1. Introduction
Nowadays, the contaminants removing from wastewater is a crucial approach for environmental remediation and public health protection, and this process helps to stop the mitigation of adverse effects on ecosystems and human health [
1].
Human activities significantly contribute to environmental contamination by heavy metals. This pollution arises from various sources, including mining operations and the widespread use of fertilizers, herbicides, and pesticides. Additionally, irrigating agricultural land with untreated sewage and industrial waste further exacerbates the problem [
2,
3].
Ultimately, the composition of wastewater differs based on their source. Typically, it includes pathogens, along with both suspended and dissolved organic and inorganic pollutants, all of which can significantly impact environmental health [
4]. The widespread contamination of water by heavy metals represents a critical environmental challenge that requires urgent attention. These metals are not only toxic but also non-biodegradable, allowing them to persist in ecosystems. Additionally, their potential for biological accumulation poses significant risks to aquatic organisms and, ultimately, human health due to their carcinogenic nature. Therefore, reduction in heavy metal pollution is essential for the protection of both environmental integrity and public health [
5].
Heavy metals, in contrast to organic pollutants, do not break down over time. When these metals are released into the environment, they accumulate in living organisms, posing a significant threat to the health of all life forms, including humans, animals, and plants. It is crucial that we take action to prevent further contamination and protect our ecosystem from these harmful substances [
6,
7,
8]. It is absolutely essential to remove heavy metals from water to effectively combat their harmful effects on the environment [
9,
10,
11,
12]. Implementing effective strategies for removal/recovery of heavy metals is vital not only for the sustainable management of these precious resources but also for significantly reducing the dangerous effects of heavy metal pollution on our environment [
13,
14,
15,
16,
17,
18,
19,
20,
21,
22,
23,
24,
25,
26]. A thorough understanding of the adsorbent structure, as well as its physical and chemical properties, the pollutant removal mechanisms, and other relevant characteristics, is essential for developing effective innovative methods to treat heavy metals contaminated effluents. Additionally, various analytical techniques are utilized to enhance our understanding of how the pollutants are retained on the tested adsorbent materials [
13,
27,
28,
29,
30,
31,
32,
33,
34,
35,
36].
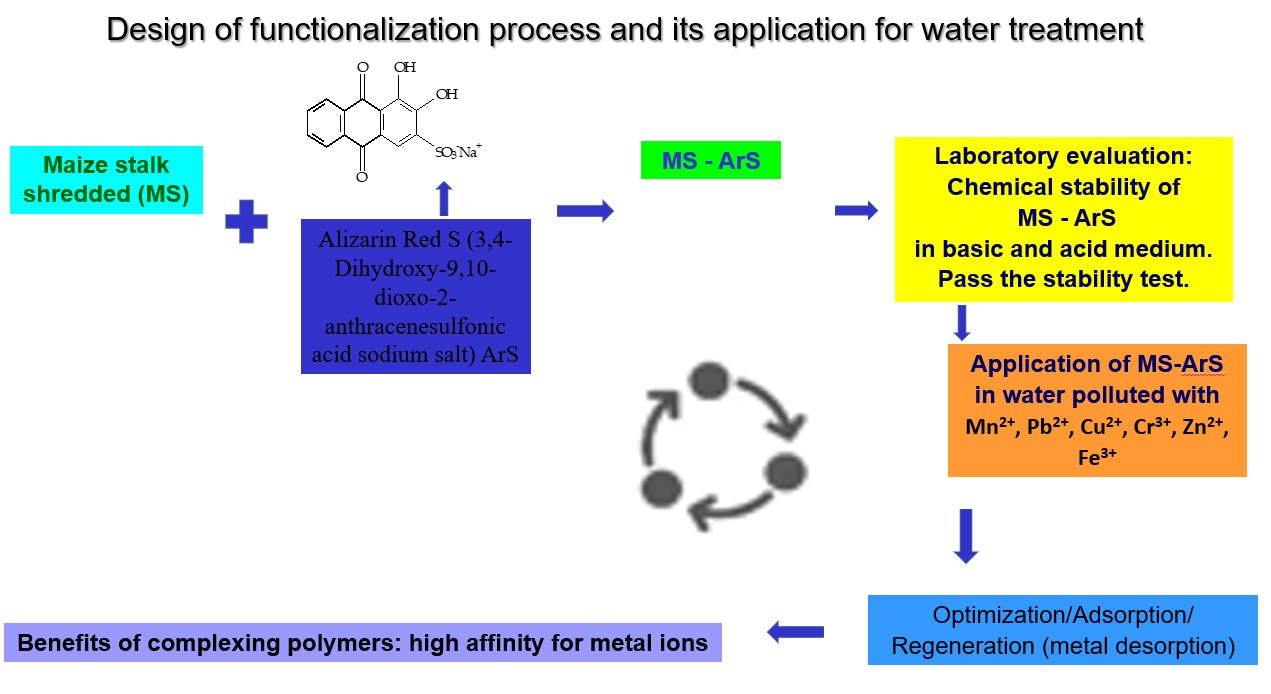
The novelty and originality of this study consisted in obtaining a new functionalized cellulose material by which the shredded maize stalk was functionalized with Alizarine Red S to obtain a new complexing polymer material, which to the authors’ best knowledge is presented for the first time in the scientific literature. Taking into account the fact that there is no literature data in which the cellulosic material of agricultural origin obtained from the maize stalk (MS) was to be functionalized with ArS to retain metal ions, all adsorption studies were performed by the batch method on the cellulosic material resulting from the shredding and activation of the maize stalk that had reached maturity. To investigate the Mn+ adsorption, a series of adsorption experiments were conducted using MS-ArS. The pH influence for metal ions adsorption into MS-ArS was tested at pH 4 and 10. The metal concentration influence in 0.5-5 mg/L range was tested to evaluate the adsorption capacity of MS-ArS. Also, regeneration studies were applied in order to metal ions recovery from exhausted material.
2. Materials and Methods
2.1. Reagent
ArS (CAS 130–22-3, Carl Roth, Karlsruhe, Germany) was used as a reagent for complexing shredded maize stalk. Mono-element solutions from Merck of Mn(NO3)2, Pb(NO3)2, Cu(NO3)2, Cr(NO3)3, Zn(NO3)2 and, Fe(NO3)3 were used for preparing calibration curves and for obtaining solutions used in adsorption experiments. NaCl solid (for analysis) and 37 % HCl were purchased from Merck and were used in regeneration experiments.
2.2. Analytical Methods
For UV-VIS spectrometric analysis, an Agilent Cary 60 UV–vis spectrophotometer (Penang, Malaysia) was employed. For the metal ions analysis, a Perkin Elmer PinAcle 900T atomic absorption spectrometer (Perkin Elmer, Norwalk, CT, USA) was utilized. The HI 255 pH-meter (Hanna Instruments, Nijverheidslaan, Belgium) was used for pH measurement of the supernatant and buffer solutions. For the preparation of the complexing material and for metal ions adsorption, a GFL 3017 horizontal mechanical shaker (Bremen, Germany) was used.
2.3. Experimental Methodology Used in Adsorption Study
In this paper, all experimental data were conducted in duplicate, and only the average values were employed to represent the experimental data given in Results and discussion
Section 3. Furthermore, synthetic samples were developed to closely simulate environmental conditions, allowing us to thoroughly assess the performance of the complexing polymers produced.
2.4. Procedures for Testing the Complex Formation Between ArS and Mn+ in Solution at Different pH Buffer
For recorded ArS spectrum the ArS solution was prepared by combining 0.75 mL ArS (500 mg/L) with 0.75 mL buffer solution at pH 4 in a 10 mL volumetric flask. After mixing, ultrapure water was added to bring the total volume to the mark. For recorded ArS-Mn+ spectrum the mixed solution of ArS-Mn+ was obtained by adding 0.5 mL of Mn+ (100 mg/L) that was combined with 0.75 mL of ArS (500 mg/L) and 0.75 mL of acetate buffer at pH 4 in a 10 mL volumetric flask, which was then filled to the mark with ultrapure water. Each solution after preparation was placed in cuvette of UV-Vis spectrometer and the spectra was recorded after 10 min of reaction in the 200-800 nm range. The same experimental procedure was followed to study complex formation at pH 10.
2.5. Procedure for Functionalization of Shredded Maize Stalk with ArS at Different pH Values
Over 0.05 g MS, 1.5 mL ArS 500 mg/L, and 1.5 mL buffer solutions, together with 7 mL of ultrapure water, were added to Erlenmeyer flasks.
The buffer solutions tested were: pH=2.0 (phosphate buffer); pH=4.0 (acetate buffer); pH=6.0 (acetate buffer); pH=8.0 (phosphate buffer), and pH=10 (carbonate buffer).
The obtained mixtures were stirred at 175 rpm, 60 min at T = 25 ± 2 °C. At the end of the stirring, the mixtures were filtered, and the amount of ArS that was not retained on the cellulose mass was spectrometrically determined. Shredded maize stalk was prepared for functionalization as presented in a previous study [
37].
All experimental data were done in duplicate, and the mean data were used to determine the adsorption capacity (
Qe) as well as the percentages of ArS retained on the MS (R(%)), using Equation 1,2.
where
Ci represents the initial concentration of ArS and
Ce (mg/L) was concentration at equilibrium, m(g) is the mass of dry MS, and
V(L) is the volume of ArS used in adsorption experiment.
2.6. Procedure for Testing the Stability of Complexing Material (MS-ArS)
The stability of the complexing cellulose in the MS-ArS form was also assessed. For this, 0.05 g MS-ArS samples (each loaded with 14.2 mg ArS/g) were tested for stability assessment. Subsequently, 10 mL of 0.5 M (HCl and NaCl) solution was added over the weighed samples. The resulting mixture was shaken in Erlenmeyer flasks for 30 min at 175 rpm at T = 25 ± 2 °C. After 30 min of shaking, the samples were kept in stand by for 5 min and then filtered. The filtered solutions were spectrometrically analyzed in the 200-800 nm range to detect the ArS released in the supernatant solution. The metals studied in this article are the most harmful pollutants and are of interest due to their toxicity to the aquatic environment. Their physical and chemical properties are presented in
Table 1.
2.7. Procedures Used to Evaluate the Influence of pH on Mn+ Adsorption onto MS-ArS
Samples of 0.05 g MS-ArS were weighed and transferred into 100 mL Erlenmeyer flasks. Subsequently, 0.01 L buffer solution (pH = 4, and 10) containing 3.5 mg/L Mn+ was added to the MS-ArS samples. The buffer solutions used were citrate buffer solution for pH = 4.0 and carbonate buffer for pH = 10.0, respectively.
2.8. Procedure Regarding Adsorption of Mn2+, Pb2+, Cu2+, Cr3+, Zn2+and Fe3+ onto MS-ArS at pH=10 in Function of Contact Time
Samples of 0.05 g ArS were weighed on an analytical balance and transferred into 100 mL Erlenmeyer flasks. Then, 0.01 L of carbonate buffer solutions with pH=10 containing 3.5 mg/L M
n+ were added to MS-ArS samples. The mixtures obtained were stirred at times that was ranging from 10, 20, 30, 40, 50, and 60 min, 175 rpm (T = 25 ±2 °C). At the end of the each stirring time, samples were filtered and all filtrate were analyzed using AAS method to detect metal ions concentration that was not retained on complexing material. The quantity of M
n+ adsorbed at time(t)
Qt(mg/g) on the ArS-MS mass was determined using Equation (3).
where
Ct (mg/L) represents the concentration of metal ions in the solution at time
t.
2.9. Procedure Regarding Adsorption of Mn2+, Pb2+, Cu2+, Cr3+, Zn2+and Fe3+ using MS-ArS at pH=10
For adsorption of Mn2+, Pb2+, Cu2+, Cr3+, Zn2+, Fe3+ onto MS-ArS, ≈ 0.05 g of MS-ArS (14.2 mg ArS/g) were stirred with 0.01 L solution, in which the concentration of Mn2+, Pb2+, Cu2+, Cr3+, Zn2+, Fe3+ was varied from 0.5; 1.5; 1.5; 2.5; 2.5; 3.5; to 5 mg/L and the pH was adjusted by adding 0.75 mL buffer solution pH=10. The mixtures were stirred for 45 min at 175 rpm (T = 25 ± 2°C). After stirring, the mixtures were filtered, and the metal concentration was determined by atomic absorption spectrometry (AAS).
2.10. Procedures for Desorption of Metal Ions Loaded onto MS-ArS
Samples of 0.05 g MS-ArS loaded with 0.65 mg Mn2+, 0.54 mg/g Pb2+, 0.83 mg/g Cu2+, 0.41 mg/g Cr3+, 0.75 mg/g Zn2+ and 0.87 mg/g Fe3+ were stirred for 30 min at 175 rpm with 0.01L (0.5M HCl, NaCl and hot water (H2O), respectively). After desorption study the concentration of Mn+ was determined and the desorption rate of those was calculated.
3. Results and Discussion
The ability of metal ions to form complexes with different ligands and ability of the ligands to be selective under certain experimental conditions, as well as the characterization of the resulted complex structure, is a continuous research interest topic. It is also known that metal ions, regardless of their position in the periodic table, can form coordination complex. This is based on the observation that a coordinate bond is formed between chemical species that accept (metal ion) and, respectively, donate electrons to the ligand (organic compound).
Starting from the premise that complexation processes in solution exhibit similarities to those occurring in solid materials, this study was initiated first by examining the formation of complexes in solution as a function of the pH medium. This initial investigation provided a robust foundation for the subsequent analysis, which focused on the adsorption characteristics of six metal ions in the aqueous medium following the functionalization of MS with ArS. This comprehensive approach enhances our understanding of complexation study and their applications in material science 38.
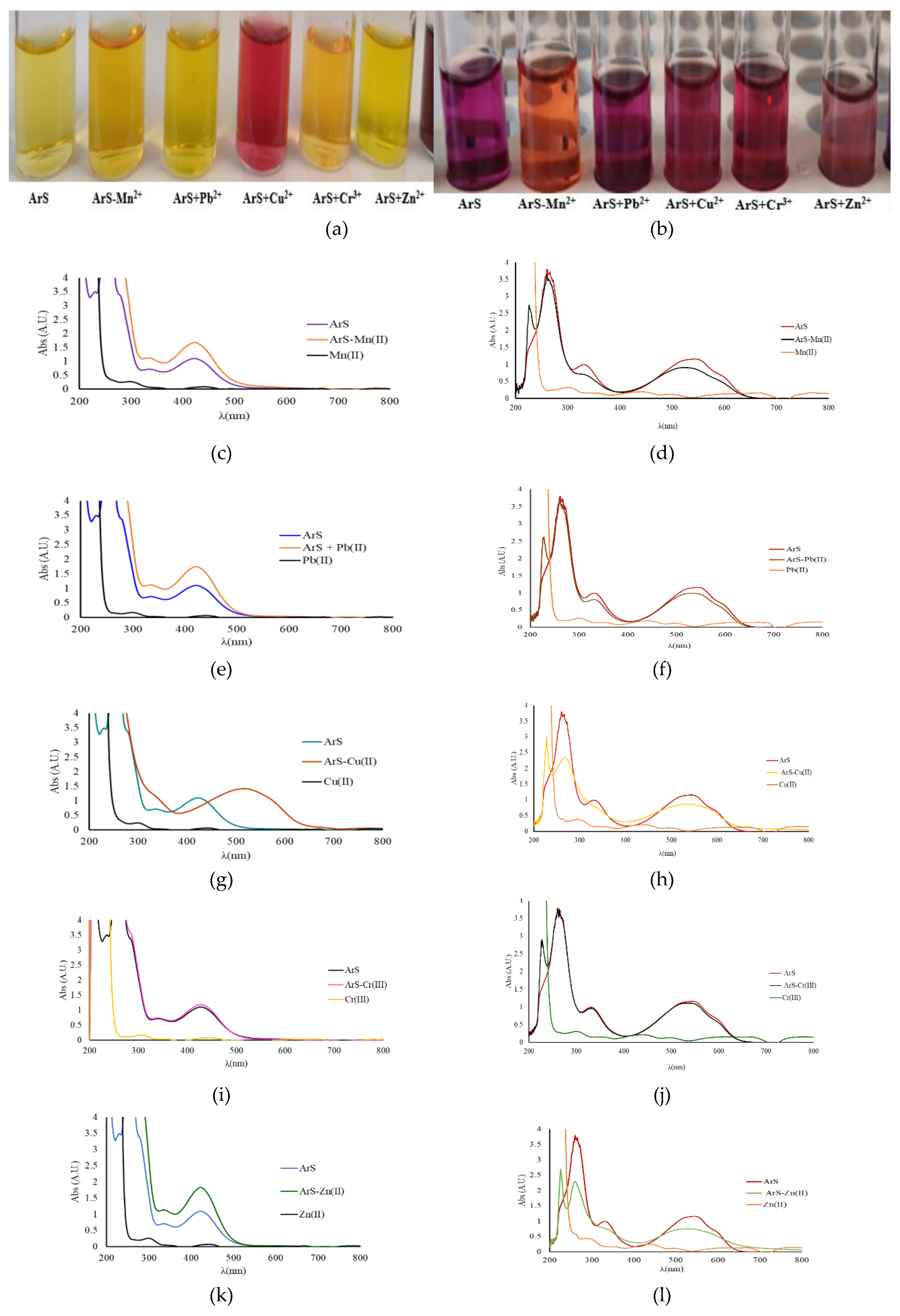
3.1. Studies on the Complex Formation (Mn+-ArS) in Buffer Solutions at pH 4 and 10
To evaluate the complex formation between ArS and M
n+, two pH levels were examined: pH 4 and pH 10. The interaction of ArS with M
n+ was monitored spectrometrically across the 200-800 nm wavelength range. The results are presented in
Figure 1a for pH=4 and in
Figure 1b for pH=10.
The ArS solution was obtained from 0.75 mL ArS 500 mg/L and 0.75 mL buffer solution of pH = 4 in a 10 mL volumetric flask that was brought to the mark with ultrapure water. The ArS-Mn+ in mixed solution was obtained from 0.5 mL Mn+ (100 mg/L) together with 0.75 mL ArS (500 mg/L) and 0.75 mL acetate buffer pH = 4 in a 10 mL volumetric flask brought to the mark with ultrapure water. For testing adsorption at pH = 10 studies, the same experimental procedure was applied.
Following UV-Vis studies for the mixed solutions of ArS-Mn+, a new maximum of intensity is observed at pH = 4 and 10. The most significant interaction regarding complex formation is observed, where all ArS-Mn+ mixture spectra are shifted to lower wave numbers and lower intensity, suggesting the formation of a complex together with a change in color.
At the same time, if we analyze each mixed solution presented in
Figure 1a, at pH = 4, the above-mentioned behavior is only observed in the case of Cu
2+ and Fe
3+, both from spectra analysis and visual analysis of color (
Figure 1a).
At pH = 10, the above-mentioned behavior is observed for all ArS-M
n+ mixture spectra, and also from colors of solutions obtained, both suggesting the formation of a complexes for the mixtures tested (
Figure 1b).
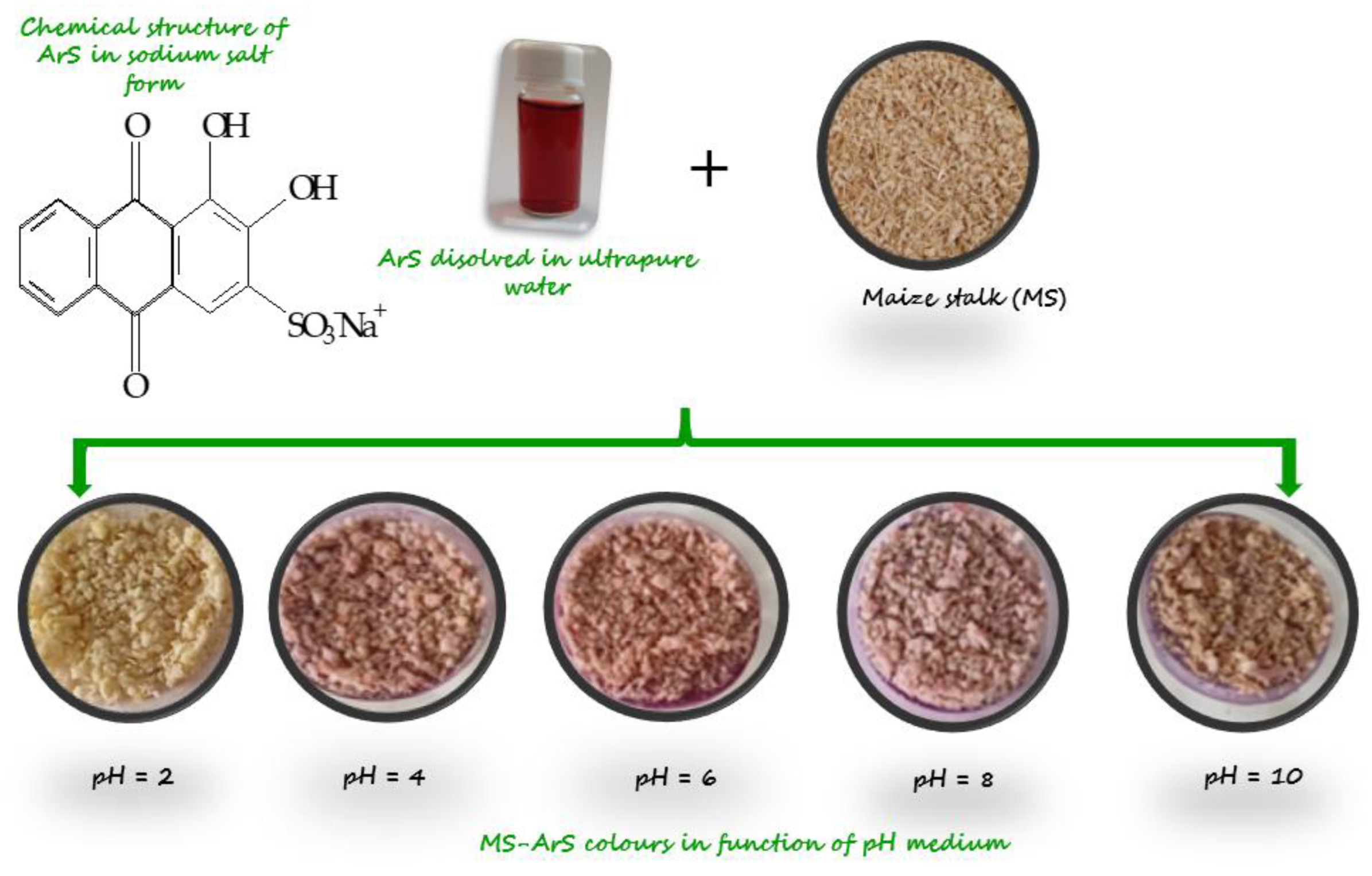
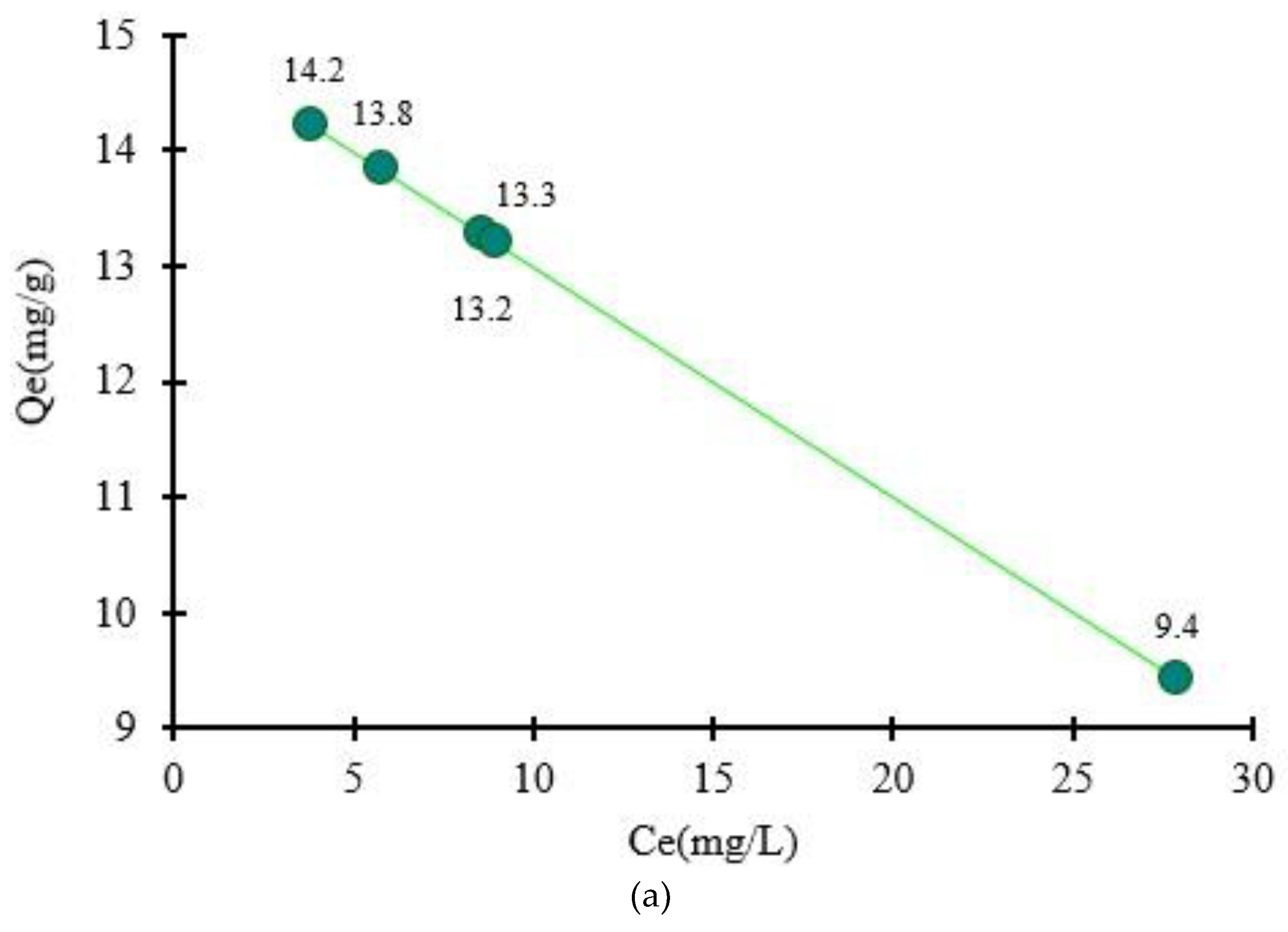
3.2. Functionalization of Shredded Maize Stalk with ArS in Function of pH Solution
Because the organic compound ArS changes its structure when the pH changes, it was studied to see how much the pH change affects the retention of the functionalizing agent onto MS mass. Functionalization of MS with ArS using different buffer solution with pH = 2, 4, 6, 8, and 10 was studied and the resulting solid phases in
Figure 2 are presented. During experimental studies the following adsorption capacities in function of pH medium was obtained for adsorption of ArS onto MS mass (
Figure 3a) and were at pH = 2 (
Qe = 14.2 mg/g) > pH = 4 (
Qe = 13.8 mg/g) > pH = 6 (
Qe = 13.3 mg/g) > pH = 8 (
Qe = 13.2 mg/g) > pH = 10 (
Qe = 9.4 mg/g). Hence, the percentage removal of ArS gradually decreased from 95 to 63 % with increasing the pH from 2 to 10. Analyzing the results obtained, the most significant value of the adsorption of ArS on MS was obtained at pH = 2 (
Qe = 14.2 mg/g), and this pH value was used to obtain MS-ArS material for metal ions adsorption.
Taking into consideration the results obtained, the complexing mechanism can be explained as: ArS is an anionic compound having a sulfonic group which is totally dissociated even at pH = 2. This behavior can be attributed to the fact that, in a strongly acidic environment, the hydroxyl groups present in the cellulose structure are protonated and engage in electrostatic interactions with the sulfonic group (SO32-) of the complexing agent ArS.
As the pH of the buffer solutions increases, deprotonation of the hydroxyl groups takes place, leading to a decrease in the degree of adsorption observed due to the electrostatic repulsions that occur when considering the behavior of ionizable groups at pH 4 and 6.
At a pH greater than 7, the adsorption process can be explained by considering the mass of the adsorbent. Aside from the specific adsorption processes associated with the ionizable groups present in the structure of the tested materials—particularly the hydroxyl groups—the overall charge of the adsorbent is close to zero. Therefore, the adsorption of ArS occurs primarily through physical interactions or diffusion within the structure of the MS material. This may explain why the adsorption capacity of ArS on the MS mass was higher at pH 2 compared to the other pH values studied.
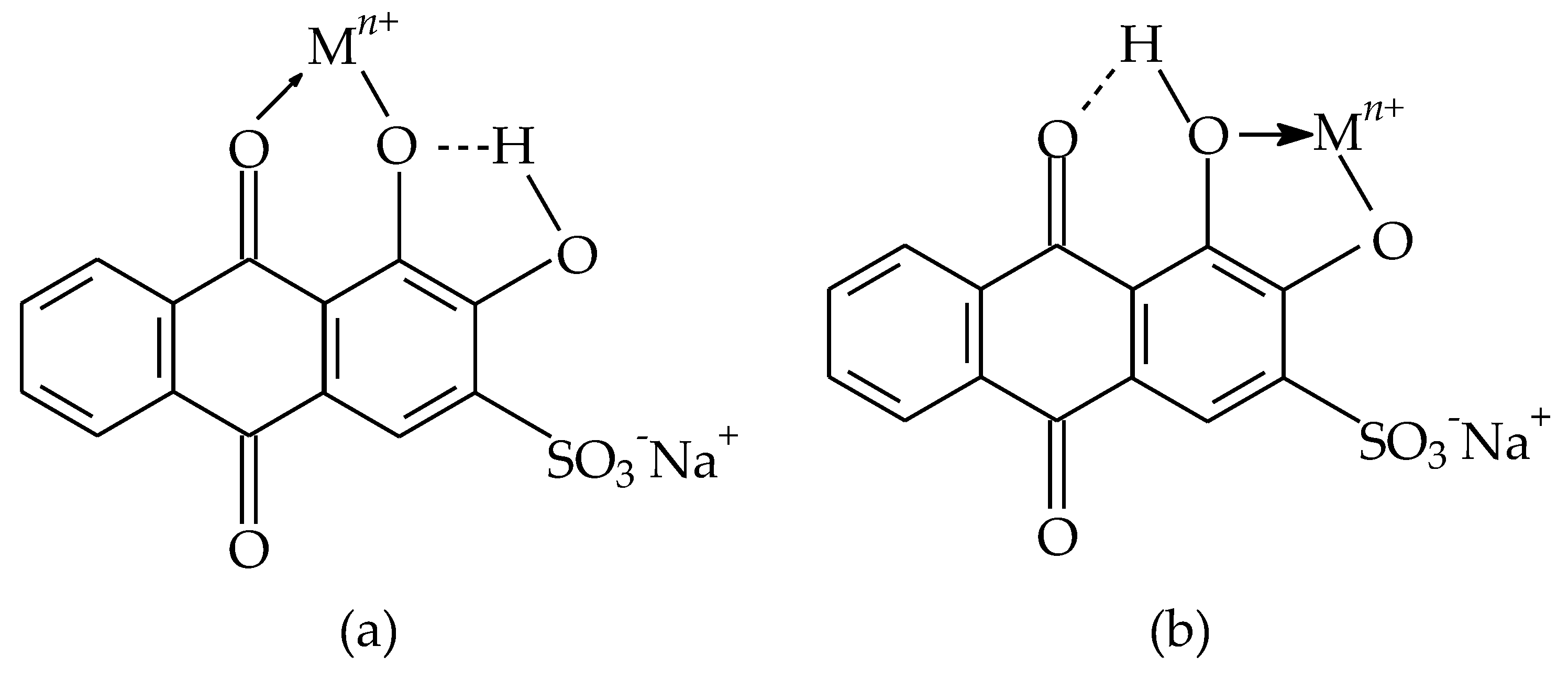
3.3. Proposal Mechanism of Metal ion Adsorption onto MS-ArS
The functional groups present in the structure of the complexing agent can exhibit an affinity for metal ions by binding metal ions through a complexation or chelating mechanism [
38,
39].
Thus, the adsorption mechanism between the metal and the complexing material can be explained by considering the following hypotheses:
- (i)
the carbonyl (-C=O) and hydroxyl (HO
-) groups present in the ArS structure may be responsible for the complexation of the Mn
2+, Pb
2+, Cu
2+, Cr
3+, Zn
2+ and Fe
3+ (
Figure 4a,b);
- (ii)
by diffusion into the porous structure of the complexing material, taking into consideration the ionic radius;
- (iii)
by ion exchange mechanism between the -SO32- group of ArS, which is not involved in the first adsorption step with MS;
- (iv)
Also, MS has in its structure functional groups such as carboxyl, hydroxyl, sulfhydryl, and amide, enabling them to bond with metal ions in water.
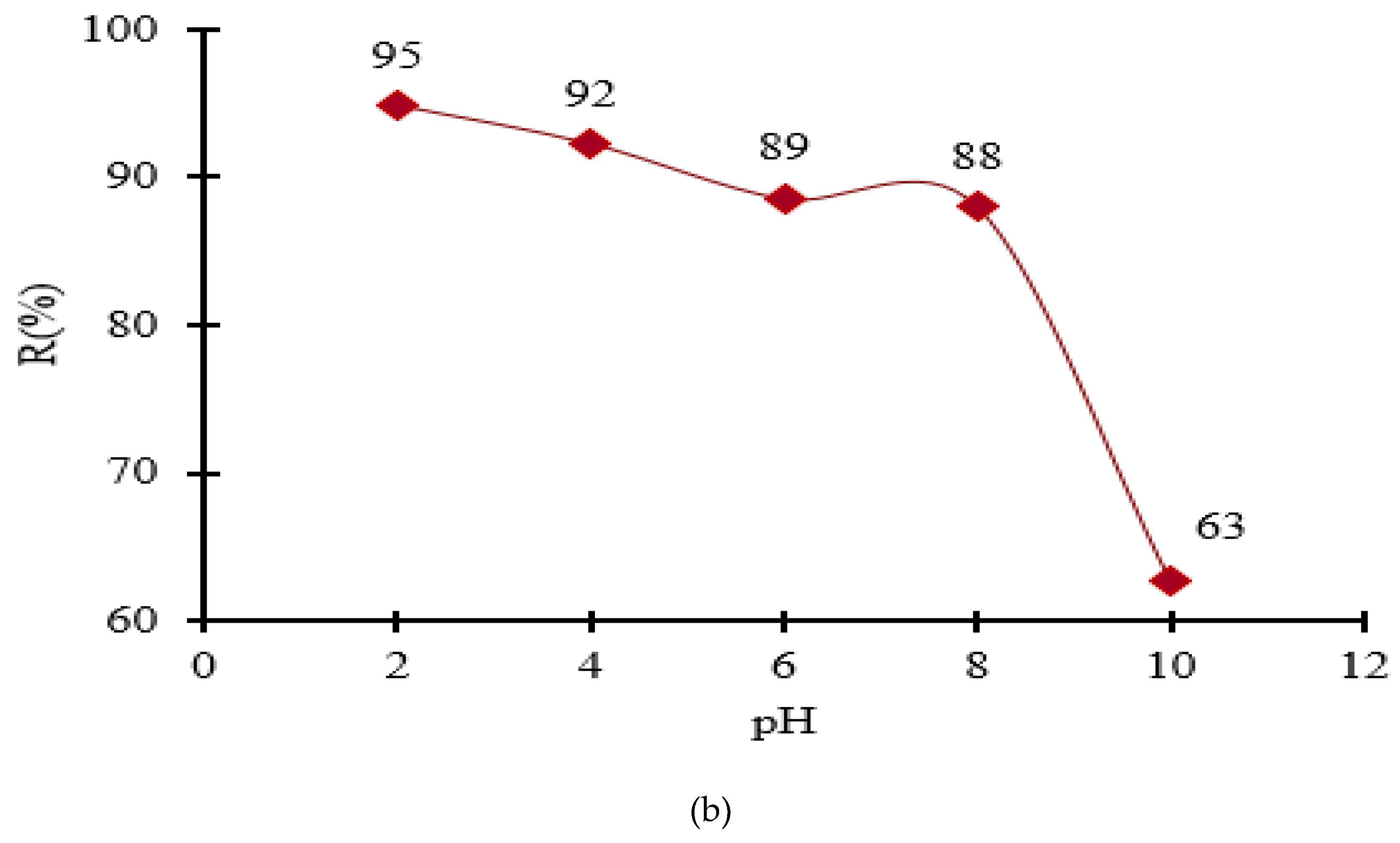
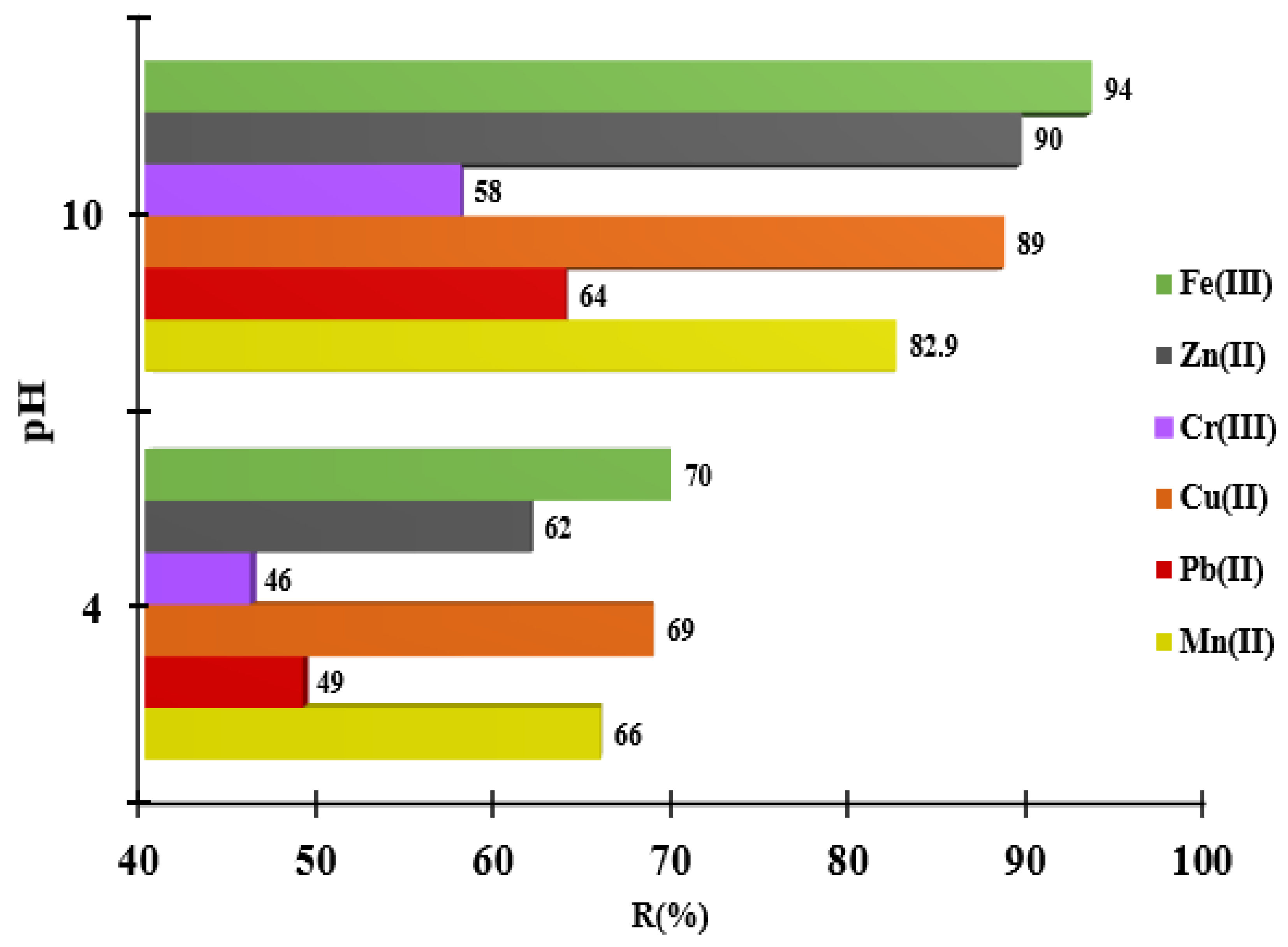
3.4. The pH Influence for the Metal Ions Adsorption onto MS-ArS
Literature studies have shown that most materials retain pollutants at certain experimentally determined pH values [
16]. This is because the pH of the solution influences the solubility of metal ions and the degree of dissociation of the groups existing on the materials structure. Thus, the functionalizing agent (ArS) contains -OH and -C=O groups in its structure, which change their degree of dissociation in function of the pH medium. Thus, at pH > 7, the -C=O groups and at pH > 9, the -OH groups are dissociated and can bind the metal ions present in the solution.
In order to avoid metal precipitation at very high pH values, a pH buffer solution were used. Herein, the metal adsorption value increased from 66 to 82.9% for Mn
2+, from 49 to 64 % for Pb
2+, from 69 to 89% for Cu
2+, from 46 to 58 for Cr
3+, from 62 to 90% for Zn
2+ and from 70 to 94 for Fe
3+ when the pH of the solution increased from 4 to 10, using MS-ArS (
Figure 5).
As one can observe, at higher pH there is an increase in the removal rate of metals from the solution by complexation, and this can only take place at a certain pH value experimentally established.
Taking into account the results obtained at pH influence, the next experimental study was focused on the adsorption of metal ions only at pH=10, when high amounts of metal ions have been adsorbed.
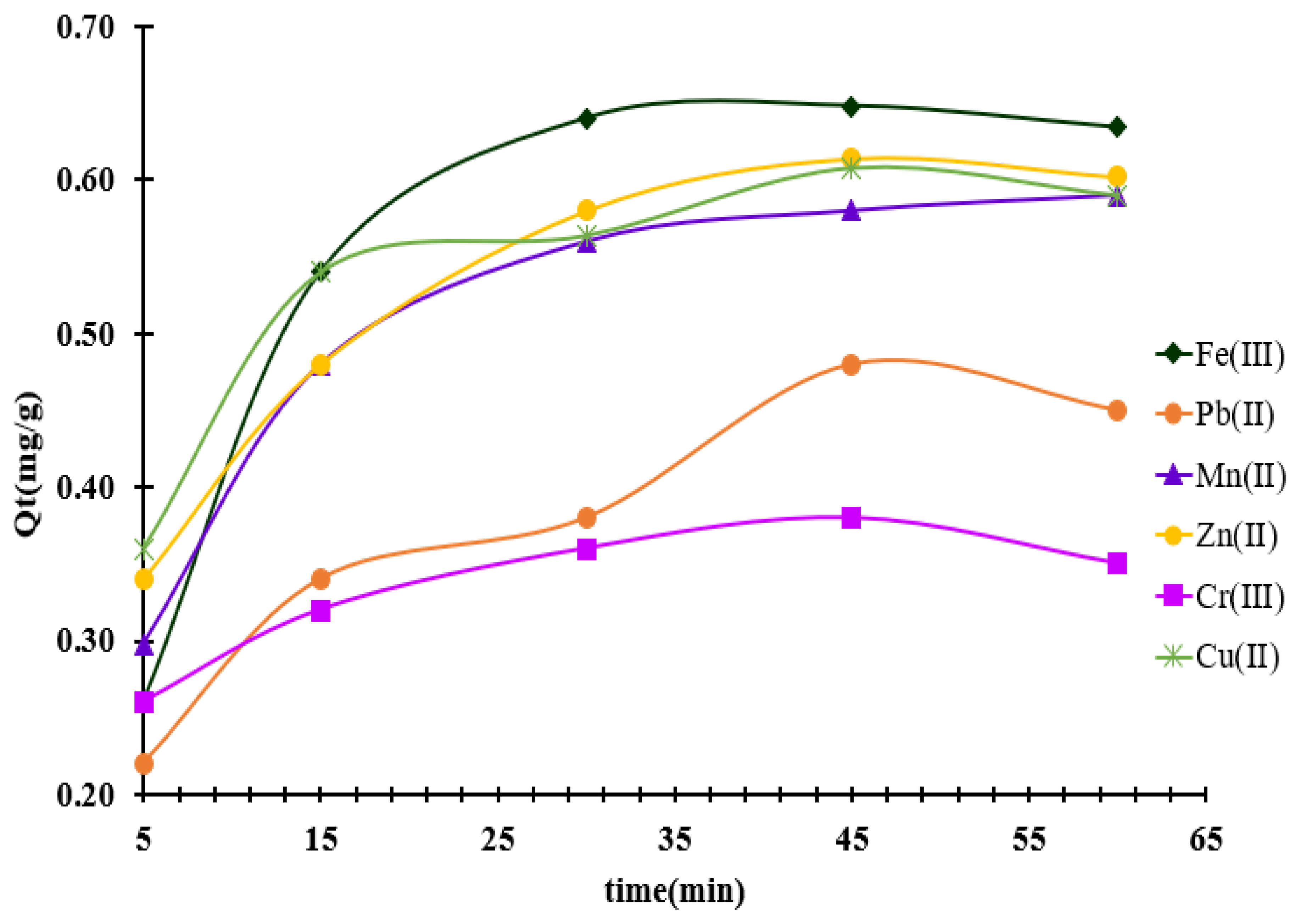
3.5. Influence of Contact Time on Metal Ion Adsorption on MS-ArS
Metal ion retention was investigated at a pH = 10 in the liquid phase to establish the optimum contact time, using an initial concentration of 3.5 mg/L of mixed metal ions.
The amount of metal ions retained at various time intervals was calculated using Equation (3). The results indicate that adsorption equilibrium was reached after 45 min for all the metal ions tested (see
Figure 6).
3.6. Adsorption of Metals Ions onto MS-ArS Mass
To represent the distribution profile of metal ions between the liquid and solid phases studied, adsorption isotherms were experimentally plotted. For this purpose, the
Qe values (mg/g) were graphically represented versus the concentration remaining in the supernatant solution at equilibrium (Ce, mg/L) [
40,
41,
42,
43,
44]. Analyzing the isotherms presented in
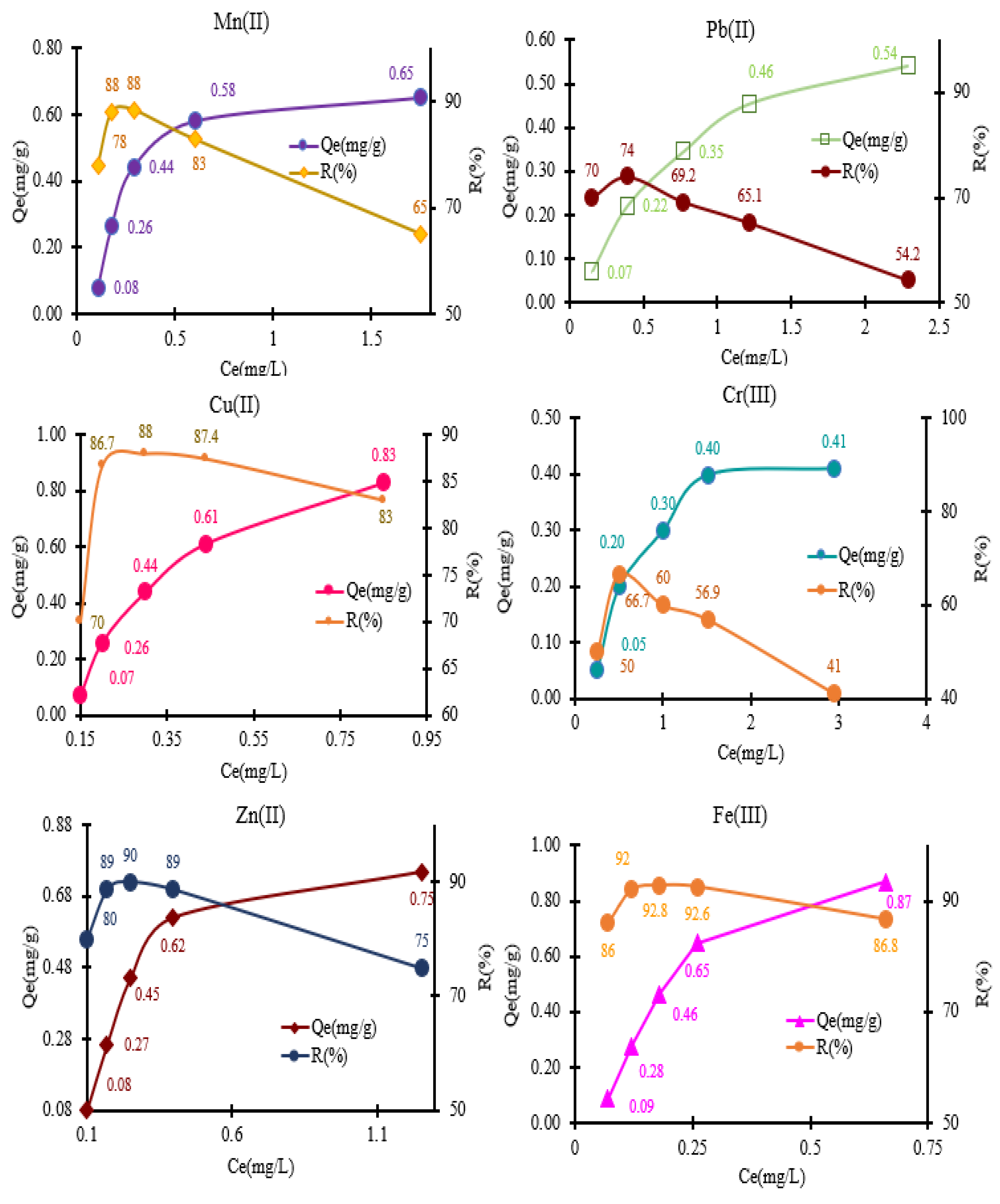
Figure 7, it can be observed that the adsorption of metal ions is nonlinear over the whole range of concentrations tested, which was varied from 0.5 to 5 mg/L in the mixture solution for all metals studied.
The testing of the complexing material was conducted using batch method, following the experimental conditions outlined in the experimental section. The adsorption behavior for each metal studied in mixed solutions was as follows: for Mn²⁺ ions, the amounts retained were 0.08, 0.26, 0.44, 0.58, and 0.65 mg/g. For Pb²⁺, the obtained adsorption values were 0.07, 0.22, 0.35, 0.46, and 0.54 mg/g. In the case of Cu²⁺, the amounts recorded were 0.07, 0.26, 0.26, 0.44, 0.61, and 0.83 mg/g. For Cr³⁺ ions, the calculated adsorption capacities ranged from 0.05 to 0.41 mg/g, with values of 0.05, 0.20, 0.30, 0.40, and 0.41 mg/g, respectively. Additionally, during the investigation of Zn²⁺ retention on MS-ArS, the following Qe values (mg/g) were obtained: 0.08, 0.26, 0.45, 0.62, and 0.75 mg/g. The amounts (Qe, mg/g) of metal ions retained on 0.05 g of MS-ArS were 0.09, 0.28, 0.46, 0.65, and 0.87 mg/g for Fe³⁺.
The adsorption capacities of the tested metal ions varied, as shown in
Figure 7. The highest adsorption capacity was observed for Fe
3+ at 0.87 mg/g, while the lowest was for Cr
3+ at 0.44 mg/g. Taking into account the results presented, in a mixed solution, the order of adsorption capacity for the metals was as follows: Fe
3+ (0.87 mg/g) > Cu
2+ (0.83 mg/g) > Zn
2+ (0.75 mg/g) > Mn
2+ (0.65 mg/g) > Pb
2+ (0.54 mg/g) > Cr
3+ (0.41 mg/g).
3.7. Studies on the Regeneration of Complexing Material Loaded with Mn2+, Pb2+, Cu2+, Cr3+, Zn2+ and Fe3+
One of the key opportunities for improvement in the adsorption process lies in enhancing the methods for regenerating and recovering saturated adsorbents. By focusing on these aspects, we can increase efficiency and sustainability in the overall process [
45].
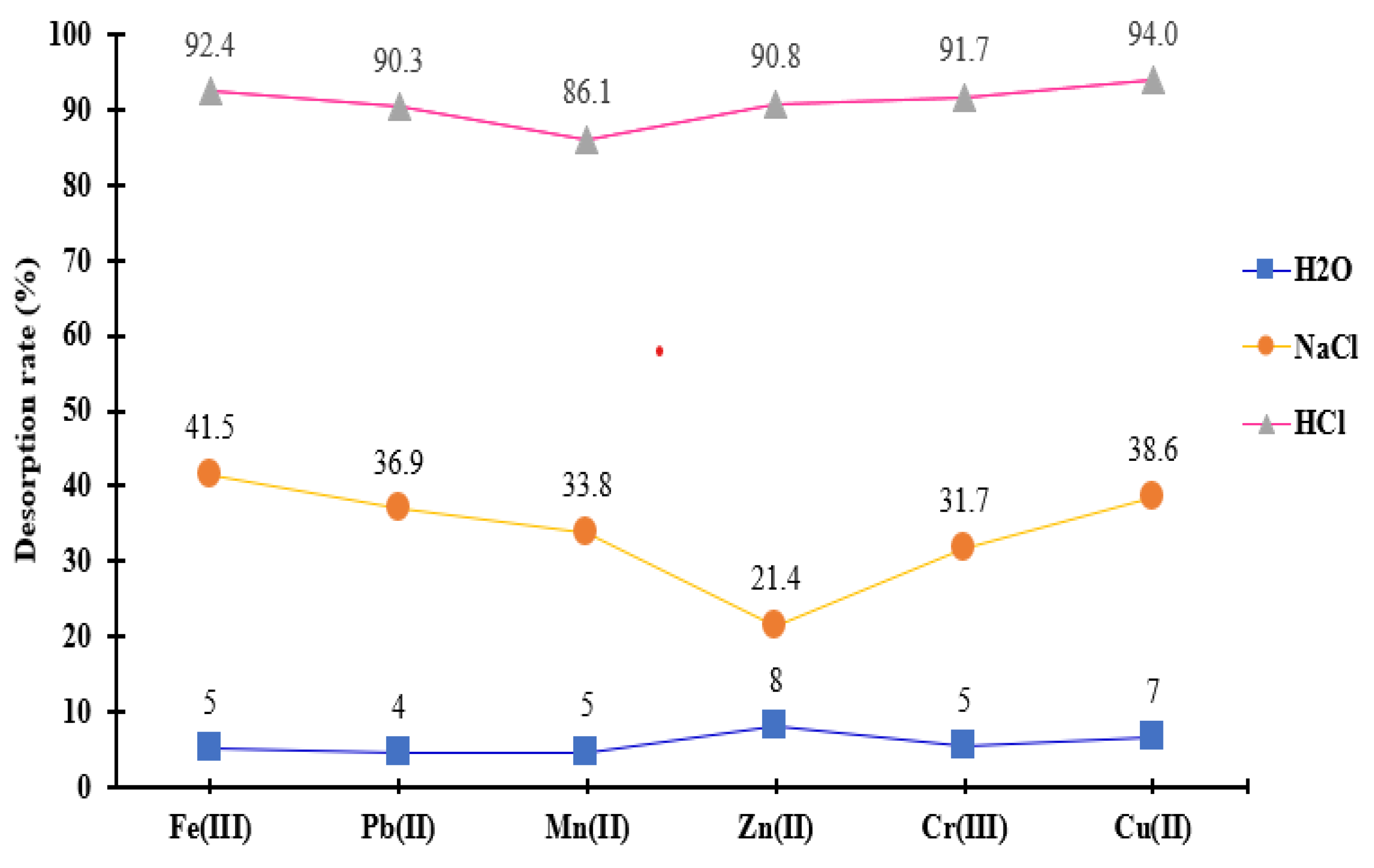
In this study, metal recovery from saturated material was achieved through chemical means using 0.5 M NaCl and HCl, as well as through heat treatment in hot water maintained at 45°C throughout the desorption experiment. It was observed as is presented in
Figure 8 that the desorption rate of retained metals was lower when using hot water for regeneration.
In contrast, metal desorption began to increase significantly with the application of the 0.5 M NaCl and HCl solutions. The desorption percentages obtained with 0.5 M NaCl were below 50%.
Notably, the 0.5 M HCl solution exhibited the most pronounced desorption effect, yielding percentages of Mn2+, Pb2+, Cu2+, Cr3+, Zn2+, and Fe3+ that reached up to 94%. These results indicate that the chemical adsorption resulting from the complexation reaction between metals and functional groups is the predominant mechanism at work, which explains the low desorption efficiency with hot water.
The regeneration mechanisms for NaCl and HCl are fundamentally similar. In both cases, Na+ and H+ ions compete to replace the metal ions that are adsorbed on the surface of the complexing material. However, the HCl solution demonstrated a greater desorption capacity compared to the NaCl solution. Among the three desorption agents tested, 0.5 M HCl was identified as the most effective reagent for regenerating the MS-ArS exhausted with metal ions, suggesting that the complexing material shows a higher affinity for H+ ions compared to Na+ ions.
4. Conclusions
During this study we successfully synthesized a novel material, MS-ArS, known for its complexing properties. The process involved mixing MS with ArS, an organic reagent characterized by its complex-forming groups, in a batch mode.
This approach allowed us to carry out a chemical reaction with metal ions at pH levels of 4 and 10, resulting in significant structural and color changes that confirmed the formation of complex compounds.
The findings reveal that the adsorptive capacity of the material increases in correlation with the concentration gradient, and we noted a relatively short time required to reach equilibrium.
So, the pH of the aqueous solution influences the dissociation of functional groups in the structure of the adsorbent material as well as the speciation of metals in the aqueous solution. Thus, at pH<4, the retention of metal ions is slightly favored due to the competitive sorption of H+ ions on the functionalized material, whose groups are protonated and unavailable for retaining metal ions from the solution. At the same time, as the pH of the solution increases, it intensifies the dissociation of functional groups, so that the surface of the material becomes more negative and adsorption is influenced by electrostatic interactions between metal ions and the mass of the tested material. This is explained by the fact that the adsorption capacities determined at pH 10 were significantly higher than those determined at pH=4 for all studied metal ions.
These results highlight the effectiveness of functionalization in enhancing the performance of the cellulosic material, paving the way for further applications and improvements in cellulosic material performance.
Author Contributions
For research articles with several authors, a short paragraph specifying their individual contributions must be provided. The following statements should be used “Conceptualization, NMM.; methodology, N.M.M., T.G and L.F.P.; software, N.M.M., validation, N.M.M., T.G and L.F.P.; formal analysis, N.-M.M.; investigation, N.M.M., T.G. and L.F.P.; resources, N.M.M.; data curation, N.M.M., T.G and L.F.P.; writing—original draft preparation, N.M.M., T.G and L.F.P.; writing—review and editing, N.M.M., T.G and L.F.P.; visualization, N.M.M., T.G and L.F.P.; supervision, N.M.M., T.G and L.F.P.; project administration, N.M.M.; funding acquisition, N.M.M. All authors have read and agreed to the published version of the manuscript.
Funding
This work was carried out via the “Nucleu” Program within the National Research Development and Innovation Plan 2022–2027 with the support of the Romanian Ministry of Research, Innovation and Digitalization, contract No. 3N/2022, Project code PN 23 22 03 01.
Institutional Review Board Statement
Not applicable.
Data Availability Statement
Data are available upon request from the authors.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Ling Felicia, W. X.; Rovina, K.; Supri, S.; Matanjun, P.; Mohd Amin, S. F.; Abdul Rahman, M. N., Next-generation sodium alginate hydrogels for heavy metal ion removal: properties, dynamic adsorption–desorption mechanisms, and sustainable application potential. Polymer Bulletin 2025, 1-51.
- Luo, H.; Wang, Q.; Guan, Q.; Ma, Y.; Ni, F.; Yang, E.; Zhang, J. Heavy metal pollution levels, source apportionment and risk assessment in dust storms in key cities in Northwest China. J. Hazard. Mater. 2022, 422, 126878. [CrossRef]
- Crini, G.; Lichtfouse, E. Advantages and disadvantages of techniques used for wastewater treatment. Environ. Chem. Lett. 2019, 17, 145–155. [CrossRef]
- Choudhary, A.; Mushtaq, A., From pollutant to valuable product: A novel reutilization strategy of wastewater. Int. J. Chem. Biochem. Sci 2023, 23, 31-37.
- Ahmadijokani, F.; Molavi, H.; Peyghambari, A.; Shojaei, A.; Rezakazemi, M.; Aminabhavi, T.M.; Arjmand, M. Efficient removal of heavy metal ions from aqueous media by unmodified and modified nanodiamonds. J. Environ. Manag. 2022, 316, 115214. [CrossRef]
- Oros, A. Bioaccumulation and Trophic Transfer of Heavy Metals in Marine Fish: Ecological and Ecosystem-Level Impacts. J. Xenobiotics 2025, 15, 59. [CrossRef]
- Abd-Elhalim, B.T.; Gideon, M.; Anton, K.; Boyi, M.O. Impact of dumpsite compost on heavy metal accumulation in some cultivated plants. BMC Res. Notes 2025, 18, 1–7. [CrossRef]
- Jomova, K.; Alomar, S.Y.; Nepovimova, E.; Kuca, K.; Valko, M. Heavy metals: toxicity and human health effects. Arch. Toxicol. 2024, 99, 153–209. [CrossRef]
- Meng, K.; Dong, Y.; Liu, J.; Xie, J.; Jin, Q.; Lu, Y.; Lin, H. Advances in selective heavy metal removal from water using biochar: A comprehensive review of mechanisms and modifications. J. Environ. Chem. Eng. 2025, 13. [CrossRef]
- Aziz, K.H.H.; Mustafa, F.S.; Hamarawf, R.F.; Omer, K.M. Adsorptive removal of toxic heavy metals from aquatic environment by metal organic framework (MOF): A review. J. Water Process. Eng. 2025, 70. [CrossRef]
- Elouali, S.; Ouydir, H.A.A.; Hamdan, Y.A.; Eladlani, N.; Rhazi, M. Chitosan from Periplaneta americana L.: a sustainable solution for heavy metals removal. Euro-Mediterranean J. Environ. Integr. 2024, 10, 25–36. [CrossRef]
- Yuan, F.; Yan, D.; Song, S.; Zhang, J.; Yang, Y.; Chen, Z.; Lu, J.; Wang, S.; Sun, Y. Removal of heavy metals from water by adsorption on metal organic frameworks: Research progress and mechanistic analysis in the last decade. Chem. Eng. J. 2025, 506. [CrossRef]
- Castellanos, H.G.; Aryanfar, Y.; Mohtaram, S.; Keçebaş, A.; Karaca-Dolgun, G.; Ahmad, S.; Asiri, A.N.M.; Islam, S. The efficacy of nano-cellulose-based composites in heavy metal removal from wastewater: a comprehensive review. J. Chem. Technol. Biotechnol. 2024, 100, 291–312. [CrossRef]
- Feng, J.; Yu, Y.; Huang, S.; Zhu, N.; Mojiri, A.; Ge, D. Tannic acid as a green chemical for the removal of various heavy metals: A critical review of recent developments. J. Environ. Manag. 2025, 375, 124390. [CrossRef]
- Tan, S.; Zhang, T.; Cheng, C.; Wang, Z.; Li, H.; Zhao, Y. Efficient removal and stepwise recovery of various heavy metals from water by using calcium carbonate with different activity. Sep. Purif. Technol. 2024, 354. [CrossRef]
- Macena, M.; Pereira, H.; Cruz-Lopes, L.; Grosche, L.; Esteves, B. Competitive Adsorption of Metal Ions by Lignocellulosic Materials: A Review of Applications, Mechanisms and Influencing Factors. Separations 2025, 12, 70. [CrossRef]
- Kayani, K.F.; Mohammed, S.J.; Mustafa, M.S.; Aziz, S.B. Dyes and their toxicity: removal from wastewater using carbon dots/metal oxides as hybrid materials: a review. Mater. Adv. 2025, 6, 5391–5409. [CrossRef]
- Ahmad, A. An innovative step to fabricate biomass-derived reduced graphene oxide electrodes to boost energy efficiency with metal removal using an electrochemical approach. Biomass- Convers. Biorefinery 2024, 15, 5997–6012. [CrossRef]
- Arabkhani, P.; Asfaram, A. A novel biowaste-derived magnetic adsorbent for efficient removal of cadmium, cobalt and strontium ions from industrial wastewater. Inorg. Chem. Commun. 2025, 174. [CrossRef]
- Sireesha, S.; Agarwal, A.; Sopanrao, K. S.; Sreedhar, I.; Anitha, K., Modified coal fly ash as a low-cost, efficient, green, and stable adsorbent for heavy metal removal from aqueous solution. Biomass Conversion and Biorefinery 2025, 15 (15), 21685-21708.
- Ahmadzadeh, S.; Hemmati, A. Innovative approach to Pb (II) removal using zinc-based MOFs-derived carbon: In-depth analysis of adsorption mechanisms, isotherms, thermodynamics, and kinetics. Results Eng. 2025, 25. [CrossRef]
- Ghaedi, S.; Rajabi, H.; Mosleh, M.H.; Spencer, B.F.; Sedighi, M. Assessing the efficiency and reusability of zirconium-based MOF-biochar composite for the removal of Pb (II) and Cd (II) in single and multi-ionic systems. J. Environ. Manag. 2025, 380, 125122. [CrossRef]
- Inobeme, A.; Mathew, J. T.; Devolli, A.; Adetunji, C. O.; Sharma, N.; Maliki, M.; Ajai, A.; Inobeme, J.; Mann, A.; Enoyoze, G., Metal components in industrial wastes and methods for metal ions recovery. In Metal Value Recovery from Industrial Waste Using Advanced Physicochemical Treatment Technologies, Elsevier: 2025; pp 1-15. [CrossRef]
- Zhu, Y.; Hua, J.; Yuan, J.; Yuan, Z.; Dai, Y.; Zhang, T.; Qiu, F. Conversion and utilization of waste biomass into sustainability treasure: Surface modified eggplant biomass by PEI and enhanced removal of Pb(II) from aqueous solutions. Colloids Surfaces A: Physicochem. Eng. Asp. 2024, 708. [CrossRef]
- Namdeti, R.; Rao, G.B.; Lakkimsetty, N.R.; Qatan, M.A.A.; Al-Kathiri, D.S.M.S.; Al Amri, L.A.; Qahoor, N.M.S.; Joaquin, A.A. Innovative Approaches in Water Decontamination: A Critical Analysis of Biomaterials, Nanocomposites, and Stimuli-Responsive Polymers for Effective Solutions. J. Environ. Earth Sci. 2024, 7, 92–102. [CrossRef]
- Marin, N.M.; Lazar, M.N.; Popa, M.; Galaon, T.; Pascu, L.F. Current Trends in Development and Use of Polymeric Ion-Exchange Resins in Wastewater Treatment. Materials 2024, 17, 5994. [CrossRef]
- Chen, C.; He, E.; Jiang, X.; Xia, S.; Yu, L. Efficient removal of direct dyes and heavy metal ion by sodium alginate-based hydrogel microspheres: Equilibrium isotherms, kinetics and regeneration performance study. Int. J. Biol. Macromol. 2025, 294, 139294. [CrossRef]
- Gowayed, S.M.H.; Abdel-Salam, A.H.; Nassef, E.; Morsy, A. Innovative hybrid membrane: Pioneering metal oxide framework for improved elimination of heavy metals from industrial wastewater. Polym. Eng. Sci. 2025, 65, 2093–2105. [CrossRef]
- Martins, B.d.A.; Takahashi, J.A. Overview of bioremediation as a method for metal-contaminated wastewater treatment. Environ. Sci. Pollut. Res. 2025, 1–25. [CrossRef]
- Shamshad, J.; Rehman, R.U. Innovative approaches to sustainable wastewater treatment: a comprehensive exploration of conventional and emerging technologies. Environ. Sci. Adv. 2024, 4, 189–222. [CrossRef]
- Kazakis, N.A. Green approaches to heavy metal removal from wastewater: Microalgae solutions in a circular economy framework. Soc. Impacts 2025, 5. [CrossRef]
- Kumari, A.; Kamaraj, N.; Selvaraj, R.; Nanoth, R. Emerging trends and future outlook on chromium removal in the lab, pilot scale, and industrial wastewater system: an updated review exploring 10 years of research. Environ. Monit. Assess. 2025, 197, 1–46. [CrossRef]
- Marzbali, M.H.; Hakeem, I.G.; Ngo, T.; Surapaneni, A.; Shah, K. Innovative chemical functionalisation of biosolids for removing heavy metals and enhancing ammonium recovery from wastewater. Int. J. Environ. Sci. Technol. 2024, 22, 6665–6680. [CrossRef]
- Solcova, O.; Dlaskova, M.; Kastanek, F. Innovative Sorbents for the Removal of Micropollutants from Water. Molecules 2025, 30, 1444. [CrossRef]
- Abd Aziz, M. A.; Hairunnaja, M. A.; Arifin, M. A.; Isa, K. M., Advancements in Metal Recovery from Industrial Wastes: A Comprehensive Overview. Controlling Environmental Pollution: Practical Solutions 2025, 225-245. [CrossRef]
- Zhang, Z.; Lu, Y.; Gao, S.; Wu, S. Sustainable and Efficient Wastewater Treatment Using Cellulose-Based Hydrogels: A Review of Heavy Metal, Dye, and Micropollutant Removal Applications. Separations 2025, 12, 72. [CrossRef]
- Marin, N. M., Maize stalk obtained after acid treatment and its use for simultaneous removal of Cu2+, Pb2+, Ni2+, Cd2+, Cr3+ and Fe3+. Polymers 2022, 14 (15), 3141. [CrossRef]
- Marin, N.M. A New Approach of Complexing Polymers Used for the Removal of Cu2+ Ions. Polymers 2024, 16, 920. [CrossRef]
- Marin, N.M. Green Chemistry Applications Using Complexing Materials for Water Treatment. Polymers 2025, 17, 1467. [CrossRef]
- Pasquali, E.A.; Oro, C.E.D.; Bernardi, J.L.; Venquiaruto, L.D.; Treichel, H.; Mossi, A.J.; Dallago, R.M. Adsorption of Cr(VI) by wet blue leather: Sustainable solution for leather industry effluents. J. Water Process. Eng. 2024, 69. [CrossRef]
- Rahman, L.; Shamrih, S.A.; Azlyzan, N.A.; Sarjadi, M.S.; Arsad, S.E.; Sarkar, S.M.; Kumar, S. Removal of heavy metal ions from wastewater using modified cornstalk cellulose-derived poly(amidoxime) ligand. Carbohydr. Polym. Technol. Appl. 2024, 9. [CrossRef]
- Park, S.; Kim, Y.-H.; Lee, J.W.; Jang, S.; Kim, J.E.; Kang, G.; Choi, Y.-K. Adsorptive performance of rice husk-derived biochar for nodularin cyanotoxin from aqueous solution: Isotherm, kinetic, regeneration, and column studies. J. Water Process. Eng. 2025, 70. [CrossRef]
- Feng, Z.; Li, J.; Chen, N.; Feng, C. Sulfonated corn stalk enhanced hydrogel adsorption for heavy metal from metal mine gallery effluent. Sep. Purif. Technol. 2024, 357. [CrossRef]
- Houmia, I.; Fardioui, M.; El Amri, A.; Houmia, B.; Kaibous, N.; Bazhar, K.; Hammani, O.; Arhoutane, M.R.; Guedira, T. Structural characterization and ecological evaluation of natural clay mixtures for the removal of heavy metals (Cu(II), Co(II), and Zn(II)) from aqueous solutions: experimental study combined with RSM process optimization. J. Mol. Struct. 2025, 1344. [CrossRef]
- Renu; Sithole, T. A review on regeneration of adsorbent and recovery of metals: Adsorbent disposal and regeneration mechanism. South Afr. J. Chem. Eng. 2024, 50, 39–50. [CrossRef]
Figure 1.
a,b,c,d,e,f,g,h,i,j,k,l,m. Image of the liquid solutions used in the spectral analysis (a,b) and UV Vis absorption spectra overlapped for ArS, Mn+, and for ArS-Mn+ mixture (c,d,e,f,g,h,i,j,k,l, and m).
Figure 1.
a,b,c,d,e,f,g,h,i,j,k,l,m. Image of the liquid solutions used in the spectral analysis (a,b) and UV Vis absorption spectra overlapped for ArS, Mn+, and for ArS-Mn+ mixture (c,d,e,f,g,h,i,j,k,l, and m).
Figure 2.
Image of the complexing material obtained for adsorption of ArS on MS at different pH values.
Figure 2.
Image of the complexing material obtained for adsorption of ArS on MS at different pH values.
Figure 3.
Quantity of ArS adsorbed onto MS mass in function of pH medium (a) and as a function of R(%) (b).
Figure 3.
Quantity of ArS adsorbed onto MS mass in function of pH medium (a) and as a function of R(%) (b).
Figure 4.
Chemical structure of ArS (a,b) and its forms in which it can complexing the metals (Mn2+, Pb2+, Cu2+, Cr3+, Zn2+, Fe3+).
Figure 4.
Chemical structure of ArS (a,b) and its forms in which it can complexing the metals (Mn2+, Pb2+, Cu2+, Cr3+, Zn2+, Fe3+).
Figure 5.
Adsorption of Mn2+, Pb2+, Cu2+, Cr3+, Zn2+ and Fe3+ in function of pH solution onto MS-ArS mass.
Figure 5.
Adsorption of Mn2+, Pb2+, Cu2+, Cr3+, Zn2+ and Fe3+ in function of pH solution onto MS-ArS mass.
Figure 6.
Effect of contact time on the metal ions removal onto MS-ArS mass.
Figure 6.
Effect of contact time on the metal ions removal onto MS-ArS mass.
Figure 7.
Adsorption of Mn2+, Pb2+, Cu2+, Cr3+, Zn2+ and Fe3+ in the MS-ArS mass at pH = 10 of the tested solution.
Figure 7.
Adsorption of Mn2+, Pb2+, Cu2+, Cr3+, Zn2+ and Fe3+ in the MS-ArS mass at pH = 10 of the tested solution.
Figure 8.
Desorption capacities for metal ions removal from ArS-MS mass after adsorption studies.
Figure 8.
Desorption capacities for metal ions removal from ArS-MS mass after adsorption studies.
Table 1.
Characteristics of Mn+ studied.
Table 1.
Characteristics of Mn+ studied.
| Metal |
Mn2+ |
Pb2+ |
Cu2+ |
Cr3+ |
Zn2+ |
Fe3+ |
| Electron Configuration |
[Ar] 3d5 4s2
|
[Xe] 4f14 5d10 6s2 6p2
|
[Ar] 3d10 4s1
|
[Ar] 3d5 4s1
|
[Ar] 3d10 4s2
|
[Ar] 3d6 4s2
|
| Electronegativity |
1.55 |
2.33 |
1.90 |
1.66 |
1.65 |
1.83 |
| Oxidation Number |
+2 |
+2 |
+2 |
+3 |
+2 |
+3 |
| Atomic radius (Å) |
1.79 |
1.9 |
1.57 |
1.85 |
1.53 |
1.72 |
| Ionic radius (Å) |
0.67 |
1.19 |
0.73 |
0.62 |
0.74 |
0.55 |
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).