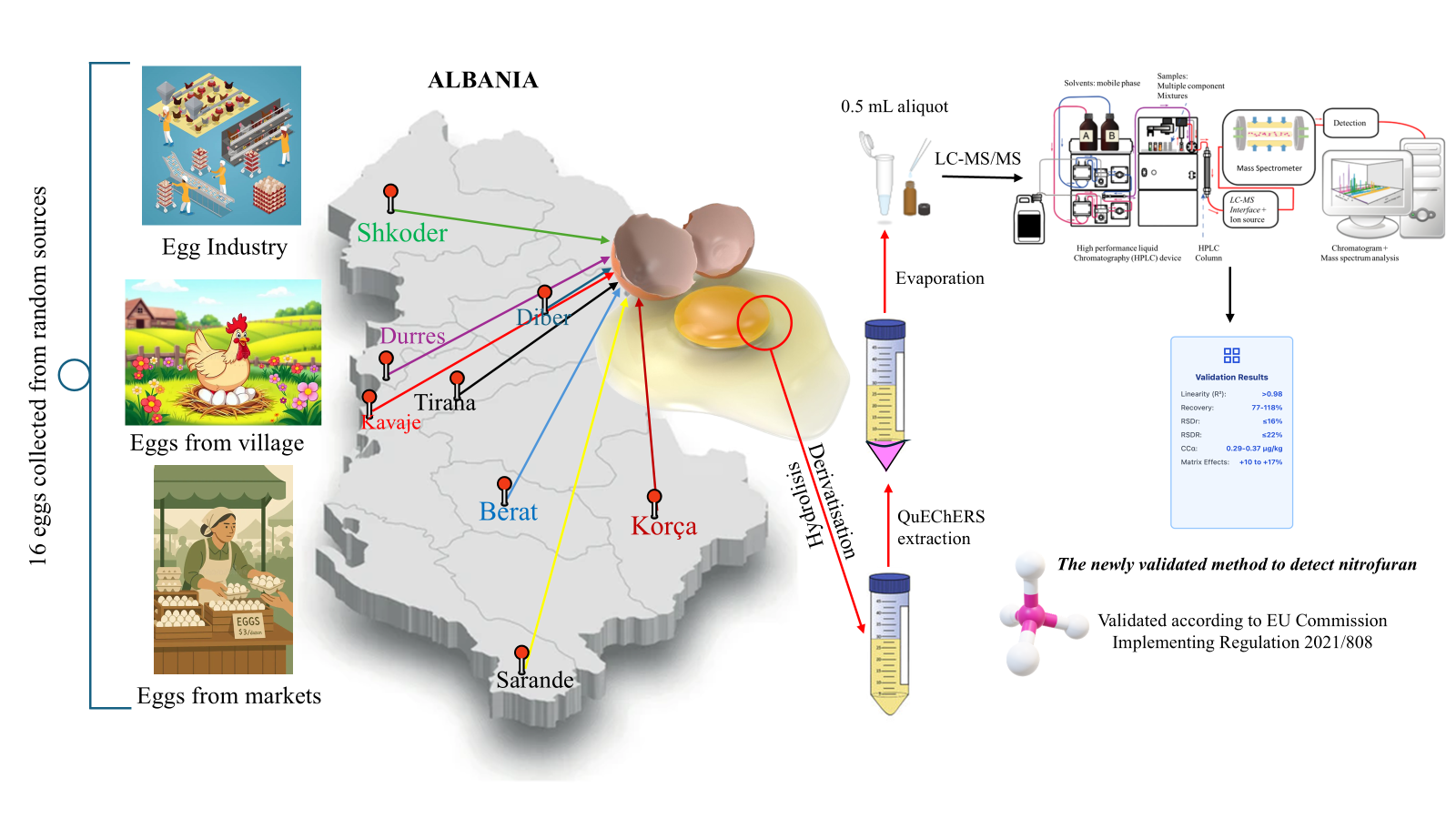
Nitrofurans are banned veterinary medicinal products due to their carcinogenic and mutagenic properties; however, their protein-bound metabolites (AOZ, AMOZ, AHD, SEM, and DNSAH) may persist in food-producing animals, particularly in eggs. Reliable confirmatory methods are therefore essential for residue monitoring under the stringent requirements of Commission Implementing Regulation (EU) 2021/808. This study reports the development and validation of a sensitive and selective LC–MS/MS method combining acid hydrolysis, 2-nitrobenzaldehyde derivatization, and QuEChERS extraction for the determination of nitrofuran metabolites in eggs. Chromatographic separation using a phenyl-hexyl column and detection by multiple reaction monitoring, supported by isotope-labeled internal standards, ensured robust identification and quantification. The method demonstrated excellent linearity (R² > 0.99), recoveries of 82–109%, and precision values below 10% (repeatability) and 22% (within-laboratory reproducibility). Matrix effects were effectively controlled, remaining within ±20% following internal standard normalization. Decision limits (CCα) ranged from 0.29 to 0.37 µg/kg, well below the EU reference point for action of 0.5 µg/kg. Method performance was further confirmed through participation in an accredited proficiency test scheme. Overall, the validated method provides a reliable analytical tool for routine official control laboratories, enabling the sensitive confirmatory detection of banned nitrofuran residues in eggs and supporting food safety and regulatory compliance.