1. Introduction
Intracerebral hemorrhage (ICH) accounts for 5-40% of stroke admissions worldwide and is a leading cause of morbidity and mortality. Patients with ICH lead to debilitating motor deficits resulting from the direct or indirect disruption of the motor cortex [
1]. Acute ICH management is rapidly advancing, with the implementation of the acute ICH care bundle and surgical hematoma evacuation. However, recovery post-ICH is variable [
2]. The severity of neurological deficits is currently assessed clinically (using the National Institute of Health Stroke Scale) and with imaging (utilizing computed tomography or Magnetic resonance imaging (MRI)). The functional outcomes are assessed using the modified Rankin scale or the Glasgow Outcome Scale [
3]. These measures have limitations as patients with similar-sized ICH or similar severity of neurological deficits have a variable prognosis. These tools primarily rely on gross clinical observations and fail to capture the underlying neural mechanisms driving recovery [
4]. There is a felt need for adjunct quantitative assessment tools that can better predict functional recovery and inform tailored rehabilitation strategies.
Neurophysiological tools, including electroencephalography (EEG), near-infrared spectroscopy (NIRS) and functional MRI (fMRI), may help in understanding complex dynamics of brain network reorganization during recovery. These modalities have higher time and spatial resolution. Acute EEG can predict post-stroke functional disability with a moderate correlation with short-term NIHSS and mRS [
5].
However, gel-based EEG application may not be feasible in acute situations. fMRI likewise, though a highly reproducible modality, is also resource-intensive and requires the availability of research MRI and stability of patients for testing. Functional NIRS (fNIRS) is developing as a preferred modality in acute stroke scenario due to ease of use [
6] fNIRS is a non-invasive, portable, and cost-effective technique that enables the monitoring of brain activity through the measurement of hemodynamic responses. Its advantages make it particularly suitable for longitudinal studies and bedside applications, bridging the gap between research and clinical practice [
7]. Recent studies have successfully employed fNIRS to assess motor cortex activity and functional connectivity in stroke patients, providing valuable insights into hemodynamic alterations associated with recovery [
8]
Resting-state functional connectivity (RSFC) and paradigm-based assessment (motor or non-motor) are useful tools in stroke research, providing insights into the temporal coherence of neural activity across distinct brain regions during rest [
9]. RSFC demonstrates blood-oxygen-level-dependent temporal coherence of signals between spatially remote brain regions [
10]. Studies have revealed disruptions in brain network dynamics following strokes, particularly within the motor cortex [
11] The motor cortex, essential for executing voluntary movements, may be susceptible to functional disruptions after ICH. These disruptions often manifest as altered connectivity within motor regions or between hemispheres, correlating closely with clinical motor impairments. Despite its utility, RSFC studies using fMRI have several limitations, including high cost, limited accessibility, and constraints on real-time applications in clinical settings [
12].
Response to the Motor task paradigm-based assessment can help understand cortical reorganization in the phases of acute, subacute, and chronic stroke. This may allow for tracking the trajectory of recovery post-stroke.
We aimed to assess RSFC in both affected and unaffected hemispheres by group-level seed-based (Primary Motor cortex, priMC) correlation analysis in patients with ICH. We also aimed to measure FT-associated relative Oxyhemoglobin (ΔHbO) changes in affected and unaffected hemispheres in patients with ICH.
We hypothesize that ICH leads to both intra- and inter-hemispheric disruptions in motor network connectivity, resulting in altered hemodynamic responses that correlate with clinical motor impairments. Specifically, we anticipate that task-induced hemodynamic changes will offer additional markers of motor network disruption, providing a nuanced understanding of how ICH affects motor control.
2. Materials and Methods
2.1. Study Design and Patient Population
The study enrolled adult patients diagnosed with acute ICH at the tertiary care centre. Participants included individuals with both left- and right-hemispheric strokes. Eligibility criteria required patients to be 18 years or older with a confirmed ICH diagnosis, while exclusion criteria included conditions that could interfere with fNIRS measurements, such as asymptomatic severe cervical or internal carotid stenosis, ongoing hemodynamic instability, antiseizure medication use, preexisting hepatic, respiratory, or renal encephalopathy and symptomatic hypothyroidism. A control group, matched by age, sex, and comorbidities, was recruited to provide baseline comparisons. Controls had no history of stroke or presented only with a transient ischemic attack (TIA) without lasting neurological impairment. Ethics approval was obtained from the Institutional Review Board and informed consent was secured from all participants or through their legally authorized representatives.
2.2. Study Procedures
Stroke severity was evaluated using the NIHSS, ranging from 0 (no symptoms) to 42 (death), at baseline (< 24 h symptom onset), 2 h, 24 h, 7 days and 90 days. Functional disability was measured at 90 days using the modified Rankin Scale (mRS), where 0 indicates no disability and 6 represents death.
2.3. fNIRS Data Acquisition
Data were collected using the NIRSport2 (NIRx Inc., Germany), a mobile fNIRS system, at a single time point. The device operated at 760 nm and 850 nm wavelengths, allowing for the quantification of oxygenated (ΔHbO) and deoxygenated hemoglobin (HbR) as markers for cerebral oxygenation and blood flow in the tissue underlying the sensors.
For each participant, fNIRS data were collected from 16 channels over the premotor, primary motor and somatosensory cortices. The eight optodes (light-emitting sources) and eight detectors (Avalanche Photodiodes) were placed on the participant’s scalp in accordance with the international 10-10 EEG electrode placement system (
Supplementary Table 1). The distance between each optode and detector was standardized at 35 mm, and the sampling rate was set at 10.2 Hz. The collected data were recorded using the NIRSite version 2.0 software (NIRx Inc., Germany). Real-time signal assessment was performed using the Aurora fNIRS software, which allowed for continuous monitoring of each optode’s signal quality. Signal-to-noise ratio (SNR) was calculated in real-time through the coefficient of variation, and only channels exhibiting an SNR below 15% were retained for further analysis. A 3-minute resting-state fNIRS recording was completed, followed by a motor paradigm that included finger-tapping exercise The motor paradigm was implemented using PsychoPy Builder (v2022.2.5, University of Nottingham, UK) and was conducted in the following sequence:
The finger tapping task required participants to sequentially tap each finger to their thumb at a rate of one tap per second. The task alternated between hands, with each hand completing 10 repetitions, resulting in a total of 5 alternations. The entire task lasted 3 minutes, with a 3-second pause between stimuli to allow brief rest intervals.
This motor protocol was administered across all participant groups, including ICH and controls. Data analysis was conducted using Satori fNIRS software version 2.0 (NIRx Inc., Germany).
2.4. fNIRS Data Processing
The raw light intensities from the fNIRS recordings were converted into relative changes in oxyhemoglobin (ΔHbO) and deoxyhemoglobin (HbR) concentrations, leveraging the modified Beer-Lambert law. This transformation from optical density (OD) to concentration chromophores (CC) enabled the quantification of hemodynamic responses within specified brain regions. Two primary areas associated with motor function—the primary motor cortex and premotor cortex—were identified as key regions of interest. Channels aligned with these regions were selected for further analysis, guided by the optode-detector layout's signal quality and spatial coverage.
All raw data were saved in the standardized .snirf format and systematically organized into structured directories specific to each patient and control subject as per the guidelines for fNIRS analyses [
13] A consistent file naming convention was applied to ensure uniformity across datasets, incorporating metadata on acquisition time, patient ID, and task type (e.g., resting state or finger tapping). Data processing involved filtering, noise reduction, and artifact correction (
Supplementary Table 2). As shown in
Figure 1, distinct preprocessing pipelines were established for the resting-state and motor-task data to accommodate the differing analytical requirements of each task.
2.5. Workflow Manager Data Pipeline
Scalp Coupling Index (SCI): An SCI threshold of 75% ensured reliable fNIRS recordings, with channels below this threshold excluded to reduce noise from poor signal contact with the scalp [
14]. This threshold was critical for maintaining data quality, especially for patients with ICH who may have scalp conditions affecting sensor coupling.
Pre-Whitening: A 5-second buffer was removed around each event to mitigate baseline effects from pre-task variations, optimizing the accuracy of fNIRS signal comparisons across tasks. By accounting for baseline shifts, this step also minimized potential signal drifts common in fNIRS data from acute ICH patients.
Spike Removal: Motion artifacts were minimized using a spike-removal algorithm, with settings customized per task [
15]
Monotonic Interpolation and Temporal Derivative Distribution Repair (TDDR): Monotonic interpolation was used to smooth the data, followed by TDDR, which corrected for motion artifacts that may otherwise distort hemodynamic measurements [
16]. This method was particularly relevant for motor tasks where head and body movement could influence sensor readings.
Temporal Filtering: A Butterworth filter was applied to reduce noise with a low-pass cutoff set at 0.15 Hz and a high-pass at 0.01 Hz for motor tasks (and at 0.10 Hz for resting data). These parameters were optimized to maintain frequency ranges that reflect meaningful hemodynamic changes without the interference of high-frequency noise.
Global Component Regression (GCR) and Correlation-Based Signal Improvement (CBSI): GCR was used to filter global noise from the fNIRS data, addressing artifacts arising from systemic physiological changes like heart rate and respiration. Additionally, CBSI corrected for head movement artifacts, essential for isolating the hemodynamic activity related to motor cortex responses with minimal interference from external factors [
17].
Normalization: Z-transform and Percent Signal Change (PSC) normalizations were implemented to standardize data and adjust for motion artifacts, allowing for reliable inter-participant and inter-hemispheric comparisons [
18]. These normalizations ensure data consistency, critical for analyzing the effects of ICH on bilateral motor cortex functionality.
Patients with at least one symmetric sensory channel were retained for further analysis. This ensured fNIRS data accurately captured bilateral oxygenation and hemodynamic responses. Patients with data not meeting these criteria, due to poor signal quality or asymmetry between the sensory channels, were excluded from the data analysis. This approach ensured data reliability and validity when comparing interhemispheric hemodynamics.
2.6. fNIRS Analyses
fNIRS analysis was conducted for resting-state functional connectivity (RSFC) and motor paradigm.
2.6.1. Resting-State Analyses
RSFC was assessed via seed-based correlation; priMC served as the seed region with the analysis focusing on the connectivity between the affected and unaffected hemispheres. Seed-based correlation is particularly suited for examining connectivity patterns between a predefined region of interest (the "seed") and other brain regions, enabling targeted analysis of synchronized activity across brain networks.
2.6.2. Motor Paradigm Analyses
A general linear model (GLM) analyzed motor task data and stroke laterality (left or right hemisphere affected) served as a model predictor. For left-hemispheric stroke patients, the contrast was set to Left Hand > Baseline (1 > Baseline), and for right-hemispheric stroke patients, it was set to Right Hand > Baseline (2 > Baseline). Beta (β) coefficients from GLM results quantified motor task-related brain activation.
The GLM provides an established analytical framework for quantifying brain activity in response to task conditions by modeling the relationship between experimental predictors and observed neural responses, accounting for both fixed and random effects to detect significant hemodynamic responses while controlling for inter-subject variability [
19].
2.6.3. Outcome Measures
The primary outcome measure is RSFC and relative changes in oxyhemoglobin (ΔHbO) during finger tapping tasks in patients with ICH. RSFC evaluates disruptions in coherence between motor-related brain regions in the bilateral hemispheres. A reduction in ΔHbO concentrations in affected motor regions indicates impaired neural reactivity, whereas increased values in other areas may suggest compensatory activity.
2.7. Statistical Analyses
We had two groups of participants: ICH and Control participants. Categorical or nominal variables were expressed as proportions and compared with chi-square for (female sex, comorbidities). Continuous variables were expressed as means or medians and compared with the Student t test or the Kruskal-Wallis test (age, ICH volumes).
RSFC was assessed in individual affected and unaffected hemispheres by group-level seed-based correlation analysis (Seed placed on priMC). Additionally, linear regression analysis was performed with controls and stroke patients by selecting a motor channel on the affected and unaffected hemispheres separately. Finger tapping-associated relative Oxyhemoglobin (ΔHbO) changes were analyzed in affected and unaffected hemispheres with GLM regression. Correlations between left and right motor cortices and event-related averages were calculated for hemodynamic responses in both resting and motor-task states. Significance was determined with a threshold of p < 0.05. All analyses were conducted using STATA 18.0 BE (StataCorp LLC, Texas, USA).
3. Results
3.1. Patient Characteristics
In this cross-sectional study we enrolled 37 patients with ICH and 44 control/TIA participants. Seven (2 ICH and 5 control/TIA patients) were excluded from the study due to poor fNIRS signal quality during the analysis. The 35 patients with ICH included in the analyses were enrolled at a median (IQR) of 42:1 (22:6, 88:3) hours after symptom onset, with a median NIHSS of 10 (5, 17), 42.8% were female, and 19 (54.3%) were right hemispheric in location.
Table 1 describes the comorbidity burden between the patients with ICH and Control/TIA participants. Dyslipidemia was more common in Control/TIA participants, 30 of 39 (76.9%), and the small vessel disease burden was higher in patients with ICH, 24 of 35 (68.6%).
3.2. Resting State Functional Connectivity Analysis
The resting-state functional connectivity (RSFC) analysis in ICH was done by seed-based correlations placed on the right or left hemisphere priMC compared to the control/TIA participants.
When the seed was placed on the left hemisphere priMC in patients with left hemispheric ICH, there was an increased coherence with the affected preMC (FC4-FC2, β = 0.83, 95% CI = 0.19, 1.47, p = 0.01) and a decreased coherence with the affected primary somatosensory cortex (C3-C5, β = -0.76, 95% CI = -1.
participants. No coherence differences were observed in the remaining affected or unaffected cerebral regions between left hemispheric ICH and controls/TIA participants.
In patients with right hemispheric ICH, when the seed was placed on the left hemisphere priMC there was a decreased coherence with affected primary somatosensory cortex (CP2-FC2, β = -0.71, 95% CI = -1.32, -0.09, p = .02) and the unaffected preMC (FC3-FC1, β = -0.6, 95% CI = -1.12, -0.09, p = .02). No coherence differences were observed in the remaining affected or unaffected cerebral regions between right hemispheric ICH and controls/TIA participants.
3.3. Motor Paradigm (Finger-Tapping) Analyses
Motor paradigm (Finger tapping) analyses were done with generalized linear regression for each channel. The control subjects and TIA participants served as a comparator group (
Figure 2 and
Figure 3).
4, -0.13, p = 0.02), compared to control/TIA
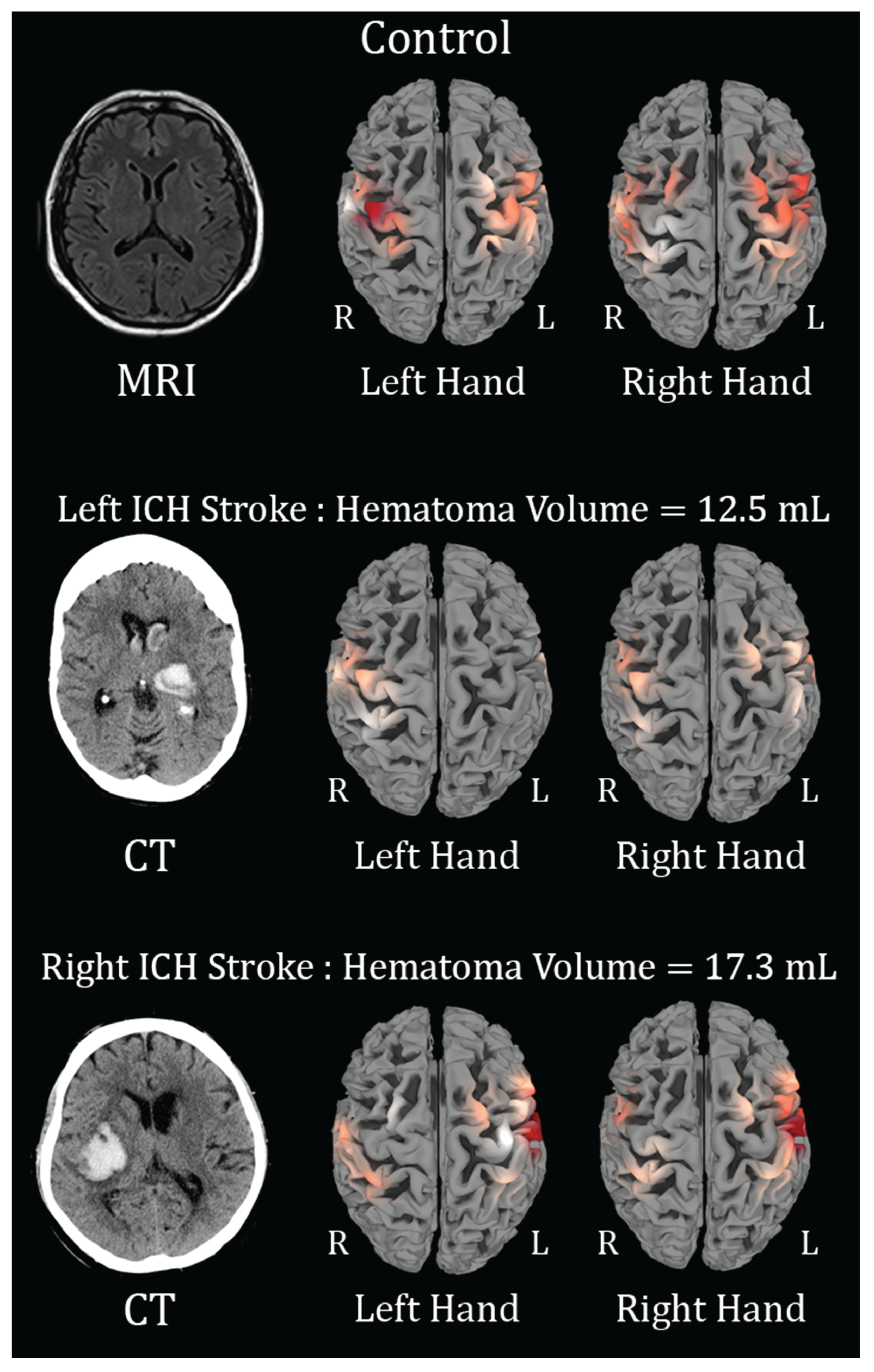
Figure 2.
Brain activation maps showing ΔHbO changes during finger tapping (FT). Red regions indicate activation; R and L denote right and left hemispheres. In the first row, the Control subject, with left-hand FT, leads to activation of the R>L hemisphere, and right-hand FT leads to activation of the L>R. In the second row, in a patient with a left hemisphere intracerebral hemorrhage (ICH), left hand FT shows activation over the R only and right hand FT shows mild activation of the R & L activation, suggestive of motor cortex re-organization and spread of activation. In the third row, in a patient with a right hemispheric ICH, the left-hand FT shows activation L>R (opposite compared to the control subject) and the right-hand FT shows activation L>R. This is suggestive of motor-cortex re-organization and spread of activation more to the L hemisphere.
Figure 2.
Brain activation maps showing ΔHbO changes during finger tapping (FT). Red regions indicate activation; R and L denote right and left hemispheres. In the first row, the Control subject, with left-hand FT, leads to activation of the R>L hemisphere, and right-hand FT leads to activation of the L>R. In the second row, in a patient with a left hemisphere intracerebral hemorrhage (ICH), left hand FT shows activation over the R only and right hand FT shows mild activation of the R & L activation, suggestive of motor cortex re-organization and spread of activation. In the third row, in a patient with a right hemispheric ICH, the left-hand FT shows activation L>R (opposite compared to the control subject) and the right-hand FT shows activation L>R. This is suggestive of motor-cortex re-organization and spread of activation more to the L hemisphere.
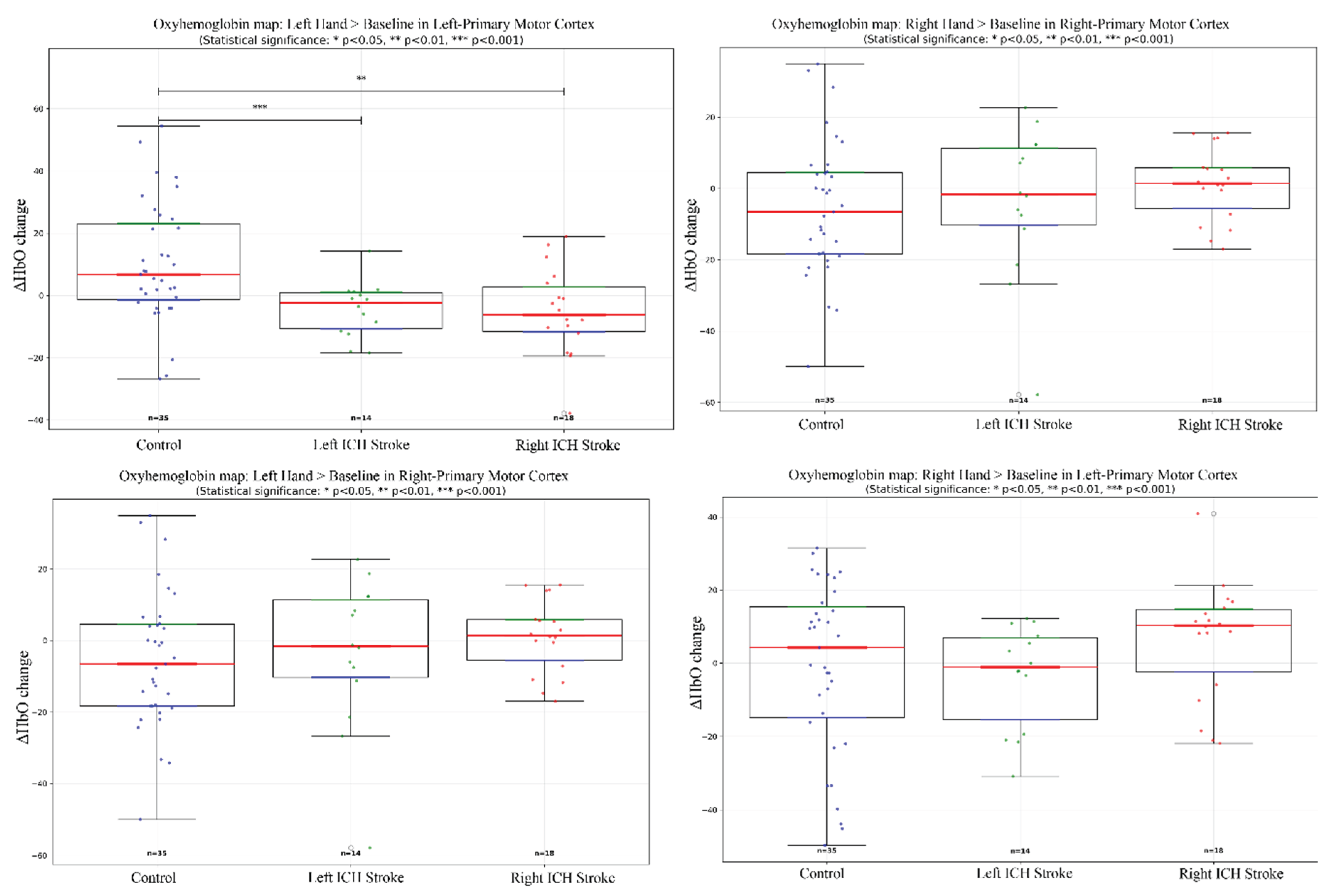
Figure 3.
General Linear Model Regression boxplots showing ΔHbO changes during left- and right-hand finger tapping across Control, Left/Right ICH Stroke. Statistical differences (p < 0.05, p < 0.001) are indicated.
Figure 3.
General Linear Model Regression boxplots showing ΔHbO changes during left- and right-hand finger tapping across Control, Left/Right ICH Stroke. Statistical differences (p < 0.05, p < 0.001) are indicated.
In left hemispheric ICH, with left hand finger-tapping, the oxyhemoglobin change (ΔHbO) was higher in the left (affected) somatosensory cortex (CP3-C3, β = 0.02, 95% CI = 0.005, 0.03, p = .008) and in the left (affected) preMC (C3-FC3, β = 0.01, 95% CI = 0.003, 0.02, p = .01).
In left hemispheric ICH, with left hand finger-tapping the deoxyhemoglobin (HbR) change was lower in the left (affected) somatosensory cortex (CP3-C3, β = -0.02, 95% CI = -0.03, -0.005, p = .01) and the left (affected) preMC (C3-FC3, β = -0.01, 95% CI = -0.02, -0.002, p = .02).
In right hemispheric ICH, with left hand finger-tapping, the oxyhemoglobin (ΔHbO) change is higher in the left (unaffected) preMC (C3-FC3, β = 0.02, 95% CI = 0.006, 0.04, p = .01). The deoxyhemoglobin (HbR) change is lower in the left (unaffected) preMC (C3-FC3, β = -0.07, 95% CI = -0.044, -0.005, p = .01).
The total hemoglobin (Hb) change is lower in the left (unaffected) somatosensory cortex (C1-C3, β = 0.02, 95% CI = -0.13, -0.007, p = .03) and higher in the right (affected) preMC (FC4-FC2, β = 0.08, 95% CI = 0.01, 0.15, p = .02).
No difference was observed in ΔHbO over either hemisphere in other channels with either right-hand or left-hand finger tapping compared to control/TIA participants. No difference was observed in HbR over either hemisphere in other channels with either right-hand or left-hand finger tapping compared to control/TIA participants. No difference was observed in total Hb over either hemisphere in other channels with either right-hand or left-hand finger tapping compared to control/TIA participants.
3.4. Motor Paradigm (Handgrip) Analysis
Motor paradigm (Finger tapping) analyses were done with generalized linear regression for each channel. The control subjects and TIA participants served as a comparator group. No differences were observed in ΔHbO, HbR and total Hb over either hemisphere in channels with either right-hand or left-hand hand grip compared to control/TIA participants
4. Discussion
This study demonstrated that RSFC over the priMC is affected early (within 48-72 hours) in patients with ICH. Furthermore, in patients with left hemispheric ICH, there is increased coherence on seed-based analysis with the affected preMC, whereas in right hemispheric ICH, there is decreased coherence with the affected preMC compared to controls/TIA participants. In the motor paradigm assessment, patients with left hemispheric ICH exhibit increased oxygenation, a surrogate for neuronal activity, over the affected preMC and affected somatosensory cortex. In contrast, those with right hemispheric ICH show increased oxygenation over the unaffected preMC alone.
Patients with ICH exhibited reduced coherence between the affected and unaffected hemispheres, highlighting impaired inter-hemispheric communication. Specifically, the negative coefficient observed for C3-C5 (Sensory/AT) in left-stroke patients indicates weakened coherence between sensory and motor areas, which may contribute to motor impairments. Conversely, increased connectivity in FC4-FC2 (preMC) indicates compensatory mechanisms, suggesting an adaptive response to motor deficits due to the increased coherence observed between the priMC and preMC.
The motor paradigm results further support these findings, as fNIRS revealed differences in hemodynamic responses between patients with ICH and control subjects. Hemodynamic activity in the affected motor regions was notably reduced in stroke patients, indicating impaired cortical activation. Specifically, left-hemispheric stroke patients showed diminished motor cortex activation, with lower oxyhemoglobin levels during movement tasks. The C4-FC4 (Motor) channel exhibited a reduction in both oxy- and deoxyhemoglobin concentrations, emphasizing impaired neural activation (
Figure 2). In contrast, right-hemispheric ICH patients exhibited increased compensatory activity, particularly in the C2-FC2 motor and premotor regions, suggesting affected side reorganization of neurons to mitigate the motor deficits caused by ICH (
Figure 2).
In previous resting state functional Magnetic Resonance Imaging (rsfMRI) studies, a dysconnectivity is observed in the subacute and chronic RSFC in acute ICH. In a cross-sectional study, a reduced coherence in the default mode network (DMN) and orbitofrontal cortex in unresponsive patients was observed using seed-based functional connectivity. This suggests early disruption in the frontal network in the acute phase [
20]. In addition, a longitudinal study compared ICH patients with matched controls using RSFC. The ICH group experienced reduced coherence between DMN and sensorimotor networks in 1 month after stroke. Over time, the ICH group experienced a minimal increase in coherence in 3 months; however, the coherence continued to increase over 12 months [
21]. These findings suggest that there are longitudinal changes from subacute to chronic phases of RSFC in fMRI studies. Comparable changes have also been found in previous fNIRS studies, in a cohort of ICH patients, RSFC in the subacute phase measured decreased coherence in functional connectivity between the dorsolateral prefrontal cortex (DLPFC) and bilateral primary motor cortex. These results demonstrate that RSFC changes by fNIRS are similar to those observed in rsfMRI, particularly in frontal-motor circuits [
22].
Building on RSFC changes, studies have also examined motor network reorganization in ICH patients. An fMRI study showed patterns of motor reorganization in acute ICH patients, characterized by disrupted temporal variability in regional brain activity that evolves across recovery stages. During the acute phase, patients exhibit reduced temporal activation in the affected precentral gyrus (PreCG). As recovery progresses to the subacute stage, activation increases in the PreCG, PreMC, and sensory regions [
23]. Notably, the degree of increased PreCG activation from acute to subacute stages correlates with long-term motor improvement. This aligns with an fNIRS study, which tracked sensorimotor reorganization in acute ICH patients. Like fMRI, fNIRS revealed early bilateral activation during paretic-arm movements, followed by progressive lateralization to the affected hemisphere [
24]. Both studies highlight an acute-phase loss of lateralization due to the unaffected hemisphere's compensation and subacute restoration of the affected hemisphere's dominance during recovery.
In this study, cw-NIRS was used as a non-invasive neuroimaging technique that measures relative changes in ΔHbO and HbR in the brain. Its simplicity, affordability, and greater temporal resolution compared to fMRI make it widely accessible and allow for multiple assessments on a subject. Importantly, fNIRS can be used during motor tasks to monitor motor network function and recovery in real time, making it particularly suitable for guiding individualized rehabilitation programs.
One limitation of this study is that short channels were not used to account for superficial signals, such as scalp blood flow and other local physiological noise, which may have introduced light contamination into the fNIRS measurements. Another limitation would be that fNIRS was collected only at a single time point, which limits the ability to measure changes in motor channels reorganization or RSFC over time. Longitudinal assessments in the future would provide a more complete picture of recovery trajectories. Lastly, this study was conducted in a single tertiary care center, so the findings may not generalize to other populations, community hospitals, or younger patients.
5. Conclusions
This study provides insights into hemisphere-based differences in motor network disruptions following ICH. Left hemispheric preMC is involved in motor cortex reorganization in acute ICH. These results highlight the importance of tailored rehabilitation strategies, with a greater emphasis on physical and occupational therapy for left hemispheric ICH patients. The left preMC may be a target for neuromodulation devices. However, larger cohort studies with standardized recruitment criteria are needed to validate these findings and further refine rehabilitation approaches for stroke patients.
Supplementary Materials
The following supporting information can be downloaded at the website of this paper posted on
Preprints.org. Supplementary Tables 1 and 2.
Author Contributions
Conceptualization, M.K., B.B., A.S.; methodology, M.K., Ni.K, G.C.D, Na.K., C.K.; software, M.K., A.S.; validation, M.K., Ni.K. and G.C.D.; formal analysis, Ni.K., G.C.D., M.K.; investigation, X.X.; resources, A.S.; data curation, Na.K..; writing—original draft preparation, Ni.K. and G.C.D.; writing—review and editing, M.K., A.S., B.B.; visualization, M.K.; supervision, M.K.; project administration, Na.K.; funding acquisition, M.K., B.B., A.S. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by Translational Research Implementation Fund (TRIF) 2023-2024, Department of Medicine, University of Alberta, Edmonton, Alberta, Canada (RSO00622419)
Institutional Review Board Statement
The study was conducted in accordance with the Declaration of Helsinki and approved by the Institutional Review Board of the University of Alberta (Pro00070689 and Date of Approval June 12, 2023)
Informed Consent Statement
Informed consent was obtained from all subjects involved in the study.
Data Availability Statement
Data will be available upon request to the corresponding author.
Acknowledgments
The funding for the Satori Software was provided by the Research funds of Dr Janis Miyasaki, Division of Neurology, Department of Medicine, University of Alberta, Edmonton, Canada.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- O’Donnell, M.J.; Chin, S.L.; Rangarajan, S.; Xavier, D.; Liu, L.; Zhang, H.; Rao-Melacini, P.; Zhang, X.; Pais, P.; Agapay, S.; et al. Global and Regional Effects of Potentially Modifiable Risk Factors Associated with Acute Stroke in 32 Countries (INTERSTROKE): A Case-Control Study. The Lancet 2016, 388, 761–775. [Google Scholar] [CrossRef]
- Magid-Bernstein, J.; Girard, R.; Polster, S.; Srinath, A.; Romanos, S.; Awad, I.A.; Sansing, L.H. Cerebral Hemorrhage: Pathophysiology, Treatment, and Future Directions. Circ Res 2022, 130, 1204. [Google Scholar] [CrossRef]
- Baker, W.L.; Sharma, M.; Cohen, A.; Ouwens, M.; Christoph, M.J.; Koch, B.; Moore, T.E.; Frady, G.; Coleman, C.I. Using 30-Day Modified Rankin Scale Score to Predict 90-Day Score in Patients with Intracranial Hemorrhage: Derivation and Validation of Prediction Model. PLoS One 2024, 19, e0303757. [Google Scholar] [CrossRef] [PubMed]
- Pożarowszczyk, N.; Kurkowska-Jastrzębska, I.; Sarzyńska-Długosz, I.; Nowak, M.; Karliński, M. Reliability of the Modified Rankin Scale in Clinical Practice of Stroke Units and Rehabilitation Wards. Front Neurol 2023, 14, 1064642. [Google Scholar] [CrossRef] [PubMed]
- Sun, L.; Yin, D.; Zhu, Y.; Fan, M.; Zang, L.; Wu, Y.; Jia, J.; Bai, Y.; Zhu, B.; Hu, Y. Cortical Reorganization after Motor Imagery Training in Chronic Stroke Patients with Severe Motor Impairment: A Longitudinal FMRI Study. Neuroradiology 2013, 55, 913–925. [Google Scholar] [CrossRef]
- Sood, I.; Injety, R.J.; Farheen, A.; Kamali, S.; Jacob, A.; Mathewson, K.; Buck, B.H.; Kate, M.P. Quantitative Electroencephalography to Assess Post-Stroke Functional Disability: A Systematic Review and Meta-Analysis. Journal of Stroke and Cerebrovascular Diseases 2024, 33. [Google Scholar] [CrossRef]
- Ferrari, M.; Quaresima, V. A Brief Review on the History of Human Functional Near-Infrared Spectroscopy (FNIRS) Development and Fields of Application. Neuroimage 2012, 63, 921–935. [Google Scholar] [CrossRef]
- Leff, D.R.; Orihuela-Espina, F.; Elwell, C.E.; Athanasiou, T.; Delpy, D.T.; Darzi, A.W.; Yang, G.Z. Assessment of the Cerebral Cortex during Motor Task Behaviours in Adults: A Systematic Review of Functional near Infrared Spectroscopy (FNIRS) Studies. Neuroimage 2011, 54, 2922–2936. [Google Scholar] [CrossRef]
- Sun, Z.; Liu, J.; Wang, K.; Zhang, J.; Liu, S.; Xue, F. Feasibility of Noninvasive Near-Infrared Spectroscopy Monitoring in Predicting the Prognosis of Spontaneous Intracerebral Hemorrhage. Front Neurol 2024, 15, 1406157. [Google Scholar] [CrossRef] [PubMed]
- Biswal, B.; Zerrin Yetkin, F.; Haughton, V.M.; Hyde, J.S. Functional Connectivity in the Motor Cortex of Resting Human Brain Using Echo-planar Mri. Magn Reson Med 1995, 34, 537–541. [Google Scholar] [CrossRef]
- Rehme, A.K.; Grefkes, C. Cerebral Network Disorders after Stroke: Evidence from Imaging-Based Connectivity Analyses of Active and Resting Brain States in Humans. Journal of Physiology 2013, 591, 17–31. [Google Scholar] [CrossRef]
- Elliott, M.L.; Knodt, A.R.; Cooke, M.; Kim, M.J.; Melzer, T.R.; Keenan, R.; Ireland, D.; Ramrakha, S.; Poulton, R.; Caspi, A.; et al. General Functional Connectivity: Shared Features of Resting-State and Task FMRI Drive Reliable and Heritable Individual Differences in Functional Brain Networks. Neuroimage 2019, 189, 516–532. [Google Scholar] [CrossRef]
- Yücel, M.A.; Lühmann, A. v.; Scholkmann, F.; Gervain, J.; Dan, I.; Ayaz, H.; Boas, D.; Cooper, R.J.; Culver, J.; Elwell, C.E.; et al. Best Practices for FNIRS Publications. Neurophotonics 2021, 8, 012101. [Google Scholar] [CrossRef]
- Prodoehl, J.; Yu, H.; Little, D.M.; Abraham, I.; Vaillancourt, D.E. Region of Interest Template for the Human Basal Ganglia: Comparing EPI and Standardized Space Approaches. Neuroimage 2007, 39, 956. [Google Scholar] [CrossRef] [PubMed]
- Hernandez-Sarabia, J.A.; Schmid, A.A.; Sharp, J.L.; Stephens, J.A. Intervention-Induced Changes in Balance and Task-Dependent Neural Activity in Adults with Acquired Brain Injury: A Pilot Randomized Control Trial. Sensors 2024, 24, 4047. [Google Scholar] [CrossRef] [PubMed]
- Fishburn, F.A.; Ludlum, R.S.; Vaidya, C.J.; Medvedev, A. V. Temporal Derivative Distribution Repair (TDDR): A Motion Correction Method for FNIRS. Neuroimage 2018, 184, 171. [Google Scholar] [CrossRef]
- von Au, S.; Helmich, I.; Kieffer, S.; Lausberg, H. Phasic and Repetitive Self-Touch Differ in Hemodynamic Response in the Prefrontal Cortex–An FNIRS Study. Frontiers in Neuroergonomics 2023, 4, 1266439. [Google Scholar] [CrossRef]
- Tak, S.; Ye, J.C. Statistical Analysis of FNIRS Data: A Comprehensive Review. Neuroimage 2014, 85, 72–91. [Google Scholar] [CrossRef] [PubMed]
- von Lühmann, A.; Ortega-Martinez, A.; Boas, D.A.; Yücel, M.A. Using the General Linear Model to Improve Performance in FNIRS Single Trial Analysis and Classification: A Perspective. Front Hum Neurosci 2020, 14, 514061. [Google Scholar] [CrossRef]
- Mikell, C.B.; Banks, G.P.; Frey, H.P.; Youngerman, B.E.; Nelp, T.B.; Karas, P.J.; Chan, A.K.; Voss, H.U.; Sander Connolly, E.; Claassen, J. Frontal Networks Associated with Command Following after Hemorrhagic Stroke. Stroke 2015, 46, 49–57. [Google Scholar] [CrossRef]
- Boren, S.B.; Savitz, S.I.; Ellmore, T.M.; Arevalo, O.D.; Aronowski, J.; Silos, C.; George, S.; Haque, M.E. Longitudinal Resting-State Functional Magnetic Resonance Imaging Study: A Seed-Based Connectivity Biomarker in Patients with Ischemic and Intracerebral Hemorrhage Stroke. Brain Connect 2023, 13, 498–507. [Google Scholar] [CrossRef] [PubMed]
- Sui, Y.; Kan, C.; Zhu, S.; Zhang, T.; Wang, J.; Xu, S.; Zhuang, R.; Shen, Y.; Wang, T.; Guo, C. Resting-State Functional Connectivity for Determining Outcomes in Upper Extremity Function after Stroke: A Functional near-Infrared Spectroscopy Study. Front Neurol 2022, 13, 965856. [Google Scholar] [CrossRef] [PubMed]
- Hu, J.; Du, J.; Xu, Q.; Yang, F.; Zeng, F.; Weng, Y.; Dai, X.J.; Qi, R.; Liu, X.; Lu, G.; et al. Dynamic Network Analysis Reveals Altered Temporal Variability in Brain Regions after Stroke: A Longitudinal Resting-State FMRI Study. Neural Plast 2018, 2018. [Google Scholar] [CrossRef]
- Puig, J.; Blasco, G.; Terceño, M.; Daunis-I-Estadella, P.; Schlaug, G.; Hernandez-Perez, M.; Cuba, V.; Carbó, G.; Serena, J.; Essig, M.; et al. Predicting Motor Outcome in Acute Intracerebral Hemorrhage. AJNR Am J Neuroradiol 2019, 40, 769. [Google Scholar] [CrossRef] [PubMed]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).