Submitted:
25 August 2025
Posted:
26 August 2025
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Materials and Methods
2.1. Chemicals
2.2. Zebrafish Husbandry and Chemical Exposure
2.3. Real-Time Reverse Transcription-Polymerase Chain Reaction (RT-PCR)
2.4. Zebrafish Behavioral Tests
2.5. ROS Detection
2.6. Detection of Apoptosis
2.7. Statistical Analysis
3. Results
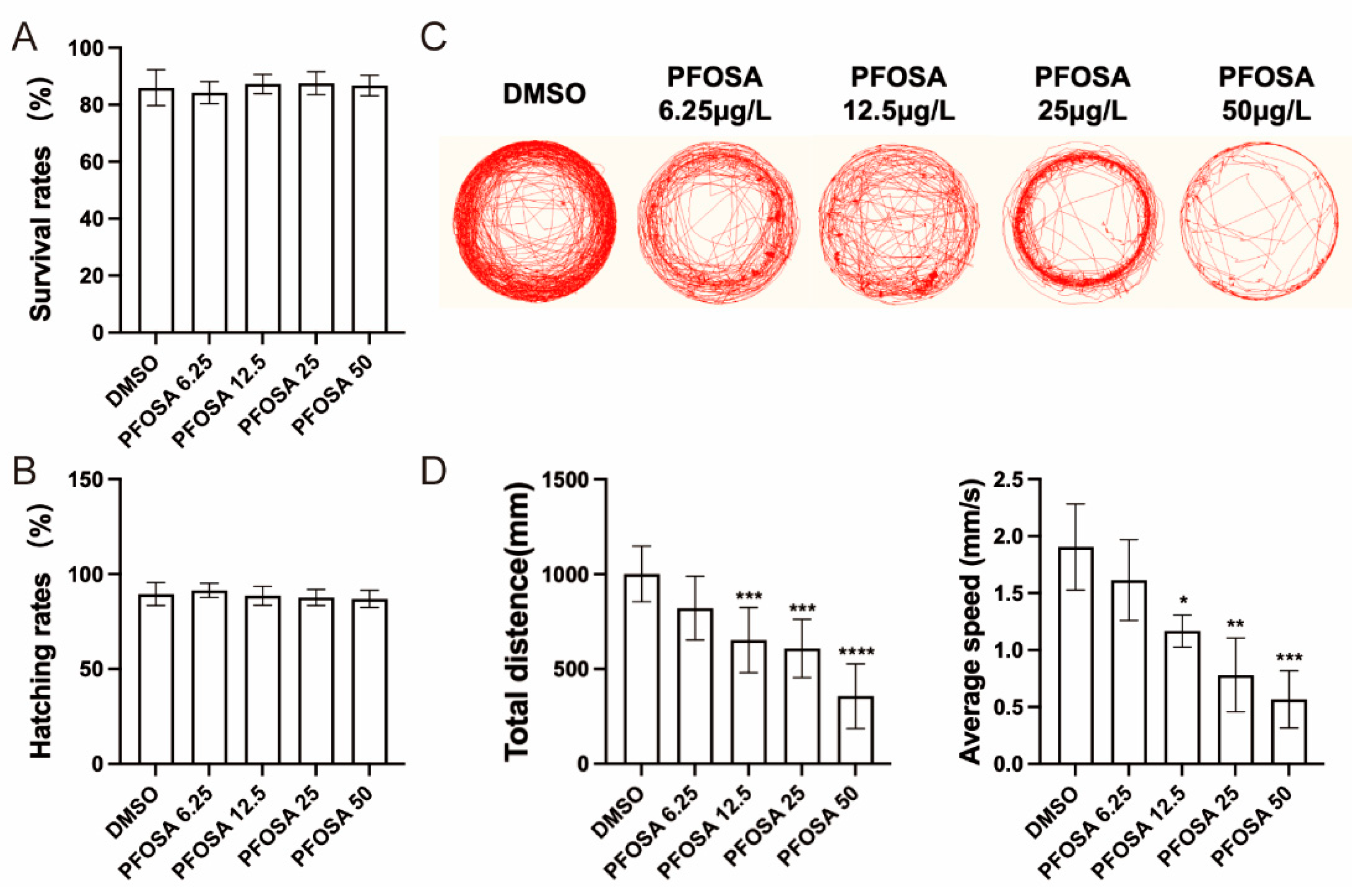
3.1. PFOSA Exposure Induces Locomotor Deficits and Behavioral Preference Alterations and in Zebrafish Larvae.
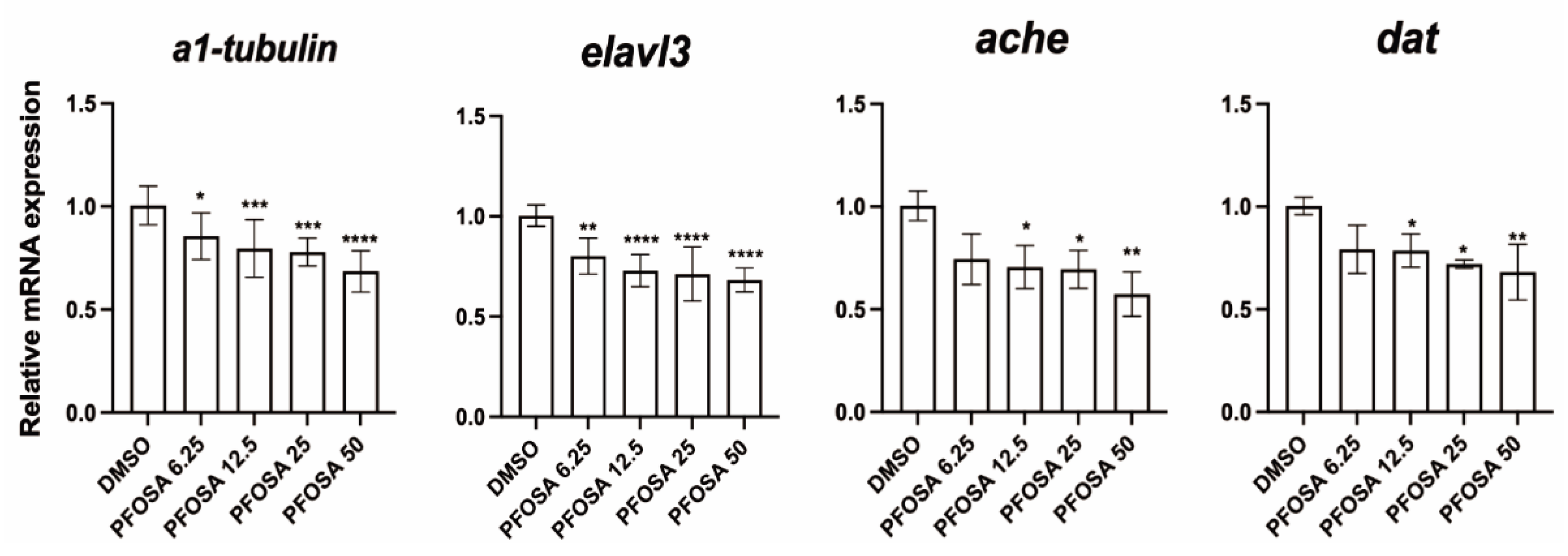
3.2. PFOSA Exposure Impairs Neuronal Differentiation
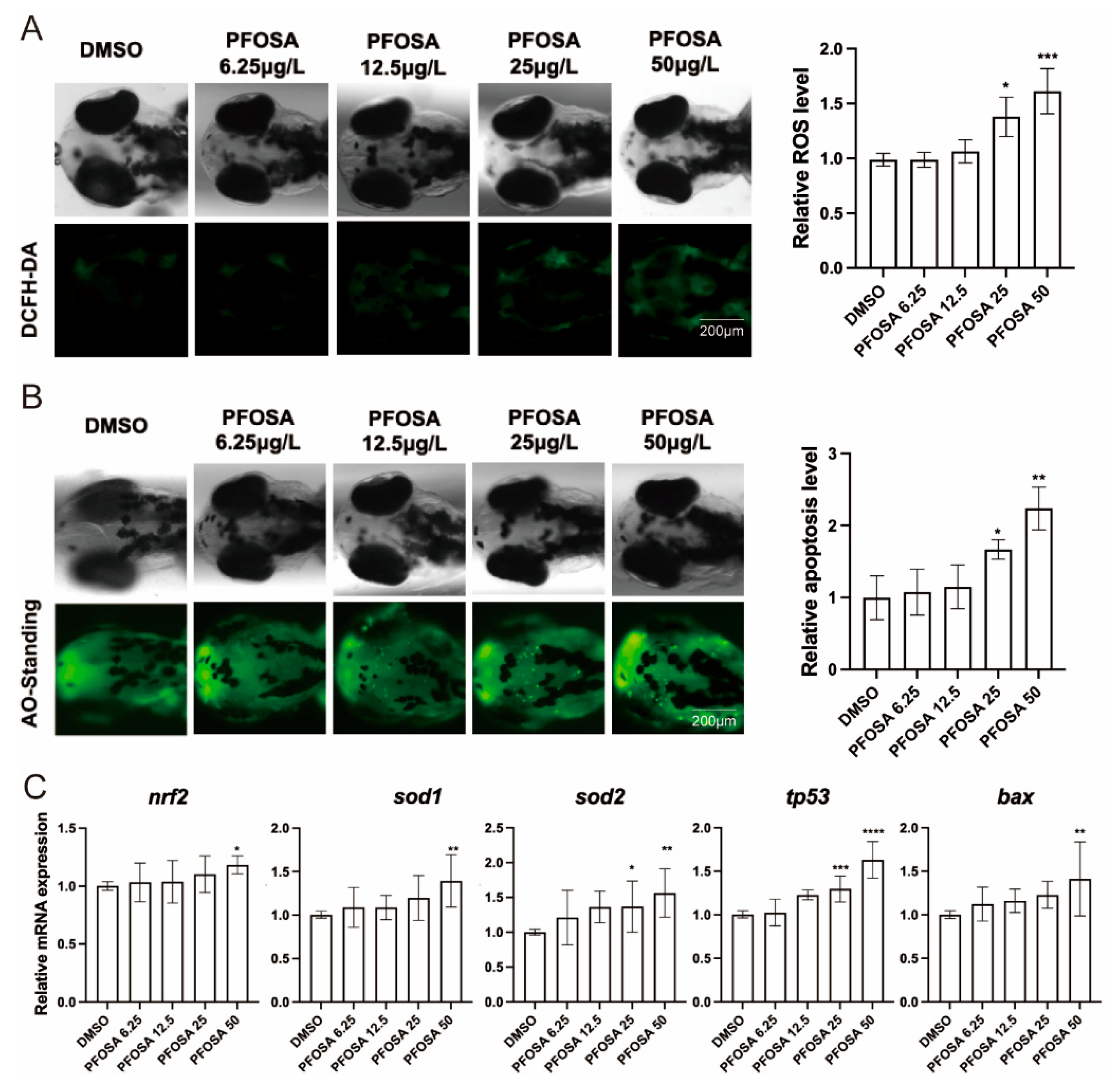
3.3. PFOSA Exposure Leads to Oxidative Stress and Apoptosis in Zebrafish Brains
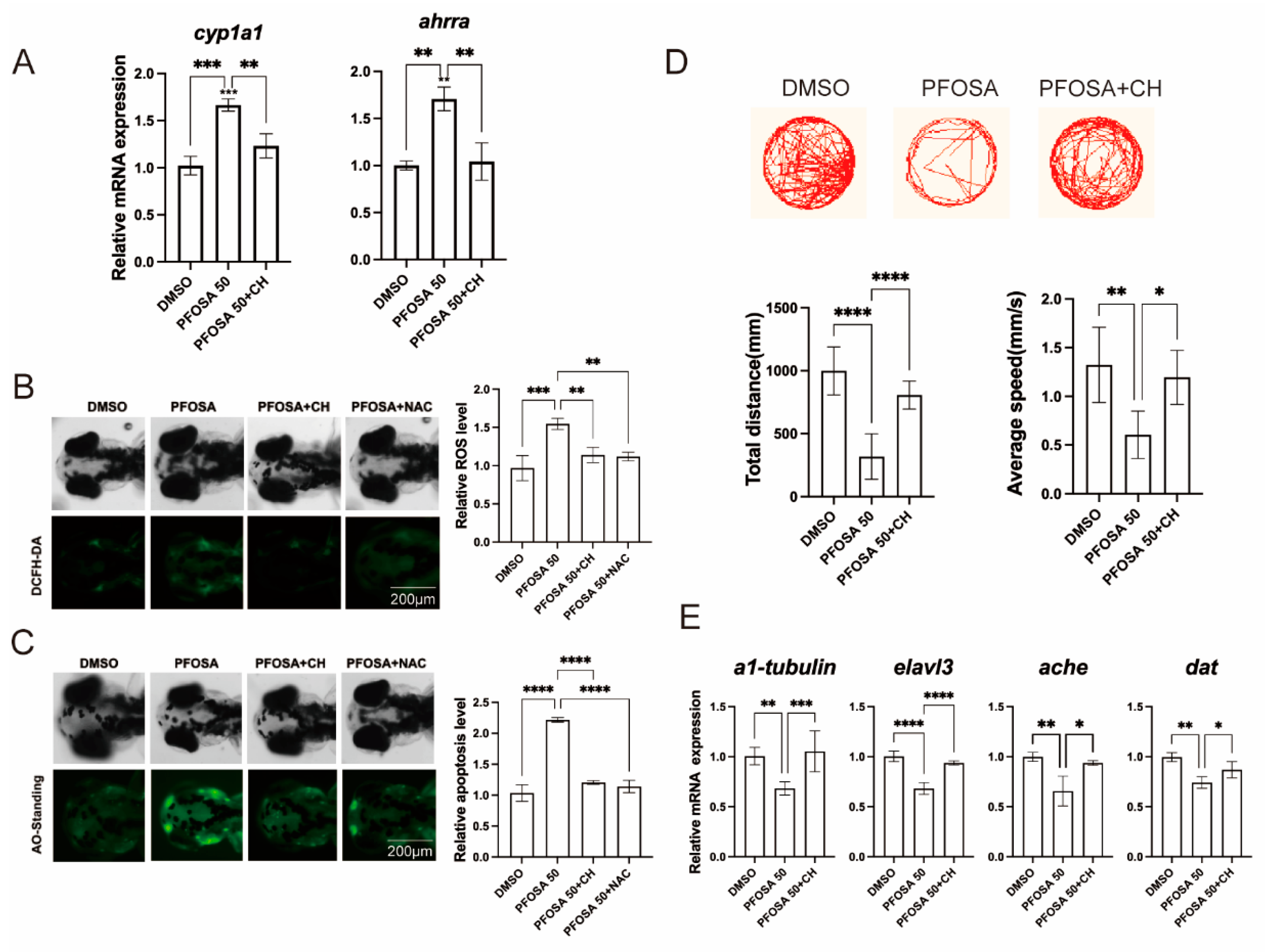
3.4. PFOSA Triggers Apoptosis via AhR-Mediated Oxidative Stress
4. Discussion
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Ateia, M. and M. Scheringer, From "forever chemicals" to fluorine-free alternatives. Science, 2024. 385(6706): p. 256-258.
- Brusseau, M.L.; Anderson, R.H.; Guo, B. PFAS concentrations in soils: Background levels versus contaminated sites. Sci. Total. Environ. 2020, 740, 140017–140017. [Google Scholar] [CrossRef]
- Anderson, R.H.; Long, G.C.; Porter, R.C.; Anderson, J.K. Occurrence of select perfluoroalkyl substances at U.S. Air Force aqueous film-forming foam release sites other than fire-training areas: Field-validation of critical fate and transport properties. Chemosphere 2016, 150, 678–685. [Google Scholar] [CrossRef]
- Anderson, R.H.; Long, G.C.; Porter, R.C.; Anderson, J.K. Occurrence of select perfluoroalkyl substances at U.S. Air Force aqueous film-forming foam release sites other than fire-training areas: Field-validation of critical fate and transport properties. Chemosphere 2016, 150, 678–685. [Google Scholar] [CrossRef] [PubMed]
- Chen, M.; Wang, Q.; Shan, G.; Zhu, L.; Yang, L.; Liu, M. Occurrence, partitioning and bioaccumulation of emerging and legacy per- and polyfluoroalkyl substances in Taihu Lake, China. Sci. Total. Environ. 2018, 634, 251–259. [Google Scholar] [CrossRef] [PubMed]
- Chen, M.; Zhu, L.; Wang, Q.; Shan, G. Tissue distribution and bioaccumulation of legacy and emerging per-and polyfluoroalkyl substances (PFASs) in edible fishes from Taihu Lake, China. Environ. Pollut. 2021, 268, 115887. [Google Scholar] [CrossRef]
- Zhang, B.; He, Y.; Yang, G.; Chen, B.; Yao, Y.; Sun, H.; Kannan, K.; Zhang, T. Legacy and Emerging Poly- and Perfluoroalkyl Substances in Finless Porpoises from East China Sea: Temporal Trends and Tissue-Specific Accumulation. Environ. Sci. Technol. 2021, 56, 6113–6122. [Google Scholar] [CrossRef]
- Ding, J.; Dai, Y.; Zhang, J.; Wang, Z.; Zhang, L.; Xu, S.; Tan, R.; Guo, J.; Qi, X.; Chang, X.; et al. Associations of perfluoroalkyl substances with adipocytokines in umbilical cord serum: A mixtures approach. Environ. Res. 2022, 216. [Google Scholar] [CrossRef]
- Liu, L.; Yan, P.; Liu, X.; Zhao, J.; Tian, M.; Huang, Q.; Yan, J.; Tong, Z.; Zhang, Y.; Zhang, J.; et al. Profiles and transplacental transfer of per- and polyfluoroalkyl substances in maternal and umbilical cord blood: A birth cohort study in Zhoushan, Zhejiang Province, China. J. Hazard. Mater. 2024, 466, 133501. [Google Scholar] [CrossRef]
- Dasgupta, S.; Reddam, A.; Liu, Z.; Liu, J.; Volz, D.C. High-content screening in zebrafish identifies perfluorooctanesulfonamide as a potent developmental toxicant. Environ. Pollut. 2020, 256, 113550–113550. [Google Scholar] [CrossRef]
- Chen, H.; Qiu, W.; Yang, X.; Chen, F.; Chen, J.; Tang, L.; Zhong, H.; Magnuson, J.T.; Zheng, C.; Xu, E.G. Perfluorooctane Sulfonamide (PFOSA) Induces Cardiotoxicity via Aryl Hydrocarbon Receptor Activation in Zebrafish. Environ. Sci. Technol. 2022, 56, 8438–8448. [Google Scholar] [CrossRef]
- Dasgupta, S.; Reddam, A.; Liu, Z.; Liu, J.; Volz, D.C. High-content screening in zebrafish identifies perfluorooctanesulfonamide as a potent developmental toxicant. Environ. Pollut. 2020, 256, 113550–113550. [Google Scholar] [CrossRef] [PubMed]
- Xuan, R.; Qiu, X.; Wang, J.; Liu, S.; Magnuson, J.T.; Xu, B.; Qiu, W.; Zheng, C. Hepatotoxic response of perfluorooctane sulfonamide (PFOSA) in early life stage zebrafish (Danio rerio) is greater than perfluorooctane sulfonate (PFOS). J. Hazard. Mater. 2023, 461, 132552. [Google Scholar] [CrossRef]
- Slotkin, T.A.; MacKillop, E.A.; Melnick, R.L.; Thayer, K.A.; Seidler, F.J. Developmental Neurotoxicity of Perfluorinated Chemicals Modeled in Vitro. Environ. Heal. Perspect. 2008, 116, 716–722. [Google Scholar] [CrossRef]
- David, N.; Ivantsova, E.; Konig, I.; English, C.D.; Avidan, L.; Kreychman, M.; Rivera, M.L.; Escobar, C.; Valle, E.M.A.; Sultan, A.; et al. Adverse Outcomes Following Exposure to Perfluorooctanesulfonamide (PFOSA) in Larval Zebrafish (Danio rerio): A Neurotoxic and Behavioral Perspective. Toxics 2024, 12, 723. [Google Scholar] [CrossRef]
- Ma, T.; Jiang, Y.; Chen, P.; Xiao, F.; Zhang, J.; Ma, Y.; Chen, T. PFOS and PFOSA induce oxidative stress-mediated cardiac defects in zebrafish via PPARγ and AHR pathways, respectively. Sci. Total. Environ. 2024, 951, 175716. [Google Scholar] [CrossRef]
- Polonio, C.M.; McHale, K.A.; Sherr, D.H.; Rubenstein, D.; Quintana, F.J. The aryl hydrocarbon receptor: a rehabilitated target for therapeutic immune modulation. Nat. Rev. Drug Discov. 2025, 24, 610–630. [Google Scholar] [CrossRef]
- Wang, X.; Hu, M.; Li, M.; Huan, F.; Gao, R.; Wang, J. Effects of exposure to 3,6-DBCZ on neurotoxicity and AhR pathway during early life stages of zebrafish (Danio rerio). Ecotoxicol. Environ. Saf. 2023, 270, 115892. [Google Scholar] [CrossRef]
- Veith, A.; Moorthy, B. Role of cytochrome P450s in the generation and metabolism of reactive oxygen species. Curr. Opin. Toxicol. 2018, 7, 44–51. [Google Scholar] [CrossRef]
- Wu, Y.; Fan, F.; Zhou, L.; Shen, Y.; Wang, A.; Qin, Y.; Wang, J.; Yao, W. ADB-FUBINACA-induced developmental toxicity, neurotoxicity, and cardiotoxicity in embryonic zebrafish (Danio rerio). Environ. Res. 2025, 276, 121517. [Google Scholar] [CrossRef]
- Lein, P.J., S. Supasai, and M. Guignet, Chapter 9 - Apoptosis as a Mechanism of Developmental Neurotoxicity, in Handbook of Developmental Neurotoxicology (Second Edition), W. Slikker, M.G. Paule, and C. Wang, Editors. 2018, Academic Press. p. 91-112.
- Rock, K.D.; Patisaul, H.B. Environmental Mechanisms of Neurodevelopmental Toxicity. Curr. Environ. Heal. Rep. 2018, 5, 145–157. [Google Scholar] [CrossRef] [PubMed]
- de Abreu, M.S., et al., Zebrafish as a Model of Neurodevelopmental Disorders. Neuroscience, 2020. 445: p. 3-11.
- Vaz, R.; Hofmeister, W.; Lindstrand, A. Zebrafish Models of Neurodevelopmental Disorders: Limitations and Benefits of Current Tools and Techniques. Int. J. Mol. Sci. 2019, 20, 1296. [Google Scholar] [CrossRef]
- Nery, L.R.; Eltz, N.S.; Hackman, C.; Fonseca, R.; Altenhofen, S.; Guerra, H.N.; Freitas, V.M.; Bonan, C.D.; Vianna, M.R.M.R.; Laks, J. Brain Intraventricular Injection of Amyloid-β in Zebrafish Embryo Impairs Cognition and Increases Tau Phosphorylation, Effects Reversed by Lithium. PLOS ONE 2014, 9, e105862. [Google Scholar] [CrossRef]
- Barreiros, M.d.O.; Barbosa, F.G.; Dantas, D.d.O.; Santos, D.d.M.L.d.; Ribeiro, S.; Santos, G.C.d.O.; Barros, A.K. Zebrafish automatic monitoring system for conditioning and behavioral analysis. Sci. Rep. 2021, 11, 1–16. [Google Scholar] [CrossRef] [PubMed]
- Zhu, X.-Y.; Wu, Y.-Y.; Xia, B.; Dai, M.-Z.; Huang, Y.-F.; Yang, H.; Li, C.-Q.; Li, P. Fenobucarb-induced developmental neurotoxicity and mechanisms in zebrafish. NeuroToxicology 2020, 79, 11–19. [Google Scholar] [CrossRef]
- Fan, C.-Y.; Cowden, J.; Simmons, S.O.; Padilla, S.; Ramabhadran, R. Gene expression changes in developing zebrafish as potential markers for rapid developmental neurotoxicity screening. Neurotoxicology Teratol. 2010, 32, 91–98. [Google Scholar] [CrossRef]
- Lionetto, M.G.; Caricato, R.; Calisi, A.; Giordano, M.E.; Schettino, T. Acetylcholinesterase as a Biomarker in Environmental and Occupational Medicine: New Insights and Future Perspectives. BioMed Res. Int. 2013, 2013, 1–8. [Google Scholar] [CrossRef]
- Du, Y.; Li, Q.; Zhou, G.; Cai, Z.; Man, Q.; Wang, W.C. Early-life perfluorooctanoic acid exposure disrupts the function of dopamine transporter protein with glycosylation changes implicating the links between decreased dopamine levels and disruptive behaviors in larval zebrafish. Sci. Total. Environ. 2024, 917, 170408. [Google Scholar] [CrossRef]
- van Leyen, K., et al., Neurogenesis and Apoptotic Cell Death. The Cell Cycle in the Central Nervous System, ed. D. Janigro. Vol. NJ. p. 2006, Humana Press: Totowa, . 71-79.
- Amaral, J.D.; Xavier, J.M.; Steer, C.J.; Rodrigues, C.M. The role of p53 in apoptosis. . 2010, 9, 145–52. [Google Scholar]
- Chipuk, J.E.; Kuwana, T.; Bouchier-Hayes, L.; Droin, N.M.; Newmeyer, D.D.; Schuler, M.; Green, D.R. Direct Activation of Bax by p53 Mediates Mitochondrial Membrane Permeabilization and Apoptosis. Science 2004, 303, 1010–1014. [Google Scholar] [CrossRef]
- Love, C.; Sominsky, L.; O’hEly, M.; Berk, M.; Vuillermin, P.; Dawson, S.L. Prenatal environmental risk factors for autism spectrum disorder and their potential mechanisms. BMC Med. 2024, 22, 1–13. [Google Scholar] [CrossRef]
- Nishimura, Y.; Kanda, Y.; Sone, H.; Aoyama, H.; Gasparovic, A.C. Oxidative Stress as a Common Key Event in Developmental Neurotoxicity. Oxidative Med. Cell. Longev. 2021, 2021, 6685204. [Google Scholar] [CrossRef]
- Kerksick, C.; Willoughby, D. The Antioxidant Role of Glutathione and N-Acetyl-Cysteine Supplements and Exercise-Induced Oxidative Stress. J. Int. Soc. Sports Nutr. 2005, 2, 38–44. [Google Scholar] [CrossRef]
- Giudice, A.; Montella, M. Activation of the Nrf2–ARE signaling pathway: a promising strategy in cancer prevention. BioEssays 2006, 28, 169–181. [Google Scholar] [CrossRef] [PubMed]
- Latchney, S.E.; Hein, A.M.; O'BAnion, M.K.; DiCicco-Bloom, E.; Opanashuk, L.A. Deletion or activation of the aryl hydrocarbon receptor alters adult hippocampal neurogenesis and contextual fear memory. J. Neurochem. 2012, 125, 430–445. [Google Scholar] [CrossRef] [PubMed]
- Juricek, L.; Coumoul, X. The Aryl Hydrocarbon Receptor and the Nervous System. Int. J. Mol. Sci. 2018, 19, 2504. [Google Scholar] [CrossRef]
- Kajta, M.; Wnuk, A.; Rzemieniec, J.; Lason, W.; Mackowiak, M.; Chwastek, E.; Staniszewska, M.; Nehring, I.; Wojtowicz, A.K. Triclocarban Disrupts the Epigenetic Status of Neuronal Cells and Induces AHR/CAR-Mediated Apoptosis. Mol. Neurobiol. 2018, 56, 3113–3131. [Google Scholar] [CrossRef] [PubMed]
- Stading, R.; Chu, C.; Couroucli, X.; Lingappan, K.; Moorthy, B. Molecular role of cytochrome P4501A enzymes in oxidative stress. Curr. Opin. Toxicol. 2020, 20-21, 77–84. [Google Scholar] [CrossRef]
- Huang, Y.; Zhang, J.; Tao, Y.; Ji, C.; Aniagu, S.; Jiang, Y.; Chen, T. AHR/ROS-mediated mitochondria apoptosis contributes to benzo[a]pyrene-induced heart defects and the protective effects of resveratrol. Toxicology 2021, 462, 152965. [Google Scholar] [CrossRef]
- Jenny, M.J.; Karchner, S.I.; Franks, D.G.; Woodin, B.R.; Stegeman, J.J.; Hahn, M.E. Distinct Roles of Two Zebrafish AHR Repressors (AHRRa and AHRRb) in Embryonic Development and Regulating the Response to 2,3,7,8-Tetrachlorodibenzo-p-dioxin. Toxicol. Sci. 2009, 110, 426–441. [Google Scholar] [CrossRef]
| Genes | GenBank No. | Forward (5’-3’) |
| β-actin | NM_131031.2 | CGAGCAGGAGATGGGAACC CAACGGAAACGCTCATTGC |
| elavl3 | NM_131449.1 | TGGTCTGCAGTTTGAGACCGTTGA |
| α1-tubulin | NM_194388.3 | AATCACCAATGCTTGCTTCGAGCC TTCACGTCTTTGGGTACCACGTCA |
| dat | NM_131755.1 | AGACATCTGGGAAGGTGGTG ACCTGAGCATCATACAGGCG |
| ache | NM_131846.3 | CCCTCCAGTGGGTACAAGAA GGGCCTCATCAAAGGTAACA |
| nrf2 | NM_182889.1 | TCGGGTTTGTCCCTAGATG AGGTTTGGAGTGTCCGCTA |
| sod1 | NM_131294.1 | CCGGACTATGTTAAGGCCATCT ACACTCGGTTGCTCTCTTTTCTCT |
| sod2 | NM_199976.1 | GTCGTCTGGCTTGTGGAGTG TGTCAGCGGGCTAGTGCTT |
| tp53 | NM_001271820.1 | CCCGGCGATCATGGATTTAG CCACATGCTCGGACTTCTTATAG |
| bax | NM_131562.2 | GGCTATTTCAACCAGGGTTCC TGCGAATCACCAATGCTGT |
| cyp1a1 | NM_131879.2 | GCATTACGATACGTTCGATAAGGAC GCTCCGAATAGGTCATTGACGAT |
| ahrra | NM_001035265.2 | GCGCATCAAGAGCTTCTGCAGCGTGTT CCACTGACGACCAGCGCAAACCCT |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





