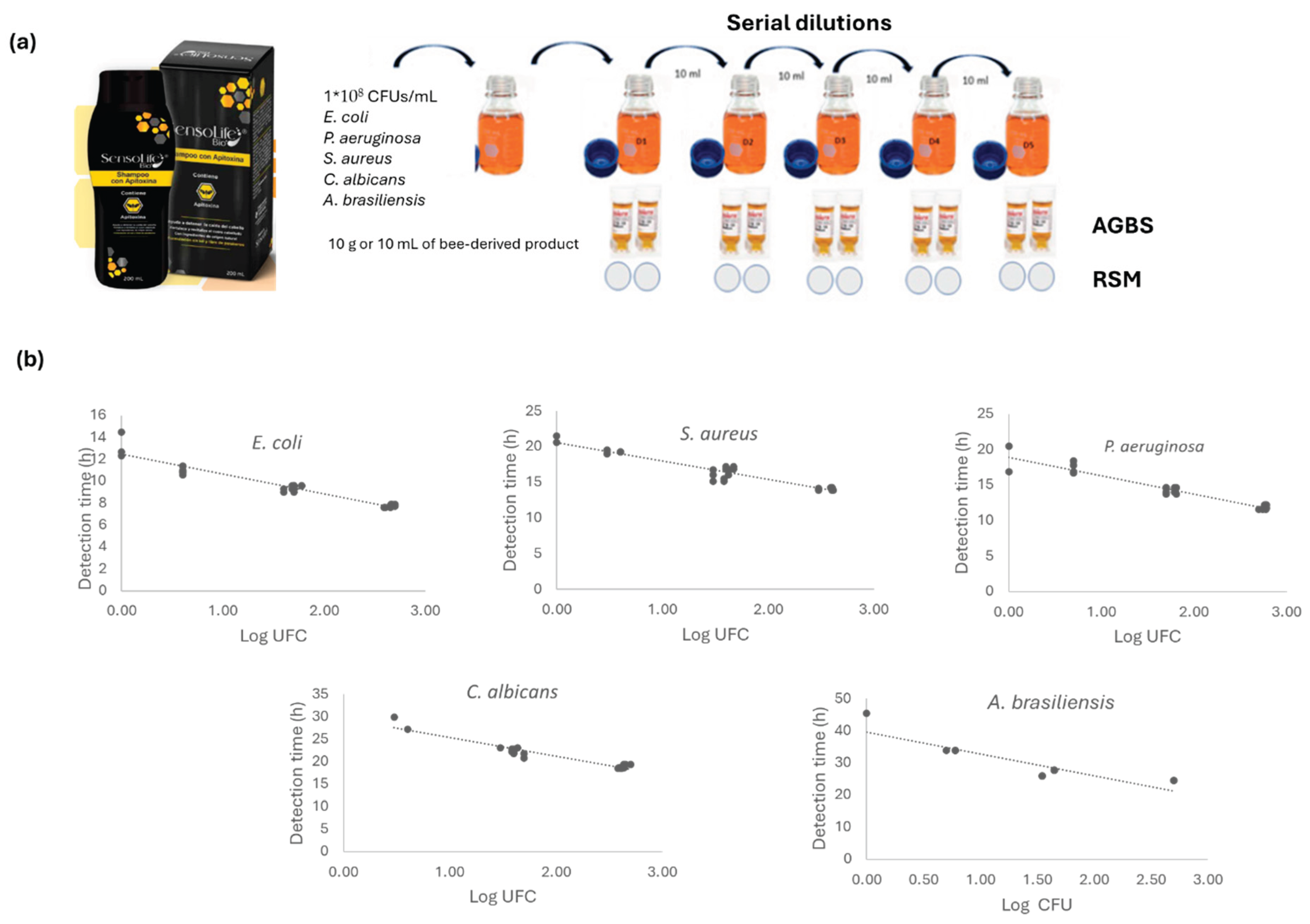
1. Introduction
Natural products such as apitoxin, royal jelly, propolis, bee pollen, and honey are increasingly being used in the cosmetic industry, because the wide spectrum of bioactive compounds such as phenols, flavonoids, enzymes, proteins, and fatty acids have wound-healing properties, high nutritional values, and regenerative potential that enable them to be included in several personal care formulations [
1,
2,
3,
4,
5,
6,
7,
8,
9,
10,
11,
12,
13]. These biological compounds are active in fighting oxidative stress, boosting collagen production, reducing inflammation, diminishing facial line expression, and inducing capillary cellular regeneration [
1,
2,
3,
4,
5,
6,
7,
8,
9,
10,
11,
12,
13]. The present investigation aims to assess the performance of an automated growth-based system (AGBS) to carry out a quantitative examination of the aerobic mesophiles from bee-derived personal care products. This cutting-edge technology brings an array of benefits, such as reducing microbiological process time for total aerobic mesophile assessment from 7 days, as with the reference standard method (RSM), to just 2 days, with reliable outcomes, since the AGBS meets all the requirements outlined in general chapter <1223>, which are suitability of the method, linearity, equivalence of results, operative range, precision, accuracy, robustness, specificity, limit of detection, and limit of quantification [
13,
14,
15,
16,
17,
18,
19]. Previously, we have proven the good performance of the AGBS as a quantitative method using as a pharmaceutical matrix an antacid oral suspension targeting objectionable pathogens like the
Burkorderia cepacia complex, yeasts, and molds [
13,
14]. However, its performance using bee-made products such as apitoxin-royal jelly-based anti-aging creams, propolis-honey-based toothpaste, and bee pollen, apitoxin, and royal jelly-based creams have been unexplored for quantitative total aerobic mesophile assessment. Regarding the antimicrobial properties of honey, apitoxin, royal jelly, bee pollen, and propolis, which prevent microbiological harvesting, it is interesting to test polysorbate as a chemical neutralizer of these bioactive compounds [
1,
2,
3,
4,
5,
6,
7,
8,
9,
10,
11,
12,
13]. Therefore, tests of the suitability of the method were carried out for all the bee-derived personal care products under study by means of chemical neutralization. Mesophile examination was performed by testing
Staphylococcus aures,
Escherichia coli,
Pseudomonas aeruginosa,
Candida albicans, and
Aspergillus brasiliensis as suitable representatives of mesophiles.
Regarding this matter, personal care products are not required to be completely aseptic, but a rapid and accurate microbiological assessment is required to examine the microbiological product’s quality in order to guarantee that the product complies with microbiological specifications. Therefore, in microbiological specification terms, cosmetic products with bioregulator activity must have a total viable aerobic microorganism count <10
2 CFU/mL or g., and harmful pathogens such as
S. aureus,
P. aeruginosa, and
E. coli must be undetectable in 1 mL or g [
16].
To assess the AGBS performance, the suitability of the method with apitoxin-royal jelly-based anti-aging creams, propolis-honey-based toothpaste, and bee pollen and apitoxin-royal jelly-based creams was tested and was shown to provide chemical neutralization of the all of these antimicrobials agents. Once the suitability of the method was proven, calibration curves were constructed by effecting a simultaneous recovery of the microorganisms by means of the RSM and AGBS for each bee-made product using
S. aures,
E. coli,
P. aeruginosa,
C. albicans, and
A. brasiliensis as representatives of all aerobic mesophiles’ viable count in order to demonstrate compliance with essential validation criteria such as linearity, equivalence of results, operative range, precision, accuracy, ruggedness, limit of detection, and limit of quantification [
13].
The AGBS is based on microbial metabolism using a nutrient-based liquid medium with dextrose as the carbon source (NF-TVC vials)
. NF-TVC vials are divided into two zones, growing and reading. Microorganisms are allowed to grow in the growing zone (liquid-enriched medium), which permits the growth of a broad spectrum of aerobic microorganisms including bacteria, yeasts, and molds. In this way, mesophiles grow in the NF-TVC vial yielding carbon dioxide, which diffuses through a gas-permeable layer from the growth medium toward the reading zone, which is a soft agar plug containing a dye indicator of the Soleris NF-TVC vial. Only gases can enter the reading zone; microorganisms, medium, and particulates are blocked. When carbon dioxide reaches the reading zone of the vial, it will trigger a color change from green (absence of microorganisms) to yellow (presence of microorganism) [
13,
14,
15,
16,
17,
18,
19]. This colorimetric change is detected and recorded by the equipment’s software and corresponds to the detection time (DT, h), indicative of a positive test result [
13,
14,
15,
16,
17,
18,
19].
2. Materials and Methods
(a) Reagents
The bee-made products chosen to carry out the present investigation, as well as their antimicrobial agents, are summarized in
Table 1, as described in the literature. For each bee-made product, three different lots were chosen to carry out microorganism recovery for both the AGBS and the RSM. The strains
A. brasiliensis (Cat. No. ATCC 16404),
C. albicans (Cat. No. ATCC 10231),
P. aeruginosa (Cat. No. ATCC 9027),
E. coli (Cat. No. ATCC 8739), and
S. aureus (Cat. No. ATCC 6538) were studied. Test microorganisms were from frozen stocks using culture maintenance techniques to ensure that viable microorganisms used for inoculation were not more than five passages removed from the master seed-lot. The reagents, namely tryptic soy broth, TSB (Scharlab, code 02-200); sabouraud dextrose agar, SDA (Neogen, cat. No. NCM0008); tryptic soy agar, TSA (Scharlab, code 02-200); and Tween® 80 (Scharlab, code 73625), were used as provided.
(b) Automated Growth-Based System
The Soleris® 128 instruments and supplies were acquired from Neogen. This system includes four incubator drawers (128 vial places) with a precise temperature control for each drawer (28.5 ± 0.5 °C) with dedicated software and computer. The design qualification (DQ), installation qualification (IQ), operational qualification (OQ), and performance qualification (PQ) of the system were satisfactorily fulfilled by both user and supplier. NF-TVC vials containing a nutrient-based medium with dextrose as the carbon source were used to quantify the S. aures, E. coli, P. aeruginosa, C. albicans, and A. brasiliensis as representatives of the total aerobic viable count.
(c) Inoculum Standardization
S. aures, E. coli, and P. aeruginosa were reactivated from frozen stocks in TSA and incubated for 48 h at 30–35 °C. C. albicans and A. brasiliensis were reactivated from frozen stocks in SDA and incubated for 48 hours at 20–25 °C. After the growth time was completed, isolated colonies were taken and resuspended in 0.9% saline solution until they reached a McFarland standard of 2 (equivalent to 1.0x108 CFU/mL).
(d) Suitability of the Method
The bee-derived product’s preservatives (phenols, flavonoids, enzymes, fatty acids, melittin-peptide, and other antimicrobial agents) were neutralized using polysorbate 80 to obtain a successful microbiological determination by means of RSM and AGBS, in order to set up an equivalence between the methods and generate a calibration curve for apitoxin-royal jelly-based anti-aging creams, propolis-honey-based toothpaste, and bee pollen, apitoxin, and royal jelly-based creams for the validation. Thus, carrying out independent assays, 1 mL of inoculum dilution from each microorganism (S. aures, E. coli, P. aeruginosa, C. albicans and A. brasiliensis) was added to a Schott bottle and mixed with 90 mL of tryptic soy broth in the presence of the selected neutralizing agent (1 mL /L of Tween® 80 in TSB). Then 10 g or 10 mL of the corresponding bee-derived product was added and vigorously shaken to ensure homogenization of the sample.
(f) Calibration Curve
Once proven the efficacy of the neutralizing polysorbate on bioactive compounds of apitoxin, royal jelly, propolis, bee pollen, and honey-based products, calibration curves were built by testing the 5 strains. From this, a serial dilution was performed. From each dilution (D1–D5), 1 mL was placed directly into each NF-TVC vial, and they were incubated for 48 hours at 28.5 °C in the Soleris® equipment. The NF-TVC vials were inoculated in duplicate. Simultaneously, TSA plates were also inoculated in duplicate with 1 mL of each dilution by means of the spread plate technique. The plates were incubated at 30 °C for 7 days. This series of experiments was performed using at least three different lots for each bee-derived product. An uninoculated NF-TVC vial was used as a negative control, and an inoculated vial with 1 mL of the dilution (count of 10–100 CFU/mL) was used as a positive control for each strain tested. Each detection time (DT) recorded by the Soleris® system within the incubation period (48 h), also confirmed by color change, was an indication of the presence of microorganisms. Data generated by both the AGBS (detection times, DT) and RSM (colony forming unit, CFU) were plotted in Soleris@ software to generate calibration curves by plotting DTs relative to the corresponding log CFU values.
(g) Linearity and Equivalence of Results
The plotted values in the calibration curve were fit to a least-squares regression, and the coefficient of determination (R2) was calculated. The linearity was determined using the chi-square (x2) goodness-of-fit test model in order to evaluate the relationship between the CFUs and the DT data obtained from the RSM and the AGBS, respectively. In this way, for each bee-product tested, 5 calibration curves were constructed, corresponding to the S. aures, E. coli, P. aeruginosa, C. albicans, and A. brasiliensis strains tested.
(h) Accuracy
The coefficient of correlation (CC) obtained from the calibration curves was a measure of accuracy, according to USP general chapter <1223>. Additionally, DT values and their log 10 equivalent CFU counterparts were statistically analyzed using the Pearson goodness-of-fit test based on a Poisson distribution. In this way, DT yields with AGBS were automatically converted into CFU by means of calibration curves constructed for each microorganism tested. Thus using the Pearson goodness-of-fit test, it was possible to predict CFU from DT with a 95% confidence interval.
(i) Limit of Detection and Limit of Quantification
The limit of detection (LOD) and limit of quantification (LOQ) were determined from the calibration curves based on the fewest number of recoverable microorganisms (<10 CFU). The LOD and LOQ for both methodologies (RSM and AGBS) were calculated using the following equations: LOD=3.3*SD/m and LOQ=10*SD/m, where SD is the standard deviation and m is the slope of the linear regression obtained for each calibration curve, as stated in the International Council of Harmonization (Q2) R2 and USP guidelines [
21].
(j) Precision
S. aures, E. coli, P. aeruginosa, C. albicans, and A. brasiliensis were used to determine the suitability of the method with apitoxin-royal jelly-based anti-aging creams, propolis honey-based toothpastes, and bee pollen, apitoxin, and royal jelly-based creams using three different lots for each bee-derived product tested. Serial dilutions were carried out, and the microorganisms were recovered simultaneously via RSM and AGBS. Dilutions that recovered microorganisms in the 10 to 300 CFU range via the RSM and AGBS were analyzed in order to determine the standard deviation (SD) and coefficient of variation (CV).
3. Results
3.1. Suitability of the Method (Antimicrobial Neutralization)
Antimicrobial activity has been widely described in apitoxin, honey, royal jelly, propolis, and bee pollen because of the existence of bioactive compounds with antimicrobial activity, which prevent microbial harvesting [
1,
2,
3,
4,
5,
6,
7,
8,
9,
10,
11,
12,
13] (
Table 1). Hence it is important to consider that suppression of antimicrobial activity needs to be done in all the bee-made products in order to successfully recover microorganisms and guarantee the product’s microbiological quality assessment. Therefore, polysorbate 80 was used to neutralize the antimicrobial activity of the bee-derived products (
Table 1). As outlined in USP <61>, polysorbate is able to neutralize quaternary ammonium compounds (QACs), iodine, and parabens [
23]. In the current investigation, it was shown that polysorbate 80 can neutralize bioactive preservative compounds such as flavonoids, phenols, enzymes, royal jelly major proteins, melittin, and fatty acid such as 10 HDA, permitting a suitable microorganism recovery, as observed for the S. aures, E. coli, P. aeruginosa, C. albicans and A. brasiliensis calibration curves (
Table 1).
Figure 1.
(a) Experimental design used for the validation testing of the AGBS. Construction of correlation curves. The suitability of the method test was carried out for each bee-derived product. From this, a serial dilution was performed. From each dilution (D1–D5), 1mL was placed directly into each NF-TVC vial, and then incubated for 48 h at 28.5 °C in the Soleris equipment. Simultaneously, agar plates were also inoculated in duplicate with 1mL of each dilution by means of the pour-plate technique. The plates were incubated at 25 °C for 7 days for yeast and molds recovery and at 30 °C for 5 days for mesophiles recovery. Data generated by both the alternative and conventional method were plotted to generate calibration curves by plotting DTs relative to the corresponding log CFU values. For each bee-product tested, 5 calibration curves were constructed, corresponding to the S. aures, E. coli, P. aeruginosa, C. albicans, and A. brasiliensis.
Figure 1.
(a) Experimental design used for the validation testing of the AGBS. Construction of correlation curves. The suitability of the method test was carried out for each bee-derived product. From this, a serial dilution was performed. From each dilution (D1–D5), 1mL was placed directly into each NF-TVC vial, and then incubated for 48 h at 28.5 °C in the Soleris equipment. Simultaneously, agar plates were also inoculated in duplicate with 1mL of each dilution by means of the pour-plate technique. The plates were incubated at 25 °C for 7 days for yeast and molds recovery and at 30 °C for 5 days for mesophiles recovery. Data generated by both the alternative and conventional method were plotted to generate calibration curves by plotting DTs relative to the corresponding log CFU values. For each bee-product tested, 5 calibration curves were constructed, corresponding to the S. aures, E. coli, P. aeruginosa, C. albicans, and A. brasiliensis.
3.2. Linearity, Operative Range, and Equivalence of Results
Considering that the RSM and AGBS yield quantitative data with different units (CFU vs. DT), an equivalence of results correlation needs to be done through building calibration curves. Thus calibration curves for all bee-made products were derived by plotting DT values with their respective equivalents in log CFU. Linear regression analysis yielded the relationship between DTs and log CFU values, as shown in
Table 2. The linearity observed in all bee-derived products was consistent with USP <1223> (R
2 ≥ 0.9025), and linearity for all bee-made products tested ranged from 1 CFU to around 1000 CFU (
Table 2).
3.3. Accuracy
The Pearson goodness-of-fit test was chosen to assess the comparison of the results obtained by AGBS and those by RSM for each bee-made product for all the strains tested. As shown in
Table 3 , DT values can be calibrated in CFU values according to the Pearson goodness-of-fit test, P ≥ 0.05, (
Table 3).
3.4. Limit of Detection and Limit of Quantification
The LOD and LOQ were calculated using the standard deviation of data obtained for the fewest number of recoverable microorganisms (<10 CFUs) and the slope of the corresponding calibration curves constructed for S. aures, E. coli, P. aeruginosa C. albicans, and A. brasiliensis. As is shown in
Table 4, the LOD and the LOQ of the AGBS for all bee-made products tested were < 10 CFU for all the strains tested, showing that it is not inferior in terms of sensibility compared to the RSM (< 10 CFUs).
3.5. Intermediate Precision and Ruggedness
The precision was measured based on a dilution that recovered CFUs within a range of 10–300 using AGBS and its equivalent in RSM for all the strains tested (S. aures, E. coli, P. aeruginosa C. albicans, and A. brasiliensis). As shown in
Table 5, for all bee-made products tested, the coefficient of variation (CV) was below USP specification, <15% (30–300 CFUs).
For this experiment, the ruggedness was interpreted as intermediate precision, a type of intra-laboratory precision involving the effect of different lots on the test result variability, as well as on the repeatability (CV <15%).
4. Discussion and Conclusions
We have shown that polysorbate 80 can be used as a suitable chemical neutralizer of bioactive compounds with well-known antimicrobial potential, such as melittin, phenols, flavonoids, enzymes, and fatty acids such 10 HDA. This is a notable finding, since chemical neutralization is pivotal in routine pharmaceutical analyses to assess the microbiological quality through a total aerobic mesophile enumeration. Therefore, thanks to the suitability of the method, an optimal microorganism’s recovery was possible from the three different bee-made matrices tested by means of AGBS and RSM. Hence, using S. aures, E. coli, P. aeruginosa C. albicans, and A. brasiliensis as suitable representatives of all aerobic mesophiles, it was possible to prove essential validation criteria such as linearity, operative range, equivalence of results, precision, accuracy, ruggedness, limit of detection, and limit of quantification.
Statistical analysis of the x2 goodness-of-fit test demonstrated that there exists a strong relationship between threshold bioburden (dilutions) in NF-TVC vials and DTs. This occurs because carbon dioxide is a direct measure of the microbial burden in the NF-TVC, so a high concentration of aerobic mesophiles in AGBS yields low DT values, while at low bioburden this automated system yields high DT values.
Using the Pearson goodness-of-fit test, the AGBS was able to predict the CFUs from the DT values with a 95% confidence interval in the calibration curves constructed for S. aures, E. coli, P. aeruginosa, C. albicans, and A. brasiliensis (P ≥ 0.05,
Table 3). Therefore, AGBS is able to predict an equivalent CFU result from a DT through the calibration curve. Each tested product should have its own calibration curve, since the active chemical and biological principle of the bee-made produtc may have a strong impact on the kinetic of the microorganism’s recovery. Even though AGBS yielded quantitative results in DT, those results will be converted automatically into equivalent CFU, meaning that the AGBS would have the same specification as described by the RSM (CFU/mL).
The use of this broth-based technique (with dextrose as the carbon source) for routine analyses of bee-made products such as apitoxin-royal jelly-based anti-aging creams, propolis-honey-based toothpaste, and bee pollen, apitoxin, and royal jelly-based creams enables reducing the microbiological assessment from 7 days, as is usual with the RSM, to just 2 days. This fact was clearly demonstrated by means of the calibration curves obtained for each bee-made product, in which the AGBS was able to detect 1 CFU for total viable counts in a maximum incubation time of 48 h, while the RSM was able to detect 1 CFU within a maximum time of 7 days of incubation. The implementation of this ground-breaking method is supported by USP guidelines, since it provides benefits such as being less labor intensive, reducing company warehousing costs, improving efficiency in inventory control, increasing the ability to respond more quickly to adverse microbiological results, and allowing faster product release into the market, as well as being more sensitive than the RSM.
Author Contributions
Authors’ Contributions: All authors contributed equally to the writing of the article. Additionally, all authors have 544 reviewed and approved the published version of the article.
Funding
No funding was received for this study.
Ethical Approval Statement
This article has been prepared in accordance with ethical standards and principles. All research conducted for this study was approved by the relevant ethics committee, ensuring that the rights and welfare of participants were safeguarded. Information consent was obtained from all participants involved in the study, and confidentiality was maintained throughout the research process. The authors declare that there are no conflicts of interest related to this publication.
Informed Consent Statement
All the authors give consent for the manuscript to be published, including individual’s data or image.
Data Availability Statement
The data underlying this article will be shared at reasonable request to the corresponding author
Acknowledgments
The authors thank Vital Health Solution for their technical support and helpful advice for the validation testing.
Conflicts of Interest
The authors declare no conflict of interest. All the research was funded by Laboratorios Coaspharma S.A.S.
References
- Rodica, M.; Mirela, S.; Alina, V.; Erkan, T.; Banu, Y.; Mihaiela, C.-C.; Maria, G.-C; Dan, C.-V. Bee Collected Pollen and Bee Bread: Bioactive Constituents and Health Benefits. Antioxidants. 2019, 8, 568. [Google Scholar] [CrossRef]
- Lilla, B.; Baci, G.-M.; Dezmirean, D.-S. Royal Jelly as a Nutraceutical Natural Product with a Focus on Its Antibacterial Activity. Pharmaceutics. 2022, 14, 1142. [Google Scholar] [CrossRef]
- Buitrago, D.; Perdomo, S.; Silva, F.; Cely-Veloza, W.; Lafaurie, I. Physicochemical Characterization, Antioxidant, and Proliferative Activity of Colombian Propolis Extracts: A Comparative Study. Molecules. 2024, 29, 1643. [Google Scholar] [CrossRef] [PubMed]
- Rim, W.; Jacinthe, F.; Mohamad, R.; Dany, E.-O.; Jean, M.; Ziad, F. Bee Venom: Overview of Main Compounds and Bioactivities for Therapeutic Interests. Molecules. 2019, 24, 2997. [Google Scholar] [CrossRef]
- Aida, E.; Shaden, K.; Mohamed, E.; Syed, M.; Aamer, S.; Alfi, K.; Haroon, T.; Xiaobo, Z.; Yahya, N.; Arshad, M.; Kai, W.; Hesham, E. Cosmetic Applications of Bee Venom. Toxins. 2021, 13, 810. [Google Scholar] [CrossRef]
- Collazo, N.; Carpena, M.; Nuñez, E.-B.; Otero, S.-G.; Prieto, M.-A. Health Promoting Properties of Bee Royal Jelly: Food of the Queens. Nutrients. 2021, 13, 543. [Google Scholar] [CrossRef]
- Soukaïna, E.-G.; Alexandra, M.-M.; Smail, A.; Badiaâ, L.; Maria, G.-M.; Maria, C.-M. ; Figueiredo,C.-A. Chemical Characterization and Biological Properties of Royal Jelly Samples From the Mediterranean Area. Natural Product Communications. [CrossRef]
- Nada, Oršolić. ; Maja, J.-J. Royal Jelly: Biological Action and Health Benefits. Int J Mol Sci. 2024, 25, 6023. [Google Scholar] [CrossRef]
- Joanna, Kocot. ; Małgorzata, K.; Dorota, L.-K., Jacek, Kurzepa., Eds.; Irena, Musik. Antioxidant Potential of Propolis, Bee Pollen, and Royal Jelly: Possible Medical Application. Oxid Med Cell Longev. 2018 May 2, 7074209. [Google Scholar] [CrossRef]
- Hiroshi, K.; Amira, M.-A. ; Royal Jelly and Its Components Promote Healthy Aging and Longevity: From Animal Models to Humans. Int J Mol Sci. 2019, 20, 4662. [Google Scholar] [CrossRef]
- Nicolas, Collazo. ; Maria, Carpena.; Bernabe, N.-E.; Paz, Otero.; Jesus, S.-G.; Prieto, M.-A. Health Promoting Properties of Bee Royal Jelly: Food of the Queens. Nutrients. 2021, 13, 543. [Google Scholar] [CrossRef] [PubMed]
- Filippo, F.; Giovanni, C.; Simone, M.; Antonio, F. Royal Jelly: An ancient remedy with remarkable antibacterial properties. Microbiol Res. 2016, 192, 130–141. [Google Scholar] [CrossRef]
- Shanshan, L.; Lingchen, T.; Xinyu, Y.; Huoqing, Z.; Jianping, W.; Fuliang, H. Royal Jelly Proteins and Their Derived Peptides: Preparation, Properties, and Biological Activities. J Agric Food Chem. 2021, 69, 14415–14427. [Google Scholar] [CrossRef]
- Prada, H.-A.; Beltran, A.-U.; Celeita, S.-P.; Fonseca, J.-C. Performance equivalence and validation of a rapid microbiological method for detection and quantification of yeast and mold in an antacid oral suspension. PDA J Pharm Sci Technol, 2023, 77, 1–14. [Google Scholar] [CrossRef]
- Prada, H.-A.; Celeita, S.-P.; Fonseca, J.C. Validation of a rapid microbiological method for the detection and quantification of Burkholderia cepacia complex in an antacid oral suspension. J AOAC Int, 1294. [Google Scholar] [CrossRef]
- Prada, H.-A.; Celeita, S.-P.; Fonseca, J.C. Efficacy of an automated growth- based system and plate count method on the detection of yeasts and molds in personal care products. J AOAC Int, 2023, 6, 1564–1573. [Google Scholar] [CrossRef] [PubMed]
- Prada, H.A. Review on enforcement of alternative microbiological method in the pharmaceutical industry. Syst Rev Pharm, 2023, 10, 616–621. [Google Scholar] [CrossRef]
- Limberg, B.-J.; Johnstone, K.; Filloon, T.; Catrenich, C. Performance equivalence and validation of the soleris automated system for quantitative microbial content testing using pure suspension cultures. J AOAC Int. 2016, 99, 1331–1337. [Google Scholar] [CrossRef] [PubMed]
- Mozola, M.; Gray, L.-R.; Feldpausch, J.; Alles, S.; McDougal, S.; Montei, C. ; Montei, C. Validation of the Soleris® NF-TVC method for determination of total viable count in a variety of foods. J AOAC Int. 2013, 96, 399–403. [Google Scholar]
- Validation of alternative microbiological methods (Chapter-1223). United States Pharmacopeia (USP). 2025.
- Validation of compendial methods (Chapter-1225). United States Pharmacopeia (USP). 2025.
- United State Pharmacopeia Convention 42 (2021) Microbiological Examination of Nonsterile Products: Microbial Enumeration Test. Rockville, MD, Ch. 8363; 61.
- Prada, H.-A.; Raquel, G.-P.; Willy, C.; Ericsson, C.-B.; Sandra, G.; Rodrigo, P.-B.; Juan, M.-T.; Romel, P.-R.; David, D.-B.; Lafaurie, G.-I.; Zardo, H. Investigation of microbiological and organoleptic properties of bee products (bee venom, solid pollen, and royal jelly) through water activity quantification during 8 days of storage. Journal of Food Science and Technology.
- Prada, H.-A.; Willy, C.; Raquel, G.-P.; Ericsson, C.-B.; Santiago, R.-C.; Sandra, G.; Rodrigo, P.-B.; Juan, M.-T.; Romel, P.-R.; Zardo, H.; David, D.-B.; Lafaurie, G.-I. Identification and Quantification of Melittin in honeybee (Apis mellifera) by High Performance Liquid Chromatography (HPLC). A Comparative Study from Three Colombian Regions. Corresponding author Prada, H. Bogotá, Colombia, 2025 (manuscript in preparation; to be submitted).
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).