1. Introduction
The analysis of solute transport in porous media is important for understanding and managing environmental processes such as groundwater pollution [
1], nutrient leaching in agricultural soils [
2,
3,
4], and the migration of pollutants in aquifers [
5,
6]. Porous media systems act as natural filters, but their effectiveness in controlling the dispersion of substances depends on the interaction between physical transport mechanisms and chemical-biological transformations and degradation [
7]. In particular, the behavior of degraded solutes, including fertilizers, pesticides, and industrial pollutants, is of increasing interest due to their direct impact on soil and water quality, sustainable land management, and ecosystem conservation [
8,
9].
The main processes that affect the movement of solutes in porous media include hydrodynamic dispersion, convection, adsorption, and degradation [
1,
2,
3,
10,
11]. Hydrodynamic dispersion, which combines molecular diffusion and mechanical mixing, governs the distribution of solute plumes under flow conditions. In contrast to idealized constant dispersion models, in natural subsurface environments the dispersion coefficient is variable and depends on the flow velocity, pore structure, and medium heterogeneity [
12,
13,
14]. Taking into account the variability of dispersion is essential for adequate modeling of contaminant transport.
Furthermore, adsorption also plays a key role in regulating solute concentrations in porous media [
11,
15]. Through reversible or irreversible binding to the solid surface, adsorption can slow the transport of solutes and reduce peak concentrations. For degradable solutes, the interplay between adsorption and degradation is particularly important: adsorption can either slow the mobility of solutes, increasing degradation time, or protect pollutants from transformation processes, prolonging their existence in the environment.
In order to represent the combined effects of advection, dispersion, and degradation in porous media, researchers have recently developed sophisticated modeling techniques [
15,
16]. These models shed light on how pollutants in the environment proliferate, endure, or decompose in many natural systems. However, a lot of research still makes the oversimplified premise that dispersion is constant, which can either overestimate or underestimate the spreading and modification of solutes. This restriction presents difficulties for environmental management, especially when evaluating remediation initiatives, groundwater protection plans, and the long-term viability of soil-water systems.
By examining the transport of degradable solutes in porous media under circumstances of varying hydrodynamic dispersion, the current study fills this knowledge gap. This work helps to improve the depiction of contaminant migration in subterranean environments by creating and evaluating a mathematical model that takes into account both spatially variable dispersion and solute degradation. Policymakers, hydrogeologists, and environmental engineers involved in pollution remediation, sustainable agriculture, and groundwater protection will find value in the findings.
2. Problem Statement and Mathematical Model
A two-zone porous medium comprising active and passive regions is examined. The solute transport equation, considering adsorption and decay, can be expressed as [
11,
17]
where
c denotes the volumetric concentration,
represents the concentration of the adsorbed substance in the active zone,
signifies the concentration of the adsorbed substance in the passive zone,
J indicates the flow density of the solute,
refers to the porosity,
is the first-order decay coefficient (degradation), and
denotes the velocity. The terms
and
represent the adsorption processes in the active and passive zones, respectively, and pertain to the mass transfer between the liquid phase and the solid surface resulting from adsorption phenomena.
By substituting equation (
2) into equation (
1), one can see that:
The following can be derived from (
3) in the case of a one-dimensional system
where
is the coordinate of the velocity along the
axis.
If the medium is homogeneous, that is,
, the filtration rate is also constant, it is obtained that
where
is the physical velocity of the fluid.
As mentioned above, in most cases hydrodynamic dispersion coefficient should be variable. As mentioned in [
18] dispersion coefficient can be found in the form
where
is the spatial variance of the tracer or solute distribution.
For longitudinal dispersivity
Several suggested dispersivity function types (denoted linear, parabolic, asymptotic, and exponential) along with the corresponding theoretical variance functions obtained from (
9) in [
18] are
Exponential:
where
longitudinal dispersivity,
spatial variance of solute distribution,
x mean travel distance,
constants,
asymptotic or maximum dispersivity value,
B characteristic half length (equals mean travel distance corresponding to
).
Hydrodynamic dispersion coefficient taken in following form in [
12]
where
represents the diffusion coefficient of a porous medium, which is frequently disregarded in field-scale tracer transport, and
denotes the magnitude of the macroscopic pore water velocity and
represent the dispersivity, which is traditionally regarded as a scale-invariant property of the porous medium.
These data indicate that longitudinal dispersivity,
, can be approximated by an empirical hyperbolic distance-dependent model of the following form
where
is an asymptotic dispersivity [L] that is achieved at great distances,
is a scale factor [L0] that describes the linear growth of the dispersion process when it is close to the origin, and x is the distance from the injection site.
In this paper used following expressions for hydrodynamic dispersion
To determine the concentration of adsorbed substance in the active zone, following multistage kinetics [
17,
19] is used
where
is the maximum concentration of adsorbed substance that can be achieved in the active zone, and
is the decay coefficient of the adsorbed substance in the liquid zone, The kinetic coefficients are represented by the variables
,
, and
, whereas the variable
indicates the highest concentration at which the "charging" effect comes to a stop.
The following is the equation for the kinetics of adsorption in the passive zone [
17,
19]
where
is coefficient of decay of the adsorbed substance in the passive zone,
is the concentration at which the "aging" effect begins.
Equations (
5), (
23), (
24) are solved with the following initial and boundary conditions
3. Numerical Solution
To solve problems (
5), (
23)-(
26), with different expressions for hydrodynamic dispersion (
20)-(
22) we use the finite difference method [
20,
21]. In the domain
, we introduce a grid
, where
T is the maximum time in the process under study. On the
axis, we divide the interval
into pieces with a step of
h and the interval
into pieces with a step of
along the time. To approximate the problem, we introduce the following grid
.
Instead of the functions , , we consider the specific functions whose values at the nodes determine , , respectively.
In the case of for
D used (
20), in (
5), we have
After some simplifications can be obtained
or
Which can be approximated on the grid
in the following form
Which can be written in following short form
where
Equation (
33) can be solved using Tridiagonal matrix algorithm [
20,
21]
In the case of for
D used (
21), in (
5), we have
or
Equation (
36) can be solved as (
30). Only difference will in equation (
33) coefficients
will have following form
In the case of for
D used (
22), in (
5), we have
or
In this case coefficients
in equation (
33) will have following form
The differential schemes for equations (
23) and (
24) are as follows:
Initial and boundary conditions (
25) and (
26) have the form
4. Results and Discussion
To carry out numerical experiments we developed a program in Python.
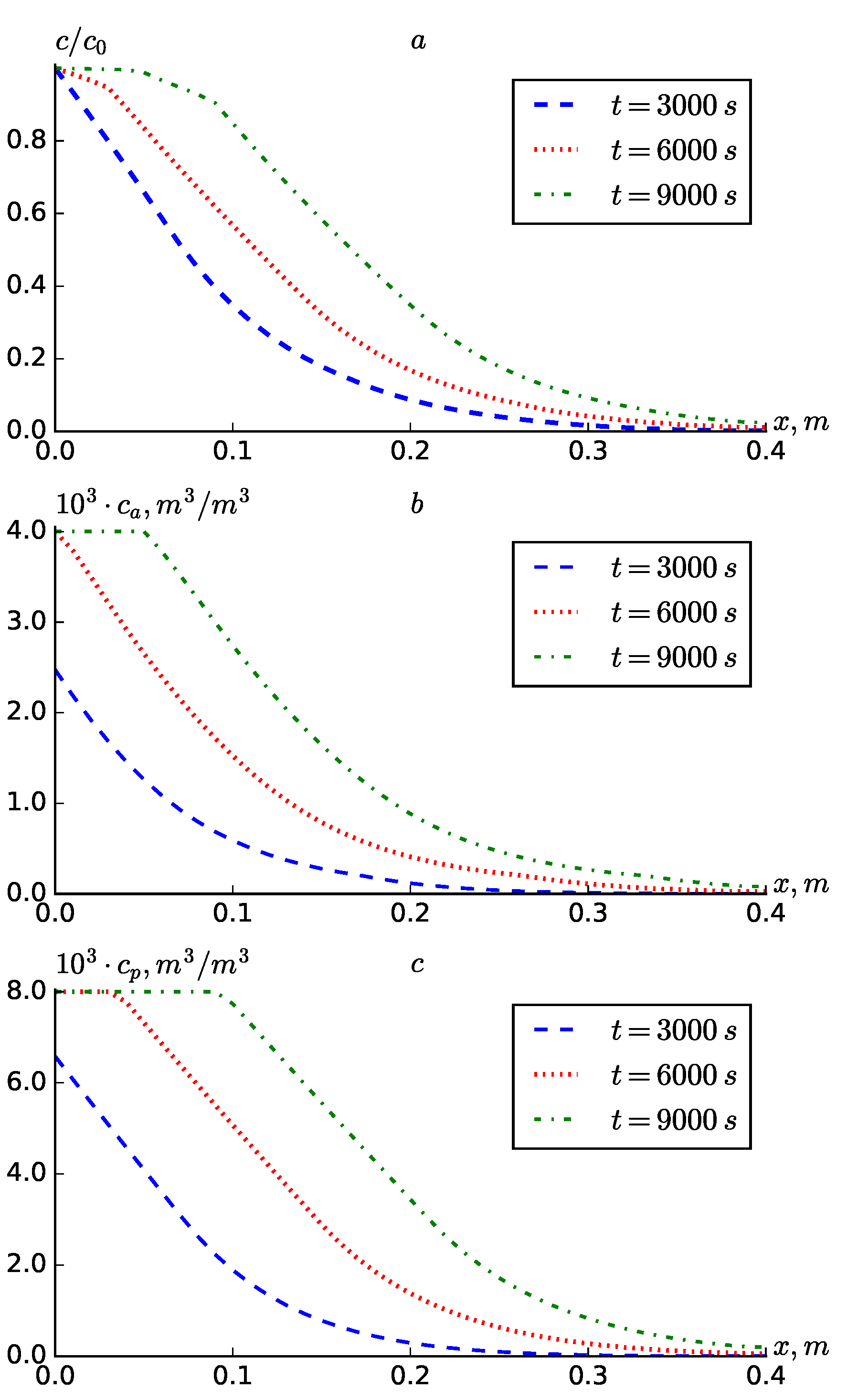
Figure 1 shows the results for the case where
, i.e., diffusion and hydrodynamic dispersion are not taken into account. The results are presented in the form of concentration profiles
and
. Over time, the values of
and
at fixed points in the reservoir increase (
Figure 1). It can be seen from the results that the solute transport process occurs more slowly when diffusion is not taken into account.
Figure 1.a shows that at
the substance concentration has only spread over a distance of
. At this time, the adsorption in both zones has approached
.
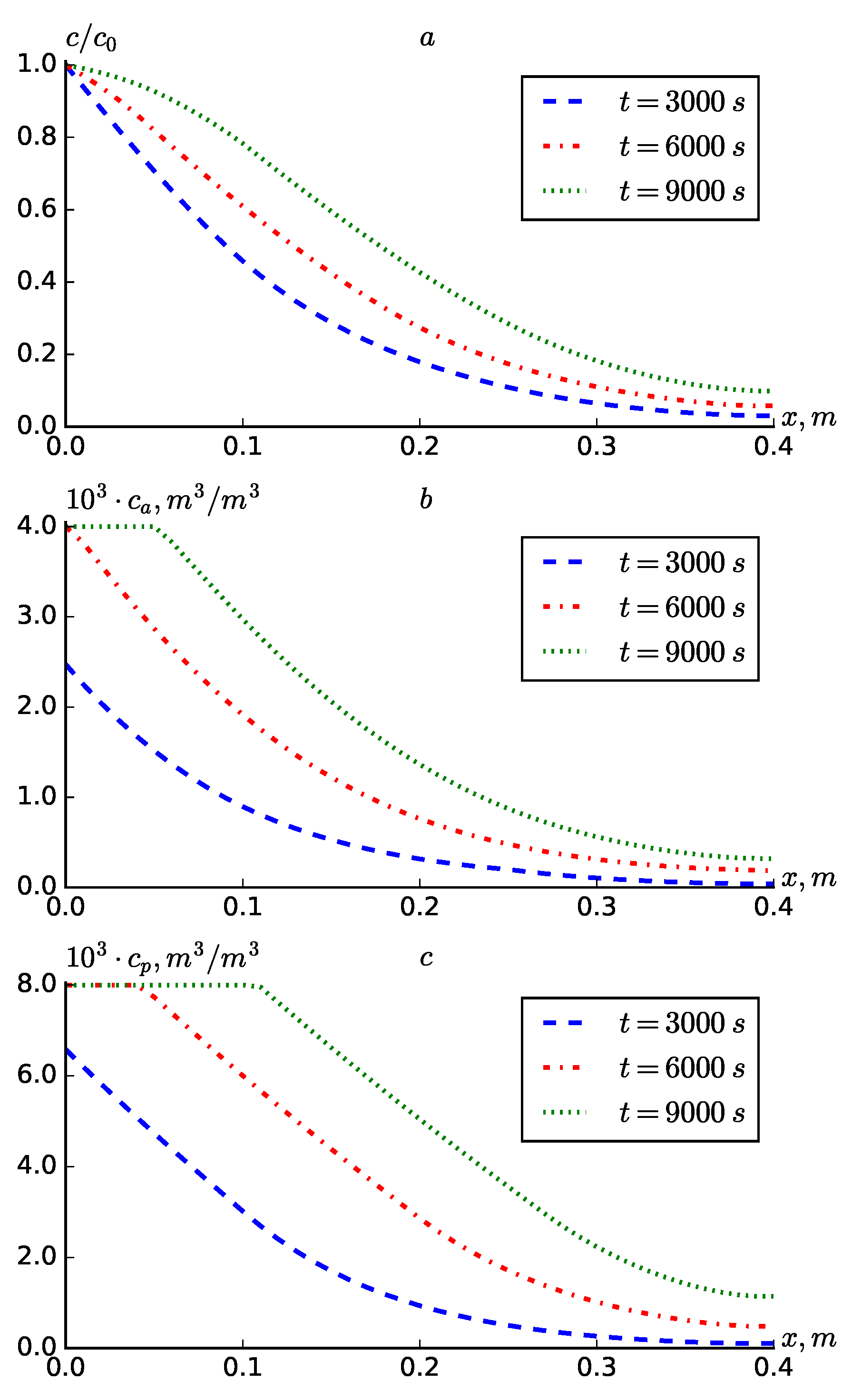
Figure 2 shows the case where
is constant diffusion. Comparing with
Figure 1, it can be concluded that taking into account diffusion significantly accelerates the solute transport and adsorption. In particular, at
in
Figure 2.a, it can be observed that the substance concentration reaches the end of the medium (
). This, in turn, is observed with an increase in the concentration of the adsorbed substance in both zones (
Figure 2b,
Figure 2c).
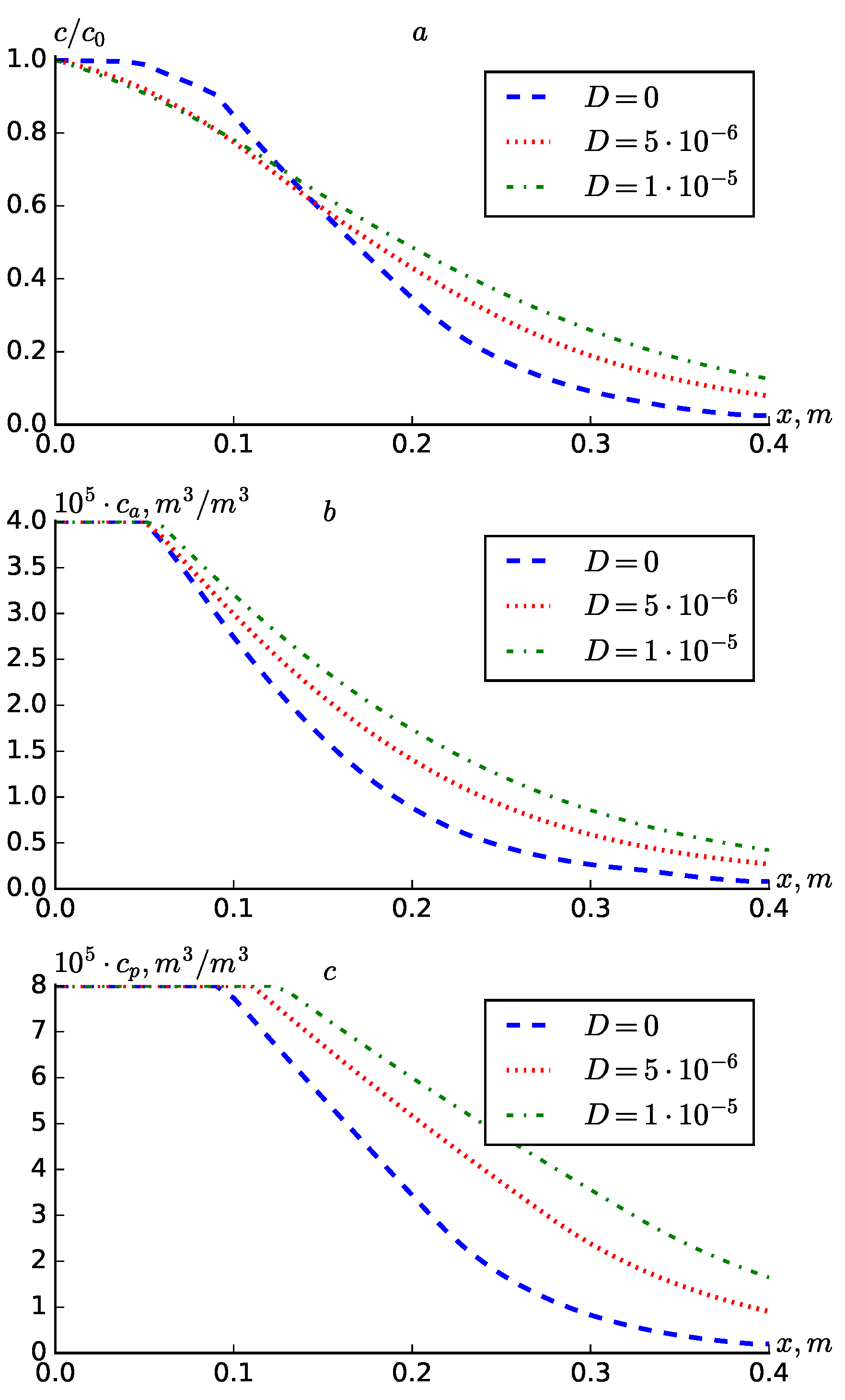
Figure 3 presents comparative graphs for different values of
. It can be seen from the graphs that an increase in the value of diffusion leads to a wider spread of the concentration profiles towards the interior of the medium. Since the scattering is less at
, the concentration
at points closer to the point
is greater than at the point
, and vice versa at points further from the point
. This was not observed for the concentrations
and
, i.e. their values increased significantly at all points of the medium with increasing diffusion.
Figure 4 presents the results of numerical experiments conducted according to formula (
20), where an exponential expression depending on the coordinate for the dispersion coefficient is obtained. The results show that using formula (
20) instead of the expression
increases the concentration distribution, albeit partially. This, in turn, increases the amount of adsorbed substance in both the active and passive zones.
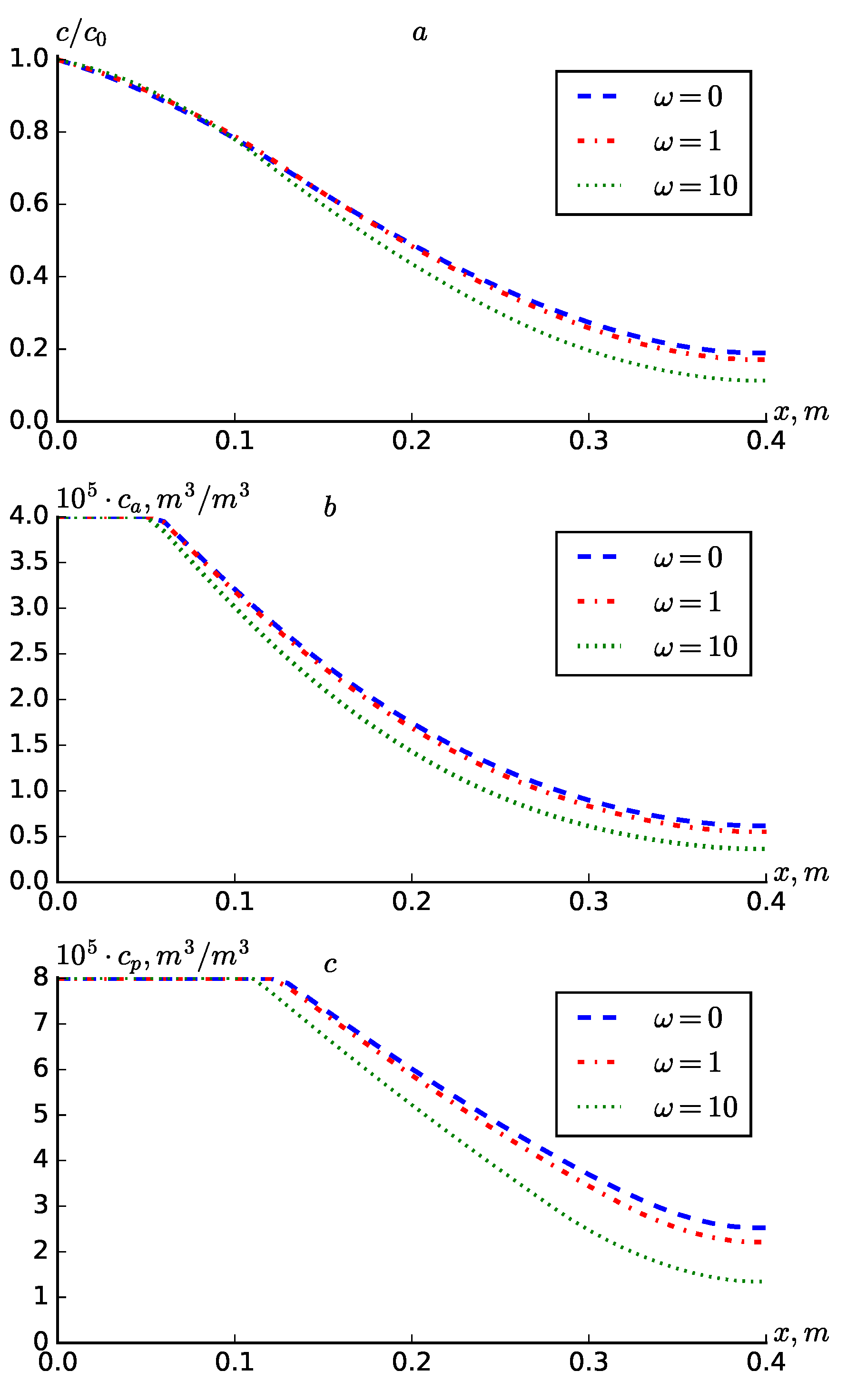
In
Figure 5 shown the solute transport and adsorption changes for different values of
(
). It can be seen from the graphs that the cases with
and
do not differ much from each other. When
, it can be observed that all three concentrations increase by a very small amount compared to when
. However, when
, it can be observed that the concentrations decrease sharply at points further from the point
.
Figure 6 illustrates the outcomes of numerical experiments performed in accordance with formula (
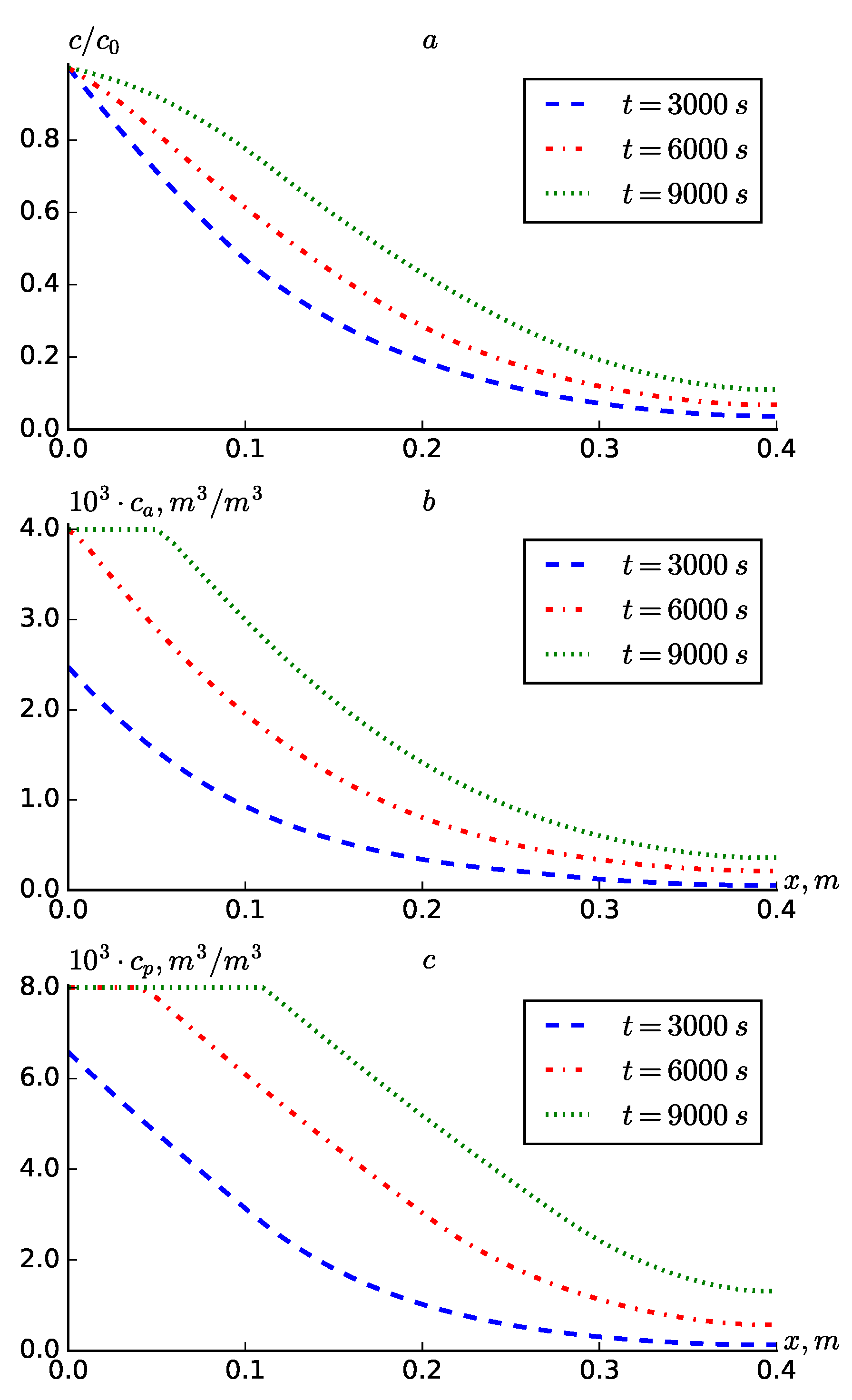
21), resulting in a linear increasing expression dependent on the coordinate for the dispersion coefficient. The results indicate that employing formula (
21) in place of the expression
leads to a minimal increase in the concentration distribution, which may not be immediately apparent upon initial observation.
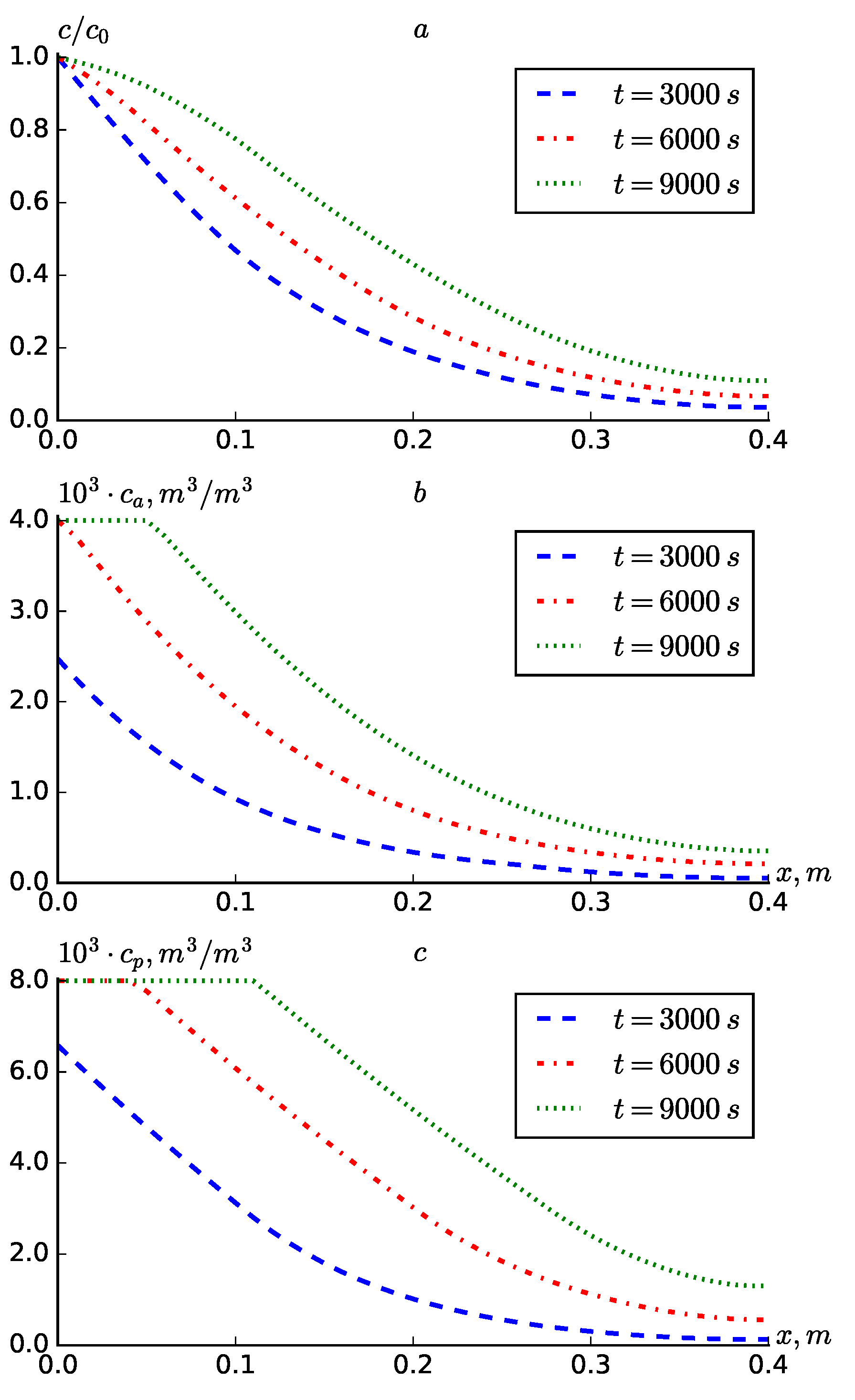
Figure 7 presents the results of numerical experiments conducted in alignment with formula (
22), yielding a parabolic decreasing function related to the coordinate for the dispersion coefficient. The findings demonstrate that utilizing formula (
22) instead of the expression
yields results that are highly comparable.
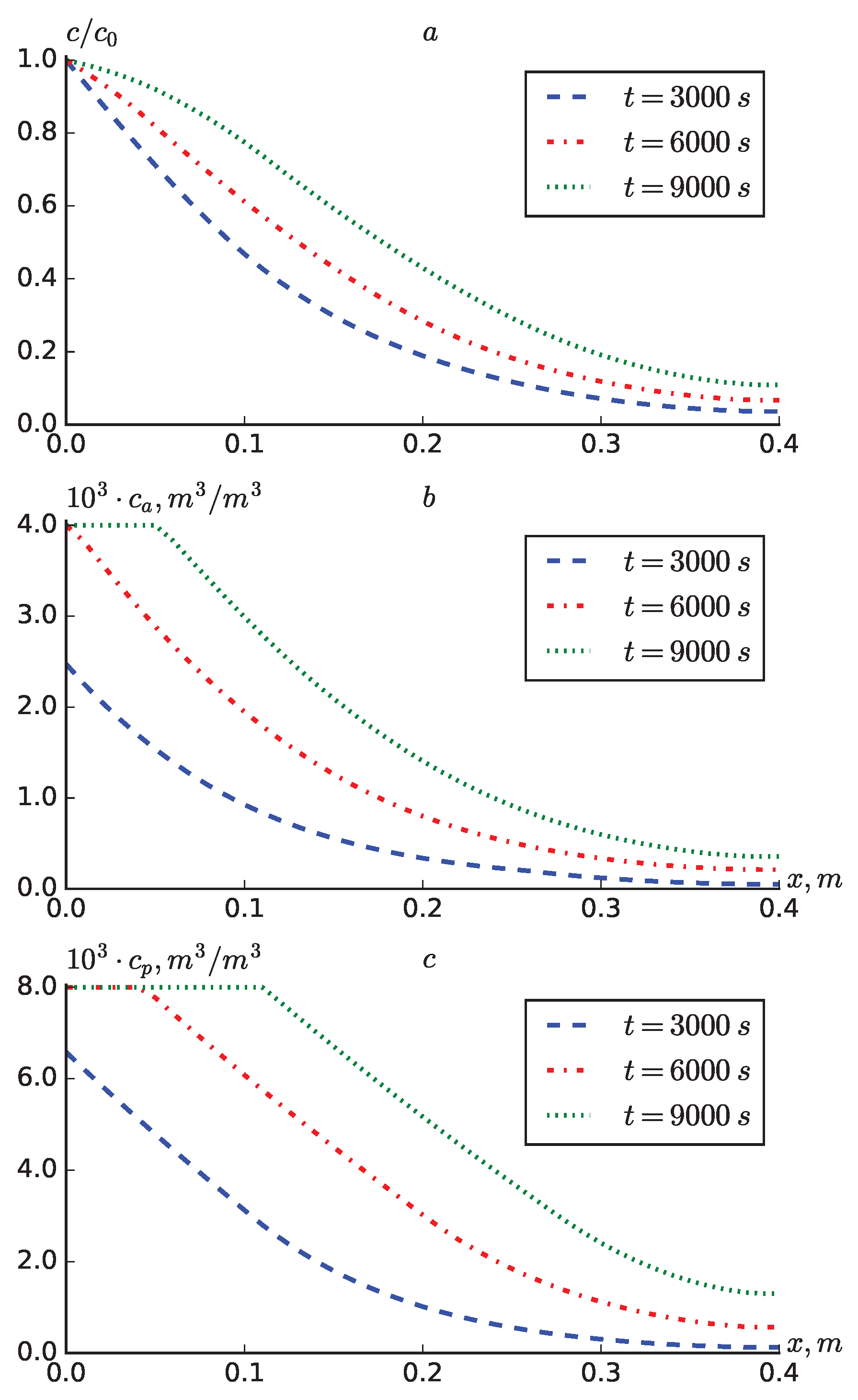
Figure 8 compares the results obtained for expressions (
20), (
21), (
21) at
. It can be seen from the results that the results obtained for expressions (
21) and (
22) do not differ much. In the results obtained for expression (
20), the values of
and
are significantly larger. The values of
are smaller at points closer to the point
for expression (
20) than for (
21) and (
22), and vice versa, the distance at
is larger.
Comparative
Table 1,
Table 2 and
Table 3 are presented for a more in-depth analysis of the results obtained using the different expressions proposed for dispersion. It can be seen from
Table 1 that when the expression (
20) is used, the concentrations at all fixed points are larger in the cases of
and
than when
is constant. When
, the difference is not so great. When the expression (
21) is used, there is a difference, although it is small compared to
, but for the expression (
22) the difference is almost imperceptible in the results obtained with 4 digits of accuracy. This can be explained by the fact that due to the small values of
x, its square becomes even smaller.
Tables
Table 2,
Table 3 present the results for larger values of time, and the conclusions made for
Table 1 are once again confirmed here.
5. Conclusions
This study formulated and examined a degradable-solute transport model inside a two-zone porous medium that explicitly integrates advection, spatially-dependent hydrodynamic dispersion, multistage adsorption in active and passive zones, and first-order decay. A finite-difference solution method utilizing a tridiagonal solver was employed to address coordinate-dependent diffusion terms and piecewise adsorption kinetics. Numerical tests were conducted for constant dispersion and three representative spatially variable forms—exponential, linear, and parabolic—using identical hydraulic and kinetic parameters.
The simulations indicate that disregarding diffusion and dispersion significantly hinders plume progression and leads to an underestimation of both aqueous concentrations and sorbed inventory. The introduction of even minimal dispersion enhances transport throughout the domain and amplifies adsorption in both areas, resulting in reduced aqueous peaks at the input and elevated concentrations further downstream. Among the variable-dispersion forms, the exponential law dispersion exhibited the most significant deviations from the constant dispersion scenario. Conversely, the linear and parabolic dispersion forms produced minimal variations at the examined length and velocity scales, indicating their relatively modest variation within specified boundaries. The sensitivity to the exponential-form parameter indicated that a quick decay of the elevated inlet dispersion () inhibits far-field accumulation while just slightly influencing the near-inlet profile. These results collectively indicate that the shape and scale of the dispersion function govern both the timing of breakthrough and the distribution between dissolved and sorbed masses.
The findings suggest that utilizing scale-dependent or coordinate-dependent dispersion models can significantly impact forecasts of pollutant arrival timings and retention in remedial or agricultural contexts. Augmented dispersion near sources typically results in a more extensive distribution of solute, hence enhancing sorptive absorption and potentially reducing local peak water concentrations; nevertheless, it may also expedite downstream exposure if the greater dispersion endures across significant distances. Consequently, depending on a fixed dispersion coefficient may inaccurately represent danger and cleanup timelines in systems where dispersion fluctuates with distance or hydraulic conditions.
This study is confined to one-dimensional, steady-flow scenarios with uniform porosity and simplified boundary conditions, excluding calibration to laboratory or field data. Future endeavors should broaden the framework to encompass two and three dimensions with heterogeneous characteristics, integrate it with variable-density or transient flows, investigate alternative (nonlinear) adsorption/desorption and biodegradation kinetics, and conduct systematic sensitivity and uncertainty evaluations. Integrating data-driven calibration or inverse modeling with tracer experiments would enhance the quantification of the suitable form and parameters of for site-specific forecasts. Notwithstanding these constraints, the study unequivocally demonstrates that the careful selection of dispersion models is crucial for accurate predictions of degradable-solute behavior and movement in porous surfaces.
Conflicts of Interest
Declare conflicts of interest or state “The authors declare no conflicts of interest.”
References
- Sethi, R.; Di Molfetta, A. Mechanisms of Contaminant Transport in Aquifers. Springer Tracts in Civil Engineering 2019, p. 193 – 217. [CrossRef]
- Rashmi, I.; Shirale, A.; Kartikha, K.; Shinogi, K.; Meena, B.; Kala, S. Leaching of plant nutrients from agricultural lands; 2017; p. 465–489. [CrossRef]
- Wang, K. Exploration of methods for Cr VI pollution remediation in karst groundwater. Hydrogeology and Engineering Geology 2025, 52, 248–254. [Google Scholar] [CrossRef]
- Bergström, L.F.; Djodjic, F. Soil as an important interface between agricultural activities and groundwater: Leaching of nutrients and pesticides in the vadose zone. Geological Society Special Publication 2006, 266, 45–52. [Google Scholar] [CrossRef]
- Luan, M.T.; Zhang, J.L.; Yang, Q. One-dimensional numerical analyses of migration processes of pollutants through a clay liner considering sorption of aquifer. Yantu Gongcheng Xuebao/Chinese Journal of Geotechnical Engineering 2005, 27, 185–189. [Google Scholar]
- Mayank, M.; Sharma, P.K. Numerical and experimental study on solute transport through physical aquifer model. Water Supply 2022, 22, 137–155. [Google Scholar] [CrossRef]
- Zhang, J.L.; Luan, M.T.; Yang, Q. One-dimensional numerical analyses of pollutant migration process in solid waste considering bio-degradation effect of contaminants. Dalian Ligong Daxue Xuebao/Journal of Dalian University of Technology 2004, 44, 870–876. [Google Scholar]
- Li, B.; Zhang, H.; Long, J.; Fan, J.; Wu, P.; Chen, M.; Liu, P.; Li, T. Migration mechanism of pollutants in karst groundwater system of tailings impoundment and management control effect analysis: Gold mine tailing impoundment case. Journal of Cleaner Production 2022, 350. [Google Scholar] [CrossRef]
- Ji, H.; Chiogna, G.; Richieri, B.; Fan, X.; Huang, K.; Chen, C.; Yang, H.; Luo, M.; Zhao, H. High-frequency dual-tracer approach to identify contaminant transport pathways and quantify migration behaviors in karst underground river system. Journal of Hydrology 2025, 662. [Google Scholar] [CrossRef]
- Ogram, A.V.; Jessup, R.E.; Ou, L.T.; Rao, P.S. Effects of sorption on biological degradation rates of (2,4-dichlorophenoxy) acetic acid in soils. Appl. Environ. Microbiol. 1985, 49, 582–587. [Google Scholar] [CrossRef]
- van Genuchten, M.T.; Wagenet, R.J. Two-site/two-region models for pesticide transport and degradation: Theoretical development and analytical solutions. Soil Sci. Soc. Am. J. 1989, 53, 1303–1310. [Google Scholar] [CrossRef]
- Mishra, S.; Parker, J.C. Analysis of solute transport with a hyperbolic scale-dependent dispersion model. Hydrol. Process. 1990, 4, 45–57. [Google Scholar] [CrossRef]
- Gupta, K.R.; Sharma, P.K. Unraveling two-dimensional modeling of multispecies reactive transport in porous media with variable dispersivity. Groundw. Sustain. Dev. 2025, 28, 101404. [Google Scholar] [CrossRef]
- Madie, C.Y.; Togue, F.K.; Woafo, P. Analysis of the importance of the dispersion coefficient depending on the distance for the transport of solute in porous media. Sadhana 2022, 47. [Google Scholar] [CrossRef]
- Khuzhayorov, B.K.; Viswanathan, K.K.; Kholliev, F.B.; Usmonov, A.I. Anomalous solute transport using adsorption effects and the degradation of solute. Computation (Basel) 2023, 11, 229. [Google Scholar] [CrossRef]
- Wu, M.C.; Hsieh, P.C. Analytical modeling of solute transport in a two-zoned porous medium flow. Water (Basel) 2022, 14, 323. [Google Scholar] [CrossRef]
- Khuzhayorov, B.; Fayziev, B.; Sagdullaev, O.; Makhmudov, J.; Saydullaev, U. A model of the degrading solute transport in porous media based on the multi-stage kinetic equation. Eng. Technol. Appl. Sci. Res. 2025, 15, 20919–20926. [Google Scholar] [CrossRef]
- Pickens, J.F.; Grisak, G.E. Modeling of scale-dependent dispersion in hydrogeologic systems. Water Resour. Res. 1981, 17, 1701–1711. [Google Scholar] [CrossRef]
- Gitis, V.; Rubinstein, I.; Livshits, M.; Ziskind, G. Deep-bed filtration model with multistage deposition kinetics. Chem. Eng. J. 2010, 163, 78–85. [Google Scholar] [CrossRef]
- Fayziev, B.; Ibragimov, G.; Khuzhayorov, B.; Alias, I.A. Numerical study of suspension filtration model in porous medium with modified deposition kinetics. Symmetry (Basel) 2020, 12, 696. [Google Scholar] [CrossRef]
- Samarskii, A.A. The theory of difference schemes; CRC Press: Boca Raton, FL, 2001. [Google Scholar]
Figure 1.
Profiles of , at .
Figure 1.
Profiles of , at .
Figure 2.
Profiles of , at .
Figure 2.
Profiles of , at .
Figure 3.
Profiles of , at different values of D.
Figure 3.
Profiles of , at different values of D.
Figure 4.
Profiles of , according to the diffusion formula: .
Figure 4.
Profiles of , according to the diffusion formula: .
Figure 5.
Profiles of , at different values of and .
Figure 5.
Profiles of , at different values of and .
Figure 6.
Profiles of , according to the diffusion formula: .
Figure 6.
Profiles of , according to the diffusion formula: .
Figure 7.
Profiles of , according to the diffusion formula: .
Figure 7.
Profiles of , according to the diffusion formula: .
Figure 8.
Profiles of , for various expressions of the diffusion function .
Figure 8.
Profiles of , for various expressions of the diffusion function .
Table 1.
Comparison of results of spreading concentration in fixed points for different expression of dispersion at .
Table 1.
Comparison of results of spreading concentration in fixed points for different expression of dispersion at .
| Variation of dispersion |
Fixed points |
| |
|
|
|
|
|
|
1.0000 |
0.4684 |
0.1890 |
0.0715 |
0.0363 |
|
1.0000 |
0.5214 |
0.2400 |
0.1092 |
0.0666 |
|
1.0000 |
0.5329 |
0.2533 |
0.1210 |
0.0779 |
|
1.0000 |
0.4721 |
0.1921 |
0.0735 |
0.0377 |
|
1.0000 |
0.4695 |
0.1900 |
0.0721 |
0.0368 |
|
1.0000 |
0.4683 |
0.1890 |
0.0715 |
0.0363 |
Table 2.
Comparison of results of spreading concentration in fixed points for different expression of dispersion at .
Table 2.
Comparison of results of spreading concentration in fixed points for different expression of dispersion at .
| Variation of dispersion |
Fixed points |
| |
|
|
|
|
|
|
1.0000 |
0.6122 |
0.2841 |
0.1186 |
0.0670 |
|
1.0000 |
0.6478 |
0.3453 |
0.1670 |
0.1098 |
|
1.0000 |
0.6516 |
0.3575 |
0.1804 |
0.1240 |
|
1.0000 |
0.6158 |
0.2886 |
0.1215 |
0.0693 |
|
1.0000 |
0.6131 |
0.2853 |
0.1195 |
0.0678 |
|
1.0000 |
0.6122 |
0.2840 |
0.1186 |
0.0670 |
Table 3.
Comparison of results of spreading concentration in fixed points for different expression of dispersion at .
Table 3.
Comparison of results of spreading concentration in fixed points for different expression of dispersion at .
| Variation of dispersion |
Fixed points |
| |
|
|
|
|
|
|
1.0000 |
0.7751 |
0.4303 |
0.1914 |
0.1093 |
|
1.0000 |
0.7881 |
0.4841 |
0.2583 |
0.1710 |
|
1.0000 |
0.7822 |
0.4898 |
0.2738 |
0.1897 |
|
1.0000 |
0.7795 |
0.4357 |
0.1960 |
0.1127 |
|
1.0000 |
0.7759 |
0.4315 |
0.1926 |
0.1104 |
|
1.0000 |
0.7751 |
0.4303 |
0.1913 |
0.1093 |
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).