Submitted:
19 August 2025
Posted:
20 August 2025
You are already at the latest version
Abstract
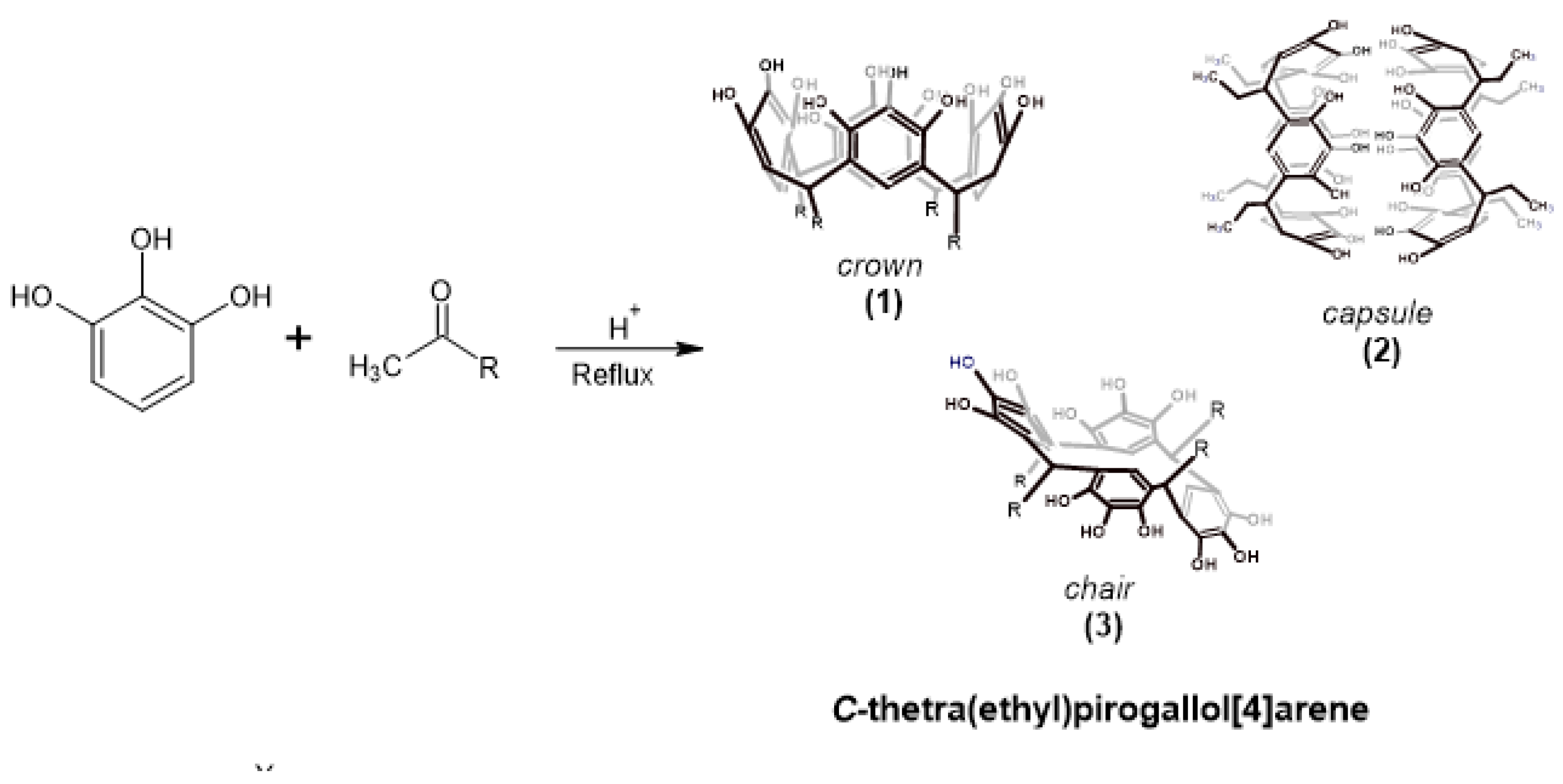
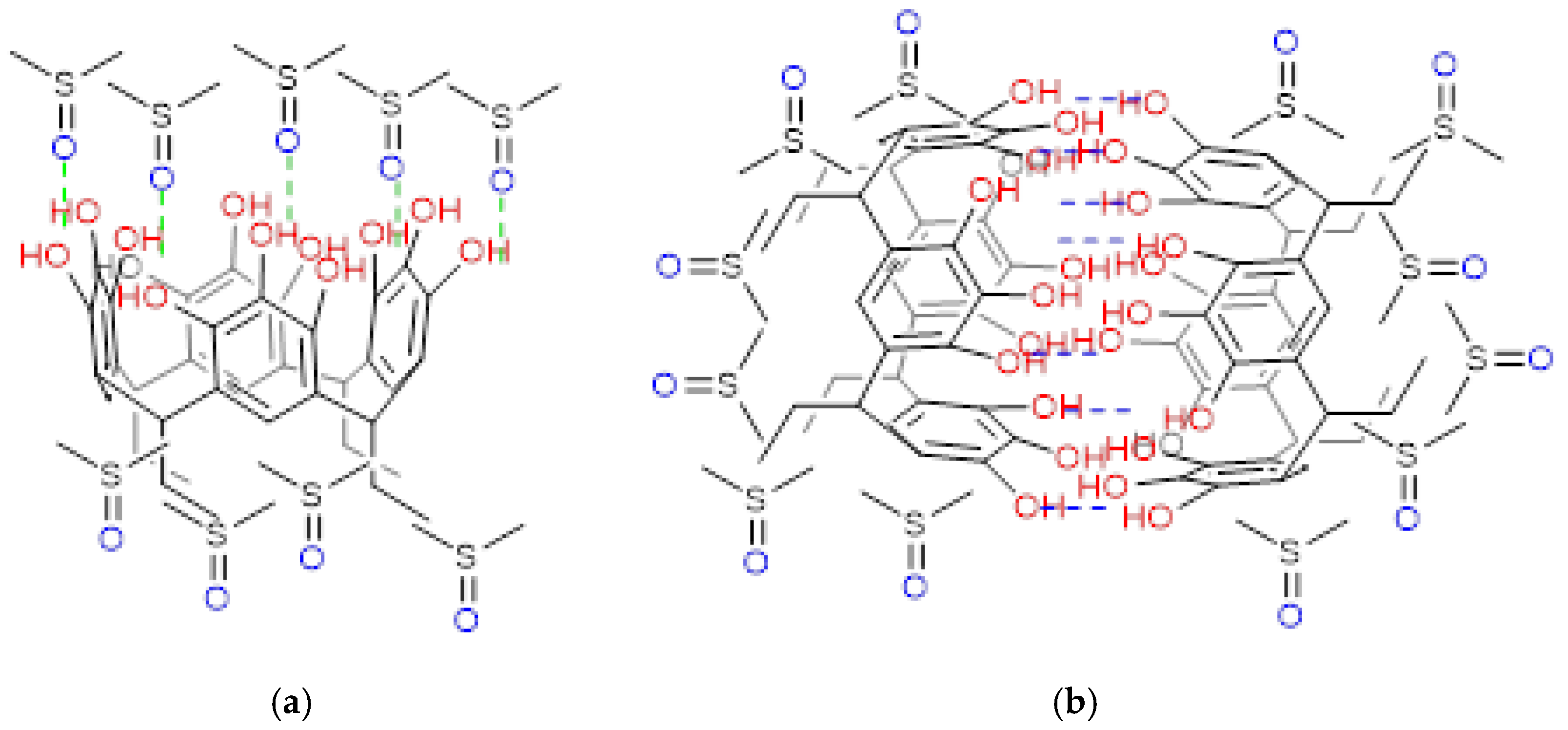
Pyrogallol[4]arenes are polyhydroxylated compounds obtained by condensation between pyrogallol and different aldehydes. Depending on both the type of aldehyde (aromatic or aliphatic) and the reaction time, these compounds can be obtained in different conformations, the most common being the crown and chair conformations. Using the conventional synthesis method, it is possible to obtain, in addition to the chair or crown conformers, other molecular associations, such as dimer capsules. The research in this study focuses on the synthesis products obtained from the condensation between pyrogallol and propanal. These products obtained were characterized using spectroscopic methods, finding that it is possible to obtain, in addition to the crown conformation, the dimer capsule of the macrocycle. Finally, the volumetric properties of these conformers were evaluated in dimethylsulfoxide (DMSO) solution and at several temperatures.
Keywords:
1. Introduction
2. Results and Discussion
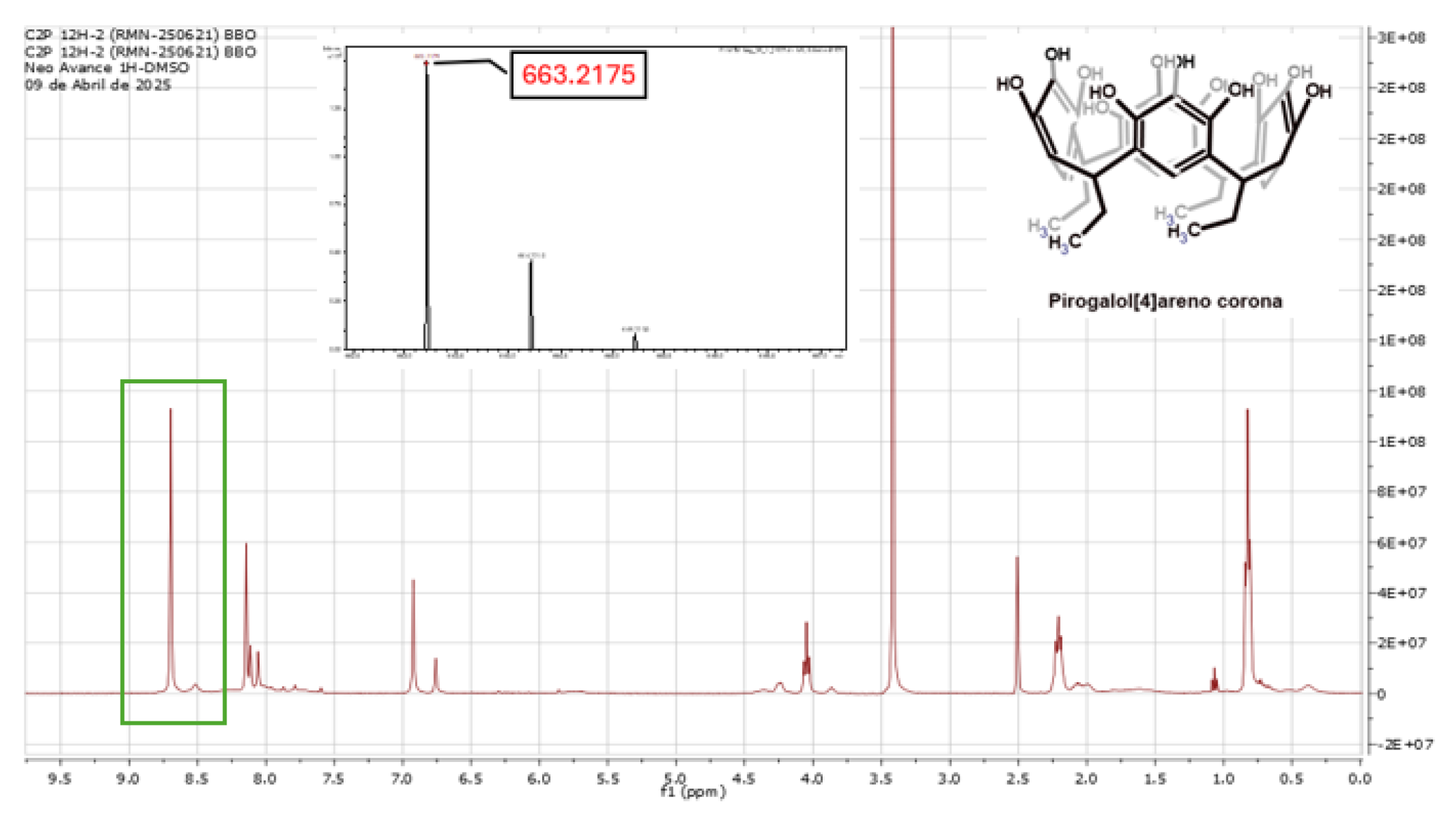
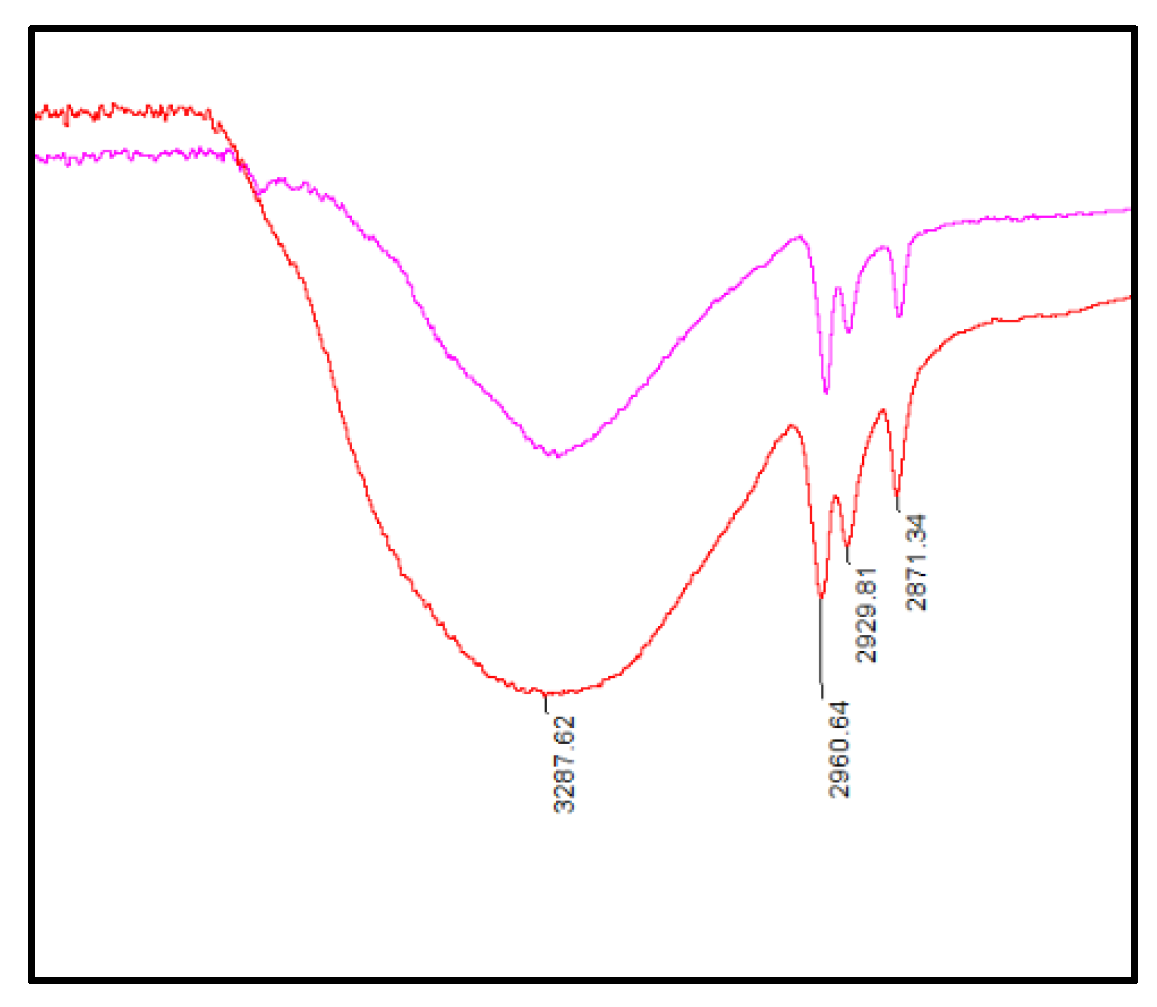
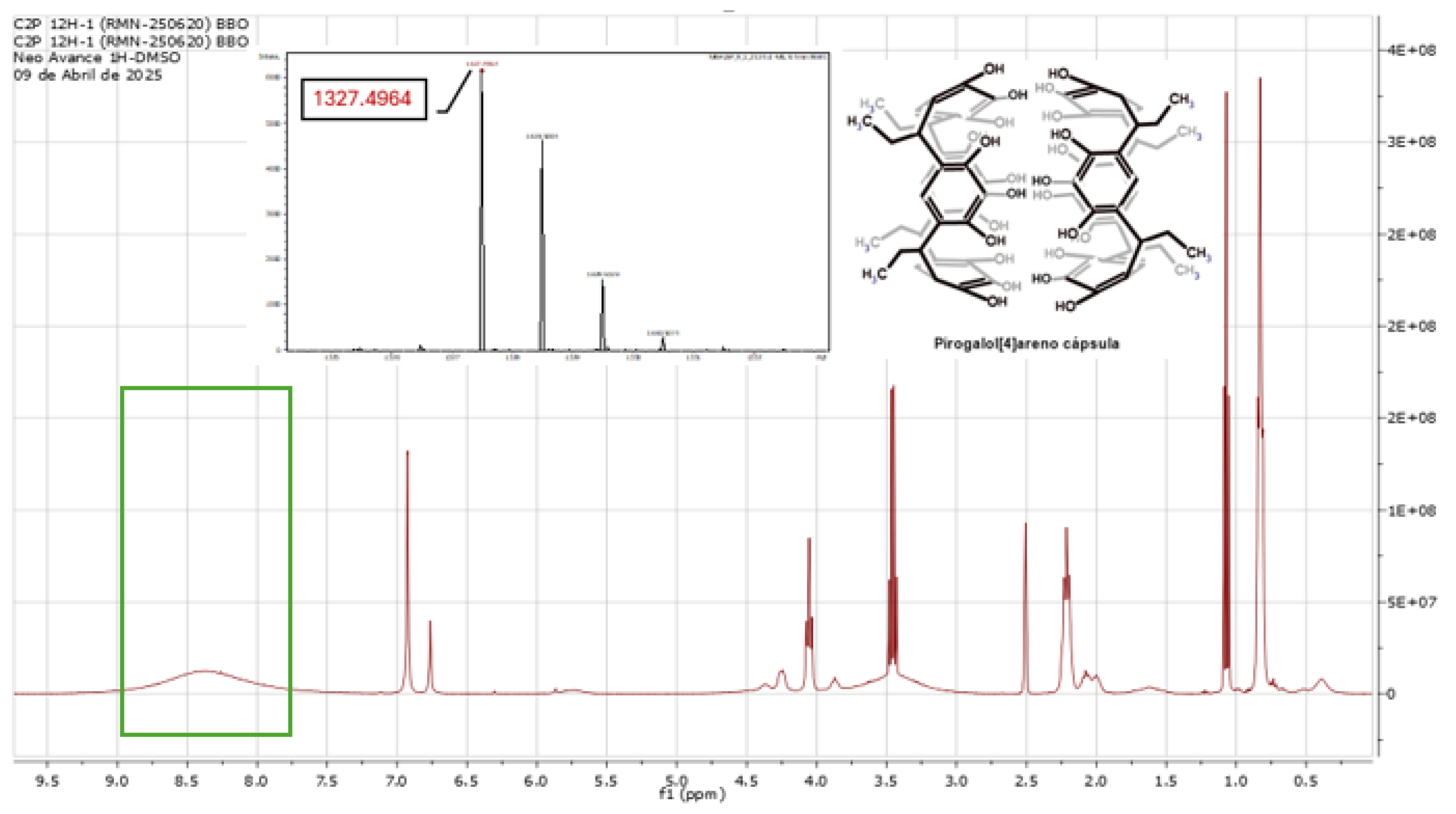
2.1. Reaction of Propionaldehyde with Pyrogallol
2.2. Calculation of Apparent Molar Volume and Standard Molar Expansibility
3. Materials and Methods
3.1. Synthesis of C-Tetra(Ethyl)Pyrogallol[4]Arene
3.2. Volumetric Properties of Monomer and Capsule Pyrogallolarene in DMSO Solution
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| FT-IR | Fourier Transform Infrared Spectroscopy |
| 1H NMR | 1H-Nuclear Magnetic Resonance |
| 13C NMR | 13C-Nuclear Magnetic Resonance |
| ESI-MS | Electrospray Ionization Mass Spectrometry |
References
- Zambrano, C.H.; Manzano, C.A.; Saltos, A.A.; Dueno, E.E.; Zeller, M. Síntesis de 2,8,14,20-tetra-n-butilpirogalol[4]areno y estudio computacional conformacional. ACI Av. Cienc. Ing. (Quito) 2010, 2, junio. [Google Scholar] [CrossRef]
- Davis, F.; Higson, S. Calixarenes. In Macrocycles: Constructions, Chemistry and Nanotechnology Applications; John Wiley & Sons, Ltd, Hoboken, Nueva Jersey, U.S., 2011; chapter 4, pp. 77-125.
- Bowley, N. Synthetic and structural studies of calix[4]pyrogallol[4]arenes: towards biological applications. Ph.D. Thesis, Nottingham Trent University, UK, 2008. [Google Scholar]
- Griffin, P. Pyrogallo4arenes: a synthetic investigation. Ph.D. Thesis, Dublin City University, 2007.
- Casas-Hinestroza, J.L.; Cifuentes, A.; Ibáñez, E.; Maldonado, M. Effect of the formation of capsules of tetra(propyl) pyrogallol[4]arene on the host-guest interaction with neurotransmitters, J. Mol. Struct. 2020, 1210, 128063. [Google Scholar] [CrossRef]
- Alshahateet, S.F.; Kooli, F.; Messali, M.; Judeh, Z.M.A.; ElDouhaibi, A.S. Synthesis and Supramolecularity of C -Phenylcalix[4] Pyrogallolarenes: Temperature Effect on the Formation of Different Isomers, J. Mol. Cryst. Liq. Cryst. 2007, 474, 89–110. [Google Scholar] [CrossRef]
- Gibb, B.C.; Chapman, R. G.; Sherman, J. C. Synthesis of Hydroxyl-Footed Cavitands. J. Org. Chem. 1996, 61, 1505–1509. [Google Scholar] [CrossRef]
- Barrett, E.S.; Dale, T.J.; Rebek Jr., J. Synthesis and Assembly of Monofunctionalized Pyrogallolarene Capsules Monitored by Fluorescence Resonance Energy Transfer. Chem. Commun. 2007, 4224–4226. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Swager, T. Main-chain calixarene polymers: conformational effects on polymerization. Macromolecules 2006, 39, 2013–2015. [Google Scholar] [CrossRef]
- Grannas, M.; Hoskins, B.; Robson, R. Synthesis and x-ray crystal structures of a calixarene-related, tetraamino, tetraphenolic, polynucleating macrocyclic ligand and its ZnII4 and CoIII3 derivates. Inorg.Chem. 1994, 33, 1071–1079. [Google Scholar] [CrossRef]
- Cave, G.; Dalgarno, S.; Antesberger, J.; Ferrarelli, M.; McKinlay, R.; Atwood, J. Investigations into chain length control over solid-state pyrogallol[4]arene nanocapsule packing. Supramol. Chem. 2008, 2), 157–159. [Google Scholar] [CrossRef]
- Hof, F.; Trembleau, L.; Ullrich, E.C.; Rebek Jr, J. Acetylcholine Recognition by a Deep, Biomimetic Pocket. Angew. Chemie Int. Ed. 2003, 42, 3150–3153. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.K.; Kang, B.; Koh, H.S.; Yoon, Y.J.; Jung, S.J.; Jeong, B.; Lee, K.; Yoon, J. A New Imidazolium Cavitand for the Recognition of Dicarboxylates. Org. Lett. 2004, 6, 4655–4658. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.; Adams, R.D.; Fenske, D. Stable hydrogen-bonded spherical capsules formed from self-assembly of pyrogallol[4]arenes, J. Inclusion Phenom. Macrocycl. Chem. 2005, 53, 275–279. [Google Scholar] [CrossRef]
- Gangemi, C.M.A.; Pappalardo, A.; Sfrazzetto, G.T. Applications of supramolecular capsules derived from resorcin[4]arenes, calix[n]arenes and metalloligands: from biology to catalysis, RSC Adv. 2015, 5, 51919-51933.
- Krause, T.; Gruner, M.; Kuckling, D.; Habicher, W.D. Novel starshaped initiators for the controlled radical polymerization based on resorcin[4]- and pyrogallol[4]arenes, Tetrahedron Lett. 2004, 45, 9635-9639. 45.
- Späth, A.; König, B. Molecular recognition of organic ammonium-ions in solution using synthetic receptors, Beilstein J. Org. Chem. 2010, 6, 132–133. [Google Scholar]
- Negin, S.; Li, R.; Kulikov, O.V.; Daschbach, M.M.; Gokel, G.W. Ion transport through bilayer membranes mediated by pyrogallol[4]arenes, Inorg. Chim. Acta 2014, 417, 177–185. [Google Scholar]
- Buschmann, H.J.; Schollmeyer, E. Improved adhesion of polyurethane coating to polyester fabrics due to the surface fixation of calixarenes, J. Adhes. Sci.Technol. 2010, 24, 113–121. [Google Scholar] [CrossRef]
- Casas-Hinestroza, J. L.; Pérez-Redondo, A.; Maldonado, M. Inclusion complexation between neurotransmitters with polyacetylated calix[4]pyrogallol arenes: 1H-NMR and crystallographic analysis. Res. Chem. Intermed. 2022, 48, 3091–3107. [Google Scholar] [CrossRef]
- Casas-Hinestroza, J.L.; Maldonado, M. Conformational Aspects of the O-acetylation of C-tetra(phenyl)calixpyrogallol[4]arene. Molecules 2018, 23, 1225. [Google Scholar] [CrossRef] [PubMed]
- Shivanyuk, A.; Rebek Jr., J. Hydrogen-bonded capsules in polar, protic solvents, Chem. Commun. 2001, 2374 -2375.
- Kumari, H.; Good, A.J.; Smith, V.J.; Barbour, L.J.; Deakyne, C.A.; Atwood, J.L. 18-Crown-6 templates offset-linked pyrogallol[4]arene dimers, Supramol. Chem. 2013, 25, 591–595. [Google Scholar]
- NanJi, S.; Marriot, R. Apparent molar volumes of isoprene and limonene in dense-phase CO2. J. Supercrit. Fluids 2025, 221, 106544. [Google Scholar] [CrossRef]
- Taylor, N.B.; Kuyatt, C.E. Guidelines for Evaluating and Expressing the Uncertainty of NIST Measurement Results. National Institute of Standards and Technology, Gaithersburg, U.S.; NIST Technical Note 1297.
- Tiantian, M.; Haiyun, H.; Mengjiao, Z.; Renzhong, L.; Junru, W.; Yanping, D. Effect of temperature on the physicochemical properties and solute–solvent interactions of a dilute solution of [Bmim][OAc] in DMSO. J. Mol. Liq. 2025, 418, 126717. [Google Scholar]
- Parvinder, V.; Kirtanjot, K. Thermodynamic insight of DMSO-DMA and DMSO-DMF binary mixtures across varying temperatures. J. Mol. Liq. 2024, 415, 126286. [Google Scholar]
 ), 298.15 K (
), 298.15 K ( ), 303.15 K (
), 303.15 K ( ), 308.15 K (
), 308.15 K ( ) and 313.15 K (
) and 313.15 K ( ) for: (a) crown pyrogalollarene, and (b) dimer capsule pyrogalollarene.
) for: (a) crown pyrogalollarene, and (b) dimer capsule pyrogalollarene.
 ), 298.15 K (
), 298.15 K ( ), 303.15 K (
), 303.15 K ( ), 308.15 K (
), 308.15 K ( ) and 313.15 K (
) and 313.15 K ( ) for: (a) crown pyrogalollarene, and (b) dimer capsule pyrogalollarene.
) for: (a) crown pyrogalollarene, and (b) dimer capsule pyrogalollarene.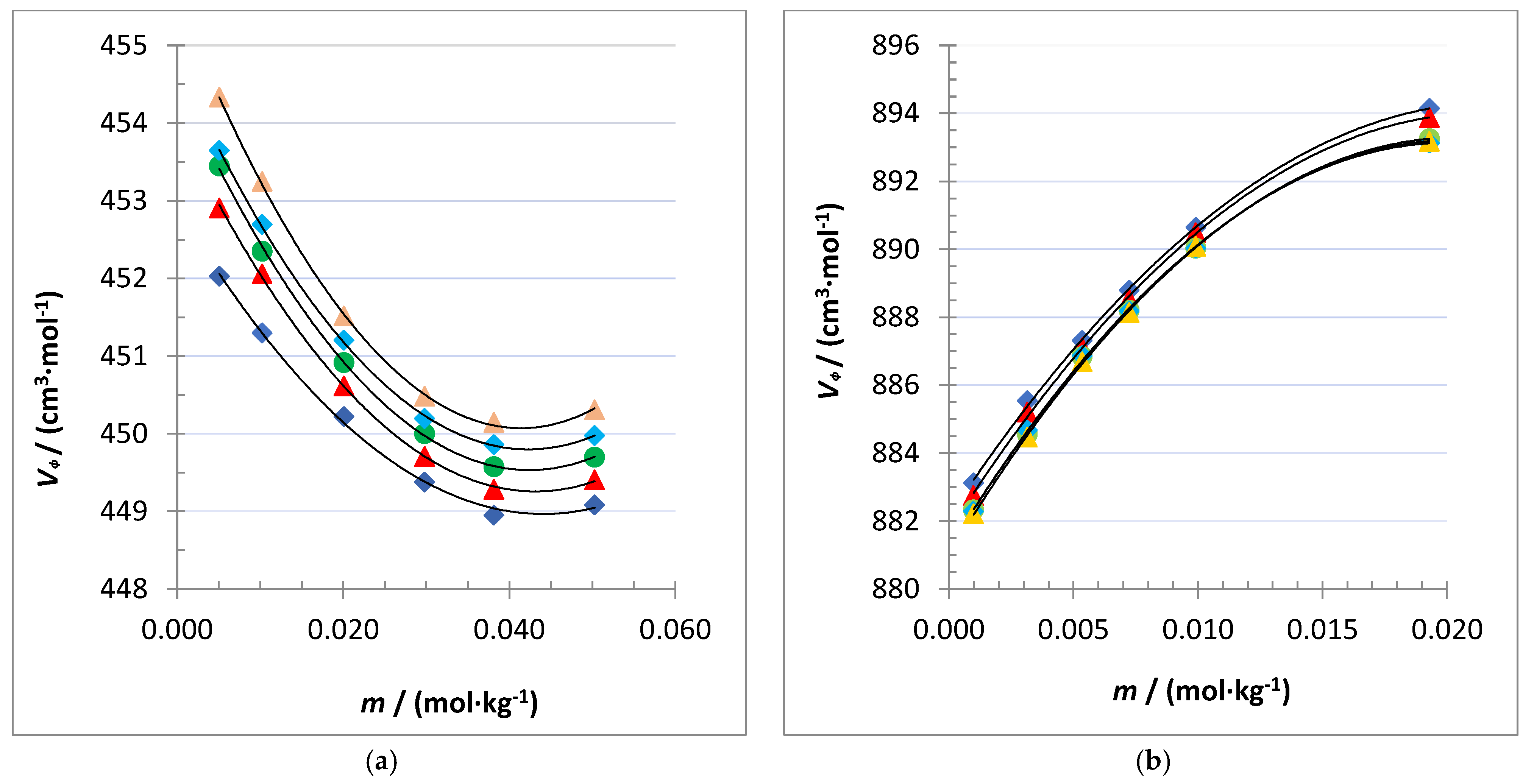
| Entry | Retention time (min) | Solvent EtOH/H2O |
||
|---|---|---|---|---|
| Crown (1) | 10.3 | (22%) | ||
| Dimer capsule (2) | 10.7 | (40 %) | - | |
| Chair (3) | 9.9 | (30%) | ||
| T/K | 293.15K | 298.15K | 303.15K | 308.15K | 313.15K | |||||
| m / (mol∙Kg-1) |
ρ / (g∙cm-3) |
Vϕ / (cm3∙mol-1) |
ρ / (g∙cm-3) |
Vϕ / (cm3∙mol-1) |
ρ / (g∙cm-3) |
Vϕ / (cm3∙mol-1) |
ρ / (g∙cm-3) |
Vϕ / (cm3∙mol-1) |
ρ / (g∙cm-3) |
Vϕ / (cm3∙mol-1) |
| 0.0050758 | 1.101341 | 452.03 | 1.096327 | 452.91 | 1.091316 | 453.45 | 1.086307 | 453.65 | 1.081298 | 454.34 |
| 0.010235 | 1.102292 | 451.34 | 1.097283 | 452.06 | 1.092280 | 452.35 | 1.087276 | 452.71 | 1.082273 | 453.23 |
| 0.020099 | 1.104116 | 450.22 | 1.099122 | 450.62 | 1.094131 | 450.92 | 1.089140 | 451.20 | 1.084151 | 451.52 |
| 0.029782 | 1.105906 | 449.38 | 1.100925 | 449.71 | 1.095946 | 450.00 | 1.090970 | 450.19 | 1.085993 | 450.49 |
| 0.038165 | 1.107444 | 448.95 | 1.102473 | 449.28 | 1.097504 | 449.58 | 1.092535 | 449.86 | 1.087568 | 450.15 |
| 0.050296 | 1.109618 | 449.08 | 1.104662 | 449.40 | 1.099708 | 449.70 | 1.094754 | 449.98 | 1.089799 | 450.31 |
| T/K | 293.15K | 298.15K | 303.15K | 308.15K | 313.15K | |||||||
| m / (mol∙Kg-1) |
ρ / (g∙cm-3) |
Vϕ / (cm3∙mol-1) |
ρ / (g∙cm-3) |
Vϕ / (cm3∙mol-1) |
ρ / (g∙cm-3) |
Vϕ / (cm3∙mol-1) |
ρ / (g∙cm-3) |
Vϕ / (cm3∙mol-1) |
ρ / (g∙cm-3) |
Vϕ / (cm3∙mol-1) |
||
| 0.00099709 | 1.100766 | 883.13 | 1.095749 | 882.75 | 1.090732 | 882.33 | 1.085719 | 882.29 | 1.080707 | 882.21 | ||
| 0.0031561 | 1.101603 | 885.55 | 1.096593 | 885.21 | 1.091585 | 884.56 | 1.086578 | 884.68 | 1.081573 | 884.48 | ||
| 0.0053671 | 1.102448 | 887.32 | 1.097445 | 887.10 | 1.092443 | 886.85 | 1.087443 | 886.87 | 1.082445 | 886.69 | ||
| 0.0072539 | 1.103159 | 888.80 | 1.098163 | 888.53 | 1.093168 | 888.20 | 1.088174 | 888.19 | 1.083181 | 888.14 | ||
| 0.0099236 | 1.104152 | 890.65 | 1.099164 | 890.49 | 1.094180 | 890.03 | 1.089194 | 890.04 | 1.084208 | 890.10 | ||
| 0.019301 | 1.107575 | 894.15 | 1.102621 | 893.88 | 1.097675 | 893.26 | 1.092721 | 893.13 | 1.087763 | 893.18 | ||
| Pyrogallorarene | T / K | Bv | ||
| Crown (monomer) | 293.15 | 452.92 | -178.96 | 2027.7 |
| 298.15 | 453.99 | -219.79 | 2548.7 | |
| 303.15 | 454.53 | -235.81 | 2778.8 | |
| 308.15 | 454.72 | -233.36 | 2765.4 | |
| 313.15 | 455.60 | -267.27 | 3227.8 | |
| a = 424.02 (cm3∙mol-1) 0.1004 (cm3∙mol-1∙K-1) | ||||
| Dimer capsule | 293.15 | 882.13 | 1108.5 | -25197 |
| 298.15 | 881.71 | 1147.8 | -26810 | |
| 303.15 | 881.19 | 1181.9 | -28845 | |
| 308.15 | 881.19 | 1191.1 | -29694 | |
| 313.15 | 881.01 | 1209.1 | -29971 | |
| a = 898.24(cm3∙mol-1) -0.0552 (cm3∙mol-1∙K-1) | ||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





