Submitted:
17 August 2025
Posted:
18 August 2025
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Materials and Methods
Box 1. NCHS Urban-Rural Classification Scheme (2013)
| Category | Category Description |
| Large Central Metro | NCHS-defined “central” counties, MSAs 1 million+ population |
| Large Fringe Metro | NCHS defined MSA fringe areas, 1 million+ population |
| Medium Metro | Counties within MSAs of 250,000 to 999,999 |
| Small Metro | Counties within MSAs of 50,000 to 249,999 |
| “Micropolitan” | Counties in NCHS-defined Micropolitan statistical areas |
| Non-core | Counties not in Micropolitan statistical areas |
Statistical Analysis
3. Results
4. Discussion
Confounding by Smoking and Diet
New Dimensions in Exposure Assessment
New Dimensions in Exposure Assessment
Strengths, Limitations and Future Directions
Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| ACS | American Community Survey |
| BKMR | Bayesian Kernel Machine Regression |
| BRFSS | Behavioral Risk Factor Surveillance System |
| CDC | Centers for Disease Control |
| CHAMACOS | a Spanish slang term referring to children or young people |
| EIM | Effect Importance Measure |
| FIPS | Federal Information Processing Standards |
| NCHS | National Center for Health Statistics |
| OMB | Office of Management and Budget |
| PUR | Pesticide Use Reporting |
| USGS | United States Geological Survey |
Appendix A
Imputing Missing Data
Appendix B
Additional Figures and Tables
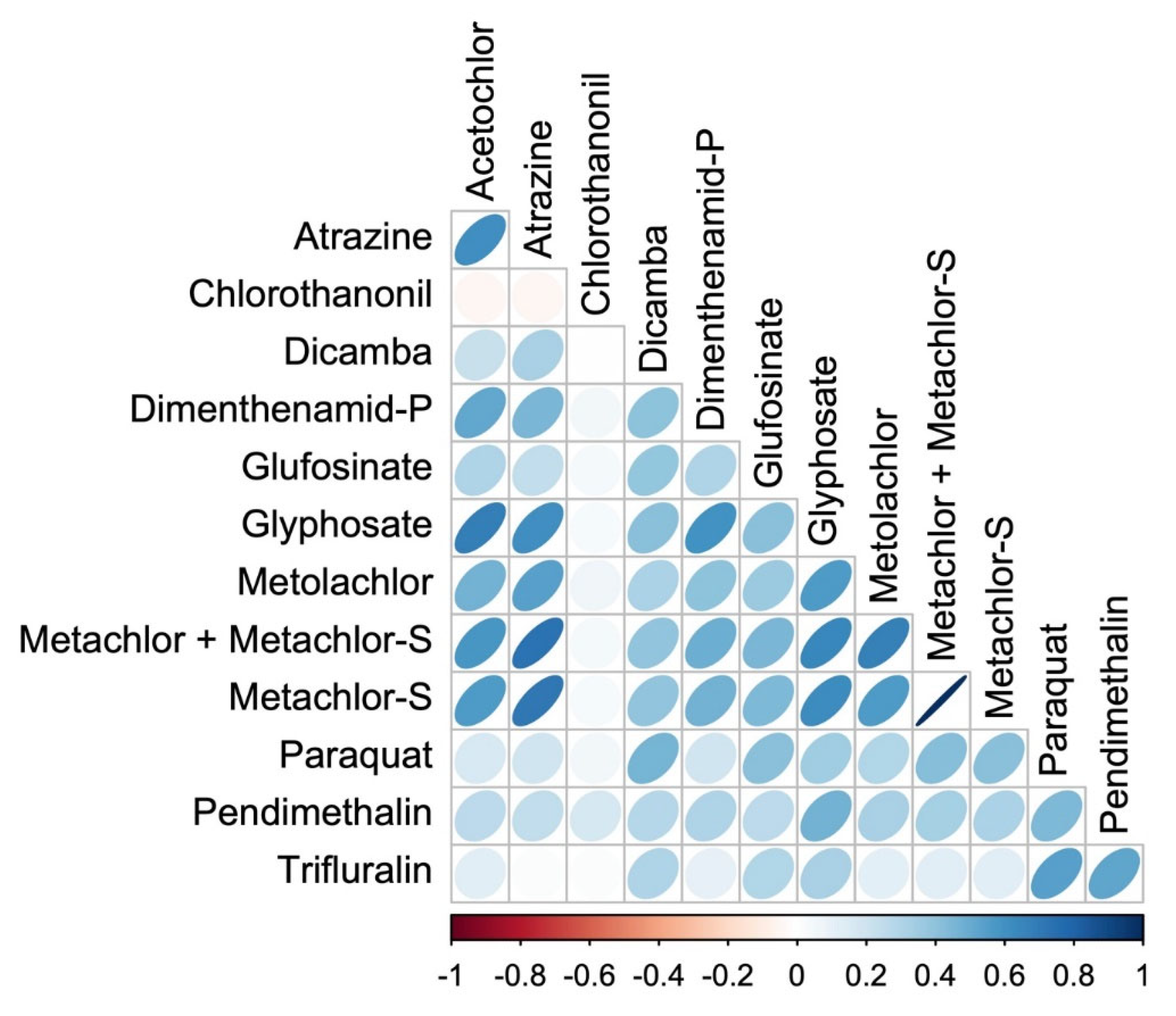
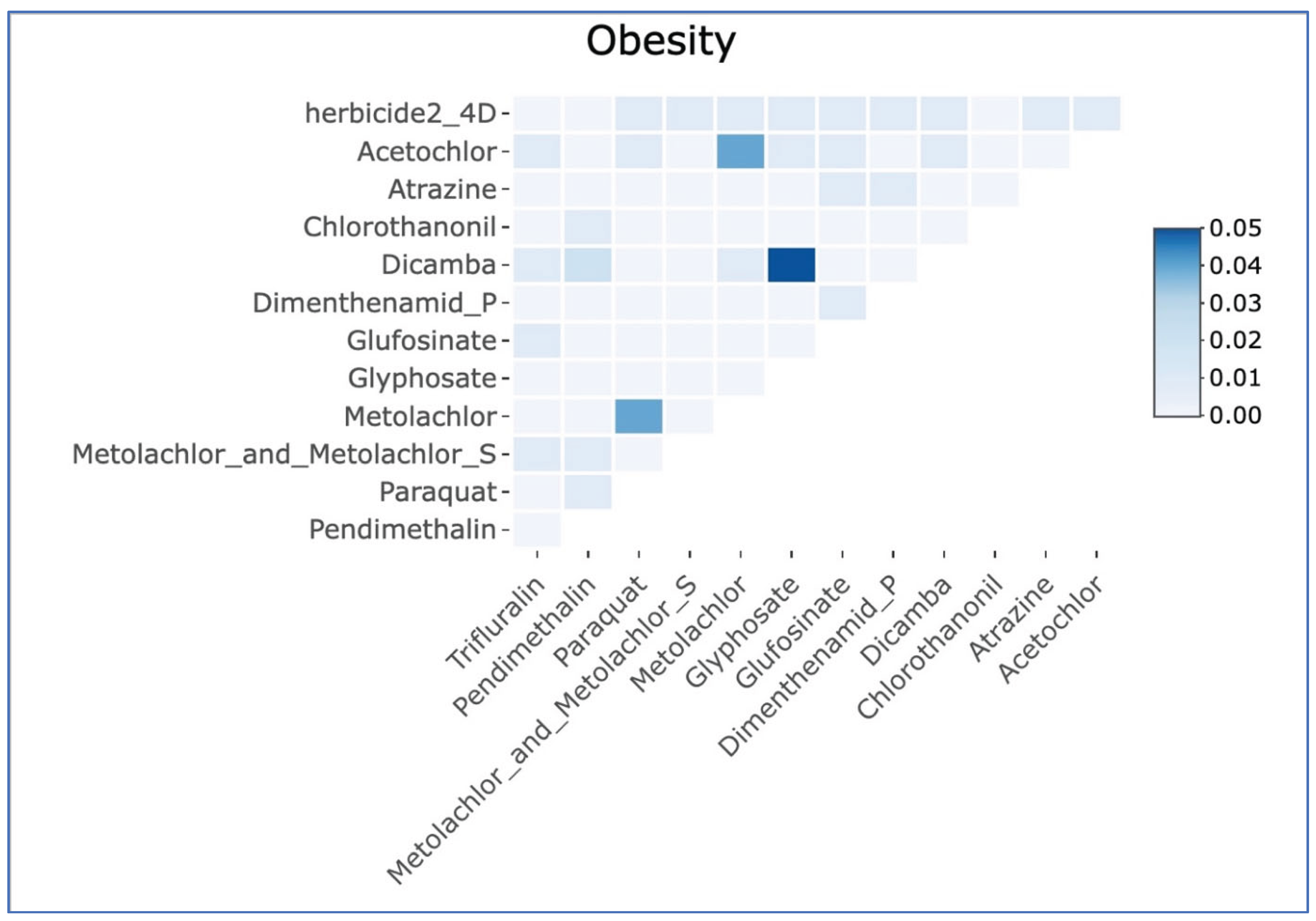
| Herbicide | Min | Q25 | Median | Q75 | Max |
| trifluralin | 0 | 47.5 | 290.2 | 1063.1 | 118984.0 |
| pendimethalin | 0 | 152.1 | 705.6 | 1940.6 | 127641.6 |
| paraquat | 0 | 95.7 | 424.9 | 1418.0 | 87700.0 |
| metolachlor-S | 0 | 264.1 | 2049.6 | 11095.6 | 193956.5 |
| metolachlor + metolachlor-S | 0 | 365.8 | 2727.5 | 13709.0 | 202600.2 |
| metolachlor | 0 | 86.4 | 608.8 | 2280.8 | 70608.4 |
| herbicide 2-4D | 0 | 802.9 | 2884.3 | 8000.0 | 199793.4 |
| glyphosate | 0.1 | 2015.7 | 15359.3 | 65956.5 | 594336.0 |
| glufosinate | 0 | 29.8 | 277.9 | 1456.4 | 87516.1 |
| dimenthenamid-P | 0 | 47.8 | 429.2 | 1807.8 | 49778.35 |
| dicamba | 0 | 91.4 | 369.1 | 1318.4 | 112221.9 |
| chlorothanonil | 0 | 21.8 | 127.5 | 661.7 | 176201.4 |
| atrazine | 0 | 312.4 | 2300.6 | 15193.0 | 743835.8 |
| acetochlor | 0 | 139.2 | 1335.5 | 9354.0 | 153269.7 |
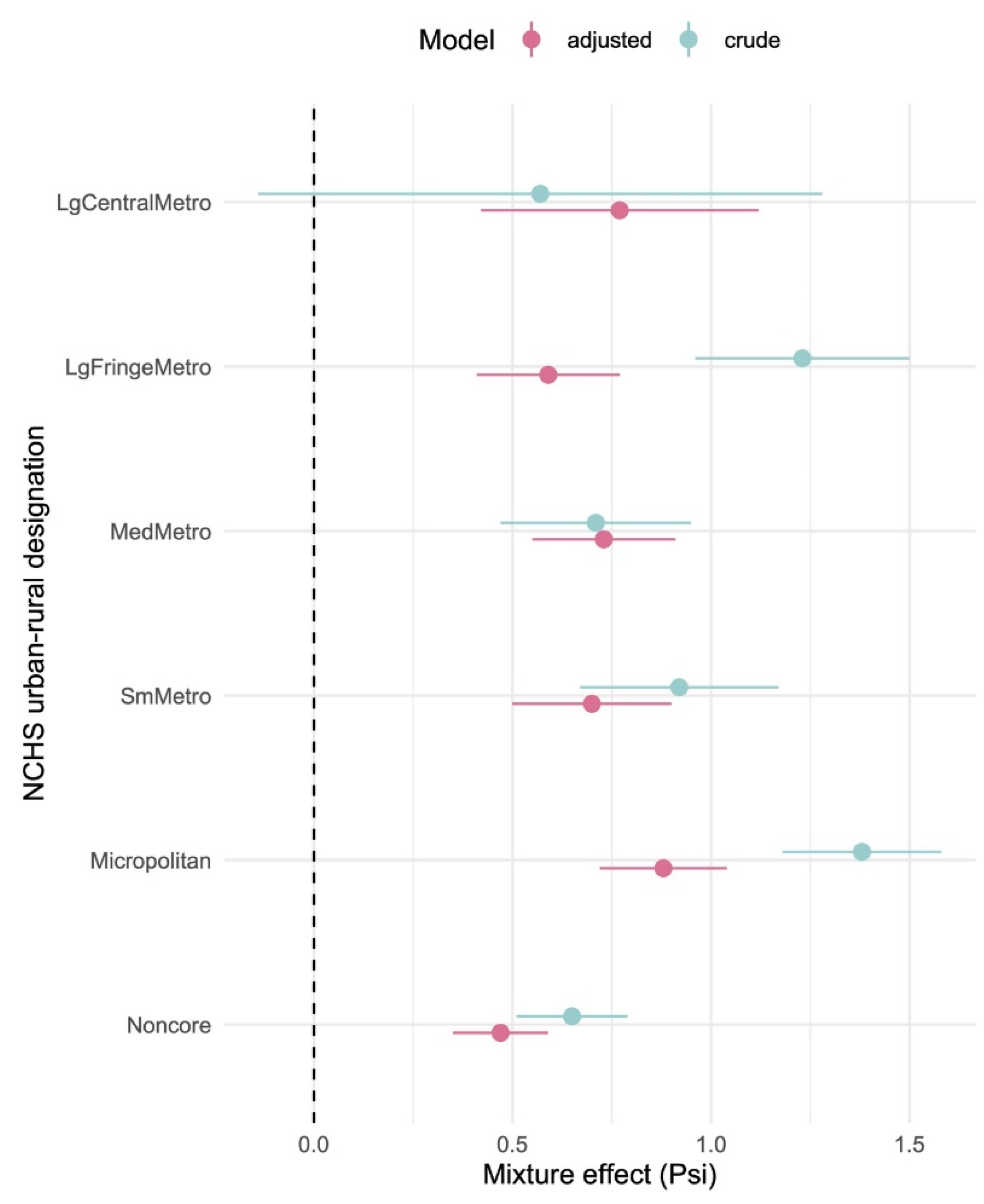
| NCHS Designation | Model | Coefficient | Estimate | 95% CI | p-value |
| Large Central Metro | Crude | intercept | 25.4 | 24.4, 26.5 | <0.0001 |
| Psi () | 0.57 | -0.14, 1.3 | 0.11 | ||
| Adjusted | intercept | 25.8 | 24.4, 27.3 | <0.0001 | |
| Psi () | 0.77 | 0.42, 1.12 | <0.0001 | ||
| Large Fringe Metro | Crude | intercept | 27.8 | 27.4, 28.2 | <0.0001 |
| Psi () | 1.23 | 0.96, 1.50 | <0.0001 | ||
| Adjusted | intercept | 27.8 | 27.2, 28.4 | <0.0001 | |
| Psi () | 0.59 | 0.4, 0.77 | <0.0001 | ||
| Medium Metro | Crude | intercept | 29.5 | 29.1, 29.9 | <0.0001 |
| Psi () | 0.71 | 0.47, 0.95 | <0.0001 | ||
| Adjusted | intercept | 28.8 | 28.3, 29.4 | <0.0001 | |
| Psi () | 0.73 | 0.55, 0.9 | <0.0001 | ||
| Small Metro | Crude | intercept | 29.5 | 29.1, 29.9 | <0.0001 |
| Psi () | 0.92 | 0.67, 1.2 | <0.0001 | ||
| Adjusted | intercept | 29.5 | 28.9, 30.1 | <0.0001 | |
| Psi () | 0.70 | 0.50, 0.90 | <0.0001 | ||
| Micropolitan | Crude | intercept | 29.4 | 29.1, 29.7 | <0.0001 |
| Psi () | 1.38 | 1.2, 1.6 | <0.0001 | ||
| Adjusted | intercept | 30.0 | 29.5, 30.4 | <0.0001 | |
| Psi () | 0.88 | 0.72, 1.04 | <0.0001 | ||
| Noncore | Crude | intercept | 30.6 | 30.35, 30.8 | <0.0001 |
| Psi () | 0.65 | 0.5, 0.8 | <0.0001 | ||
| Adjusted | intercept | 30.4 | 30.1, 30.7 | <0.0001 | |
| Psi () | 0.47 | 0.35, 0.59 | <0.0001 |
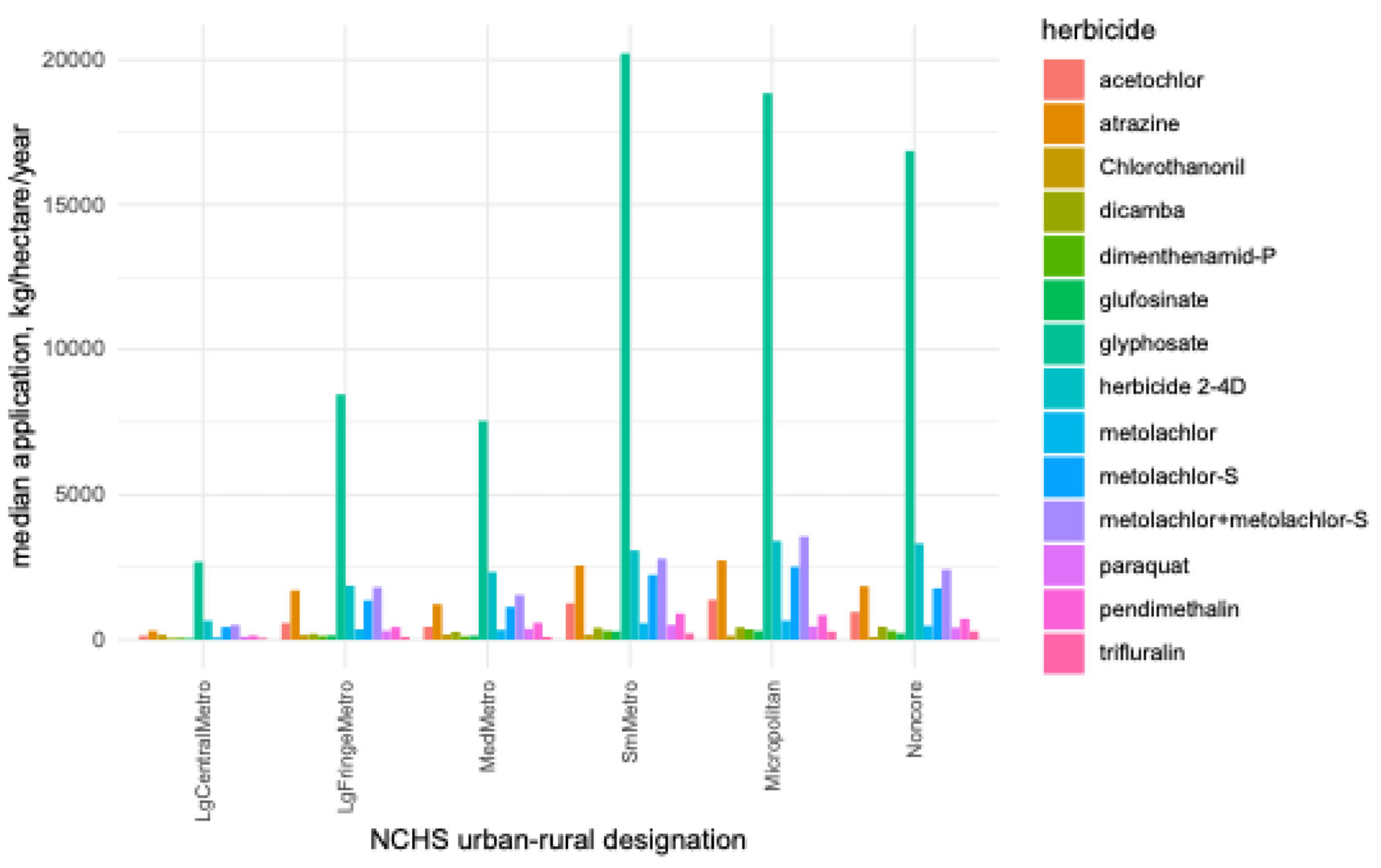
| Feature | 1NCHS Urban-Rural Designation | |||||
| Large Central Metro | Large Fringe Metro | Medium Metro | Small Metro | Micropolitan | Noncore | |
| Rurality (%) | 2.4 | 40.3 | 40.5 | 46.5 | 52.3 | 79.1 |
| Obesity rate (%) | 26.2 | 29.5 | 30.5 | 30.8 | 31.3 | 31.5 |
| Median Herbicide Application (kg/hectare) | ||||||
| acetochlor | 143 | 580 | 440 | 1290 | 1374 | 959 |
| atrazine | 321 | 1617 | 1241 | 2611 | 2734 | 1858 |
| chlorothanonil | 170 | 168 | 184 | 172 | 134 | 95 |
| dicamba | 53 | 201 | 267 | 411 | 428 | 444 |
| dimenthenamid-P | 61 | 134 | 113 | 293 | 356 | 309 |
| glufosinate | 36 | 154 | 135 | 286 | 301 | 220 |
| glyphosate | 2686 | 8449 | 7538 | 20220 | 18851 | 16838 |
| herbicide 2-4D | 656 | 1858 | 2338 | 3073 | 3404 | 3316 |
| metolachlor | 73 | 351 | 336 | 552 | 662 | 480 |
| metolachlor+metolachlor-S | 494 | 1808 | 1554 | 2800 | 3590 | 2411 |
| metolachlor-S | 421 | 1352 | 1139 | 2251 | 2581 | 1769 |
| paraquat | 91 | 310 | 376 | 509 | 456 | 412 |
| pendimethalin | 136 | 441 | 587 | 902 | 856 | 728 |
| trifluralin | 53 | 102 | 96 | 221 | 277 | 292 |
References
- Ng, M.; Fleming, T.; Robinson, M.; Thomson, B.; Graetz, N.; Margono, C.; Mullany, E.C.; Biryukov, S.; Abbafati, C.; Abera, S.F.; et al. Global, regional, and national prevalence of overweight and obesity in children and adults during 1980-2013: a systematic analysis for the Global Burden of Disease Study 2013. Lancet 2014, 384, 766–781. [Google Scholar] [CrossRef]
- Cho, N.H.; Shaw, J.E.; Karuranga, S.; Huang, Y.; da Rocha Fernandes, J.D.; Ohlrogge, A.W.; Malanda, B. IDF Diabetes Atlas: Global estimates of diabetes prevalence for 2017 and projections for 2045. Diabetes Res Clin Pract 2018, 138, 271–281. [Google Scholar] [CrossRef]
- Stackpoole, S.M.; Shoda, M.E.; Medalie, L.; Stone, W.W. Pesticides in US Rivers: Regional differences in use, occurrence, and environmental toxicity, 2013 to 2017. Sci Total Environ 2021, 787, 147147. [Google Scholar] [CrossRef] [PubMed]
- Medalie, L.; Baker, N.T.; Shoda, M.E.; Stone, W.W.; Meyer, M.T.; Stets, E.G.; Wilson, M. Influence of land use and region on glyphosate and aminomethylphosphonic acid in streams in the USA. Sci Total Environ 2020, 707, 136008. [Google Scholar] [CrossRef] [PubMed]
- Otaru, S.; Jones, L.E.; Carpenter, D.O. Associations between urine glyphosate levels and metabolic health risks: insights from a large cross-sectional population-based study. Environ Health 2024, 23, 58. [Google Scholar] [CrossRef]
- Hongoeb, J.; Tantimongcolwat, T.; Ayimbila, F.; Ruankham, W.; Phopin, K. Herbicide-related health risks: key mechanisms and a guide to mitigation strategies. J Occup Med Toxicol 2025, 20, 6. [Google Scholar] [CrossRef]
- Jayasumana, C.; Gunatilake, S.; Siribaddana, S. Simultaneous exposure to multiple heavy metals and glyphosate may contribute to Sri Lankan agricultural nephropathy. BMC Nephrol 2015, 16, 103. [Google Scholar] [CrossRef] [PubMed]
- Mesnage, R.; Defarge, N.; Spiroux de Vendômois, J.; Séralini, G.E. Potential toxic effects of glyphosate and its commercial formulations below regulatory limits. Food and chemical toxicology : an international journal published for the British Industrial Biological Research Association 2015, 84, 133–153. [Google Scholar] [CrossRef]
- Hayes, T.B.; Khoury, V.; Narayan, A.; Nazir, M.; Park, A.; Brown, T.; Adame, L.; Chan, E.; Buchholz, D.; Stueve, T.; et al. Atrazine induces complete feminization and chemical castration in male African clawed frogs (Xenopus laevis). Proc Natl Acad Sci U S A 2010, 107, 4612–4617. [Google Scholar] [CrossRef]
- Myers, J.P.; Antoniou, M.N.; Blumberg, B.; Carroll, L.; Colborn, T.; Everett, L.G.; Hansen, M.; Landrigan, P.J.; Lanphear, B.P.; Mesnage, R.; et al. Concerns over use of glyphosate-based herbicides and risks associated with exposures: a consensus statement. Environmental health : a global access science source 2016, 15. [Google Scholar] [CrossRef]
- Winchester, P.D.; Huskins, J.; Ying, J. Agrichemicals in surface water and birth defects in the United States. Acta Paediatr 2009, 98, 664–669. [Google Scholar] [CrossRef]
- Suppa, A.; Kvist, J.; Li, X.; Dhandapani, V.; Almulla, H.; Tian, A.Y.; Kissane, S.; Zhou, J.; Perotti, A.; Mangelson, H.; et al. Roundup causes embryonic development failure and alters metabolic pathways and gut microbiota functionality in non-target species. Microbiome 2020, 8. [Google Scholar] [CrossRef]
- Walsh, L.; Hill, C.; Ross, R.P. Impact of glyphosate (Roundup) on the composition and functionality of the gut microbiome. Gut Microbes 2023, 15, 2263935. [Google Scholar] [CrossRef]
- Jayaraman, S.; Krishnamoorthy, K.; Prasad, M.; Veeraraghavan, V.P.; Krishnamoorthy, R.; Alshuniaber, M.A.; Gatasheh, M.K.; Elrobh, M.; Gunassekaran. Glyphosate potentiates insulin resistance in skeletal muscle through the modulation of IRS-1/PI3K/Akt mediated mechanisms: An in vivo and in silico analysis. Int J Biol Macromol 2023, 242, 124917. [Google Scholar] [CrossRef]
- Prasad, M.; Gatasheh, M.K.; Alshuniaber, M.A.; Krishnamoorthy, R.; Rajagopal, P.; Krishnamoorthy, K.; Periyasamy, V.; Veeraraghavan, V.P.; Jayaraman, S. Impact of Glyphosate on the Development of Insulin Resistance in Experimental Diabetic Rats: Role of NFκB Signalling Pathways. Antioxidants (Basel) 2022, 11. [Google Scholar] [CrossRef]
- Ferris Pasquini, V.; Hurtazo, H.; Quintanilla, F.; Cruz-Soto, M. 2,4-Dichlorophenol Shows Estrogenic Endocrine Disruptor Activity by Altering Male Rat Sexual Behavior. Toxics 2023, 11. [Google Scholar] [CrossRef] [PubMed]
- See, W.Z.C.; Naidu, R.; Tang, K.S. Cellular and Molecular Events Leading to Paraquat-Induced Apoptosis: Mechanistic Insights into Parkinson's Disease Pathophysiology. Mol Neurobiol 2022, 59, 3353–3369. [Google Scholar] [CrossRef]
- McCormack, A.L.; Atienza, J.G.; Johnston, L.C.; Andersen, J.K.; Vu, S.; Di Monte, D.A. Role of oxidative stress in paraquat-induced dopaminergic cell degeneration. J Neurochem 2005, 93, 1030–1037. [Google Scholar] [CrossRef] [PubMed]
- Dinić, S.; Arambašić Jovanović, J.; Uskoković, A.; Mihailović, M.; Grdović, N.; Tolić, A.; Rajić, J.; Đorđević, M.; Vidaković, M. Oxidative stress-mediated beta cell death and dysfunction as a target for diabetes management. Front Endocrinol (Lausanne) 2022, 13, 1006376. [Google Scholar] [CrossRef]
- Yesupatham, A.; Saraswathy, R. Role of oxidative stress in prediabetes development. Biochem Biophys Rep 2025, 43, 102069. [Google Scholar] [CrossRef] [PubMed]
- Carpenter, D.O.; Arcaro, K.; Spink, D.C. Understanding the human health effects of chemical mixtures. Environ Health Perspect 2002, 110, 25–42. [Google Scholar] [CrossRef] [PubMed]
- Heys, K.A.; Shore, R.F.; Pereira, M.G.; Jones, K.C.; Martin, F.L. Risk assessment of environmental mixture effects. RSC Advances 2016, 6, 47844–47857. [Google Scholar] [CrossRef]
- Lagunas-Rangel, F.A.; Linnea-Niemi, J.V.; Kudłak, B.; Williams, M.J.; Jönsson, J.; Schiöth, H.B. Role of the Synergistic Interactions of Environmental Pollutants in the Development of Cancer. Geohealth 2022, 6, e2021GH000552. [Google Scholar] [CrossRef] [PubMed]
- Wagner, V.; Antunes, P.M.; Irvine, M.; Nelson, C.R. Herbicide usage for invasive non-native plant management in wildland areas of North America. J Appl Ecol 2017, 54, 198–204. [Google Scholar] [CrossRef]
- Rojas, J.; Dhar, A.; Naeth, M. Urban naturalization for green spaces using soil tillage, herbicide application, compost amendment and native vegetation. Land 2021, 10. [Google Scholar] [CrossRef]
- Curl, C.L.; Hyland, C.; Spivak, M.; Sheppard, L.; Lanphear, B.; Antoniou, M.N.; Ospina, M.; Calafat, A.M. The Effect of Pesticide Spray Season and Residential Proximity to Agriculture on Glyphosate Exposure among Pregnant People in Southern Idaho, 2021. Environ Health Perspect 2023, 131, 127001. [Google Scholar] [CrossRef]
- De Troeyer, K.; Casas, L.; Bijnens, E.M.; Bruckers, L.; Covaci, A.; De Henauw, S.; Den Hond, E.; Loots, I.; Nelen, V.; Verheyen, V.J.; et al. Higher proportion of agricultural land use around the residence is associated with higher urinary concentrations of AMPA, a glyphosate metabolite. Int J Hyg Environ Health 2022, 246, 114039. [Google Scholar] [CrossRef] [PubMed]
- Hyland, C.; Spivak, M.; Sheppard, L.; Lanphear, B.P.; Antoniou, M.; Ospina, M.; Calafat, A.M.; Curl, C.L. Urinary Glyphosate Concentrations among Pregnant Participants in a Randomized, Crossover Trial of Organic and Conventional Diets. Environ Health Perspect 2023, 131, 77005. [Google Scholar] [CrossRef]
- USGS. Pesticide National Synthesis Project. Available online: https://water.usgs.
- Remington, P.L.; Catlin, B.B.; Gennuso, K.P. The County Health Rankings: rationale and methods. Popul Health Metr 2015, 13, 11. [Google Scholar] [CrossRef]
- Hood, C.M.; Gennuso, K.P.; Swain, G.R.; Catlin, B.B. County Health Rankings: Relationships Between Determinant Factors and Health Outcomes. Am J Prev Med 2016, 50, 129–135. [Google Scholar] [CrossRef]
- Carlson, S.A.; Watson, K.B.; Rockhill, S.; Wang, Y.; Pankowska, M.M.; Greenlund, K.J. Linking Local-Level Chronic Disease and Social Vulnerability Measures to Inform Planning Efforts: A COPD Example. Prev Chronic Dis 2023, 20, E76. [Google Scholar] [CrossRef]
- Vo, A.; Tao, Y.; Li, Y.; Albarrak, A. The Association Between Social Determinants of Health and Population Health Outcomes: Ecological Analysis. JMIR Public Health Surveill 2023, 9, e44070. [Google Scholar] [CrossRef]
- Ibrahim, R.; Habib, A.; Terrani, K.; Ravi, S.; Takamatsu, C.; Salih, M.; Ferreira, J.P. County-level variation in healthcare coverage and ischemic heart disease mortality. PLoS One 2024, 19, e0292167. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Holt, J.B.; Lu, H.; Wheaton, A.G.; Ford, E.S.; Greenlund, K.J.; Croft, J.B. Multilevel regression and poststratification for small-area estimation of population health outcomes: a case study of chronic obstructive pulmonary disease prevalence using the behavioral risk factor surveillance system. Am J Epidemiol 2014, 179, 1025–1033. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Holt, J.B.; Yun, S.; Lu, H.; Greenlund, K.J.; Croft, J.B. Validation of multilevel regression and poststratification methodology for small area estimation of health indicators from the behavioral risk factor surveillance system. Am J Epidemiol 2015, 182, 127–137. [Google Scholar] [CrossRef]
- McCullough, M.L.; Chantaprasopsuk, S.; Islami, F.; Rees-Punia, E.; Um, C.Y.; Wang, Y.; Leach, C.R.; Sullivan, K.R.; Patel, A.V. Association of Socioeconomic and Geographic Factors With Diet Quality in US Adults. JAMA Netw Open 2022, 5, e2216406. [Google Scholar] [CrossRef] [PubMed]
- Rubin, D.B. Multiple Imputation for Nonresponse in Surveys; Wiley-Interscience: 1987, 2004; p. 258. [Google Scholar]
- Rubin, D.B. Multiple Imputation for Nonresponse in Surveys; Wiley: 2009.
- Benjamini, Y.; Hochberg, Y. Controlling the False Discovery Rate: A Practical and Powerful Approach to Multiple Testing. Journal of the Royal Statistical Society: Series B (Methodological) 1995, 57, 289–300. [Google Scholar] [CrossRef]
- Bobb, J.F.; Valeri, L.; Claus Henn, B.; Christiani, D.C.; Wright, R.O.; Mazumdar, M.; Godleski, J.J.; Coull, B.A. Bayesian kernel machine regression for estimating the health effects of multi-pollutant mixtures. Biostatistics 2015, 16, 493–508. [Google Scholar] [CrossRef]
- Bobb, J.F.; Claus Henn, B.; Valeri, L.; Coull, B.A. Statistical software for analyzing the health effects of multiple concurrent exposures via Bayesian kernel machine regression. Environ Health 2018, 17, 67. [Google Scholar] [CrossRef]
- Keil, A.P.; Buckley, J.P.; O'Brien, K.M.; Ferguson, K.K.; Zhao, S.; White, A.J. A Quantile-Based g-Computation Approach to Addressing the Effects of Exposure Mixtures. Environ Health Perspect 2020, 128, 47004. [Google Scholar] [CrossRef]
- Hornung, R.; Boulesteix, A.-L. Interaction forests: Identifying and exploiting interpretable quantitative and qualitative interaction effects. Comput Stat Data Anal 2022, 171, 107460. [Google Scholar] [CrossRef]
- Peluso, A.; Rastogi, D.; Klasky, H.B.; Logan, J.; Maguire, D.; Grant, J.; Christian, B.; Hanson, H.A. Environmental determinants of health: Measuring multiple physical environmental exposures at the United States census tract level. Health Place 2024, 89, 103303. [Google Scholar] [CrossRef]
- Madani, N.A.; Carpenter, D.O. Patterns of Emergency Room Visits for Respiratory Diseases in New York State in Relation to Air Pollution, Poverty and Smoking. Int J Environ Res Public Health 2023, 20. [Google Scholar] [CrossRef]
- Doogan, N.J.; Roberts, M.E.; Wewers, M.E.; Stanton, C.A.; Keith, D.R.; Gaalema, D.E.; Kurti, A.N.; Redner, R.; Cepeda-Benito, A.; Bunn, J.Y.; et al. A growing geographic disparity: Rural and urban cigarette smoking trends in the United States. Prev Med 2017, 104, 79–85. [Google Scholar] [CrossRef]
- Parker, M.A.; Weinberger, A.H.; Eggers, E.M.; Parker, E.S.; Villanti, A.C. Trends in Rural and Urban Cigarette Smoking Quit Ratios in the US From 2010 to 2020. JAMA Netw Open 2022, 5, e2225326. [Google Scholar] [CrossRef] [PubMed]
- Eskenazi, B.; Gunier, R.B.; Rauch, S.; Kogut, K.; Perito, E.R.; Mendez, X.; Limbach, C.; Holland, N.; Bradman, A.; Harley, K.G.; et al. Association of Lifetime Exposure to Glyphosate and Aminomethylphosphonic Acid (AMPA) with Liver Inflammation and Metabolic Syndrome at Young Adulthood: Findings from the CHAMACOS Study. Environ Health Perspect 2023, 131, 37001. [Google Scholar] [CrossRef] [PubMed]
- Glover, F.; Jean-Baptiste, O.; Del Giudice, F.; Belladelli, F.; Seranio, N.; Muncey, W.; Eisenberg, M. The association between glyphosate exposure and metabolic syndrome among US adults. Human and Ecological Risk Assessment: An International Journal. 2023, 29, 1212–1225. [Google Scholar] [CrossRef]
| Covariate (%) | Mean | Median | IQR | |
| Outcome | Obese | 30.9 | 31.0 | [29.0, 34.0] |
| Risk factors | Smoking | 19.4 | 19.0 | [16.0, 22.0] |
| Uninsured | 17.0 | 16.9 | [12.0, 21.0] | |
| Unemployed | 6.7 | 6.3 | [4.7, 8.1] | |
| Rurality | 60.3 | 60.7 | [35.2, 88.9] | |
| Food Insecure | 13.6 | 14.0 | [11.0, 16.0] | |
| education | High School Graduate | 84.1 | 86.0 | [79.0, 91.0] |
| Some College | 55.8 | 55.8 | [47.6, 64.3] | |
| Age | Population < 18 years | 22.7 | 22.6 | [20.7, 24.4] |
| Population > 64 years | 17.4 | 17.1 | [11.5, 13.8] | |
|
Race (percent) |
Non-Hispanic White | 77.9 | 86.3 | [68.2, 93.9] |
| Black/African American | 8.8 | 2.0 | [0.6, 9.3] | |
| Asian | 1.3 | 0.6 | [0.4, 1.1] | |
| Hispanic | 8.9 | 3.5 | [1.9, 8.0] | |
| Native American | 1.9 | 0.6 | [0.3, 1.1] | |
| Pacific Islander | 0.08 | 0.0 | [0.0, 0.10] | |
| Level | Number counties | (Percent) | ||
|
1NCHS Urban-Rural Designation |
Large Central Metro | 351 | (1.9%) | |
| Large Fringe Metro | 2130 | (11.6) | ||
| Medium Metropolitan | 2190 | (11.9) | ||
| Small Metro | 2094 | (11.4) | ||
| Micropolitan | 3803 | (20.7) | ||
| Noncore | 7814 | (42.5) | ||
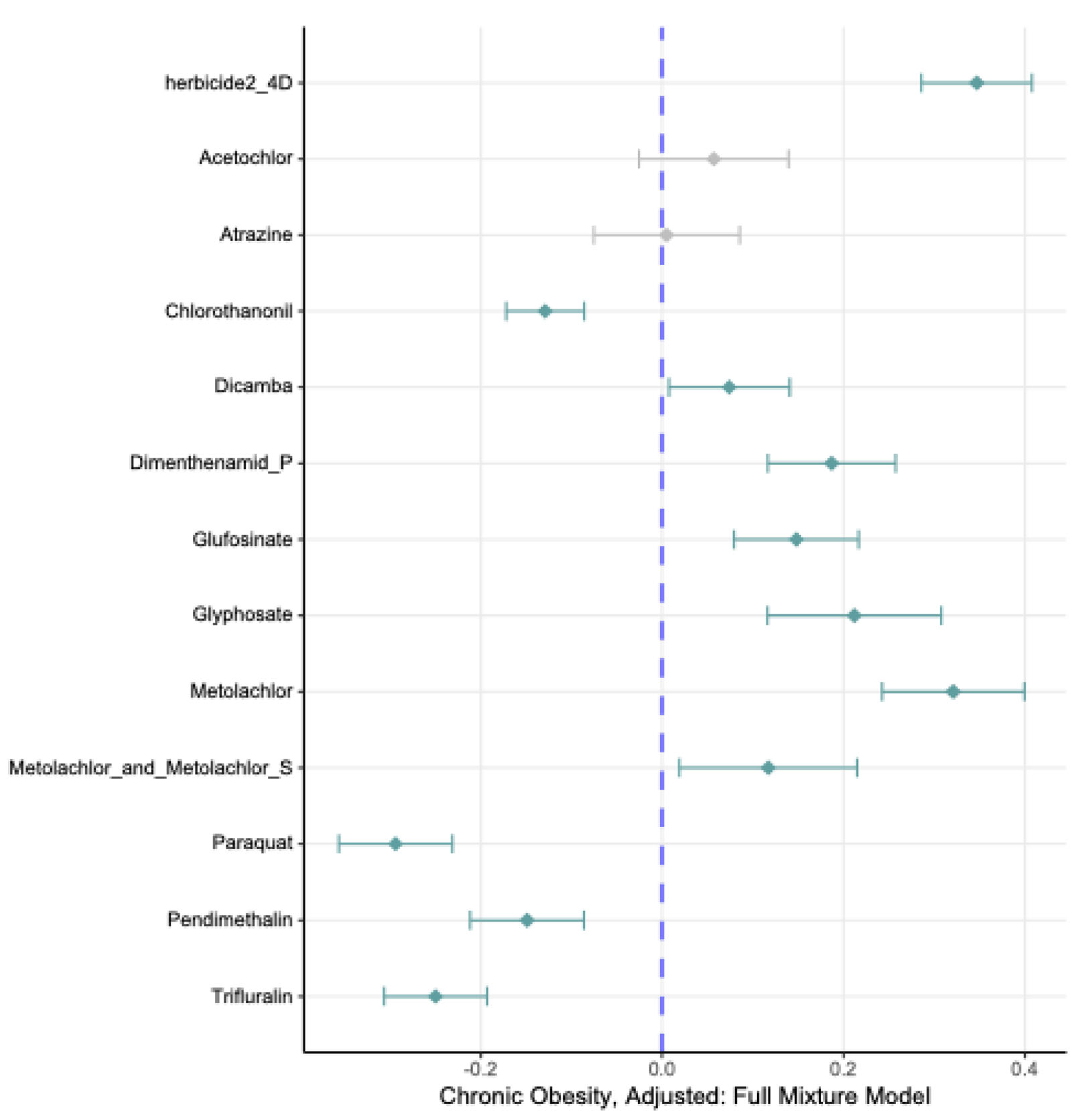
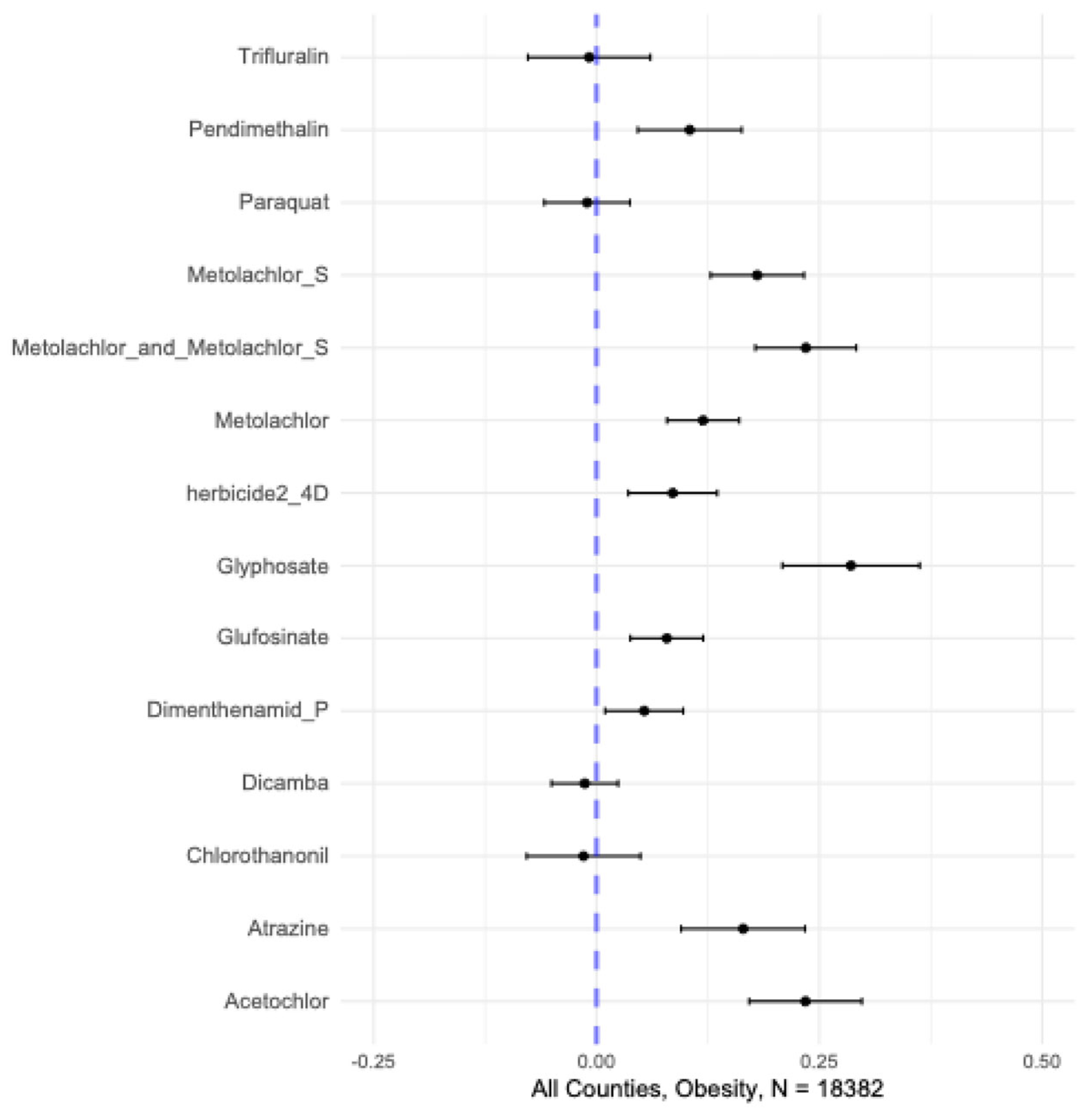
| Herbicide | Estimate | 95% Confidence Interval | p-value |
FDR adjusted |
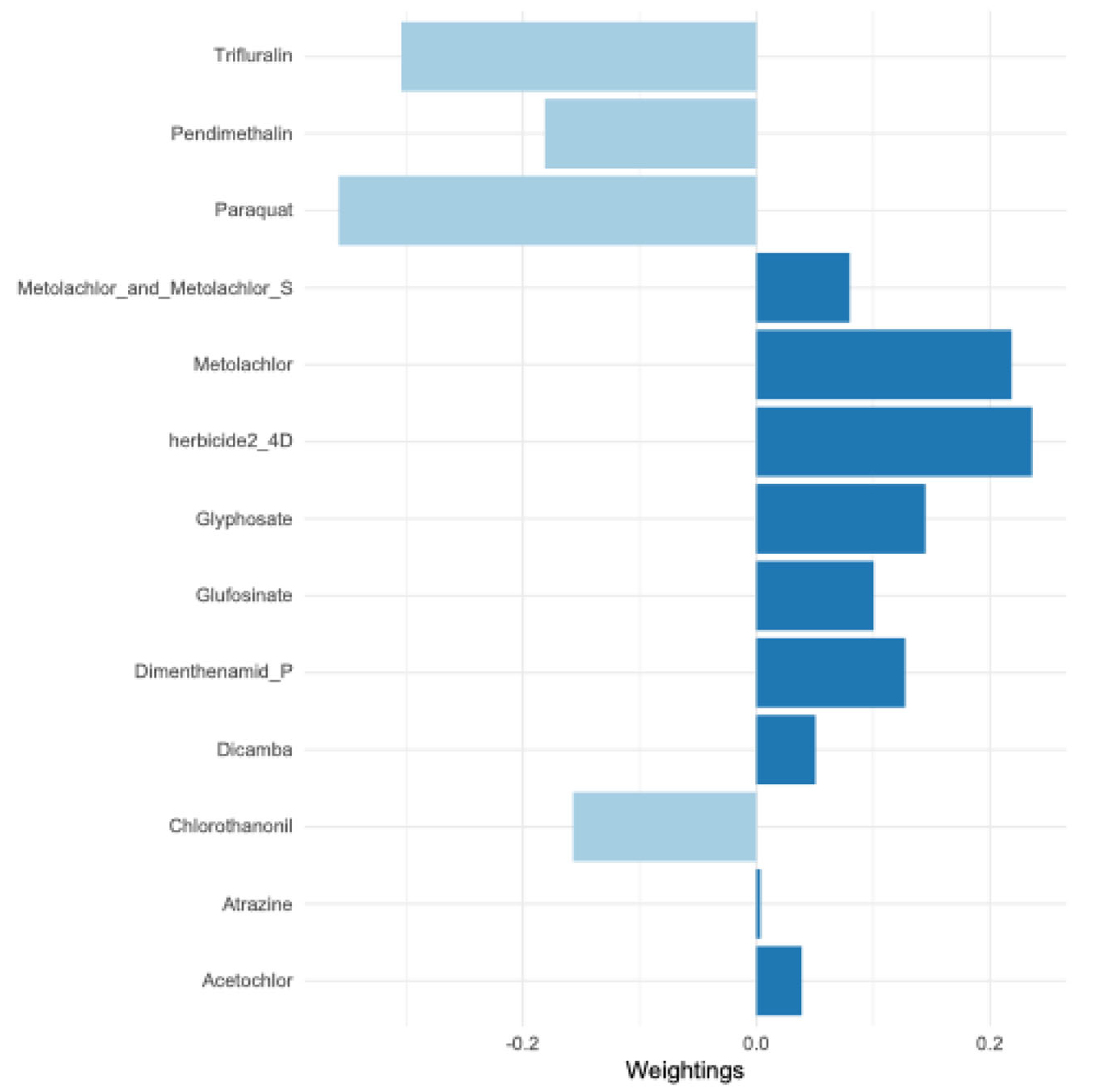
| acetochlor | 0.23 | 0.17, 0.30 | <0.0001 | <0.0001 |
| atrazine | 0.16 | 0.095, 0.23 | <0.0001 | <0.0001 |
| glufosinate | 0.08 | 0.038, 0.12 | <0.0001 | <0.0001 |
| glyphosate | 0.29 | 0.21, 0.36 | <0.0001 | <0.0001 |
| metolachlor | 0.12 | 0.08, 0.16 | <0.0001 | <0.0001 |
| metolachlor + metolachlor-S | 0.24 | 0.18, 0.29 | <0.0001 | <0.0001 |
| metolachlor-S | 0.18 | 0.13, 0.23 | <0.0001 | <0.0001 |
| pendimethalin | 0.11 | 0.046, 0.16 | <0.0001 | <0.0001 |
| herbicide 2-4D | 0.09 | 0.036, 0.135 | 0.001 | 0.002 |
| dimenthenamid-P | 0.05 | 0.01, 0.097 | 0.02 | 0.04 |
| dicamba | -0.013 | -0.05, 0.024 | 0.49 | 0.62 |
| chlorothanonil | -0.02 | -0.078, 0.049 | 0.66 | 0.72 |
| paraquat | -0.01 | -0.0, 0.038 | 0.67 | 0.72 |
| trifluralin | -0.008 | -0.077, 0.06 | 0.81 | 0.81 |
| Model | Coefficient | Estimate | 95% Confidence Interval | p-value |
| Crude | intercept | 29.5 | 29.4, 29.6 | <0.0001 |
| Psi () | 1.0 | 0.94, 1.1 | <0.0001 | |
| Adjusted | intercept | 28.0 | 27.6, 28.4 | <0.0001 |
| Psi () | 0.71 | 0.65, 0.76 | <0.0001 | |
| Crude + interactants |
intercept | 29.4 | 29.3, 29.6 | <0.0001 |
| Psi () | 1.4 | 1.2, 1.6 | <0.0001 | |
| Adjusted + interactants |
intercept | 28.1 | 27.7, 28.5 | <0.0001 |
| Psi () | 0.68 | 0.50, 0.86 | <0.0001 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





