1. Introduction
Kuwait desalination facilities generate a substantial volume of brine solutions, containing a high concentration of valuable minerals [
1], which are currently discharged into the Arabian Gulf without any pretreatment. The extraction of these beneficial minerals from brine solutions is considered a promising economic opportunity. This extraction has beneficial aspects [
2]: increasing the productivity of desalination plants, reducing operating expenses, resulting in a high economic value of critical commercial minerals, reducing power consumption in desalination facilities, and mitigating the negative risk impact on the ecosystem and marine environment.
To enable efficient extraction with low operating costs, the solubility limits of these valuable minerals must be assessed in a real brine solution that has the same mineral matrix and concentration as the actual brine solution.
It is well known that the solubility limit of all minerals depends on various parameters, including temperature, pressure, pH values, ionic strength of the solvent solution, and the concentration of other minerals dissolved in the base solution [
3].
Kuwait brine water, discharged from desalination plants, is characterized by a high concentration of Ca, Mg, SO
4, Na, Cl, K, and HCO
3 ions [
3]. This favors the extraction of many valuable minerals such as magnesium hydroxide (Mg(OH)
2), sodium chloride (NaCl), potassium chloride (KCl), lithium carbonate (Li
2CO
3), calcium carbonate (CaCO
3) and sulfate minerals (SM) which include CaSO
4, BaSO
4 and SrSO
4.
High concentration and economic value are crucial parameters in selecting minerals for extraction. The solubility limit is the most critical factor that must be considered before choosing a specific mineral for extraction. Generally, not all minerals can be extracted from brine solutions unless their solubility limits are exceeded.
The minerals dissolved in the Kuwait brine solution of the Doha Research Plant (BSDRP) have high economic value and high potential for extraction because of their high concentration and low solubility limits.
Table 1 contains the solubility limits of most of the minerals in distilled water. Two of them will be considered in the present study: magnesium hydroxide and calcium sulfate.
The average price of Mg(OH)
2 per ton during the first quarter of 2025 varied from
$465 in China to
$780 in the USA and reached
$1,500 in Brazil, according to industry market analysis [
10,
11,
12,
13]. Mg(OH)
2 was recognized as the most essential mineral for extraction from brine solution due to its very low solubility limit in water of 0.00122 g/100 mL and solubility product constant of 5.61×10
−12 [
14]. This mineral is expensive, and it is used in many applications in the international market as absorbents for heat, isolation, and polymer applications [
15].
Additionally, the global price for sulfate minerals (SMs) as CaSO
4, BaSO
4, and SrSO
4 ranges from
$ 160,
$ 170, and
$ 250, respectively, per ton for standard grade [
16]. SMs are commonly used in the industrial field, such as construction, dental products, and food processing. SMs are essential minerals for glass and pigment manufacturing. X-ray, medical applications, and drilling operations in oil production are other applications for SMs. Thus, SMs are not only a challenge to be managed but can also represent an opportunity for resource recovery and economic gain in industrial settings.
Measuring the solubility limits accurately and under different operating conditions is not only essential in predicting the scaling limits of significant minerals, but it will also significantly reduce the cost of annual maintenance in desalination plants.
Based on the preliminary facts, experiments are planned to determine the solubility limits of the early-mentioned valuable minerals using high-salinity Kuwait brine solutions and under different operating conditions.
The primary objective of this paper is to determine the solubility limit of selected valuable minerals in brine under various operating conditions, with the specific aim of investigating the relationship between ionic strength and the solubility limit of selected valuable minerals in brine water generated from seawater desalination under different operating conditions.
2. Experimental Procedure
2.1. Testing Unit
A laboratory test unit was inspected and prepared for the execution of the testing program. It consists of the following parts:
- -
Double jacket incubating vessel that incubates the test base solution at a constant temperature,
- -
Condenser that ensures constant composition of the tested brine solution during the experiments and condenses any vapor produced from the brine solution during incubation,
- -
Heat exchanger that controls the temperature of the base solution inside the double jacket vessel,
- -
Automatic pressure pump that controls the pressure inside the testing vessel,
- -
Reflux section that retains any condensate vapor back to feed water.
- -
Digital controller with a digital display to control the temperature inside the incubator vessel and the heat exchanger.
- -
Stirrer mixer with adjustable control speeds.
- -
Temperature and pressure gauges to monitor the temperature and pressure values.
The Rota-vapor will be used to prepare different concentrations of brine solution at low operating temperatures. Other measuring instruments and regulators, such as pH meters, conductivity meters, dipping turbidity meters, temperature sensors, and pressure controllers, are also needed.
All measuring instruments were calibrated before starting the testing schedule to ensure proper performance and accuracy.
All the required chemicals were acquired from the local market for the execution of the tasks outlined in the proposal. Minerals were requested and received to be used in the preparation of supersaturated solutions, which will be incubated at ambient temperature under stirring conditions to stabilize before starting each experiment.
All the sample containers were cleaned with diluted hydrochloric acid, and then they were rinsed several times with distilled water just before using them for sample collection.
2.2. Brine Water Samples Collection
Samples were collected from the brine discharge of the Shuwaikh Desalination Plant in a polyethylene container weekly (starting in April 2024) to measure the induction time required for saturation and to prepare the saturated solution before initiating solubility limit experiments. The collected samples (of about 50 L of reverse osmosis (RO)) were filtered, and excess solids of the target minerals were added. Samples were then incubated in the reactor at a controlled temperature in preliminary experiments to measure the time required to reach saturation, or the equilibrium time, to be implemented in the test plan. In addition, concentrated brine solutions were prepared from the base solution using a rotary evaporator for further experiments and stored at ambient temperature in closed containers to prevent pH variation.
Sample collections were conducted in accordance with international standards for quality control/quality assurance (QA/QC) to ensure the reliability of the data, thereby ensuring accuracy, precision, representativeness, and completeness. Moreover, a written standard operating procedure (SOP) for the collection, preservation, and storage of samples is applied in the proposal, in addition to the calibration of the equipment and following the QC/QA aspects during sampling and analysis.
The collected samples were analyzed for all required ions and cations dissolved in BSSDP. The average, maximum, minimum, standard deviation,relative standard deviation (RSD), Standard Error of the Mean (SEM), and other statistical parameters were calculated to ensure the reliability of the analysis.
2.3. Minerals Analysis
Experiments have been conducted since the end of October 2025, to measure the solubility limits of the required salts at different concentrations of BSSDR and under various conditions. Solubility measurements were taken for two important minerals: Mg(OH)2 and CaSO4, respectively. Results for other minerals will be discussed in a separate work further.
The base experimental work was based on several concentrations of the base solution. The solubility of the selected minerals was determined using concentration methods with high-precision instruments available at DRP, such as ICP and ICS, to quantify concentrations in high-salinity brine solutions.
High-purity salts were used in supersaturated solutions to avoid contamination and control the ionic strength of the brine solutions. Each experiment was repeated three times to ensure reproducibility, increase confidence, and produce scientifically valid data on mineral solubility limits in such a complex brine solution.
3. Ionic Strength Calculations
It is therefore of great importance to discuss the ionic strength of seawater since it falls at the heart of marine chemistry and geochemical modeling. For this reason, the ionic strength of major ions in seawater is calculated as a function of temperature.
Ionic strength is a quantitative measure of the total dissolved anions and cations and their valence in the brine and saline solution, which usually affects ion stability, scaling tendency, fouling, precipitation, activity coefficient, and the chemical properties of the brine solution.
Generally, ionic strength is defined as mentioned in the work of Millero [
17]:
where c stands for the molar concentration of a given ion [mol/l] and z is the charge of this ion.
From this equation, it is clear that temperature has no direct influence on ionic strength. Actually, it has an indirect influence by changing the density of seawater, which in turn changes the molar concentration of the ion.
4. Results
The results of the measurements for CaSO4 and Mg(OH)2 will be presented and discussed separately, as will be detailed below.
4.1. Solubility Limits of Hydrous CaSO4
The solubility limit of this mineral was measured at concentrations of 50%, 60%, and 70%. The brine of different concentrations was expressed in terms of ionic strength (I), as it is essential to describe the behavior of dissolved ions in high-saline solutions and is commonly used in desalination systems, mineral extraction processes, and solubility measurements. Thus, the determination of the ionic strength in the brine solution analysis is essential; therefore, thereby the ionic strength of (BSSDP), and for 50%, 60%, and 70% concentrated solutions is estimated for a more efficient solubility limit investigation.
Table 2 gives the ionic strength calculation of BSSDP samples using the analytical concentrations in (mg/l) and the charge of ions separately, and then applying equation 1 above.
The concentration of ions in the brine solution was converted from mg/L to mol/L using equation 2 below:
Table 3 contains the results of the ionic strength calculated based on the converted values of the concentrations.
The ionic strength of the Shuwaikh Brine solution is calculated to range approximately from 1.25 to 1.43 mol/L, with an average value of 1.38 mol/L depending on the chemical composition and the concentration of dissolved ions, while it ranged from 1.13 to 1.22 mol/L for brine water at the Doha Research Plan (BSDRP) with an average of 1.20. The 50% concentrated solution of BSSDP displays a higher ionic strength value of 1.43 mol/L. Moreover, the ionic strength was approximately 1.68 mol/L for a 60% concentration of BSSDP and increased to reach 1.996 mol/L at a 70% concentration.
The solubility limits of CaSO
4*2H
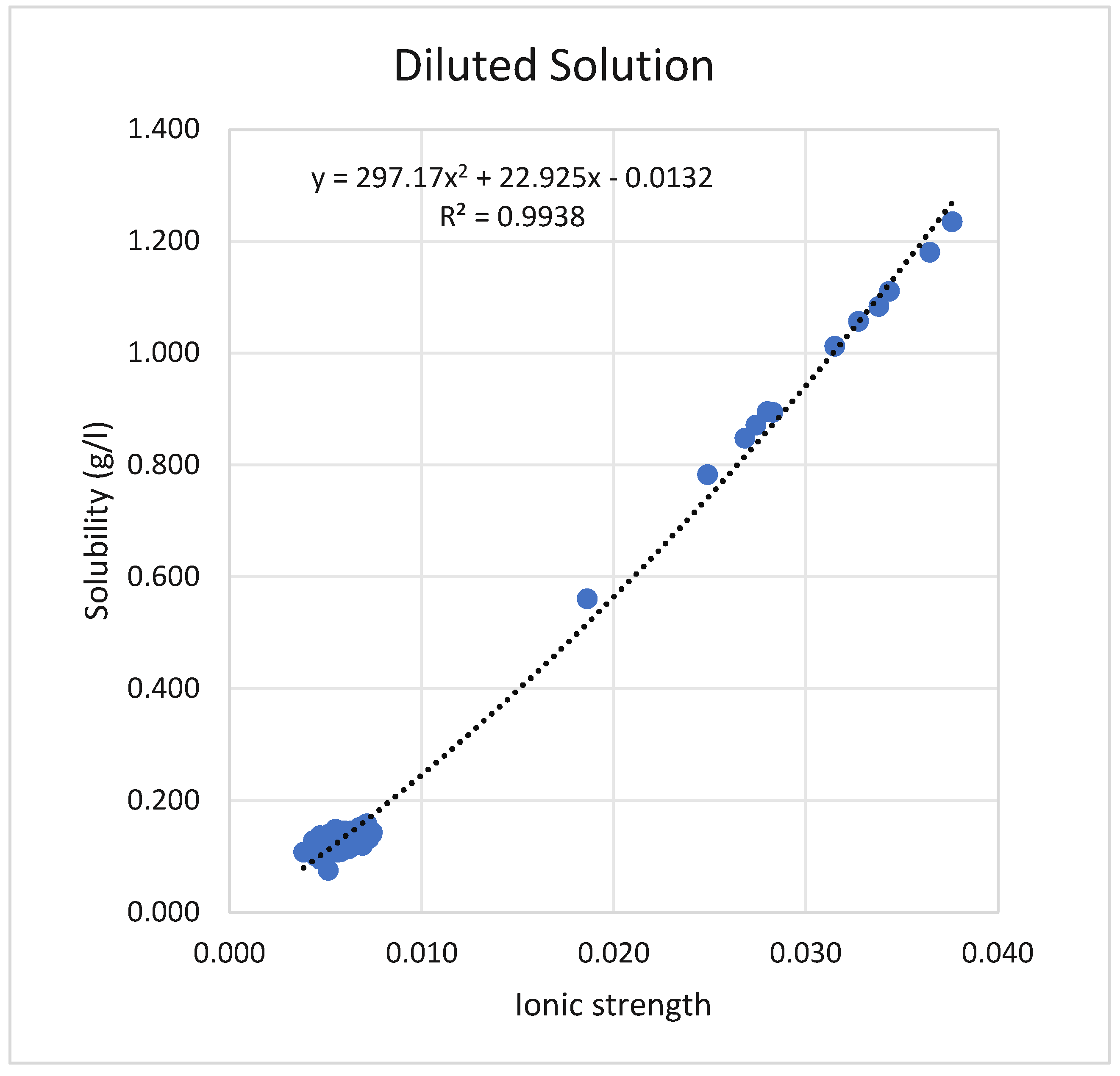
2O at ambient temperature for diluted solutions with different ionic strengths are shown in
Figure 1. It was found that the ionic strength ranged from 0.004 to 0.037 mol/L while the measured solubility ranged from 0.2 to 1.2 g/L.
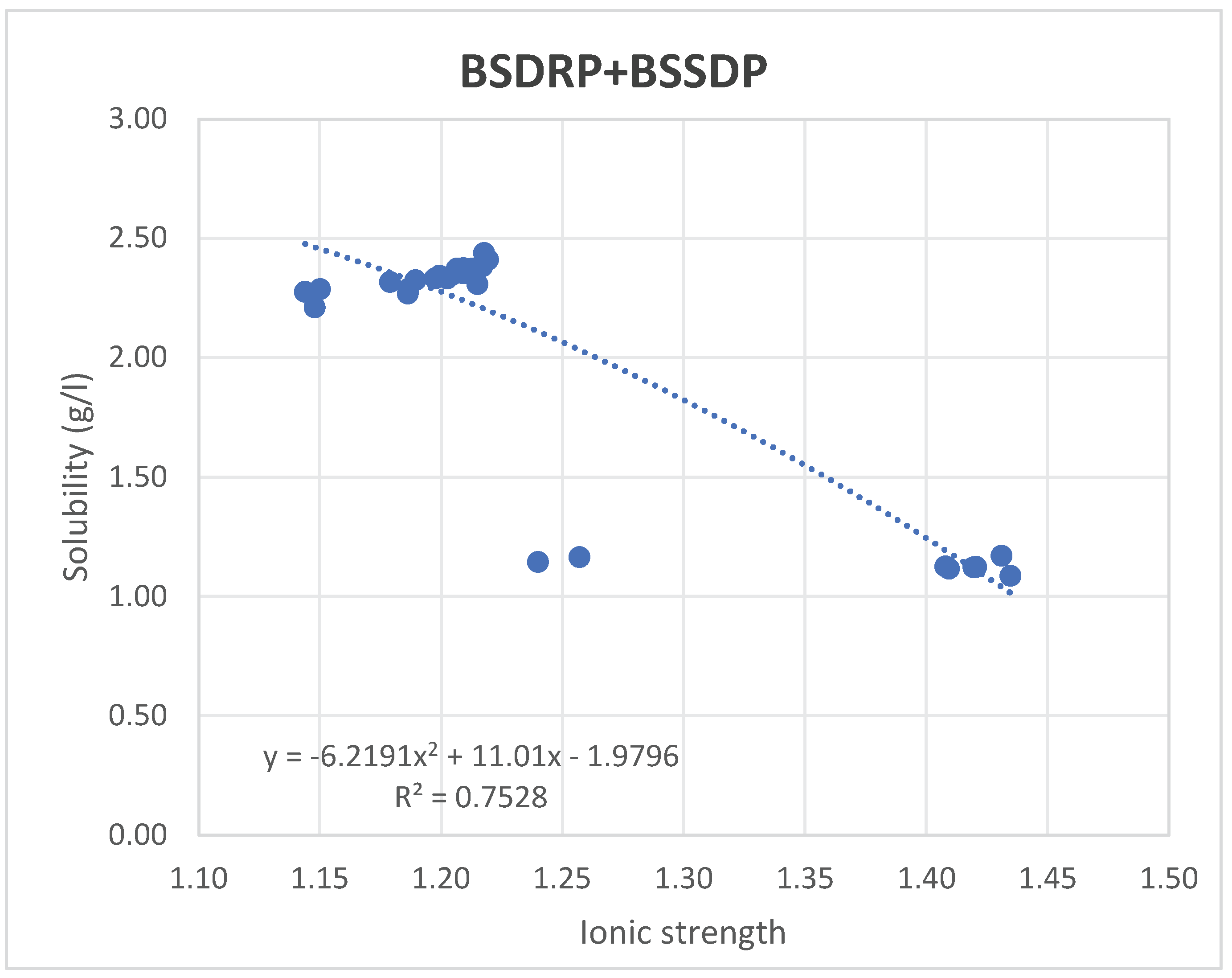
Figure 2 traces the results of the solubility limit measured in the brine solution of BSDRP and BSSDP, while in
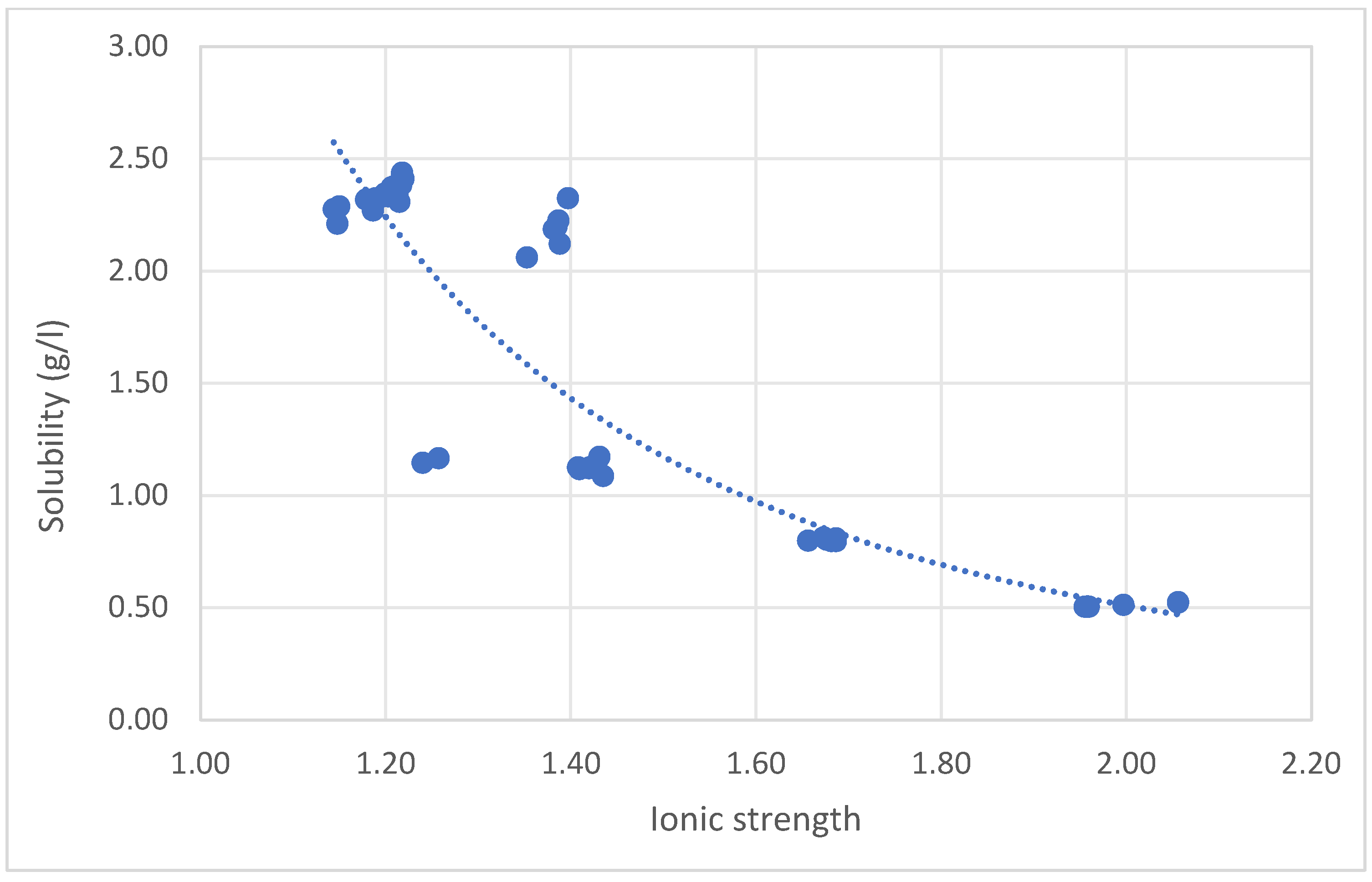
Figure 3, the results of the concentrated brine solution, which was prepared from concentrated BSSDP at 50%, 60% and 70% concentrations, are demonstrated.
The solubility of CaSO
4*2H
2O in brine solution with an ionic strength greater than 1.10 shows a distinct behavior, which is totally different from that in a diluted solution, as shown in
Figure 1. As the ionic strength increased beyond 1.1 mol/L, a distinct pattern emerged.
Figure 2 shows the solubility measured for two brine solutions collected from two RO desalination plants in Kuwait, BSSDP and BSDRP. Both represent a brine solution; the only difference is the ionic strength, which, as is evident from
Figure 2, is the dominating factor. Although the chemical composition is similar, the concentrations in the BSSDP are higher. Since the ionic strength of BSSDP ranged from 1.35 to 1.43, with an average of 1.38, while BSDRP ranged from 1.13 to 1.22, with an average value of 1.2.
The measured solubility limits of CaSO
4*2H
2O (mol/kg) in different concentration brine solutions were found to range between 2.34 g/L at an ionic strength of 1.2 and decrease to 2.19 g/L at an ionic strength of 1.38. The aforementioned solubility refers to the standard brine solution used in RO desalination plants in Kuwait. Hence, the previous solubility limit values were observed to further decrease to 1.12 at 50% concentration, then to 0.8 and 0.51 for 60% and 70% concentrations, respectively (
Figure 3), due to the increase in the solubility limit as the concentration % increased. It is clear that as the ionic strength of the tested solution increases, the solubility limit decreases, or as the content of dissolved brine increases, it reversibly affects the solubility limit of CaSO
4*2H
2O, making the minerals more suitable for extractions as the concentration increases. That pattern of solubility may be primarily due to the effect of the double layer around the ions, which reduces the ion activity coefficient (a), ion pairing, and increases the common ion effect.
Comparing the experimental solubility obtained in the current study with that from the literature, (
Table 4) shows excellent agreement, with a slight deviation of approximately 1%. The deviation of the measured value from the values in the literature does not exceed 7%. At high ionic strength, the deviation is even lower, using a 70% concentration. However, the deviation % was found to be less than 6.5% at 60% concentration. Moreover, at a standard brine solution, the deviation was less than 0.4%. Therefore, the deviation % of the result obtained from the current investigation is acceptable and within a reasonable range.
4.2. Solubility Limits of Mg(OH)2
The solubility limit of Mg(OH)2 was measured in brine solution rejected from the Shuwaikh desalination plant at ambient temperature and in different concentrated solutions: 50%, 60%, 70%.
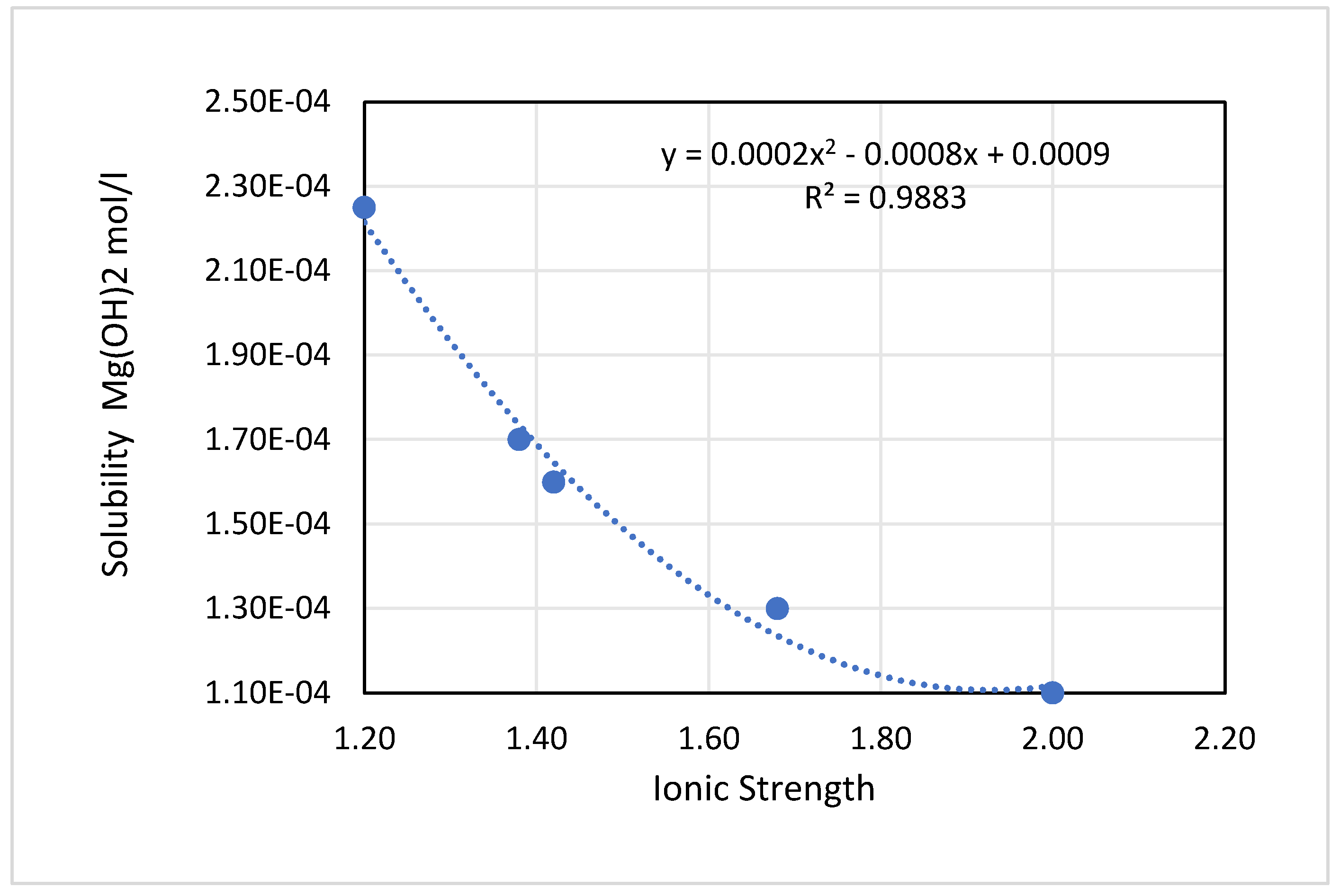
The experimental results of the measurements for Mg(OH)
2 under different ionic strength values are summarized in
Table 5 and plotted graphically in
Figure 4.
The solubility in Kuwait brine water is sensitive to the ionic strength of the tested solution, and generally, as the ionic strength increases, the measured solubility decreases.
Table 6 shows the deviation of the measured solubility limit from that reported in the literature, along with the reference.
5. Discussion
The solubility limit measurements of CaSO4 and Mg(OH)2 were prioritized among other assigned minerals because these minerals are common problematic scaling agents in desalination plants and in industrial water systems. These two minerals, when precipitated in any desalinated system, can severely reduce heat transfer efficiency, clog the membrane, and increase maintenance costs in desalination and industrial water systems. They also form multiple polymorphs in brine water and transfer between these polymorphs when the temperature and ionic strength change. Therefore, for reliable and reproducible measurement of their solubility limits, it requires an accurate analytical analysis, high-purity reagents, and precise control of testing condition.
5.1. Calcium Sulfate
The obtained tendency in results for CaSO
4 was expected since ionic strength usually determines the concentration of the solution and expresses the high concentration of dissolved ions in the brine solution, indicating the activity of dissolved ions and how much they are expected to deviate from an ideal or dilute solution. It is also noted from
Table 4 that the pH was affected by the concentration process due to the precipitation of a small amount of aragonite, although the evaporation or concentration process lasted two or three days at very low temperatures. Therefore, after each concentration, the pH must be adjusted to 7.9 as in the raw situation before the addition of excess salt, to ensure a constant pH condition and restore the original alkalinity level in the raw solution.
The results traced in
Figure 1 show a high regression ratio of up to 99% and an increasing trend with increasing solubility. The solubility in the literature was reported to be 2.1 in pure water [
30,
31], so the result obtained aligns well with the literature result for potable water and water produced from an RO plant.
The tendency of results shown in
Figure 2 implies the high effect of ionic strength on the solubility of gypsum at ambient conditions. BSSDP exhibits a lower solubility limit measurement than BSDRP, despite having a higher ionic strength, in contrast to the solubility trend observed in diluted water, as shown in
Figure 1.
That pattern of solubility was demonstrated by several studies, which reported that the addition of calcium chloride or calcium nitrate increases the concentration of the common calcium ion, thereby shifting the dissociation reaction toward the solid and decreasing gypsum solubility [
32]. Another study demonstrated that the addition of NaCl increases gypsum solubility up to 3 M, after which the solubility begins to decrease [
33]. Reiss also reported a similar trend in 2012, when the solubility of gypsum in brine solution, resulting from mixing Dead Sea brine water and standard seawater, was found to increase until 55% mixing, then decrease as the mixing ratio increased above 55% [
34].
Therefore, the initial increase in the solubility of gypsum at low ionic strength or diluted solutions is attributed to the salting-in effect, where the presence of other ions in the tested solution, even at low concentrations, surrounds the Ca and SO
4 ions, reducing their tendency to combine and precipitate as gypsum. As a result, the solubility of CaSO
4 increases to a certain point, where this phenomenon has no more effect or demolishes, and the high ionic strength reduces the activity coefficient and consequently the activity of these two ions (Ca and SO
4), the solubility begins to decrease dramatically as the ionic strength increases to above 1.2 M [
35]. This means that the decrease in solubility as ionic strength rises is mainly related to the thermodynamic effect of ionic strength on the activity coefficient, in addition to the common ion effect.
Table 4 shows a comparison between the experimental values of solubility and the corresponding values that may be found in the literature. A slightly higher value of the deviation may refer to the unique composition of Kuwait brine water, ion competition, and the complexity of the brine, which differs from a NaCl solution. Thus, the result is consistent with the established findings in the literature.
5.2. Magnesium Hydroxide
From
Figure 4, the solubility of Mg(OH)
2 in Kuwait brine water was proven to decrease as the ionic strength of the brine solution increases.
From
Table 6, a moderate deviation was observed, with a magnitude ranging from 9.7 to 4.03% when compared to the K
sp obtained by other researchers using brine water with a similar ionic strength. Although the solution used in the literature differs completely from the investigated solution in this study, which may make the comparison nonequivalent, they are using brine water with a similar ionic strength since brine water can be composed of different ions and have varying concentrations.
6. Conclusions
In this work, the solubility limits of selected valuable minerals in Kuwait brine solution generated from desalination plants for extraction purposes under different operating conditions were experimentally investigated. The aim was to establish a reference knowledge base on the extraction of selected valuable minerals from brine water and to show the influence of ionic strength, as it may lead to optimising the desalination process.
The experimental investigation was limited to two minerals viewed as the most important minerals. The ionic strength of the brine solution was first determined for four types of brine solutions. The ionic strength is used to express the high concentration of dissolved ions in each brine solution and to indicate the activity of the dissolved ions, as well as how much they are expected to deviate from an ideal or diluted solution. It is a critical parameter for comparison between solubility limits in different brine solutions. Then, the solubility limits of the assigned minerals were determined at least three times with high precision.
In order to improve data reliability and ensure the integrity of the final results, many protocols were considered, such as reducing the matrix effect, using a standard calibration procedure, implementing a highly accurate reference material, reducing the background noise, applying a standard method for analysis, using a statistical indicator for accuracy, and lowering the Relative Standard Deviation (RSD).
The analysis of the results of CaSO4*2H2O proves a special behavior for that mineral in a diluted solution or low ionic strength. The solubility limit of gypsum increases as the ionic strength increases. However, a reversed solubility pattern was observed when using a brine solution with high ionic strength, similar to brine water. In addition, it was proved that the solubility limit of gypsum in a high ionic strength solution decreases as the content of dissolved solids increases. That makes the minerals suitable for extraction in the concentrated brine solution. The observed behavior of gypsum solubility is consistent with the data published in the literature, where a similar pattern of solubility is reported.
In the case of Mg(OH)2, special attention was paid due to its very low solubility limits. The solubility of Mg(OH)2 in Kuwait brine water was shown to decrease as the ionic strength of the brine solution increases. The obtained solubility limit of Mg(OH)2 was compared to that in the literature, and a moderate deviation was observed for brine water with a similar ionic strength.
References
- Al-Sairfi, H.; Koshuryian, M.Z.A.; Ahmad, M. Membrane distillation of saline feeds and produced water: a comparative study of an air-gap and vacuum driven modules. desalination and water treatment 2024, 317, 100145. [Google Scholar] [CrossRef]
- Salman, M.A.; Ahmad, M.; Al-Sairfi, H.; Al-Foudari, Y. Mineral extraction from mixed brine solutions. Separations 2025, 12, 266. [Google Scholar] [CrossRef]
- Al-Sairfi, H.; Koshuryian, M.Z.A.; Ahmad, M. Performance feasibility study of direct membrane distillation systems in the treatment of seawater and oilfield- produced brine: the effect of hot-and-cold channel depth. Desalination and water treatment 2023, 313, 26–36. [Google Scholar] [CrossRef]
- Cheng, W.; Li, Z.; Cheng, F. Solubility of Li2CO3 in Na–K–Li–Cl brines from 20 to 90 °C. J. Chem. Thermodyn. 2013, 67, 74–82. [Google Scholar] [CrossRef]
- Guide to Best Practices for Ocean CO2 Measurements. Dickson, A.G.; Sabine, C.L.; Christian, J.R., Eds. PICES Special Publication. U.S. Department of Commerce, No-AA. 2007. Available online. https://www.pmel.noaa.gov/co2/files/dickson_the carbon dioxide system in seawater equilibrium chemistry and measurements. pp17-40.pdf (accessed on 20 May 2025).
- Meijer, J.; Van Rosmalen, G. Solubilities and supersaturations of calcium sulfate and its hydrates in seawater. Desalination 1984, 51, 255–305. [Google Scholar] [CrossRef]
-
CRC Handbook of Chemistry and Physics, 103rd ed.; Haynes, W.M., Ed.; CRC Press: Boca Raton, FL, USA, 2022. [Google Scholar]
- Ksp Table: Solubility Product Constants; Department of Chemistry, University of Massachusetts Amherst: Massachusetts, Amherst, USA. Available online: https://owl.oit.umass.edu/departments/Chemistry/appendix/Ksp.html (accessed on 8 September 2025).
- Technical Chemistry Reports. Worldwide Sodium Chloride Commodity Prices, Academic and technical studies compilation, 2025.
- Academic Market Analysis. 2025. Magnesium Hydroxide and Global Inorganic Salts Prices Q1 2025.” Retrieved from market intelligence database. Retrieved from market intelligence database.
- IMARC Group. Magnesium Hydroxide Price Trend, Chart and Index 2025. 2024. Available online: https://www.imarcgroup.com/magnesium-hydroxide-price-trend.
- Grand View Research. Magnesium Hydroxide Market Size, Share & Trends Analysis Report by Grade, Region, and Segment Forecasts, 2025-2030. 2023. Available online: https://www.grandviewresearch.com/industry-analysis/magnesium-hydroxide-market-report.
- Market Research Future. Magnesium Hydroxide Market Size, Share & Forecast 2034. 2023. Available online: https://www.marketresearchfuture.com/reports/magnesium-hydroxide-market-19261.
- Romano, S.; co-authors. The Role of Operating Conditions in the Precipitation of Magnesium Hydroxide from Brine. Crystal Growth & Design 2023, 23(8), 5303–5312. [Google Scholar]
- US Geological Survey. Mineral Commodity Summaries: Strontium. 2025. Available online: https://pubs.usgs.gov/periodicals/mcs2025/mcs2025-strontium.pdf.
- Industrial Minerals Survey. “Global Prices of Calcium Sulfate, Barium Sulfate, Strontium Sulfate, Calcium Carbonate, Potassium Chloride.” Technical review and academic databases 2025.
- F. Millero, The Physical Chemistry of seawater, Annual reviews inc, 1974.
- He, S.; Morse, J.W. The solubility of gypsum in relation to ionic strength at 25°C. Marine Chemistry 1993, 44, 153–167. [Google Scholar]
- Kopittke, P.M. Gypsum solubility in seawater, and its application to soil amelioration. Australian Journal of Soil Research 2004, 42, 903–911. [Google Scholar]
- Kumar, V.; Trivedi, R.; Shukla, S.; Chattopadhyay, K. Effect of ionic strength on gypsum solubility: NaCl and KCl systems. Journal of Chemical Thermodynamics 2004, 36, 503–508. [Google Scholar]
- Wang, W.; Chou, I.M.; Song, Y.; Song, K. Experimental determination and modeling of gypsum solubility in multicomponent brines at 25°C. Chemical Engineering Science 2013, 100, 448–457. [Google Scholar]
- Tlili, M.; Ben Amor, M.; Montiel, A.; Bounahmidi, T. Gypsum precipitation kinetics and solubility in the NaCl–MgCl2–CaSO4–H2O system. Industrial & Engineering Chemistry Research 2014, 53, 9554–9560. [Google Scholar]
- Reznik, I.J.; Ganor, J.; Kushmaro, A. Kinetics of gypsum crystal growth from high ionic strength solutions. Geochimica et Cosmochimica Acta 2011, 75, 2119–2131. [Google Scholar] [CrossRef]
- Trivedi, R.; Kumar, V.; Shukla, S.; Chattopadhyay, K. Measurement and modeling of the solubility of gypsum in NaCl and KCl solutions at 25°C. Journal of Chemical Thermodynamics 2013, 60, 13–18. [Google Scholar]
- Altmaier, M.; Freyer, D.; Pannach, M.; Gies, R.; Wolf, M.; Hofmann, A. Solid-liquid equilibria of Mg(OH)2 in aqueous solutions: experimental data and thermodynamic modeling. Frontiers in Nuclear Engineering 2023, 5, 1188789. [Google Scholar]
- Xiong, Y.; Zhou, Y. Experimental determination of solubility constant of hydromagnesite in NaCl solutions up to 7 M ionic strength. Journal of Solution Chemistry 2011, 40, 797–812. [Google Scholar]
- Palmer, D.A.; Wesolowski, D.J.; Benezeth, P. Solubility of Magnesium Hydroxide in Aqueous Solutions. Journal of Chemical & Engineering Data 2000, 45, 1192–1197. [Google Scholar]
- Alkaline earth hydroxides in water and aqueous solutions. In Solubility Data Series; Lorimer, J. W., Ed.; IUPAC. NIST, 1984; Vol. 52, pp. 119–123. [Google Scholar]
- Friis, K.; Shaw, C.; Ringham, M. C.; Carter, B. R.; Tyka, M. D.; Eisaman, M. D. Measuring magnesium hydroxide dissolution in stirred and non-stirred seawater conditions: kinetic insights and alkalinity release. Frontiers in Climate 2025, 36. [Google Scholar]
- Al-Ghouti, K.A.H.; Al-Muhtaseb, M.M.; Al-Qaradaghi, M.A. Solubility of calcium sulfate in water at various temperatures. Journal of Environmental Science and Health, Part A 2010, 45, 1834–1841. [Google Scholar]
- Smith, J.M.; Jones, L.T.; Brown, R.W. Calcium sulfate solubility in water: Implications for drinking water standards. Water Research 2015, 85, 1–10. [Google Scholar]
- Zhang, X.; Muhammed, M. Calcium sulfate solubility in aqueous solutions. Hydrometallurgy 1989, 23, 1–14. [Google Scholar]
- Taherdangkoo, R.; Tian, M.; Sadighi, A.; Meng, T.; Yang, H.; Butscher, C. Experimental data on solubility of the two calcium sulfates: Gypsum and anhydrite in aqueous solutions. Data Descriptor. 2025. Available online: https://www.scribd.com/document/626836928/data-07-00140DVADSWADSSD.
- Reiss, J.; et al. Gypsum Precipitation under Saline Conditions. Minerals 2021, 11, 141. [Google Scholar] [CrossRef]
- Berner, U.R.; Gimmi, T.; Griffault, L.; Schäfer, T. Solubility of anhydrite and gypsum at temperatures below 100°C and the gypsum-anhydrite transition temperature in aqueous solutions: A re-assessment. Frontiers in Nuclear Engineering 2023, 3, Article 1208582. Available online: https://www.frontiersin.org/journals/nuclear engineering/articles/10.3389/fnuen.2023.1208582/full.
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).