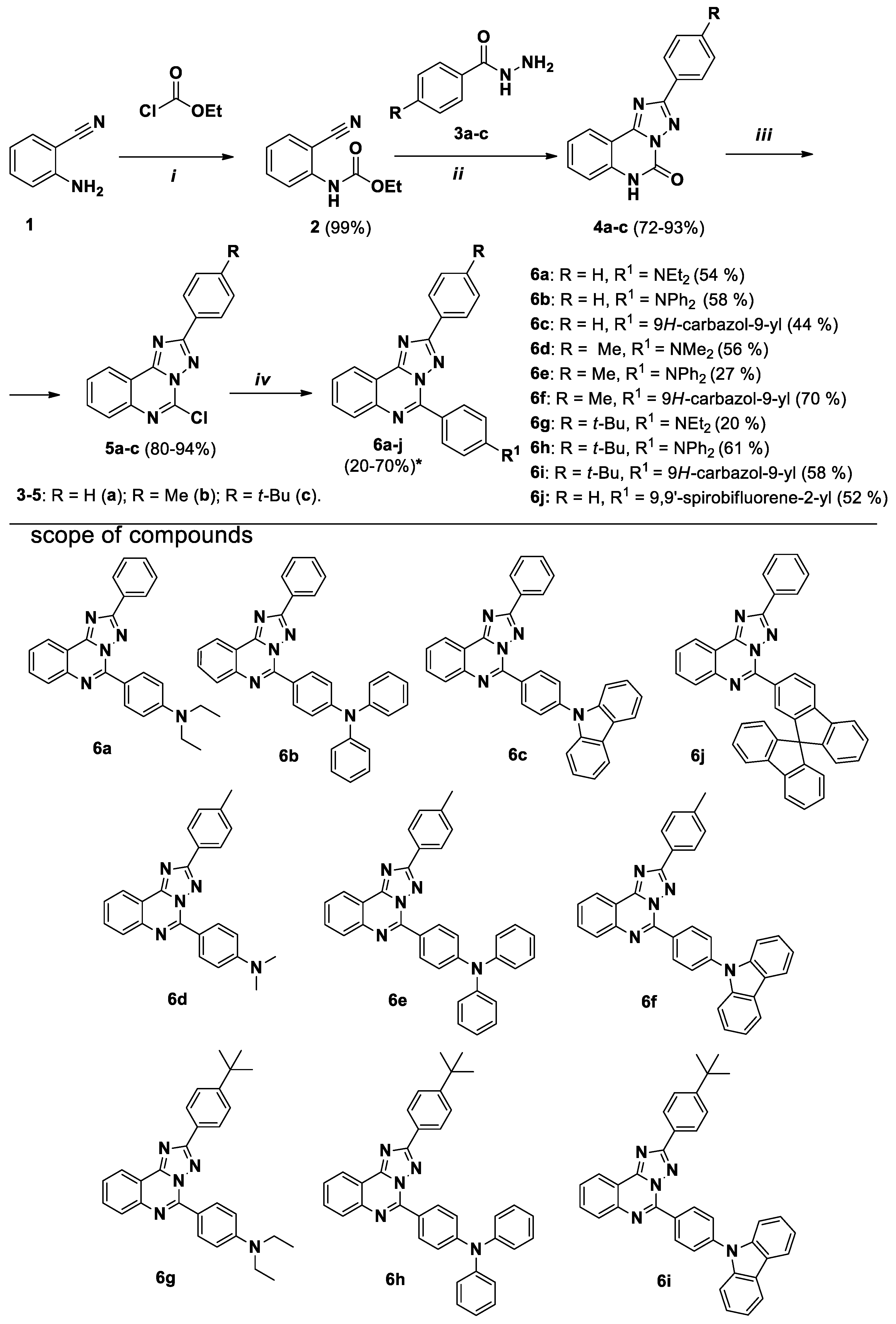
3.5.1. Synthesis of the Intermediate Products
Ethyl
N-(2-cyanophenyl)carbamate (
2) was prepared similar to describe procedure [
17]. To 2-aminobenzonitrile (2.7 g, 22.8 mmol) and potassium carbonate (9.5 g, 68.6 mmol) in tetrahydrofuran (130 ml) ethyl chloroformate (4.4 ml, 46.0 mmol) was added. The mixture was stirred at 85 °C for 16 h. After cooling the precipitate was filtered, washed with tetrahydrofuran and water. Product was used without further purification. Colourless solid, yield: 99% (4.3 g);
1Н NMR (DМSO-d
6, 400 MHz) δ 1.24 (t,
3J = 6.8, 3H, CH
3), 4.14 (q,
3J = 6.8, 2H, CH
2), 7.30–7.34 (m, 1H), 7.50–7.52 (m, 1H), 7.64–7.68 (m, 1H), 7.77‒7.79 (m, 1H), 9.7 (s, 1H, NH).
Compounds
4a-c were obtained following to described procedure [
17]. Ethyl
N-(2-cyanophenyl)carbamate
2 (1.5 g, 7.85 mmol) and corresponding hydrazide
3a-с (9.36 mmol) were stirred in N,N-dimethylformamide (10 mL) at 120 °C for 12 hours. After cooling water was added to the mixture, precipitated product was filtered off and recrystallized from MeCN (for
4a) or DMSO (for
4b and
4c).
2-Phenyl-[1,2,4]triazolo[1,5-
c]quinazolin-5(6
H)-one (
4a). Colourless solid, yield: 72% (1.5 g); mp 296–298 °C (mp lit. 311–313 °C [
32]).
1Н NMR (DМSO-d
6, 400 MHz) δ 7.39–7.66 (m, 6H), 8.24–8.25 (m, 3Н), 12.31 (s, 1H, NH); EIMS (m/z, I
rel %): 263 [M+1]
+ (18), 262 [M]
+ (100); Exact mass for C
15H
10N
4O (262.0855).
2-(p-Tolyl)-[1,2,4]triazolo[1,5-c]quinazolin-5(6H)-one (4b). Colourless solid, yield: 83% (1.80 g); mp 305–307 °C. 1Н NMR (DМSO-d6, 400 MHz) δ 2.43 (3H, s, CH3), 7.32–7.40 (3H, m, H-3’, H-5’, H-8 or H-9), 7.45–7.47 (1H, m, H-7 or H-10), 7.64–7.64 (1Н, m, H-8 or H-9), 8.12–8.14 (2Н, d, 3J = 7.5, H-2’, H-6’), 8.22–8.24, (1H, m, H-7 or H-10), 12.28 (1H, s, NH); EIMS (m/z, Irel %): 277 [M+1]+ (20), 276 [M]+ (100); Exact mass for C16H12N4O (276.1011).
2-4-(Tert-butyl)phenyl-[1,2,4]triazolo[1,5-c]quinazolin-5(6H)-one (4c). Colourless solid, yield: 93% (2.33 g); mp 243–245 °C. 1Н NMR (CDCl3, 400 MHz) δ 1.37 (9H, s, t-Bu), 7.36–7.40 (1H, m, H-8 or H-9), 7.51–7.54 (3H, m, H-3’, H-5’, H-7 or H-10), 7.59–7.61 (1Н, m, H-8 or H-9), 8.29–8.31 (2Н, d, 3J = 7.8, H-2’, H-6’), 8.35–8.37, (1H, m, H-7 or H-10), 11.4 (1H, s, NH); EIMS (m/z, Irel %): 318 (29), 304 [M-CH3 +1]+ (23), 303 [M-CH3]+ (100); Exact mass for C19H18N4O (318.1950).
Compounds
5a-c were obtained following to described procedure [
17]. To the dried [1,2,4]triazolo[1,5-
c]quinazolin-5(6
H)-one
3a-c (1.14 mmol) in phosphorus(V) oxychloride (7.2 mL, 77 mmol),
Ν,Ν-diisopropylethylamine (0.4 mL, 2.27 mmol) was added carefully and the mixture was stirred for 20 h at 110 °C. Condenser was equipped with a calcium chloride drying tube. The mixture was concentrated. Resulting product was purified with column chromatography on SiO
2 using mixture of hexane and EtOAc as eluent.
5-Chloro-2-phenyl-[1,2,4]triazolo[1,5-c]quinazoline (5a). Colourless solid, yield: 94% (300 mg); mp 170–172 °C. 1Н NMR (CDCl3, 400 MHz): δ 7.53–7.55 (3H, m, H-3’, Н-4’, H-5’), 7.75–7.79 (1H, m, H-8 or H-9), 7.85–7.89 (1H, m, H-8 or H-9), 8.01–8.02 (1Н, m, H-10), 8.39–8.42 (2Н, m, H-2’, Н-6’), 8.61 (1Н, d, 3J = 7.9, H-7). EIMS (m/z, Irel %): 282 [M + 2]+ (33), 281 [M+1]+ (21), 280 [M]+ (100), 245 (18), 163 (18), 102 (15), 89 (15). Exact mass for C15H9ClN4 (280.0516).
5-Chloro-2-(p-tolyl)-[1,2,4]triazolo[1,5-c]quinazoline (5b). Beige solid, yield: 82% (277 mg); mp 166–168 °C. 1Н NMR (DМSO-d6, 400 MHz): δ 2.45 (s, 3H, CH3), 7.38 (2H, d, 3J = 8.1, H-3’, H-5’), 7.84–7.88 (1H, m, H-8 or H-9), 7.94–7.98 (1Н, m, H-8 or H-9), 8.01–8.03 (1H, m, H-10), 8.22 (2Н, d, 3J = 8.1, H-2’, H-6’), 8.54 (1Н, d, 3J = 7.8, H-7); EIMS (m/z, Irel %): 296 [M+2]+ (35), 295 [M+1]+ (25), 294 [M]+ (100), 293 (17), 163 (11), 131 (13), 116 (12), 102 (19), 90 (13); Exact mass for C16H11ClN4 (294.0672).
5-Chloro-2-(4-(Tert-butyl)phenyl)-[1,2,4]triazolo[1,5-c]quinazoline (5c). Beige solid, yield: 80% (297 mg); mp 126–128 °C. 1Н NMR (DМSO-d6, 400 MHz): δ 1.39 (s, 9H, 3 CH3), 7.56 (2H, d,3J = 8.5, H-3’, H-5’), 7.82–7.86 (1H, m, H-8 or H-9), 7.93–7.95 (1Н, m, H-8 or H-9), 7.99–8.01 (1H, m, H-10), 8.22 (2Н, d, 3J = 8.5, H-2’, H-6’), 8.54 (1Н, d, 3J = 7.9, H-7); EIMS (m/z, Irel %): 338 [M+2]+ (10), 336 [M]+ (28), 323 (35), 322 (23), 321 [M-CH3]+ (100), 163 (11), 146 (13), 102 (11); Exact mass for C19H17ClN4 (336.1142).
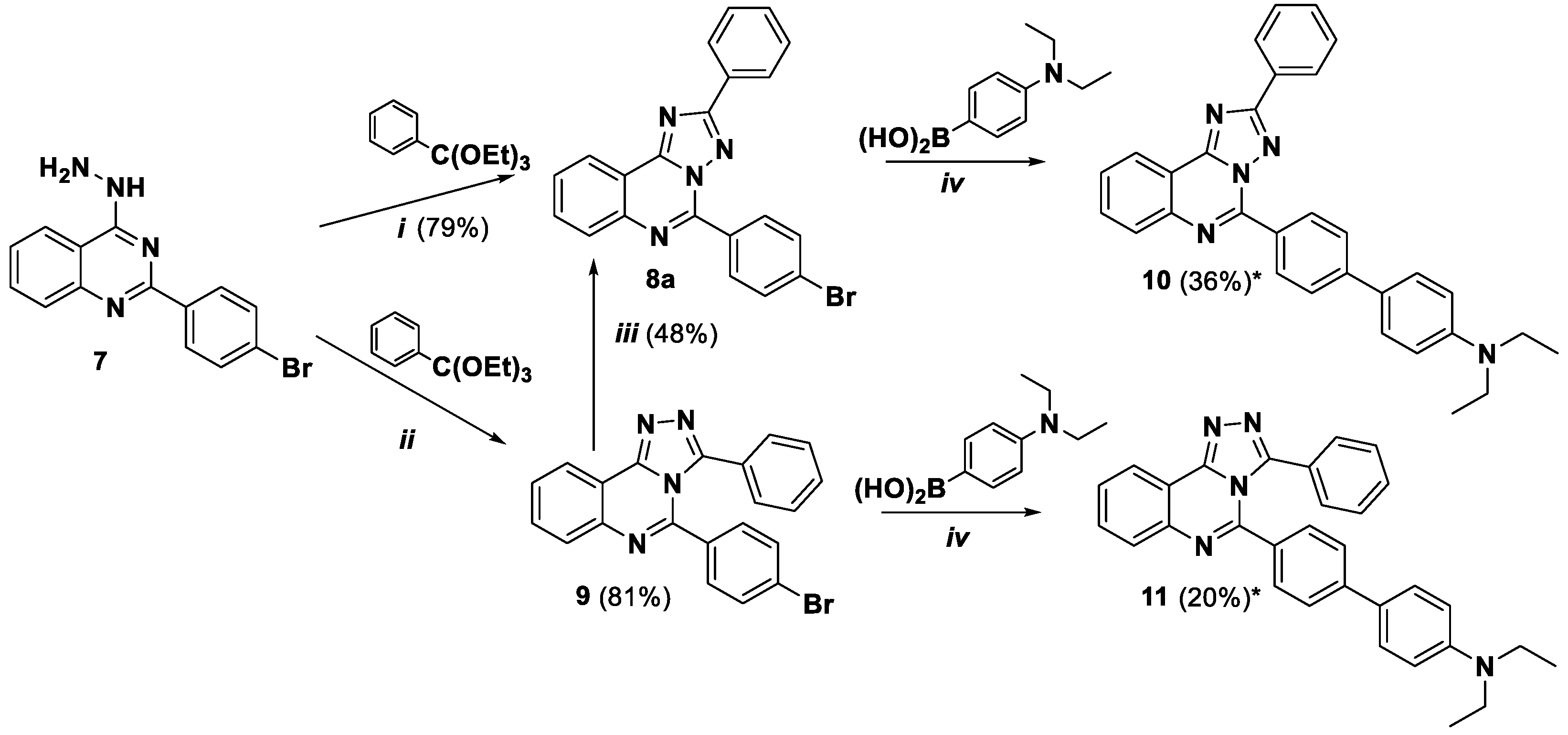
4-Hydrazino-2-(4-bromophenyl)quinazoline (
7) and 5-(4-Bromophenyl)-2-ethyl- [1,2,4]triazolo[1,5-
c]quinazoline (
8b) were prepared as described previously [
8,
9].
Synthesis of 5-(4-Bromophenyl)-2-phenyl-[1,2,4]triazolo[1,5-c]quinazoline (8a).
Method 1. In a round-bottom flask equipped with a magnetic stirred bar, 4-hydrazino-2-(4-bromophenyl)quinazoline 7 (0.27 g, 0.86 mmol) in glacial acetic acid (7 mL) triethyl orthobenzoate (0.86 mL, 4.3 mmol) were added. The mixture was refluxed for 16 h. After cooling down the water was added until the formation of precipitate. The product was filtered off and washed with water and recrystallized from DMSO.
Method 2. In a round-bottom flask equipped with a magnetic stirred bar, 5-(4-Bromophenyl)-3-phenyl-[1,2,4]triazolo[4,3-c]quinazoline 9 (0.18 g, 0.45 mmol) refluxed in glacial acetic acid (3.6 mL) for 20 h. After cooling down the water was added until the formation of precipitate. The solid was filtered off and washed with water.
Colourless powder, yield 79%, 0.27 g (method 1), yield 48%, 0,073 g (method 2); mp 190–192 °C; 1H NMR (DМSO-d6, 400 MHz) δ 7.58–7.60 (3H, m, H-3’, H-4’, H-5’), 7.85–7.91 (3H, m, H-3’’, H-5’’, H-8 or H-9), 7.96–7.99 (1H, m, H-8 or H-9), 8.13–8.15 (1H, m, H-10), 8.30–8.33 (2H, m, H-2’, H-6’), 8.53–8.55 (3H, m, H-2’’, H-6’’, H-7); 13C {1H} NMR (CDCl3, 100 MHz, 45 °C) δ 116.9, 123.3, 125.4, 127.1, 128.4, 128.8, 128.9, 129.7, 130.6, 131.3, 132.2, 132.5, 142.2, 145.0, 152.4, 162.8; EIMS (m/z, Irel %): 402 [M+2]+ (100), 401 [M+1]+ (66), 400 [M]+ (99); Exact mass for C21H13BrN4 (400.0324).
Synthesis of 5-(4-Bromophenyl)-3-phenyl-[1,2,4]triazolo[4,3-c]quinazoline (9). Starting 2-(4-bromophenyl)-4-hydrazinoquinazoline was preliminarily dried in oven at 100 oC for 4 h.
Method 1. In a round-bottom flask equipped with a magnetic stirred bar, 2-(4-bromophenyl)-4-hydrazinoquinazoline 7 (0.32 g, 1.00 mmol) in absolute ethanol (17 mL) and triethyl orthobenzoate (1 mL, 4.80 mmol) were added. The mixture was refluxed for 4 h. Condenser was equipped with a calcium chloride drying tube. After cooling down and partial evaporation the solid was filtered off, washed with EtOH (5 mL). The product was purified by column chromatography on SiO2 using EtOAc and hexane as eluent, gradually from (1:9) to pure EtOAc.
Method 2. In a round-bottom flask equipped with a magnetic stirred bar, dried 2-(4-bromophenyl)-4-hydrazinoquinazoline 7 (0.20 g, 0.62 mmol) and triethyl orthobenzoate (1 mL, 4.80 mmol) were added. The mixture was refluxed for 4 h. A condenser was equipped with a calcium chloride drying tube. After cooling down the solid was filtered off, washed with EtOH (5 mL) and dried.
Colourless powder, yield 63% (method 1), yield 81% (method 2); mp 237–239 °C; 1H NMR (DМSO-d6, 400 MHz) δ 7.13–7.23 (6H, m, H-2’’, H-3’’, H-5’’, H-6’’, H-2’, H-6’), 7.29–7.31 (3H, m, H-3’, H-4’, H-5’), 7.78–7.82 (1H, m, H-7 or H-10), 7.84–7.88 (1H, m, H-7 or H-10), 7.96–7.98 (1H, m, H-10), 8.62 (1H, d, 3J = 7.9, H-7); EIMS (m/z, Irel %): 402 [M+2]+ (94), 401 [M+1]+ (79), 400 [M]+ (100); Exact mass for C21H13BrN4 (400.0324).
3.5.1. Synthesis of the Target Products
The corresponding boronic acid or boronic acid pinacol ester (0.57 mmol), PdCl2(PPh3)2 (40 mg, 57 μmol), PPh3 (30 mg, 114 μmol), saturated solution of K2CO3 (3.1 mL) and EtOH (3.1 mL) were added to the suspension of the corresponding chloro or bromo derivative (5a-c or 8a,b, 9) (0.53 mmol) in toluene (19 mL). The mixture was stirred at 85 °C for 14–30 h in argon atmosphere in round-bottom pressure flask equipped with magnetic stirred bar. The reaction mixture was cooled to room temperature, and EtOAc/H2O (10/10 mL) mixture was added. The organic layer was separated, additionally washed with water (10 mL), and evaporated at reduced pressure. The product was purified by column chromatography.
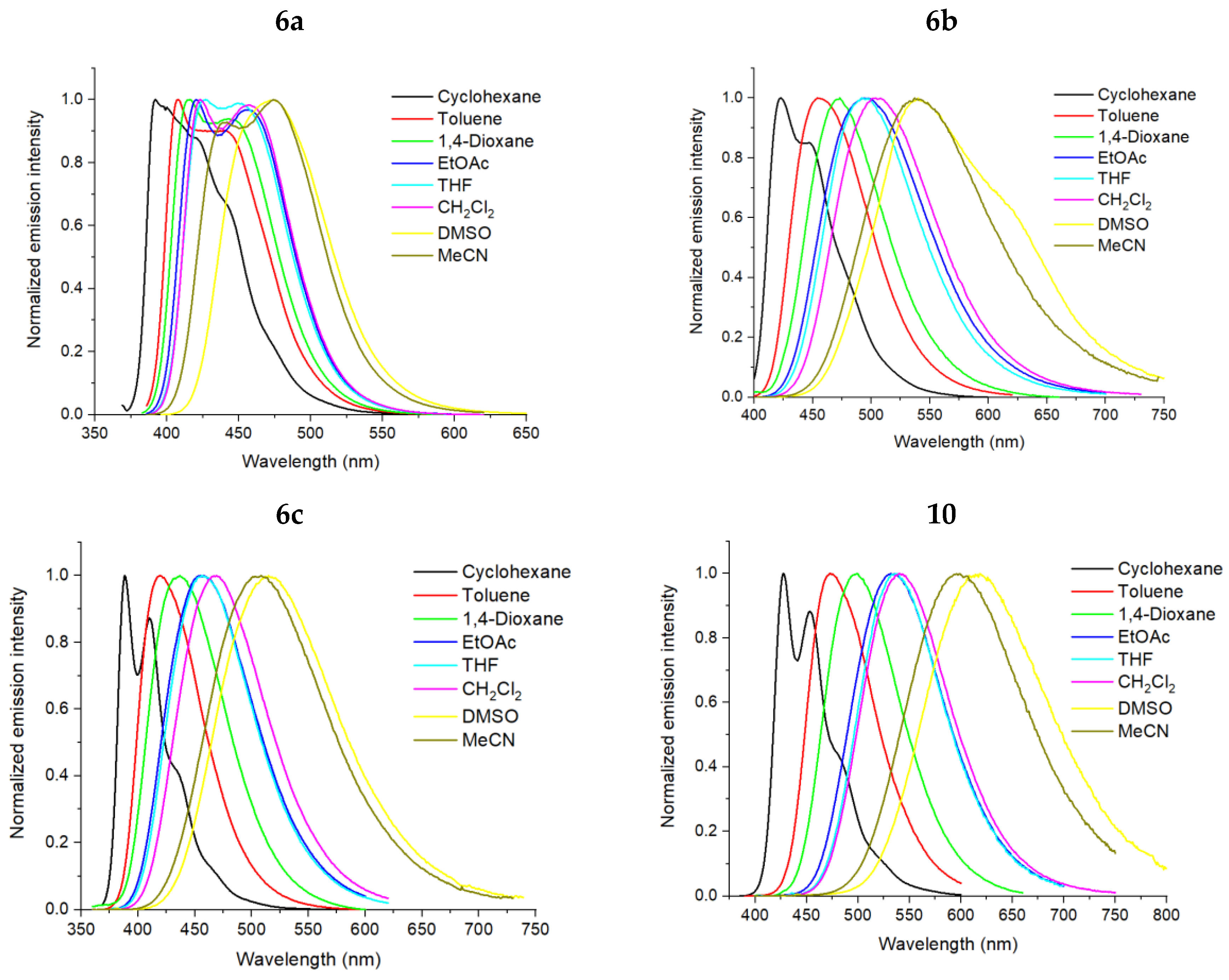
5-(4-Diethylaminophenyl)-2-phenyl-[1,2,4]triazolo[1,5-c]quinazoline (6a).
The general procedure was applied using 5-chloro-2-phenyl- [1,2,4]triazolo[1,5-c]quinazoline 5a and 4-(diethylamino)phenylboronic acid as the starting materials. Reaction time is 30 h. Column chromatography: SiO2, EtOAc and hexane (1:9) was used as an eluent. Pale-yellow solid, yield: 54% (114 mg); mp = 135–137 °C; 1H NMR (CDCl3, 400 MHz): δ 1.27 (6H, t, 3J = 7.1, 2CH3), 3.50 (4H, q, 3J = 7.1, 2CH2), 6.85 (2H, d, 3J = 9.2, H-3’’, H-5’’), 7.47–7.56 (3H, m, H-3’, H-4’, H-5’), 7.60–7.64 (1H, m, H-8), 7.77–7.81 (1Н, m, H-9), 8.04–8.05 (1H, m, H-10), 8.43–8.45 (2Н, m, H-2’, H-6’), 8.59 (1Н, d, 3J = 8.1, H-7), 8.74 (2H, d, 3J = 9.2, H-2’’, H-6’’); 13C {1H} NMR (CDCl3, 100 MHz) δ 12.8 (2 CH3), 44.7 (2 CH2), 110.8, 116.9, 118.0, 123.8, 127.0, 127.8, 128.3, 128.8, 130.3, 130.8, 131.9, 132.5, 143.6, 146.8, 150.2, 153.2, 163.7; EIMS (m/z, Irel %): 394 [M+1]+ (18), 393 [M]+ (58), 379 (29), 378 [M-CH3]+ (100), 350 (12), 189 (17); Exact mass for C25H23N5 (393.1953). Calcd: C, 76.31, H, 5.89, N, 17.80%; Found: C, 76.22, H, 6.04, N, 17.56%.
5-(4-Diphenylaminophenyl)-2-phenyl-[1,2,4]triazolo[1,5-c]quinazoline (6b). The general procedure was applied using 5-chloro-2-phenyl-[1,2,4]triazolo[1,5-c]quinazoline 5a and 4-(diphenylamino)phenylboronyc acid as the starting materials. Reaction time is 14 h. Column chromatography: SiO2, hexane and CH2Cl2 (7:3) and then EtOAc and hexane (1:9). Pale-yellow solid, yield: 58% (150 mg); mp = 191–193 °C; 1H NMR (CDCl3, 400 MHz) δ 7.13–7.16 (2H, m, Phenyl), 7.21–7.24 (6H, m, Phenyl, H-3’’, H-5’’), 7.33–7.37 (4H, m, Phenyl), 7.51–7.53 (3H, m, H-3’, H-4’, H-5’), 7.66–7.70 (1H, m, H-8), 7.81–7.84 (1Н, H-9), 8.08 (1H, d, 3J = 8.2, H-10), 8.38–8.44 (2H, m, H-2’, H-6’), 8.61–8.65 (3H, m, H-7, H-2’’, H-6’’); EIMS (m/z, Irel %): 490 [M+1]+ (39), 489 [M]+ (100), 488 (14), 77 (11); 13C {1H} NMR (CDCl3, 100 MHz) δ 117.2, 120.8, 123.9, 124.1, 124.4, 125.9, 127.8, 128.6, 128.8, 129.7, 130.5, 130.6, 131.9, 132.1, 143.3, 146.2, 147.0, 151.1, 153.2, 164.0; Exact mass for C33H23N5 (489.1953). Calcd: C, 80.96, H, 4.74, N, 14.30%. Found: C, 80.86, H, 4.87, N, 14.19%.
5-(4-(9H-carbazol-9-yl)phenyl)-2-phenyl-[1,2,4]triazolo[1,5-c]quinazoline (6c).
The general procedure was applied using 5-chloro-2-phenyl- [1,2,4]triazolo[1,5-c]quinazoline 5a and 9H-Carbazole-9-(4-phenyl) boronic acid pinacol ester as the starting materials. Reaction time is 21 h. Column chromatography: SiO2, hexane and CH2Cl2 (7:3) and then EtOAc and hexane (1:9). Pale-yellow solid, yield: 44% (113 mg); mp = 235–237 °C; 1H NMR (CDCl3, 400 MHz) δ 7.33–7.36 (2H, m, carbazolyl), 7.46–7.49 (2H, m, carbazolyl), 7.53–7.57 (3H, m, H-3’, H-4, H-5’), 7.61–7.63 (2H, m, carbazolyl), 7.75–7.79 (1H, m, H-8), 7.87–7.90 (3H, m, H-9, H-3’’, H-5’’), 8.17–8.19 (3H, m, H-10, carbazolyl), 8.46–8.48 (2H, m, H-2’, H-6’), 8.69 (1H, d, 3J = 8.2, H-7), 8.98–99.00 (2H, m, H-2’’, H-6’’); 13C {1H} NMR (CDCl3, 100 MHz) δ 110.1, 117.6, 120.6, 120.6, 123.9, 124.1, 126.3, 126.6, 127.9, 128.6, 129.0, 130.4, 130.6, 130.8, 132.4, 140.6, 141.0, 143.1, 145.7, 153.2, 164.4; EIMS (m/z, Irel %): 488 [M+1]+ (37), 487 [M]+ (100). Exact mass for C33H21N5 (487.1797). Calcd: C, 81.29; H, 4.34; N, 14.37%. Found: C, 81.21; H, 4.42; N, 14.28%.
5-(4-Dimethylaminophenyl)-2-(p-tolyl)-[1,2,4]triazolo[1,5-c]quinazoline (6d). The general procedure was applied using 5-chloro-2-(p-tolyl)-[1,2,4]triazolo[1,5-c]quinazoline 5b and 4-(dimetylamino)phenylboronyc acid as the starting materials. Reaction time is 21 h. Column chromatography: SiO2, hexane and CH2Cl2 (1:1) and then EtOAc and hexane (1:9). Colourless solid, yield: 56% (112 mg); mp = 176–178 °C; 1H NMR (400 MHz, CDCl3): δ 2.45 (3H, s, CH3), 3.12 (6H, s, N(CH3)2), 6.88 (2H, d, 3J = 9.2, H-3’’, H-5’’), 7.34 (2H, d, 3J = 8.0, H-3’, H-5’), 7.61–7.65 (1H, m, H-8), 7.77–7.81 (1Н, m, H-9), 8.04–8.06 (1H, m, H-10), 8.32 (2H, d, 3J = 8.0, H-2’, H-6’), 8.58 (1H, d, 3J = 8.0, H-7), 8.74–8.77 (2H, m, H-2’’, H-6’’); 13C {1H} NMR (CDCl3, 100 MHz) δ 21.7 (CH3), 40.3 (N(CH3)2), 111.3, 117.0, 119.0, 123.8, 127.1, 127.7, 128.0, 128.3, 129.5, 131.8, 132.3, 140.5, 143.6, 146.8, 152.6, 153.1, 163.8; EIMS (m/z, Irel %): 380 [M + 1]+ (28), 379 [M]+ (100), 378 (29), 190 (10); Exact mass for C24H21N5 (379.1797). Calcd: C, 75.97, H, 5.58, N, 18.45%. Found: C, 75.92, H, 5.63, N, 18.58%.
5-(4-Diphenylaminophenyl)-2-(p-tolyl)-[1,2,4]triazolo[1,5-c]quinazoline (6e). The general procedure was applied using 5-chloro-2-(p-tolyl)-[1,2,4]triazolo[1,5-c]quinazoline 5b and 4-(diphenylamino)phenylboronyc acid as the starting materials. Reaction time is 21 h. Column chromatography: SiO2, hexane and CH2Cl2 (1:4) and then EtOAc and hexane (1:9). Pale-yellow, yield: 27% (73 mg); mp = 198–200 °C; 1H NMR (CDCl3, 400 MHz): 2.44 (3H, s, CH3), 7.12–7.16 (2H, m, Phenyl), 7.20–7.25 (6H, m, Phenyl, H-3’’, H-5’’), 7.31–7.36 (6H, m, Phenyl, H-3’, H-5’), 7.65–7.69 (1H, m, H-8), 7.79–7.84 (1Н, m, H-9), 8.06–8.08 (1H, m, H-10), 8.29 (2H, d, 3J = 8.1, H-2’, H-6’), 8.60–8.65 (3H, m, H-7, H-2’’, H-6’’); 13C {1H} NMR (CDCl3, 100 MHz) δ 21.7 (CH3), 117.2, 120.8, 123.9, 124.2, 124.4, 125.9, 127.7, 127.8, 128.6, 129.6, 129.7, 131.9, 132.0, 140.7, 143.3, 146.2, 147.0, 151.0, 153.1, 164.1; EIMS (m/z, Irel %): 504 [M+1]+ (40), 503 [M]+ (100), 502 (13), 252 (11), 77 (11); Exact mass for C34H25N5 (503.2110). Calcd: C, 81.09, H, 5.00, N, 13.91%. Found: C, 81.55, H, 5.13, N, 13.32%.
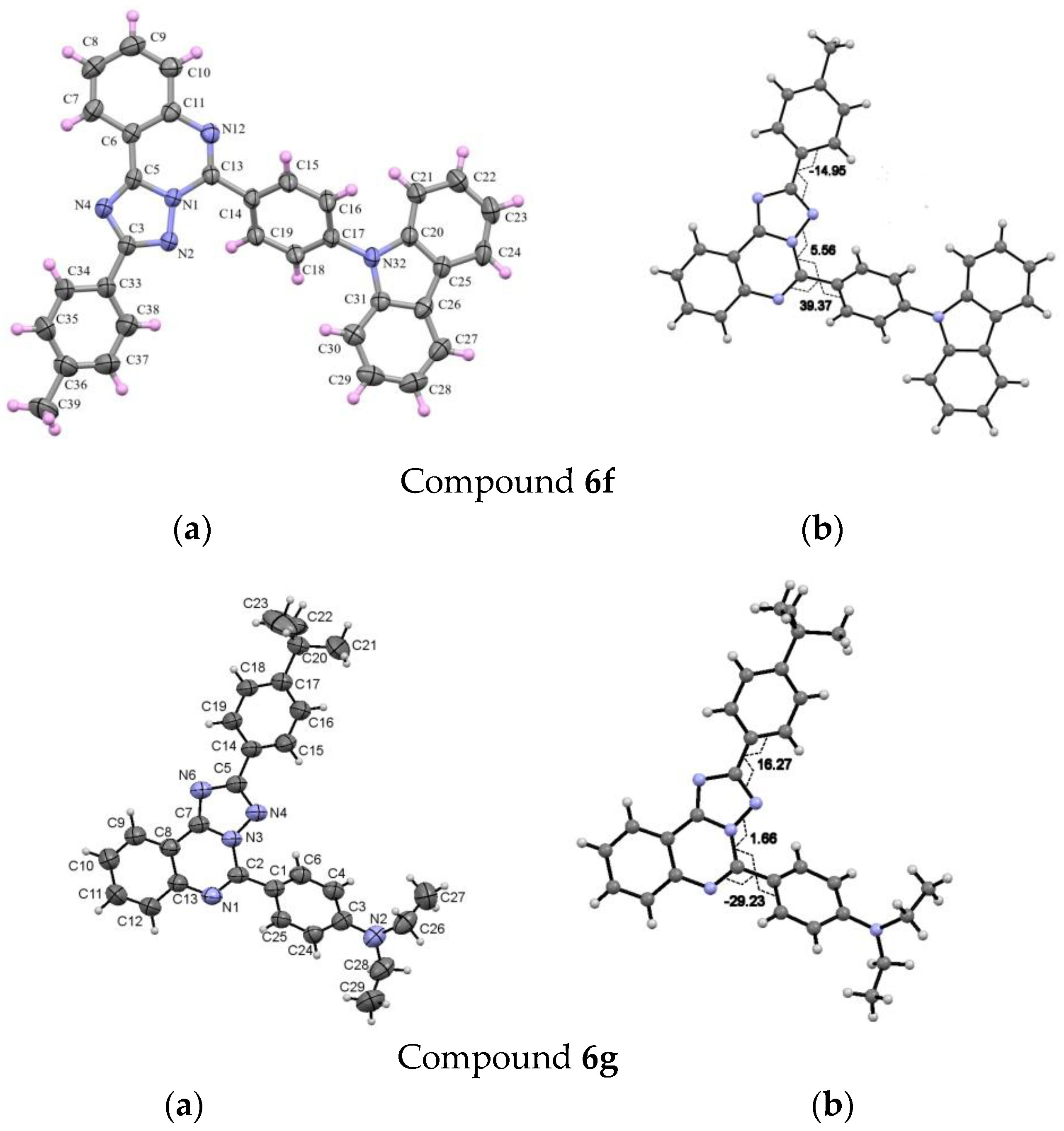
5-(4-(9H-carbazol-9-yl)phenyl)-2-(p-tolyl)-[1,2,4]triazolo[1,5-c]quinazoline (6f). The general procedure was applied using 5-chloro-2-(p-tolyl)-[1,2,4]triazolo[1,5-c]quinazoline 5b and 9H-Carbazole-9-(4-phenyl) boronic acid pinacol ester as the starting materials. Reaction time is 21 h. Column chromatography: SiO2, hexane and CH2Cl2 (7:3). Colourless solid, yield: 70% (187 mg); mp = 210–212 °C; 1H NMR (CDCl3, 400 MHz): 2.46 (3H, s, CH3), 7.33–7.38 (4H, m, carbazolyl, H-3’, H-5’), 7.46–7.50 (2H, m, carbazolyl), 7.61–7.63 (2H, m, carbazolyl), 7.75–7.79 (1H, m, H-8), 7.87–7.92 (3Н, m, H-9, H-3’’, H-5’’), 8.16–8.19 (3Н, m, H-10, carbazolyl), 8.35 (d, 3J = 8.1, 2H, H-2’, H-6’), 8.67–8.69 (1H, m, H-7), 8.97–9.01 (2H, m, H-2’’, H-6’’); 13C {1H} NMR (CDCl3, 100 MHz) δ 21.7 (CH3), 110.1, 117.6, 120.6, 120.6, 123.9, 124.1, 126.3, 126.6, 127.6, 127.8, 128.5, 128.9, 129.7, 130.7, 132.3, 132.4, 140.6, 140.9, 141.0, 143.1, 145.7, 153.2, 164.5; EIMS (m/z, Irel %): 502 [M+1]+ (38), 501 [M]+ (100), 500 (13), 251 (19); Exact mass for C34H23N5 (501.1953). Calcd: C, 81.42, H, 4.62, N, 13.96%. Found: C, 81.29, H, 4.70, N, 13.83%.
5-(4-Diethylaminophenyl)-2-(4-(tert-butyl)phenyl)-[1,2,4]triazolo[1,5-c]quinazoline (6g). The general procedure was applied using 5-chloro-2-(4-(tert-butyl)phenyl)-[1,2,4]triazolo[1,5-c]quinazoline 5c and 4-(diethylamino)phenyl boronic acid as the starting materials. Reaction time is 14 h. Column chromatography: SiO2, eluent: gradually from hexane/СH2Cl2 (8:2) to СH2Cl2. Yellow solid, yield: 20% (46 mg); mp = 135–137 °C; 1H NMR (CDCl3, 400 MHz): δ 1.27 (6H, t, 3J = 7.1, 2CH3), 1.39 (9H, s, t-Bu), 3.50 (4H, q, 3J = 7.1, 2CH2), 6.84 (2H, d, 3J = 9.1, H-3’’, H-5’’), 7.55 (2H, d, 3J = 8.4, H-3’, H-5’), 7.60–7.63 (1H, m, H-8), 7.76–7.80 (1Н, m, H-9), 8.03–8.05 (1H, m, H-10), 8.35 (2Н, d, 3J = 8.4, H-2’, H-6’), 8.59 (1Н, d, 3J = 8.4, H-7), 8.74 (2H, d, 3J = 9.1, H-2’’, H-6’’); 13C {1H} NMR (CDCl3, 100 MHz) δ 12.7 (2CH3), 31.3 (C(CH3)3), 34.9 (C(CH3)3), 44.6 (2 CH2), 110.7, 116.8, 117.9, 123.7, 125.6, 126.8, 127.4, 127.9, 128.1, 131.7, 132.4, 143.5, 146.7, 150.1, 153.0, 153.5, 163.6; EIMS (m/z, Irel %): 450 [M+1]+ (26), 449 [M]+ (72), 435 (36), 434 [M-CH3]+ (100), 390 (14), 210 (27), 196 (14), 182 (11); Exact mass for C29H31N5 (449.2579). Calcd: C, 77.47, H, 6.95, N, 15.58%; Found: C, 77.38, H, 6.85, N, 15.44%.
5-(4-Diphenylaminophenyl)-2-(4-(tert-butyl)phenyl)-[1,2,4]triazolo[1,5-c]quinazoline (6h). The general procedure was applied using 5-chloro-2-(4-(tert-butyl)phenyl)-[1,2,4]triazolo[1,5-c]quinazoline 5c and 4-(diphenylamino)phenylboronyc acid as the starting materials. Reaction time is 14 h. Column chromatography: SiO2, hexane and CH2Cl2 (7:3) and then EtOAc and hexane (1:9). Pale-yellow solid, yield: 61% (177 mg); mp = 223–225 °C; 1H NMR (CDCl3, 400 MHz): δ 1.38 (9H, s, t-Bu), 7.12–7.16 (2H, m, Phenyl), 7.20–7.24 (6H, m, Phenyl, H-3’’, H-5’’), 7.33–7.37 (4H, m, Phenyl), 7.54 (2H, d, 3J = 8.3, H-3’, H-5’), 7.66–7.69 (1H, m, H-8), 7.80–7.84 (1H, m, H-9), 8.06–8.08 (1H, m, H-10), 8.32 (2Н, d, 3J = 8.3, H-2’, H-6’), 8.61–8.65 (3Н, m, H-7, H-2’’, H-6’’); 13C {1H} NMR (CDCl3, 100 MHz) δ 31.4 (C(CH3)3), 35.0 (C(CH3)3), 117.3, 120.8, 123.9, 124.3, 124.4, 125.8, 125.9, 127.6, 127.7, 127.8, 128.6, 129.7, 131.9, 132.0, 143.3, 146.2, 147.0, 151.0, 153.1, 153.8, 164.1; EIMS (m/z, Irel %): 546 [M + 1]+ (43), 545 [M]+ (100), 530 [M-CH3]+ (15), 265 (22), 251 (11); Exact mass for C37H31N5 (545.2579). Calcd: C, 81.44, H, 5.73, N, 12.83%. Found: C, 81.50, H, 5.82, N, 12.78%.
5-(4-(9H-carbazol-9-yl)phenyl)-2-(4-(tert-butyl)phenyl)-[1,2,4]triazolo[1,5-c]quinazoline (6i). The general procedure was applied using 5-chloro-2-(4-(tert-butyl)phenyl)-[1,2,4]triazolo[1,5-c]quinazoline 5c and 9H-Carbazole-9-(4-phenyl) boronic acid pinacol ester as the starting materials. Reaction tyme is 21 h. Column chromatography: SiO2, hexane and CH2Cl2 (7:3) and then EtOAc and hexane (1:9). Colourless solid, yield: 58% (167 mg); mp = 228–230 °C; 1H NMR (CDCl3, 400 MHz): δ 1.40 (9H, s, t-Bu), 7.33–7.37 (2H, m, carbazolyl), 7.46–7.50 (2H, m, carbazolyl), 7.58–7.63 (4H, m, carbazolyl, H-3’, H-5’), 7.75–7.79 (1H, m, H-8), 7.86–7.92 (3H, m, H-9, H-3’’, H-5’’), 8.16–8.19 (3H, m, H-10, carbazolyl), 8.38 (2H, d, 3J = 8.5, H-2’, H-6’), 8.68–8.71 (1H, m, H-7), 9.00 (2Н, m, 3J = 8.7, H-2’’, H-6’’); 13C {1H} NMR (CDCl3, 100 MHz) δ 31.4 (C(CH3)3), 35.1 (C(CH3)3), 110.1, 117.6, 120.6, 120.6, 123.9, 124.1, 125.9, 126.3, 126.6, 127.6, 127.7, 128.5, 128.9, 132.3, 132.4, 140.6, 140.9, 143.1, 145.7, 153.2, 154.1, 164.5; EIMS (m/z, Irel %): 544 [M + 1]+ (44), 543 [M]+ (100), 529 (15), 528 [M-CH3]+ (36), 268 (13), 264 (27), 250 (19); Exact mass for C37H29N5 (543.2423). Calcd: C, 81.74, H, 5.38, N, 12.88%. Found: C, 81.88, H, 5.46, N, 12.65%.
5-(9,9'-Spirobi[fluoren]-2-yl)-2-phenyl-[1,2,4]triazolo[1,5-c]quinazoline (6j). The general procedure was applied using 5-chloro-2-phenyl-[1,2,4]triazolo[1,5-c]quinazoline 5a and 9,9'-spirobifluorene-2-boronic acid as the starting materials. Reaction time is 14 h. Column chromatography: SiO2, hexane and EtOAc (8:2) to pure EtOAc. Colourless solid, yield: 52% (155 mg); mp = 269–271 °C; 1H NMR (CDCl3, 400 MHz) δ 6.86–6.88 (3H, m, fluoren), 7.17–7.24 (3H, m), 7.42–7.47 (6H, m, H-3’, H-4’, H-5’), 7.64–7.68 (1H, m, H-8), 7.78–7.80 (1H, m, H-9), 7.88–7.90 (2H, m), 7.97–7.8.15 (5H, m), 8.15 (1H, d, 4J = 1.1, bisfluorenyl), 8.54–8.56 (2H, m, H-2’, H-6’); 13C {1H} NMR (CDCl3, 100 MHz) δ 117.3, 120.2, 120.3, 121.0, 123.9, 124.4, 124.5, 126.9, 127.6, 128.0, 128.1, 128.2, 128.8, 128.8, 129.0, 130.2, 130.4, 130.5, 131.0, 132.1, 141.1, 142.1, 143.1, 145.1, 146.5, 148.3, 148.8, 149.8, 152.8, 163.7; EIMS (m/z, Irel %): 561 [M+1]+ (48), 560 [M]+ (100); Exact mass for C40H24N4 (560.2001). Calcd: C, 85.69, H, 4.31, N, 9.99%. Found: C, 85.52, H, 4.45, N, 9.84%.
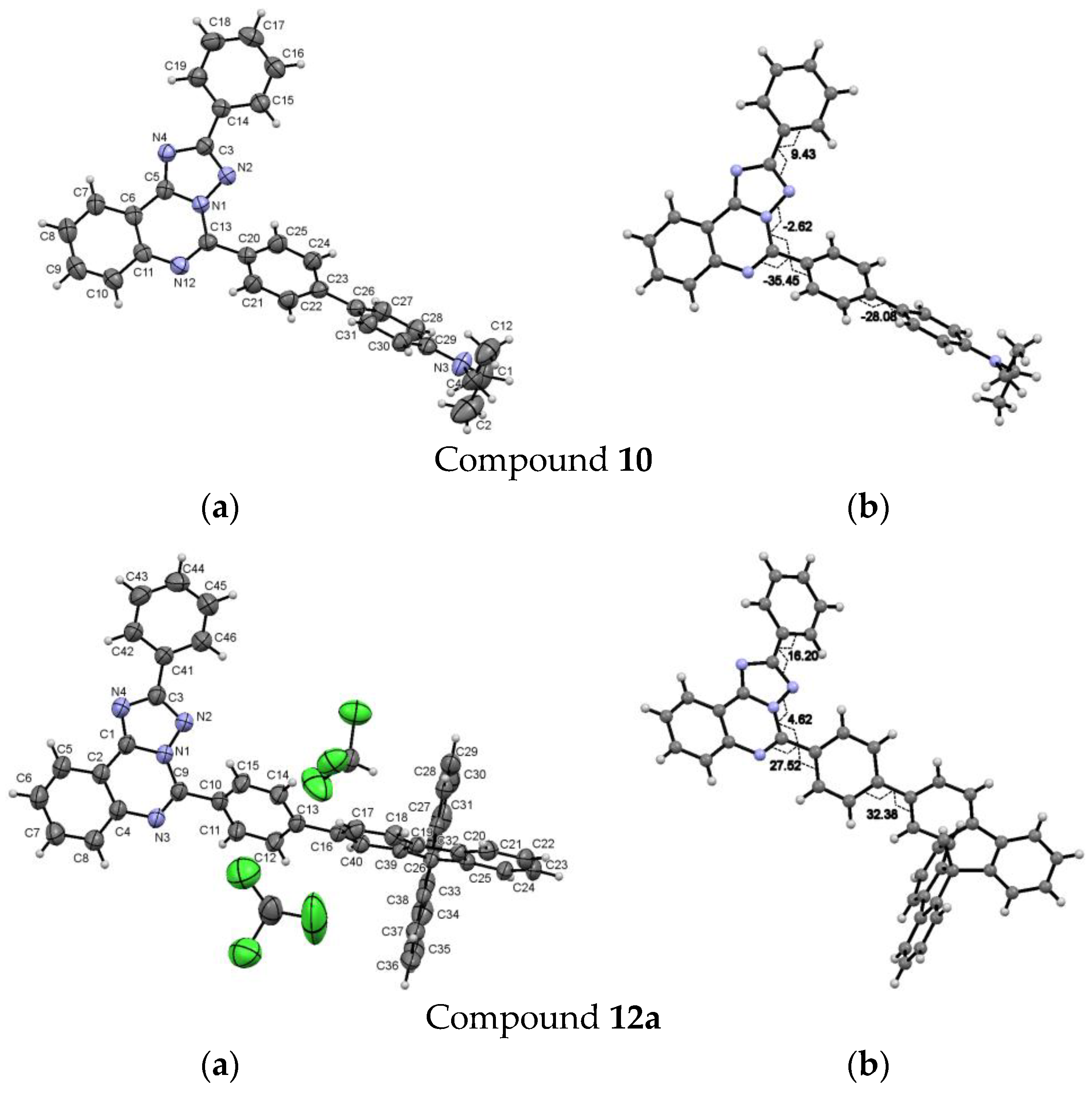
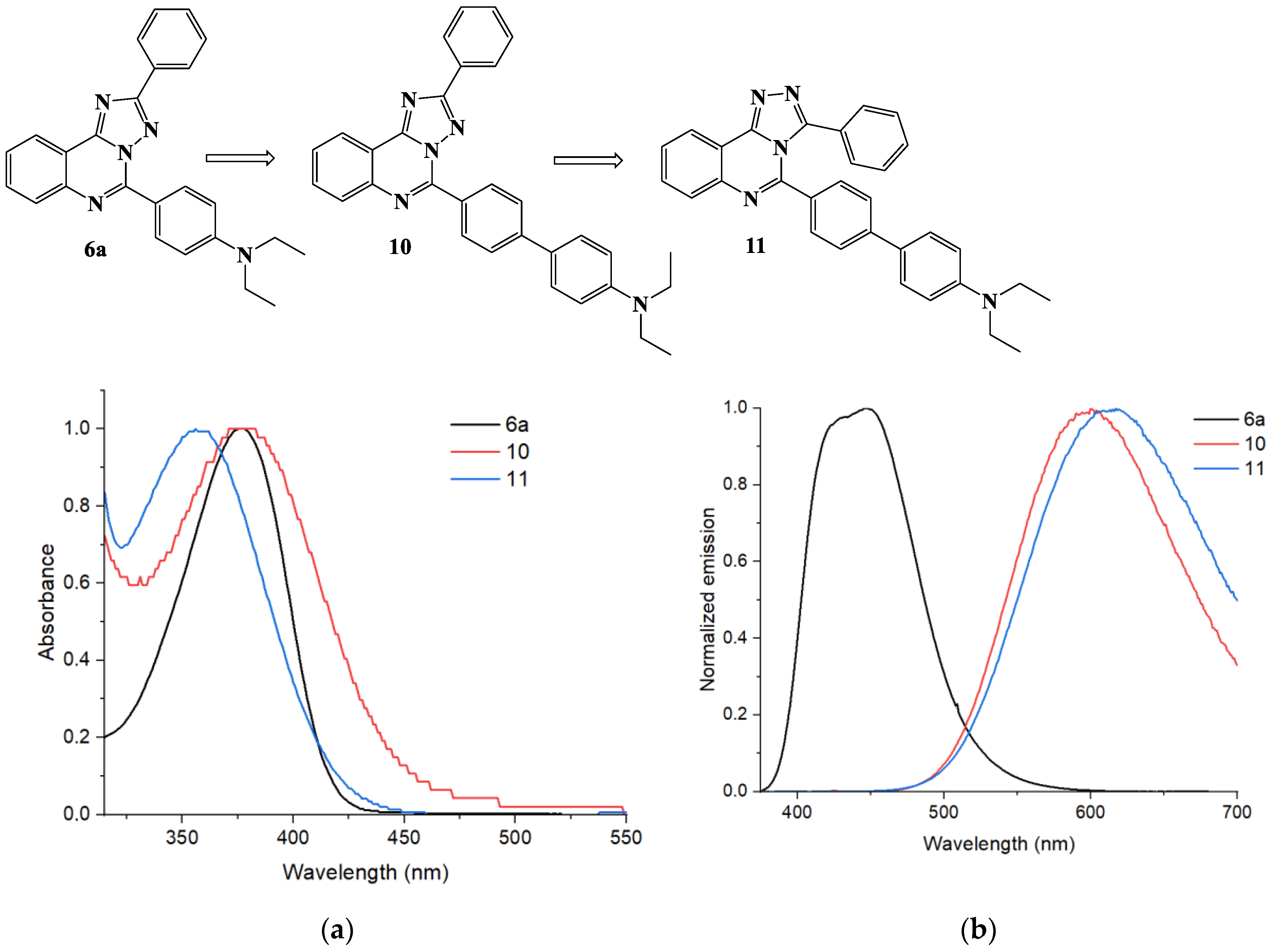
5-(4′-Diethylamino-[1,1′]-biphenyl-4-yl)-2-phenyl-[1,2,4]triazolo[1,5-c]quinazoline (10). The general procedure was applied using 5-(4-bromophenyl)-2-phenyl-[1,2,4]triazolo[1,5-c]quinazoline 8a and 4-(diethylamino)phenyl boronic acid as the starting materials. Reaction time is 16 h. Column chromatography: SiO2, hexane and EtOAc from (1:9) to (1:1). Bright yellow solid, yield: 36% (91 mg); mp = 190–192 °C; 1H NMR (CDCl3, 400 MHz): δ 1.23 (6H, t, 3J = 7.1, 2CH3), 3.44 (4H, q, 3J = 7.1, 2CH2) 6.80 (2H, d, 3J = 8.3, Et2NC6H4), 7.49–7.56 (3H, m, H-3’, H-4’, H-5’), 7.64 (2H, d, 3J = 8.3, 2H, Et2NC6H4), 7.70–7.73 (1H, m, H-8), 7.81–7.87 (3H, m, H-9, H-3’’, H-5’’), 8.12–8.14 (1H, m, H-10), 8.44 (2H, d, 3J = 7.1, H-2’, H-6’), 8.65 (1H, d, 3J = 8.3, H-7), 8.75 (2Н, d, 3J = 8.3, H-2’’, H-6’’); 13C {1H} NMR (CDCl3, 100 MHz) δ 12.8 (2CH3), 44.6 (2CH2), 112.0, 117.4, 123.9, 125.8, 126.7, 127.9, 128.1, 128.3, 128.8, 128.9, 129.0, 130.5, 130.6, 131.1, 132.1, 143.3, 144.5, 146.6, 147.9, 153.1, 164.1; EIMS (m/z, Irel %): 470 [M + 1]+ (34), 469 [M]+ (88), 455 (36), 554 [M-CH3]+ (100); Exact mass for C31H27N5 (469.2266). Calcd: C, 79.29; H, 5.80; N, 14.91%. Found: C, 79.21, H, 5.93, N, 14.85%.
5-(4′-Diethylamino-[1,1′]-biphenyl-4-yl)-3-phenyl-[1,2,4]triazolo[4,3-c]quinazoline (11). The general procedure was applied using 5-(4-bromophenyl)-3-phenyl- [1,2,4]triazolo[4,3-c]quinazoline 9 and 4-(diethylamino)phenyl boronic acid as the starting materials. Reaction time is 11 h. Column chromatography: SiO2, hexane and EtOAc (1:1) with addition of CF3COOH and then Et3N. Pale yellow solid, yield: 20% (38 mg); mp = 175–177 °C; 1H NMR (CDCl3, 400 MHz): δ 1.21 (6H, t, 3J = 7.1, 2CH3), 3.41 (4H, q, 3J = 7.1, 2CH2) 6.74 (2H, d, 3J = 8.2, Et2NC6H4), 7.08–7.11 (2H, m), 7.19–7.24 (5H, m), 7.29–7.31 (2H, m), 7.35 (2H, d, 3J = 8.4), 8.72–8.76 (1H, m, H-8), 8.80–8.83 (1H, m, H-9), 8.04–8.06 (1H, m, H-10), 8.75 (1H, d,3J = 8.2, H-7); 13C {1H} NMR (CDCl3, 100 MHz) δ 12.8 (2CH3), 44.6 (2CH2), 111.9, 116.5, 123.6, 125.3, 126.6, 127.9, 127.9, 128.1, 128.4, 129.1, 129.3, 129.5, 129.8, 131.9, 141.4, 143.6, 146.0, 147.8, 149.2, 150.2; EIMS (m/z, Irel %): 470 [M + 1]+ (30), 469 [M]+ (78), 455 (37), 554 [M-CH3]+ (100); Exact mass for C31H27N5 (469.2266). Calcd: C, 79.29; H, 5.80; N, 14.91%. Found: C, 79.23, H, 5.91, N, 14.86%.
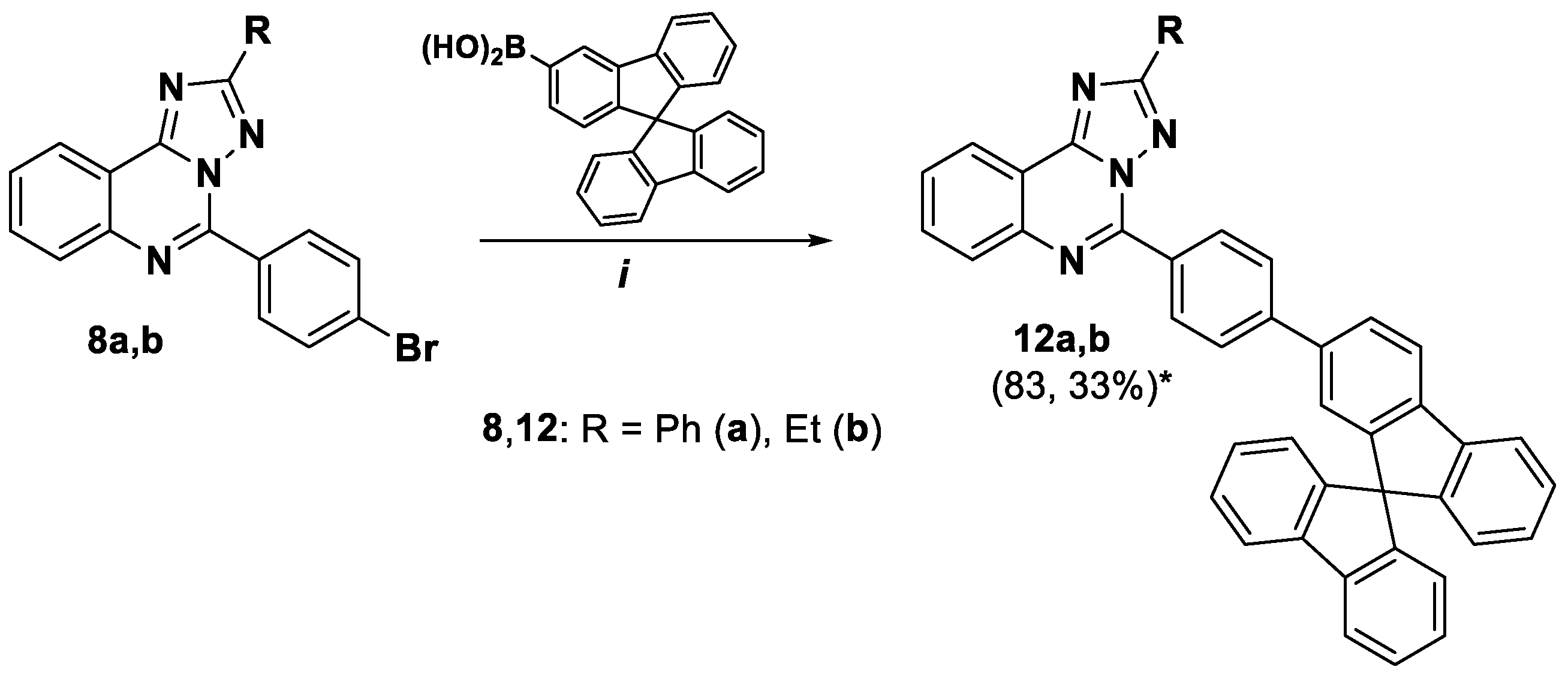
5-(4-(9,9'-Spirobi[fluoren]-2-yl)phenyl)-2-phenyl-[1,2,4]triazolo[1,5-c]quinazoline (12a). The general procedure was applied using 5-(4-bromophenyl)-2-phenyl- [1,2,4]triazolo[1,5-c]quinazoline 8a and 9,9'-spirobifluorene-2-boronic acid as the starting materials. Reaction time is 14 h. Column chromatography: SiO2, EtOAc and petroleum ether from (2:8) to (3:1). Pale yellow solid, yield: 83% (75 mg); mp = 175–177 °C; 1H NMR (DMSO-d6, 400 MHz) δ 6.62–6.64 (1H, m), 6.70–6.72 (2H, m), 7.02 (1H, d,4J = 1.0, spirobifluorene) 7.16–7.20 (3H, m), 7.42–7.44 (3H, m), 7.56–7.58 (3H, m), 7.77–7.82 (3H, m), 7.94–7.97 (2H, m), 8.08–8.12 (4H, m), 8.20–8.22 (1H, m), 8.30–8.32 (3H, m), 8.53 (1H, d, 3J = 8.2, H-7), 8.63 (2Н, d, 3J = 8.7, H-2’’, H-6’’); 13C {1H} NMR (CDCl3, 100 MHz) δ 117.5, 120.2,120.4, 120.6, 123.0, 123.9, 124.2, 124.3, 127.0, 127.2, 127.8, 128.0, 128.1, 128.2, 128.8, 128.9, 130.4, 130.5, 130.6, 131.0, 132.1, 140.0, 141.3, 142.0, 143.1, 144.0, 146.2, 148.8, 149.4, 149.9, 153.1, 164.1; EIMS (m/z, Irel %): 637 [M+1]+ (50), 636 [M]+ (100); Exact mass for C37H29N5 (636.2314). Calcd: C, 81.74, H, 5.38, N, 12.88%. Found: C, 81.86, H, 5.47, N, 12.63%.
5-(4-(9,9'-Spirobi[fluoren]-2-yl)phenyl)-2-ethyl-[1,2,4]triazolo[1,5-c]quinazoline (12b). The general procedure was applied using 5-(4-bromophenyl)-2-ethyl-[1,2,4]triazolo[1,5-c]quinazoline 8b (0.12 g, 0.34 mmol) and 9,9'-spirobifluorene-2-boronic acid as the starting materials. Reaction time is 14 h. Column chromatography: SiO2, hexane and EtOAc (2:8). Colourless solid, yield: 33% (65 mg); mp = 289–291 °C; 1H NMR (CDCl3, 400 MHz) δ 1.46 (3H, t, 3J = 8.1, CH3), 3.03 (2H, q, 3J = 8.1, CH2), 6.75—6.77 (1H, m), 6.79–6.81 (2H, m), 7.05 (1H, d,4J = 1.1, spirobifluorene) 7.12–7.16 (3H, m), 7.38–7.41 (3H, m), 7.64–7.69 (3H, m), 7.72–7.75 (1H, m), 7.79–7.83 (1H, m), 7.87–7.91 (3H, m), 7.95–7.97 (1H, m), 8.07–8.09 (1H, m), 8.52 (1H, d, 3J = 8.3, H-7), 8.56 (2Н, d, 3J = 8.8, H-2’’, H-6’’); 13C {1H} NMR (CDCl3, 100 MHz) δ 12.8 (CH3), 22.5 (CH2), 117.3, 120.2, 120.3, 120.6, 123.0, 123.7, 124.3, 127.0, 127.2, 128.0, 128.1, 128.2, 128.8, 130.7, 130.9, 132.0, 140.0, 141.4, 142.0, 143.1, 144.0, 146.1, 148.7, 149.4, 149.9, 152.6, 168.5; EIMS (m/z, Irel %): 589 [M + 1]+ (47), 588 [M]+ (100), 294 (31); Exact mass for C42H28N4 (588.2314). Calcd: C, 85.69; H, 4.79; N, 9.52 %. Found: C, 85.62, H, 4.89, N, 9.63%.