 2, 1224–1245. [Google Scholar] [CrossRef]
- Winter, M.; Brodd, R.J. What are batteries, fuel cells, and supercapacitors? Chem. Rev. 2004, 104, 4245–4270. [Google Scholar] [CrossRef] [PubMed]
- González, A.; Goikolea, E.; Barrena, J.A.; Mysyk, R. Review on supercapacitors: Technologies and materials. Renew. Sustain. Energy Rev. 2016, 58, 1189–1206. [Google Scholar] [CrossRef]
- Zhang, S.; Pan, N. Supercapacitors performance evaluation. Adv. Energy Mater. 2015, 5, 1401401. [Google Scholar] [CrossRef]
- Panchal, K.; Bhakar, K.; Sharma, K.S.; Kumar, D.; Prasad, S. Review on electrochemical impedance spectroscopy: a technique applied to hollow structured materials for supercapacitor and sensing applications. Appl. Spectrosc. Rev. 2025, 60, 30–55. [Google Scholar] [CrossRef]
- Grebel, H.; Yu, S.; Zhang, Y. Active carbon based supercapacitors with Au colloids: the case of placing the colloids in close proximity to the electrode interface. Nanoscale Adv. 2023, 5, 179–190. [Google Scholar] [CrossRef]
- Kötz, R.; Carlen, M.J.E.A. Principles and applications of electrochemical capacitors. Electrochim. Acta 2000, 45, 2483–2498. [Google Scholar] [CrossRef]
- Fic, K.; Frackowiak, E.; Béguin, F. Unusual energy enhancement in carbon-based electrochemical capacitors. J. Mater. Chem. 2012, 22, 24213–24223. [Google Scholar] [CrossRef]
- Islam, M.; Hossain, M.S.; Adak, B.; Rahman, M.M.; Moni, K.K.; Nur, A.S.; Mukhopadhyay, S. Recent advancements in carbon-based composite materials as electrodes for high-performance supercapacitors. J. Energy Storage 2025, 107, 114838. [Google Scholar] [CrossRef]
- Chen, X.; Paul, R.; Dai, L. Carbon-based supercapacitors for efficient energy storage. Natl. Sci. Rev. 2017, 4, 453–489. [Google Scholar] [CrossRef]
- Zhang, J.; Zhao, X.S. On the configuration of supercapacitors for maximizing electrochemical performance. ChemSusChem 2012, 5, 818–841. [Google Scholar] [CrossRef]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).