Introduction
The monitoring of veterinary drug residues in food of animal origin is a fundamental requirement for consumer protection and regulatory enforcement, as outlined in Commission Regulation (EU) 2019/1871. Among the many classes of banned compounds, nitrofurans(NFs) represent a particularly challenging group due to their extensive past use, their toxicological profile, and the analytical complexity associated with their detection [
28]. NF antibiotics such as furazolidone, furaltadone, nitrofurantoin, and nitrofurazone were historically employed in veterinary medicine due to their broad antimicrobial activity against
Escherichia coli,
Salmonella spp.,
Mycoplasma spp., and protozoa [
26,
34,
36].
Due to their potential carcinogenic and mutagenic effects, nitrofurans are recognized as significant health hazards in humans, as well as potential toxicity to pulmonary, cardiac, hepatic, and reproductive systems [
35,
40]. Despite their effectiveness, these compounds were prohibited in the European Union and in Albania following evidence of their carcinogenic, mutagenic, and genotoxic potential [
5]. NFs are now categorized as banned substances under a zero-tolerance policy, whereby any confirmed residue in food of animal origin renders the product non-compliant. In accordance with EU regulations, the action threshold for NF metabolites has been set at 0.5 µg/kg, necessitating the use of highly sensitive confirmatory methods [
6]. Albania has adopted all EU legislation to fully comply with EU rules [
23].
A significant analytical challenge arises from the instability of the parent NFs furazolidone, furaltadone, nitrofurantoin, nitrofurazone, and nifursol. These compounds degrade rapidly in vivo and are rarely detectable shortly after administration [
27]. Their metabolites, however, covalently bind to tissue proteins and exhibit greater stability, persisting for extended periods in edible matrices such as muscle, liver, and eggs [
10,
11,
16,
17,
18,
29]. The major metabolites include 3-amino-2-oxazolidinone (AOZ), 3-amino-5-morpholinomethyl-2-oxazolidinone (AMOZ), 1-aminohydantoin (AHD), semicarbazide (SEM), and 3,5-dinitro-salicilik Acid hydrazid (DNSAH) [
41].
Table. 1
These metabolites have therefore been designated as marker residues for monitoring the abuse of NFs in food-producing animals. Eggs are particularly relevant matrices for residue control due to their high consumption rates. Experimental studies have demonstrated that NF metabolites accumulate in eggs at concentrations exceeding those of the parent compounds, with persistence extending well beyond established withdrawal periods [
30]. For instance, AOZ residues have been detected in eggs up to three weeks after cessation of treatment [
29]. Similarly, nitrofurazone metabolites (SEM) exhibit prolonged half-lives, particularly in yolk, compared with their parent drug [
25]. Based on this, the study is conducted on whole eggs.
Food processing does not eliminate these residues. Boiling and frying have been shown to leave NF metabolites intact, and in some cases to increase measured concentrations due to enhanced extraction efficiency [
1,
28]. Additionally, SEM contamination has been reported in processed egg powders, sometimes originating from technological treatments such as chlorination during processing [
8,
15]. These findings highlight the importance of eggs and egg-derived products in monitoring programs.
Accurate detection of NF metabolites requires efficient sample preparation and highly selective instrumentation. Traditional workflows involve acid hydrolysis to release protein-bound residues, followed by derivatization with 2-nitrobenzaldehyde (NBA), producing stable nitrophenyl derivatives with improved ionization efficiency in mass spectrometry [
24,
25]. Subsequent solvent extraction removes proteins and lipids, yielding clean extracts suitable for instrumental analysis [
29].
In recent years, the QuEChERS (Quick, Easy, Cheap, Effective, Rugged, and Safe) method has emerged as a powerful approach for sample cleanup. Its combination of acetonitrile extraction and salting-out partitioning with MgSO₄ and NaCl provides efficient removal of matrix interferences while preserving analyte recovery [
21]. The QuEChERS approach provides an efficient cleanup for complex matrices like eggs, effectively removing interfering substances while preserving analyte recovery. Compared with classical liquid–liquid or solid-phase extraction, QuEChERS offers shorter processing times, lower costs, and improved reproducibility, making it especially attractive for routine applications in official control laboratories.
Liquid chromatography coupled to tandem mass spectrometry (LC-MS/MS) has become the reference technique for NF residue analysis [
3,
7,
8,
9,
14,
19,
20,
21,
22,
31,
39]. By integrating chromatographic separation with mass spectrometric analysis, the method allows quantification of NF metabolites at µg/kg levels. Most validated methods employ reversed-phase LC with electrospray ionization (ESI) and multiple reaction monitoring (MRM), ensuring both sensitivity and selectivity [
2,
13,
14].
Compared to earlier approaches, such as single quadrupole LC-MS or HPLC-UV detection, LC-MS/MS offers significantly improved performance. For SEM, for example, published methods report decision limits (CCα) between 0.20–0.37 µg/kg and detection limits (LOD) as low as 0.05 µg/kg, well below the EU action limit of 0.5 µg/kg [
12,
32,
42]. More recently, the European Union Reference Laboratory (EURL) for Residues of Antibacterial Substances has published a harmonized method for the confirmatory detection of AOZ, AMOZ, AHD, SEM, and DNSAH at an RPA of 0.5 µg/kg [
14].
Furthermore, the development of triple quadrupole instruments and harmonized protocols published by the European Union Reference Laboratory (EURL) has standardized confirmatory testing across member states [
14].
Considering the toxicological risks of NFs, their persistence in eggs, and the limitations of conventional detection approaches, there is a clear need for sensitive, reliable, and reproducible methods for residue monitoring. The combination of QuEChERS sample preparation with LC-MS/MS confirmatory detection offers a robust strategy that meets the strict performance requirements outlined in Commission Implementing Regulation (EU) 2021/808. By validating such methods for egg matrices, regulatory authorities can strengthen residue control programs, ensure compliance with EU legislation, and safeguard consumer health [
20].
Material and Methods
Chemicals and Reagents
All solvents used were of LC–MS/MS grade, and all other reagents were of analytical grade. Distilled water (18.2 MΩ·cm) was prepared using a Milli-Q system (Adrona Crystal). Hydrochloric acid was obtained from VWR International (Pennsylvania, USA). 2-Nitrobenzaldehyde (2-NBA, purity 99.93% was purchased from Apollo Scientifics (Manchester, UK). Acetonitrile 99.95% and trisodium phosphate dodecahydrate were supplied by Titol Chimica (Rome, Italy). Sodium hydroxide (97%) and ammonium formate 99 % were from Carlo Erba (Milan, Italy), and HPLC-grade methanol was purchased from Romil (Cambridge, UK). All chemicals were used without further purification and handled according to standard laboratory practices.
Standards
Analytical standards of the NF metabolites were obtained from commercial suppliers and were purchased from different producers. SEM, 3-Amino-2-oxazolidinone (AOZ) and 1-aminohydantoin (AHD) were purchased from CPA Chem (Bogomilovo, Bulgaria), 3-Amino-5-morpholinomethyl-1,3-oxazolidin-2-one (AMOZ) from HPC (Cunnersdorf, Deutschland) and 3,5-dintrosalicylic acid hydrazide (DNSAH) from Witega (Berlin, Germany) as well all nitrophenyl derivative marker residues 3-(2-nitro phenyl)methylene-amino-2-oxazolidinone (NPAOZ), 5-Methylmorfolino-3-((2-nitrophenyl)methylene)-3-amino-2-oxazolidinone (NPAMOZ), 1-((2-nitrophenyl) methylene)-amino-2-hydantoin (NPAHD), (2-nitrophenyl) methylene-semicarbazide (NPSEM), 3,5-(2-nitrophenyl)-dinitrosalicylic acid hydrazide (NPDNSAH) and internal standards 3-amino-5-morpholinomethyl-1,3-oxazolidinon-2-one-d5 (AMOZ-d5), 1-aminohydantoin-13C3 (AHD-13C3), 3,5-dinitrosalicylic acid hydrazide 13C6 (DNSAH-13C6) except 3-amino-2-oxazolidinone-d4 (AOZ-d4), semicarbazide-13C 15N2 (SEM-13C 15N2), that were purchased from HPC (Cunnersdorf, Deutschland).
Standard Preparation
A stock solution of NF metabolites (MM1) was prepared at 50 µg/ml, based on the batch used as indicated in the certificate of analysis. Nitrophenyl derivatives (NP1) and corresponding internal standards (IS1) were prepared at the same concentration.
The first mixture of each standard was prepared at 1 µg/ml by transferring 200 µL of each stock solution into a 10 mL volumetric flask. On the day of analysis, two working mixtures were freshly prepared: one at 5 µg/l (50 µl of the first mixture in a 1 ml flask) and one at 0.5 µg/l (100 µl of the 5 µg/l mixture in a 1 ml flask). All solutions were prepared in methanol.
For nitrophenyl derivatives, the first mixture (NP2) was prepared at 1 µg/ml based on free metabolites. From this, a 10 µg/ml solution was prepared for spiking at the end of evaporation and for preparing a freshly working solution used for system control at 1 µg/l. Volumes were adjusted according to the required quantities. The concentrated stock solutions MM1, NP1, and IS1 were stable for at least two years [
13].
Sample Preparation
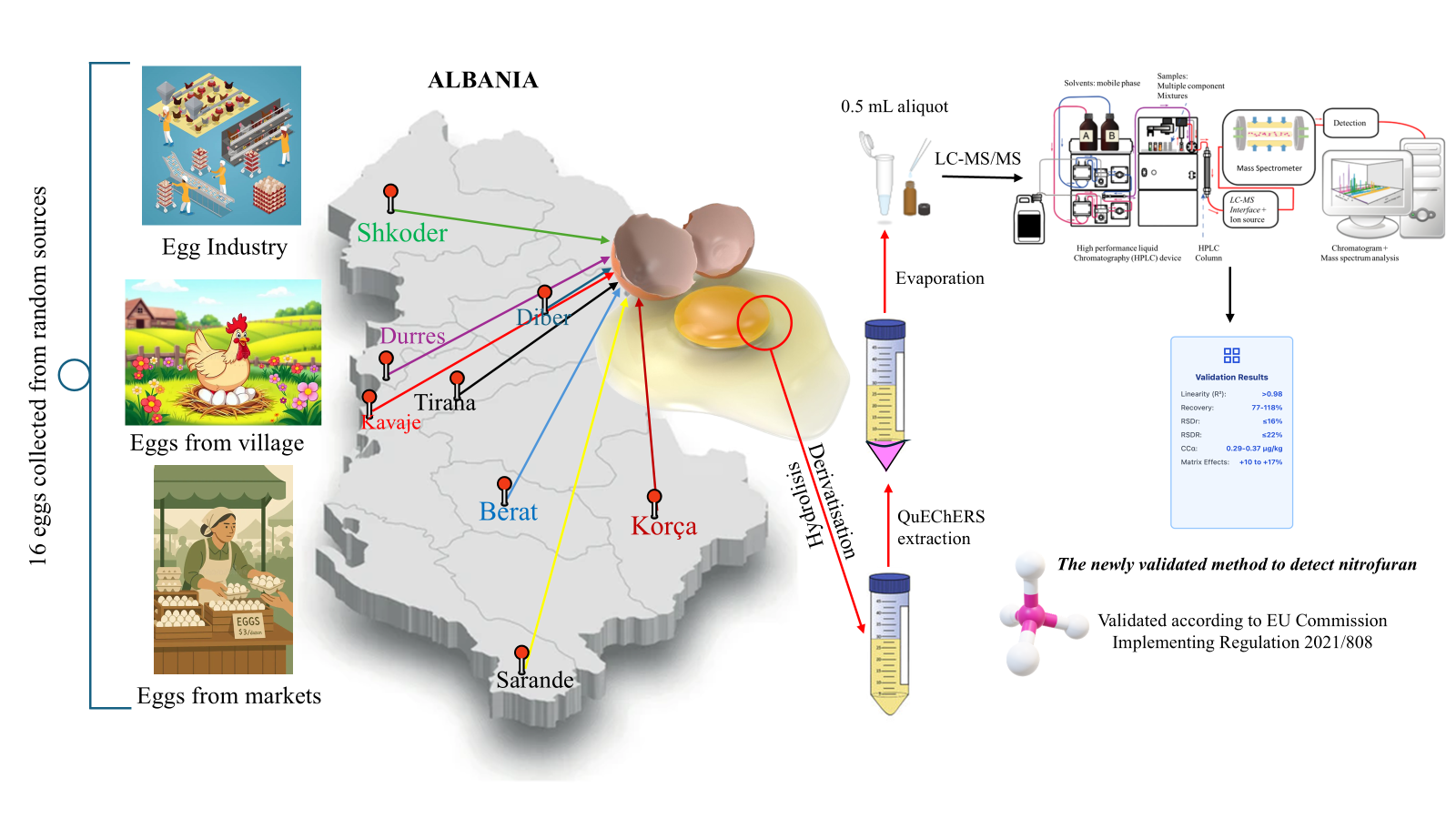
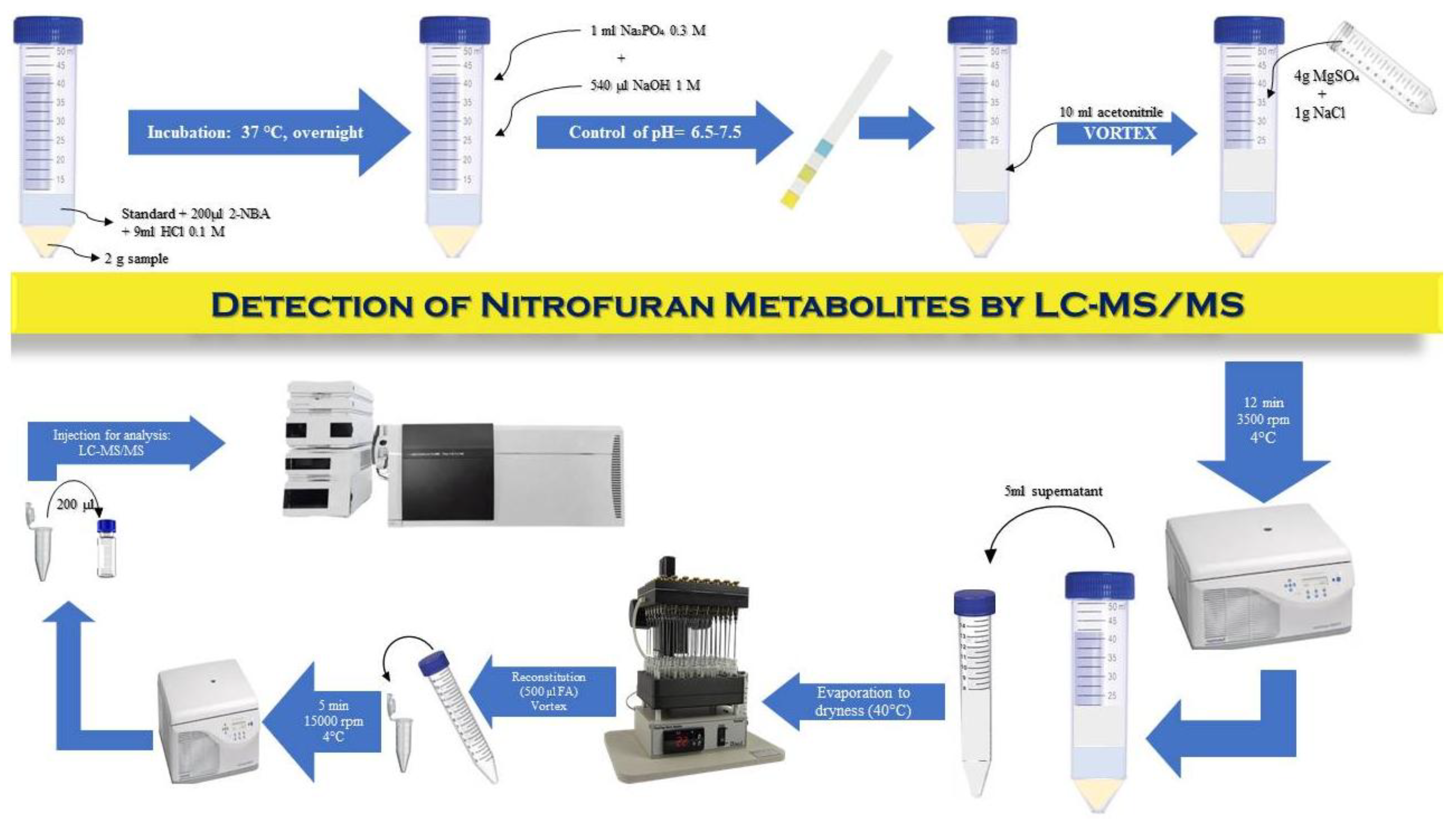
Samples are collected based on point 1, Annex II of Regulation EU2021/808, where it is emphasized that the sample size is at least 12 eggs or more, according to the analytical methods. After removing the shell, the egg samples are mixed and stored at -20ºC or analyzed immediately. Egg samples (2.0 ± 0.05 g) were homogenized and transferred into centrifuge tubes. Internal standard mixtures were added before hydrolysis. Protein-bound residues were released by adding 9 ml of 0.1 M HCl and 200 µl of 100 mM 2-NBA. The tubes were vortexed for 30 s, sealed, and incubated either overnight at 37 ± 2 °C or for 2 h at 60 ± 2 °C in a water bath. After derivatization, the samples were cooled to room temperature and neutralized to pH 6,5–7,5 with 1 M HCl or NaOH. Extraction was carried out with acetonitrile, followed by a QuEChERS cleanup using 1 g NaCl and 4 g MgSO₄. After centrifugation at 4500 rpm for 15 min, 5 ml of supernatant was collected, evaporated to dryness in a nitrogen evaporator at 40 °C, and reconstituted in 500 µl of mobile phase A. A 20 µl aliquot was injected into the LC-MS/MS system.
Figure 1.
Workflow of sample preparation and LC-MS/MS analysis of nitrofuran metabolites.
Figure 1.
Workflow of sample preparation and LC-MS/MS analysis of nitrofuran metabolites.
Method Validation
The method validation was carried out following the requirements of Commission Implementing Regulation (EU) 2021/808, which specifies the criteria for analytical methods used in official control of veterinary drug residues and is evaluated to be fully in line with
Table 5 of this regulation, “Classification of analytical methods by the performance characteristics that have to be determined”. Since NFs are classified as prohibited substances, the method was validated as a quantitative confirmatory method. Accordingly, all mandatory performance parameters were evaluated, including identification, selectivity/specificity, linearity, trueness, precision (repeatability and reproducibility), matrix effects, and decision limit (CCα). The method was validated using matrix-matched samples to ensure applicability to routine analysis under official control conditions.
Identification
For confirmatory methods, identification of analytes must meet strict requirements regarding retention times, ion ratios, and diagnostic transitions. In this study, all their nitrophenyl derivatives of NFs fulfilled these criteria. The use of isotope-labeled internal standards (AOZ-d4, AMOZ-d5, AHD-13C3, SEM-13C15N2, DNSAH-13C6) provided robust confirmation by compensating for matrix effects and signal fluctuations. The relative retention times of analytes compared with their internal standards deviated by no more than 1% during the entire validation process and routine analysis. Diagnostic ion ratios were also stable, with variations remaining within ±40% of the values obtained for calibration standards. Furthermore, the signal-to-noise S/N ratio exceeded the acceptance limit of 3 for all monitored ions. These results demonstrate that the method provides reliable confirmatory identification of NF residues in egg matrices.
Selectivity and Specificity
Selectivity refers to the ability of the method to clearly distinguish the target analytes from other compounds that may be present in the sample in the retention time windows of the target analytes. To assess this parameter, 20 blank egg samples from different sources were analyzed. None of the samples showed interfering peaks at the retention times of the target compounds. This indicates that the method is highly selective and specific for identifying NFs quantitatively from possible interferences, even in egg, which is a complex matrix.
Matrix Effects
Matrix effects represent a critical analytical challenge in the quantification of veterinary drug residues such as NF metabolites in complex food matrices like eggs. These effects arise due to co-extracted matrix components that influence the ionization efficiency of target analytes during LC-MS/MS analysis, leading to signal suppression or enhancement. The egg matrix, characterized by high lipid and protein content, is particularly prone to causing substantial matrix-induced ion suppression, which may compromise analytical accuracy and sensitivity. Eggs are a complex matrix containing proteins, lipids, and other endogenous compounds that can affect ionization efficiency in LC-MS/MS analysis. To evaluate matrix effects, 36 blank egg samples were fortified with NF standards (NP 2) at the reference point for action (RPA = 0.5 µg/kg). The responses were compared with those of equivalent concentrations in neat solvent solutions, which were derivatized according to the described procedure. The variability in matrix factors, after normalization with isotopically labeled internal standards, was consistently below 20% for all compounds. These results confirm that the method is robust against matrix-related interference and that matrix-matched calibration is appropriate for accurate quantification.
Trueness, Precision, and Decision Limit (CCα)
The high sensitivity is largely attributable to the derivatization step with 2-nitrobenzaldehyde, which forms nitrophenyl derivatives that exhibit enhanced ionization efficiency in mass spectrometry [
24]. This step significantly increases signal intensity and improves the reliability of quantification, particularly for SEM and AOZ, which are typically more challenging to detect at trace levels. As there are no certified reference materials available, it is acceptable that trueness of measurements is assessed in other ways, such as evaluating by recovery experiments using blank matrix samples fortified at three concentration levels at concentrations of 0.5, 1.0, and 1.5 RPA. For each level, not less than six replicates were prepared and analyzed under repeatability conditions, and were analyzed according to the prescribed procedure. For each sample analyzed, the trueness values (
Table 3) were calculated based on Equation 1.
Acceptable recovery values were based on the criteria outlined in Regulation 2021/808 and complementary guidelines (e.g., SANTE/2023/20), with typical acceptable ranges of 70–120%. Wider limits (60–130%) were considered acceptable at lower concentration levels or in complex matrices. Precision was assessed in terms of repeatability (RSDr) (intra-day) and within-laboratory reproducibility (RSDR) (inter-day) and was determined by analyzing not less than six replicates at each spiking level (L1, L2, and L3) under the same experimental conditions (same analyst, equipment, reagents, and day).
Within-laboratory reproducibility was determined by analyzing fortified samples over three separate weeks, using different analysts, different incubation times (37 °C overnight or 2 hours at 60 °C), and a chromatographic column using the same method and instrumentation. For each level, the mean concentration, standard deviation, and coefficient of variation (%) of the fortified samples were calculated to assess repeatability and reproducibility.
In terms of considering a result as compliant or non-compliant, the decision limit (CCα) under conditions complying with the requirements for identification or identification plus quantification as defined under ‘Performance criteria and other requirements for analytical methods’ as laid down in Chapter 1 of the Regulation (EU) 2021/808. The decision limit (CCα) is determined with Method 3 and calculated with equation 2:
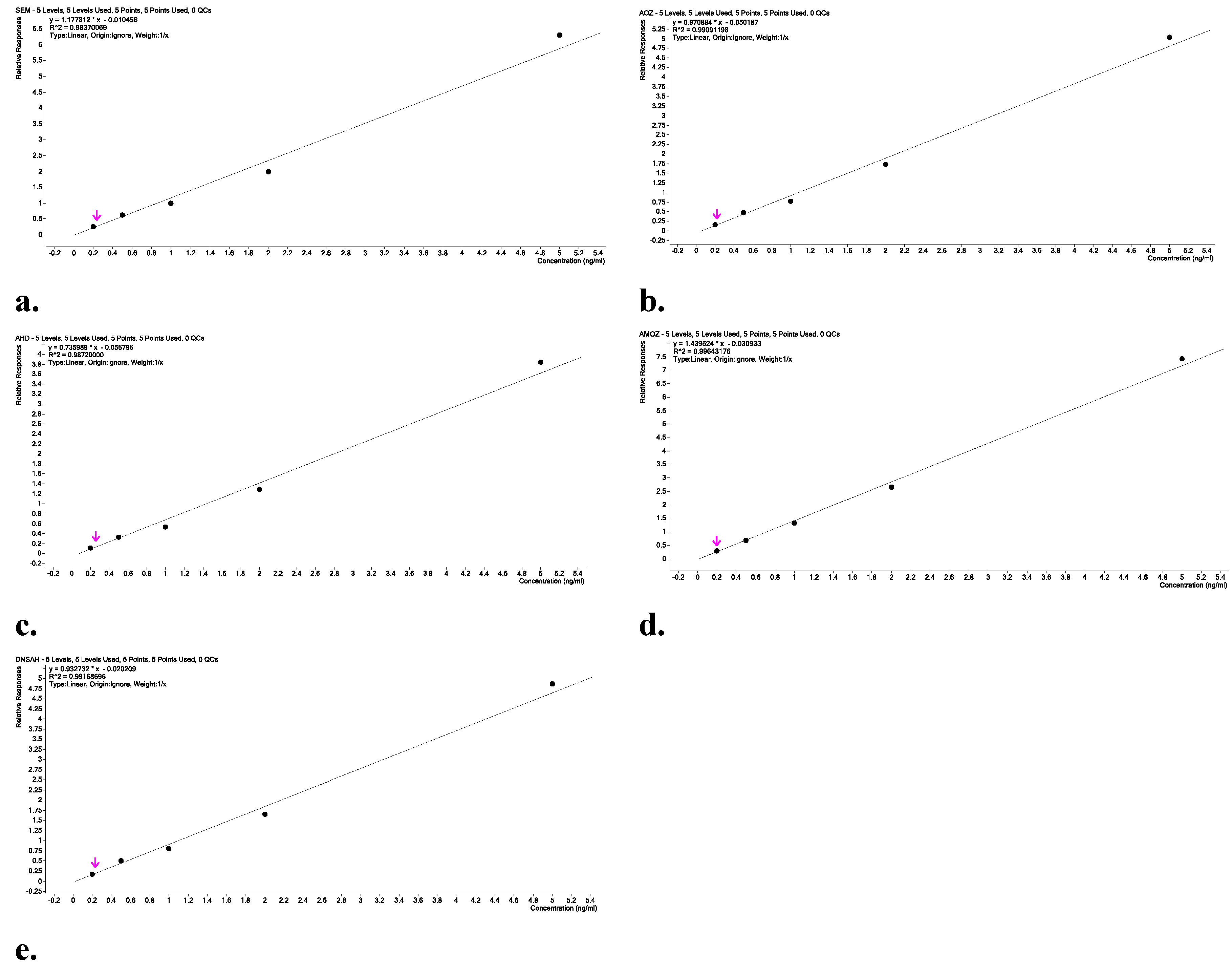
Linearity
Calibration curves (
Figure 2a-e) were prepared by fortifying blank egg samples with NF metabolites across the concentration range of 0.2, 0.5, 1.0, 2.0, and 5.0 µg/kg, spiked at the beginning of the procedure. Each calibration curve showed excellent linearity, with correlation coefficients (R²) which was evaluated during the validation and method performance verification to be higher than 0.99, with some exceptional cases for AMOZ, which could be greater than 0.98, which is higher than the values of 0.95 suggested by EURL methodology. Importantly, the use of matrix-matched calibration compensates for potential ion suppression or enhancement caused by egg components, thereby improving the reliability of quantification in real-world samples [
38].
Stability and Roughness
Method validation in accordance with European Commission Regulation 2021/808 requires assessment of analyte stability in solution and matrix. The first one was determined by Regan et al., 2021 but for the stability of NFs in eggs, no data have been reported so far. Stability testing experiments may seem to be straightforward to conduct, but differences in day-to-day measurements are challenging, and so make this study is very rarely published in the literature [
37]. Due to a lack of incurred samples for all analytes, the laboratory determined the stability of one nitrofuran metabolite in an incurred sample and the stability data for all analytes in a spiked sample.
Results and Discussion
Matrix Effect
In order to compensate for matrix-related variability, the regulation requires the use of matrix-matched calibration or stable isotope-labeled internal standards. Isotopically labeled analogues, such as
13C- or D-labeled for all 5 NF metabolites, are crucial to correct for matrix-induced signal variation during quantitative analysis. The extent of matrix effect must be assessed across representative egg samples, as required under the regulation's provisions for method performance verification. Acceptable limits for matrix effects are generally considered to be within ±20% signal variation; beyond this, analytical performance may be deemed non-compliant. Thus, a rigorous evaluation and control of matrix effects was performed to ensure the validity, comparability, and traceability of results obtained in the determination of NF residues in eggs, in full compliance with the performance criteria outlined in Regulation (EU) 2021/808 [
4].
Matrix effect is calculated by dividing the matrix effect of the standard by internal standards. Matrix effect of standards is calculated by dividing the area counts of each spiked egg sample obtained from post-extraction by the area counts obtained from standards spiked into the solvent. Matrix effect of internal standards is calculated by dividing the area counts of each spiked egg sample obtained from post-extraction by the area counts obtained from standards spiked into the solvent. For 36 different egg samples spiked at 0.5 µg/kg (RPA level) was performed the matrix effect was normalized for IS, and the coefficient of variance was calculated. Also, the application of sample clean-up procedures with QuEChERS and acid hydrolysis step, may mitigate matrix interference and improve method reliability which is clearly expressed by values of matrix effect (standard normalized for IS) achieved for each analyte: AHD +10,14 %; +AOZ 17,31%; +AMOZ 14,44%; +DNSAH 10,54% and +SEM 11,62% which are well below ±20% for all analytes.
Recovery and Precision
Recovery experiments demonstrated that the method is capable of extracting and quantifying NF metabolites with high accuracy. Mean recoveries across all concentration levels ranged between 82% and 109% (
Table 2), which falls well within the 50–120% range accepted for quantitative confirmatory methods for mass fraction ≤ 1 µg/kg. The precision data supported method reliability, like intra-day repeatability, which was below 10% (except AMOZ, which was 16 % but is still less than 20%), making it an acceptable value of precision under repeatability conditions. For inter-day reproducibility, the precision value didn’t exceed 22%, which is far below 30 % the acceptable values of precision for concentration ˂ 10 µg/kg. These findings indicate that the method is accurate and reproducible under routine laboratory conditions. The relatively low variability is particularly noteworthy considering the complexity of the egg matrix. Egg yolk contains high levels of lipids, while albumen is rich in proteins, both of which can complicate sample preparation and analysis. The QuEChERS-based cleanup, combined with derivatization to nitrophenyl derivatives, proved effective in minimizing these matrix effects, resulting in stable recoveries even at the lowest tested concentrations.
Sensitivity and Decision Limits
The values of decision limits are calculated by combining data from precision in terms of repeatability and reproducibility, which provides the possibility to satisfy the criteria of a confirmatory method, such as ion ratios and satisfactory results for signal-to-noise ratio. The data are obtained from analyzing the sample in days separated into more than one week, two different technicians (Halo and Kinetex), and two different times of incubation (37 °C overnight or 2 hours at 60 °C).
The calculated decision limits (CCα) ranged between 0.29 and 0.37 µg/kg for all analytes (
Table 3). These values are below the reference point for action (RPA) of 0.5 µg/kg set by the European Union. This demonstrates that the method is sufficiently sensitive for enforcement of the EU’s zero-tolerance policy on NF residues and is achievable under this method for egg samples.
Analysis of a Real Sample
The results obtained from this study highlight the suitability and robustness of this confirmatory method, given that it was applied to a wide range of different samples. The method was fit to analyze all NF-bound residues, and no additional interferences were observed. This method was verified with the participation in a commercial PT scheme organized by Test VERITAS (
Table 4) with sample ID E5106 and laboratory code T046.
Values of Z-score, calculated by the PT Provider, were within the range from ±1 for 3 of the analytes that were present in the spiked sample provided by Progeto Trieste, showing that this method is fit for purpose and the method performance is satisfactory. With this PT sample, the reported results were analyzed in the Kinetex column, and for internal purposes, the tests were performed in the Halo column, and the results are well comparable.
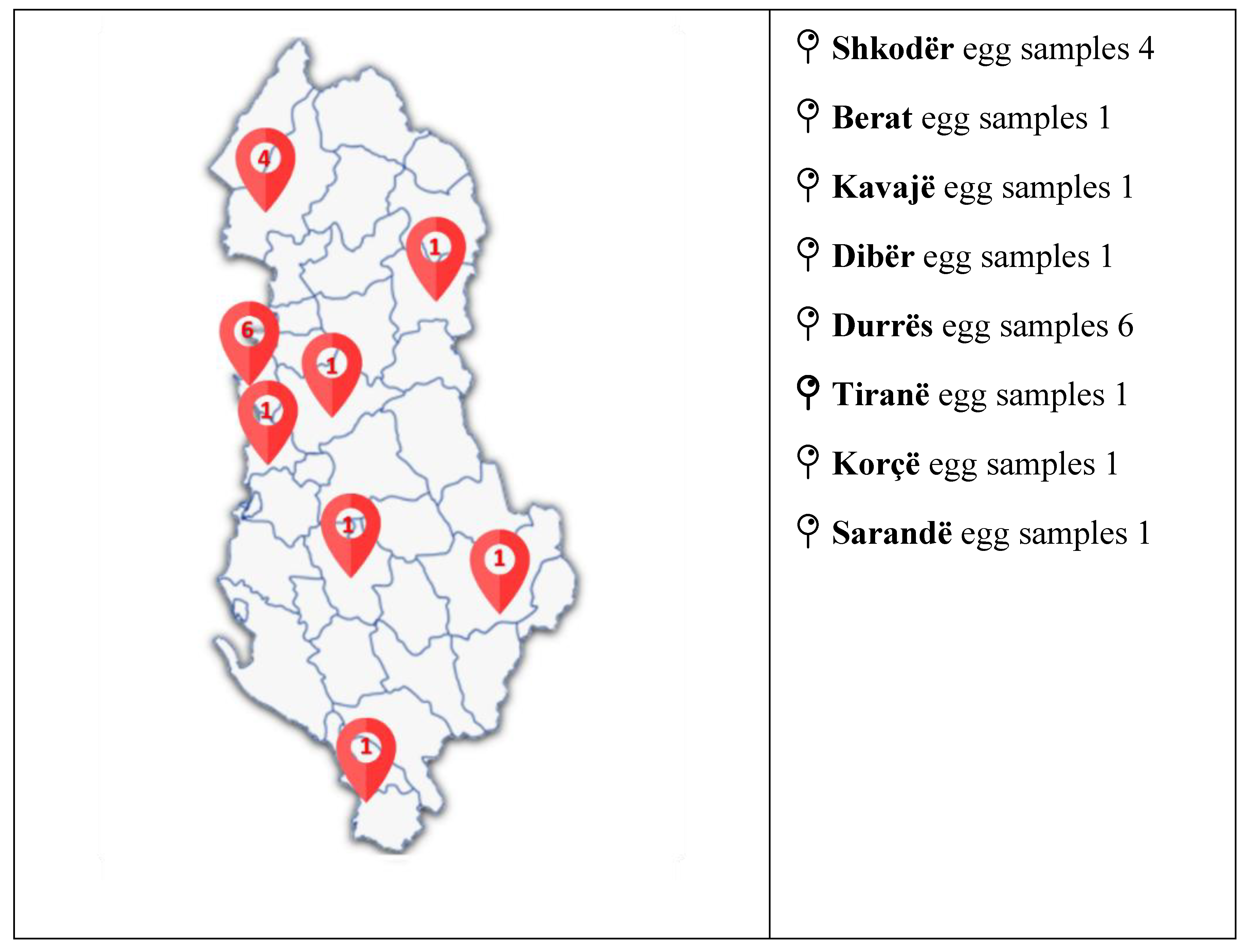
Based on the validated method described in this publication, 16 different samples from different sources, like farms, industry, or markets in different areas of Albania, are analyzed. These chromatographic and spectrometric conditions ensured reproducible retention times and stable ion ratios across multiple runs. Almost half of the sample detected some residues of SEM, but they were not detected or quantified in terms of validation and compliance with the parameters prescribed in 808. As in all annual meetings, the issue of finding an alternative marker for SEM to distinguish between the presence of SEM and other sources, or misuse of nitrofurazone, is still under discussion in EURL. Further work will be conducted in this framework, while we will analyse the samples in the framework of the project [
33].
Table 5.
Results of egg sample with the presence of SEM and AOZ.
Table 5.
Results of egg sample with the presence of SEM and AOZ.
| |
Origin |
SEM
(µg/kg)
|
AOZ
(µg/kg)
|
RPA
(µg/kg)
|
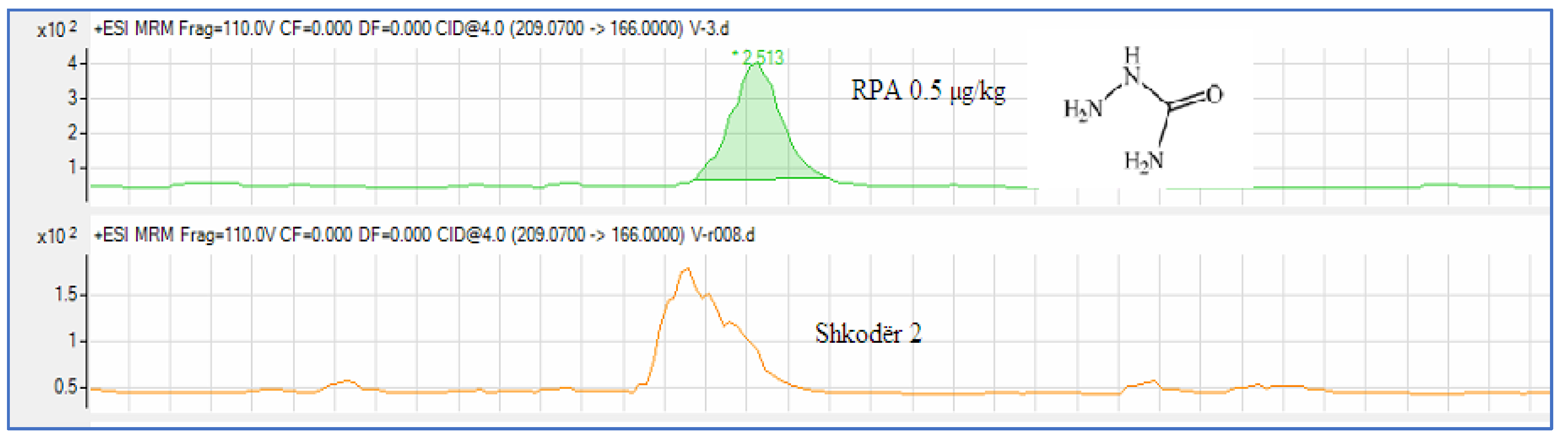
| 1 |
Shkodër 2 |
0,240 |
|
0,5 |
| 2 |
Durrës 2 |
|
0,227 |
0.5 |
| 3 |
Shkodër 3 |
|
0,271 |
0.5 |
Figure 4.
Geo-mapping of the collected eggs throughout Albania.
Figure 4.
Geo-mapping of the collected eggs throughout Albania.
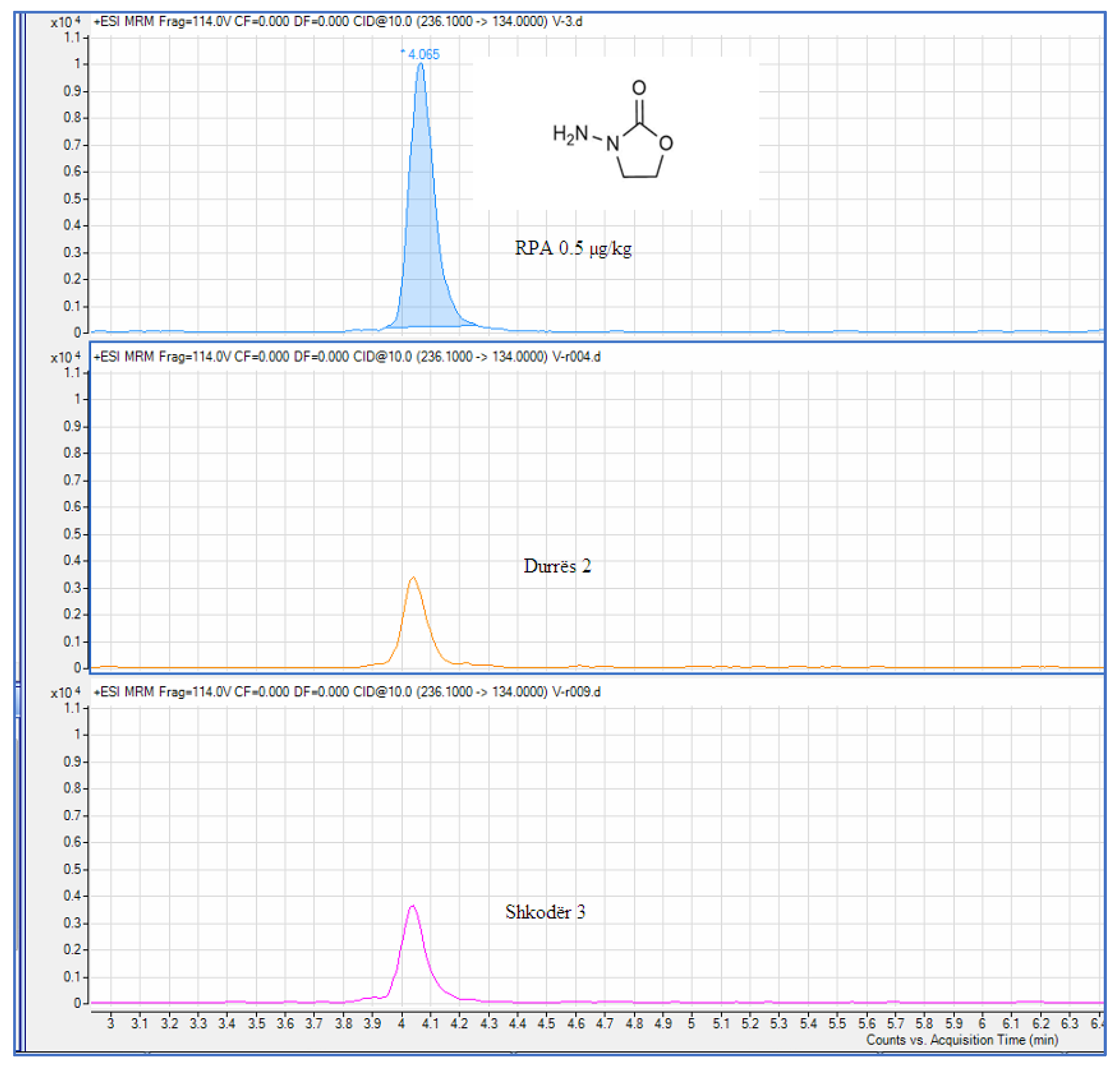
Figure 5.
Chromatographic presentation of samples with the presence of the metabolite SEM..
Figure 5.
Chromatographic presentation of samples with the presence of the metabolite SEM..
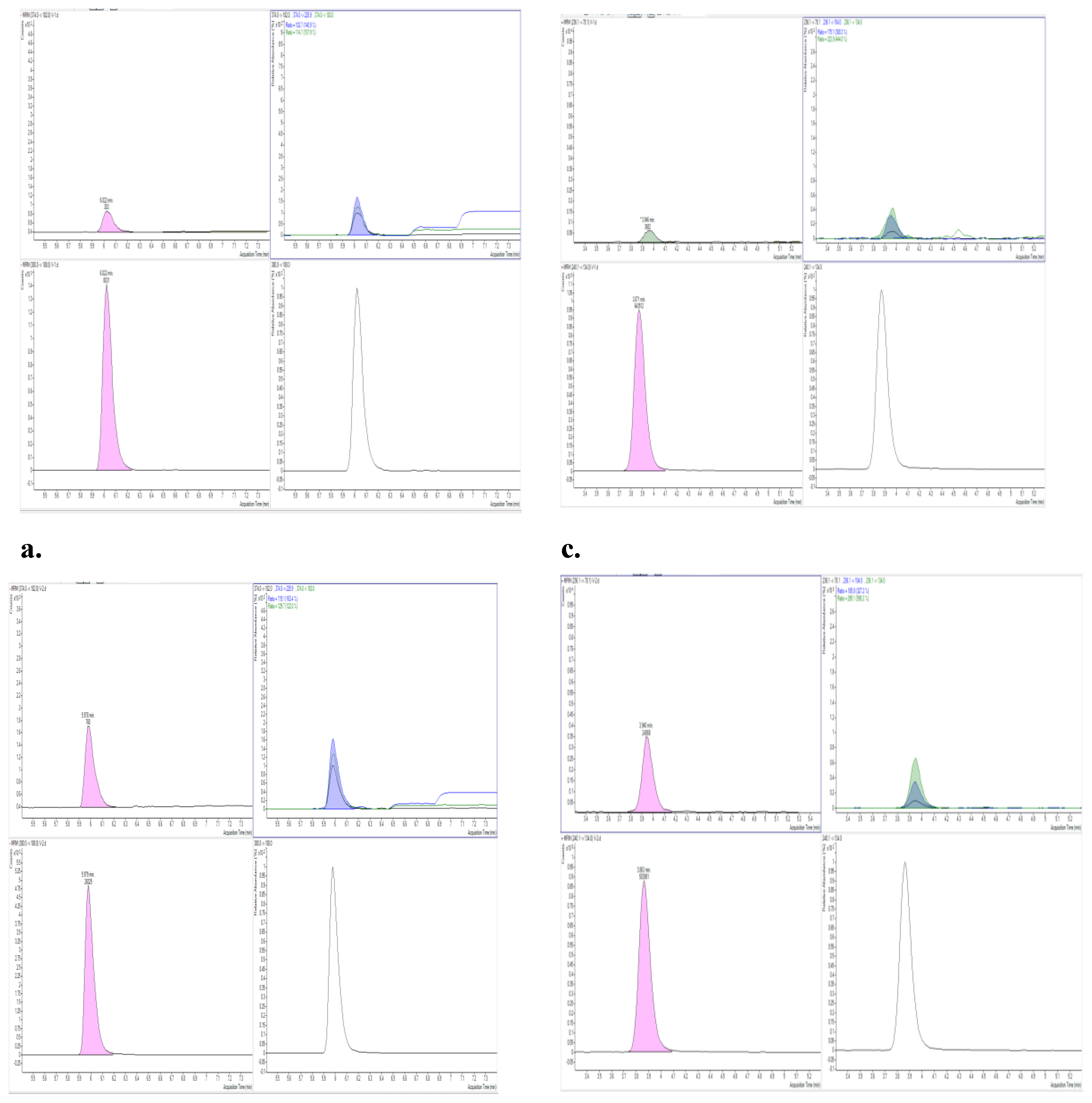
Figure 6.
Chromatographic presentation of samples with the presence of the metabolite AOZ.
Figure 6.
Chromatographic presentation of samples with the presence of the metabolite AOZ.
In three samples from different regions of Albania were detected values of SEM and AOZ in levels well below the CCα and RPA levels, but very well defined and quantitate. Some investigations were performed to evaluate the evidence of any misuse of nitrofuran, or whether it is just a contamination from the past.
Stability Data
The timeline of the stability study started on Day 0 when the spiked samples at RPA were prepared. The set of spiked and incurred egg samples was divided and stored at -20ºC. On 2 weeks and on months 1, 2, 3, 4, and 6, the appropriate batch of centrifugal tubes was removed from the storage facilities and injected 5 times, and analyzed in a new batch of analyses with the set of calibration and all other requirements. Due to literature reviews where it is emphasized that NF metabolites are stable it was performed a stability study in -20ºC.
Table 7.
Summarized details on measured concentration and loss for each NF metabolite in the spiked and incurred egg sample.
Table 7.
Summarized details on measured concentration and loss for each NF metabolite in the spiked and incurred egg sample.
| |
Day 0 |
4 Months |
6 Months |
WLR |
|
Analyte
|
Measured concentration (µg/kg) |
Measured concentration (µg/kg) |
Loss (%) |
Measured concentration (µg/kg) |
Loss (%) |
|
SEM
|
0,893 |
0,808 |
10% |
0,682 |
32% |
16,20% |
|
AHD
|
0,943 |
0,856 |
9% |
0,780 |
22% |
10,47% |
|
AOZ
|
0,893 |
0,886 |
1% |
0,835 |
16% |
8,78% |
|
AMOZ
|
0,893 |
0,849 |
5% |
0,687 |
31% |
12,47% |
|
DNSAH
|
0,866 |
0,868 |
0% |
0,861 |
14% |
6,65% |
|
AOZ incurred
|
1,305 |
1,308 |
0% |
1,306 |
0% |
N/A |
The other stability study like light, dark, or 4ºC, were not performed as it is not relevant to store the egg sample in these conditions.
This finding confirms that the protein-bound form of AOZ in incurred tissue is exceptionally stable, consistent with literature reports on the persistence of covalently bound NF metabolites in biological matrices.
The comprehensive stability study conducted over six months at minus twenty degrees Celsius revealed important differences in the degradation behavior of NF metabolites in the egg matrix. The excellent stability of AOZ and DNSAH, with only sixteen percent and fourteen percent losses, respectively, at six months, makes these compounds particularly suitable as long-term reference materials. This stability is consistent with the known chemical properties of these metabolites and supports their use in proficiency testing materials and certified reference materials.
Conclusions
This study successfully developed and validated a sensitive and selective LC–MS/MS confirmatory method for the determination of protein-bound nitrofuran metabolites in eggs, fully compliant with the performance criteria outlined in Commission Implementing Regulation (EU) 2021/808. with decision limits ranging from 0.29 to 0.37 micrograms per kilogram, well below the reference point for action of 0.5 micrograms per kilogram. The combination of acid hydrolysis, 2-nitrobenzaldehyde derivatization, and QuEChERS extraction proved highly effective for releasing and isolating nitrophenyl derivatives from the complex egg matrix. The use of a phenyl-hexyl chromatographic column and isotope-labeled internal standards ensured robust identification, minimized matrix-induced variability, and enabled reliable quantification at sub-µg/kg levels.
Stability studies conducted over six months at minus twenty degrees Celsius revealed differential degradation patterns among metabolites, with SEM and AMOZ requiring four-month retest intervals while AOZ and DNSAH exhibited excellent long-term stability suitable for reference materials. Application of the method to sixteen egg samples from across Albania demonstrated the capability to use this method for routine surveillance programs. Its application can be extended to other complex matrices, offering a template for developing similar confirmatory methods for banned veterinary drugs. The method is accredited by the General Directorate of Accreditation in Albania with code LT 112.
Author Contributions
Concept, design, and project administration K.V. and E.M. Methodology and Validation E.M., M.D., E.P., S.T. Collection of egg samples, analysis, and interpretation of data: all authors. Data processing, E.M., K.V., E.P., J.C., and I.P. Original draft preparation E.M., K.V., M.D., E.P., Critical revision of the manuscript for important intellectual content: all authors. All authors have read and agreed to the published version of the manuscript.