Submitted:
31 July 2025
Posted:
18 August 2025
You are already at the latest version
Abstract
Background: Endoscopic endonasal approach (EEA) has become a well- established approach for skull base pathologies, providing a minimally invasive corridor to access various lesions. While the technique has been extensively studied for common skull base tumors, data on extremely rare pathologies remain limited. Our study aims to present our experience with rare skull base lesions managed via the endoscopic endonasal approach, highlighting the challenges associated with preoperative diagnosis, intraoperative decision-making, and surgical strategy modifications. Methods: A retrospective analysis was conducted on patients who underwent endoscopic endonasal surgery at the tertiary center up to January 2025. Among 6,225 endoscopic endonasal procedures performed, 41 patients with exceptionally rare skull base pathologies were identified. Each tumor type was separately evaluated for differences in resection rates, complication rates, and recurrence patterns. Results: These included 6 pituitary pituicytomas, 5 plasmacytomas, 5 xanthogranulomas, 4 granular cell tumors, 3 giant cell tumors and others. Gross total resection was achieved in 68% of cases, with subtotal resection in vascular or malignant tumors. Intraoperative challenges included unexpected vascularity and fibrotic adhesions. Postoperative CSF leakage occurred in 7%, and recurrence was observed in 17%, mostly in malignant or partially resected tumors. No intraoperative mortality was recorded. Tailored adjuvant treatments ensured durable disease control in most patients. Conclusions: Even in highly experienced centers, rare skull base pathologies pose unique diagnostic and surgical challenges. The discrepancy between preoperative and intraoperative findings often necessitates real-time strategic adaptations. In these rare cases, for optimizing patient outcomes requires a comprehensive understanding of skull base anatomy, flexibility in surgical planning, readiness for sudden strategy modifications, and a multidisciplinary approach.
Keywords:
1. Introduction
2. Materials and Methods
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| SBL | Skull Base Lesions |
| PA | Pituitary Adenom |
| EEA | Endoscopic endonasale approach |
| GTR | Gross-total Resection |
| STR | Subtotal Resection |
| DI: | Diabetus Insipitus |
| CSF | Cerebrospinal Fluid |
| HPC | HEmangiomapericytoma |
References
- Kassam, A.B.; Gardner, P.; Snyderman, C.; Mintz, A.; Carrau, R. Expanded Endonasal Approach: Fully Endoscopic, Completely Transnasal Approach to the Middle Third of the Clivus, Petrous Bone, Middle Cranial Fossa, and Infratemporal Fossa. Neurosurg Focus 2005, 19, 1–10. [Google Scholar] [CrossRef]
- Hadad, G.; Bassagasteguy, L.; Carrau, R.L.; Mataza, J.C.; Kassam, A.; Snyderman, C.H.; Mintz, A. A Novel Reconstructive Technique After Endoscopic Expanded Endonasal Approaches: Vascular Pedicle Nasoseptal Flap. Laryngoscope 2006, 116, 1882–1886. [Google Scholar] [CrossRef]
- Cavallo, L.M.; Messina, A.; Cappabianca, P.; Esposito, F.; de Divitiis, E.; Gardner, P.; Tschabitscher, M. Endoscopic Endonasal Surgery of the Midline Skull Base: Anatomical Study and Clinical Considerations. Neurosurg Focus 2005, 19, 1–14. [Google Scholar] [CrossRef]
- Cappabianca, P.; Cavallo, L.M.; Esposito, F.; de Divitiis, E. Endoscopic Endonasal Transsphenoidal Surgery: Procedure, Endoscopic Equipment and Instrumentation. Child’s Nervous System 2004, 20, 796–801. [Google Scholar] [CrossRef]
- Gardner, P.A.; Kassam, A.B.; Thomas, A.; Snyderman, C.H.; Carrau, R.L.; Mintz, A.H.; Prevedello, D.M. ENDOSCOPIC ENDONASAL RESECTION OF ANTERIOR CRANIAL BASE MENINGIOMAS. Neurosurgery 2008, 63, 36–54. [Google Scholar] [CrossRef]
- Evans, A.R.; Pelargos, P.; Deel, C.D.; Dunn, I.F. Primary Diffuse Large B-Cell Lymphoma of the Clivus: Systematic Review and Illustrative Case Example. World Neurosurg 2025, 194, 123513. [Google Scholar] [CrossRef]
- ZHAO, J.; QIAN, T.; ZHI, Z.; LI, Q.; KANG, L.; WANG, J.; SUI, A.; LI, N.; ZHANG, H. Giant Cell Tumor of the Clivus: A Case Report and Review of the Literature. Oncol Lett 2014, 8, 2782–2786. [Google Scholar] [CrossRef]
- Yaprak Bayrak, B.; Özcan, E.; Vural, Ç.; Emengen, A.; Çabuk, B.; Ceylan, S. A Single-Center Experience with Giant Cell Tumors of Sphenoid Bone and Clivus. Tumori Journal 2021, 107, NP94–NP100. [Google Scholar] [CrossRef]
- Gagliardi, F.; Losa, M.; Boari, N.; Spina, A.; Reni, M.; Terreni, M.R.; Mortini, P. Solitary Clival Plasmocytomas: Misleading Clinical and Radiological Features of a Rare Pathology with a Specific Biological Behaviour. Acta Neurochir (Wien) 2013, 155, 1849–1856. [Google Scholar] [CrossRef] [PubMed]
- Giantini Larsen, A.M.; Cote, D.J.; Zaidi, H.A.; Bi, W.L.; Schmitt, P.J.; Iorgulescu, J.B.; Miller, M.B.; Smith, T.R.; Lopes, M.B.; Jane, J.A.; et al. Spindle Cell Oncocytoma of the Pituitary Gland. J Neurosurg 2019, 131, 517–525. [Google Scholar] [CrossRef] [PubMed]
- Kloub, O.; Perry, A.; Tu, P.-H.; Lipper, M.; Lopes, M.B.S. Spindle Cell Oncocytoma of the Adenohypophysis. American Journal of Surgical Pathology 2005, 29, 247–253. [Google Scholar] [CrossRef]
- Roncaroli, F.; Giannini, C. Posterior Pituitary Tumors and Other Rare Entities Involving the Pituitary Gland. Brain Pathology 2025, 35. [Google Scholar] [CrossRef] [PubMed]
- Cappabianca, P.; Cavallo, L.M.; Esposito, F.; de Divitiis, O.; Messina, A.; de Divitiis, E. Extended Endoscopic Endonasal Approach to the Midline Skull Base: The Evolving Role of Transsphenoidal Surgery. In; 2008; pp. 151–199.
- Ceylan, S.; Koc, K.; Anik, I. Extended Endoscopic Approaches for Midline Skull-Base Lesions. Neurosurg Rev 2009, 32, 309–319. [Google Scholar] [CrossRef]
- Ceylan, S.; Koc, K.; Anik, I. Endoscopic Endonasal Transsphenoidal Approach for Pituitary Adenomas Invading the Cavernous Sinus. J Neurosurg 2010, 112, 99–107. [Google Scholar] [CrossRef] [PubMed]
- Ceylan, S.; Sen, H.E.; Ozsoy, B.; Ceylan, E.C.; Ergen, A.; Selek, A.; Anik, Y.; Balci, S.; Cabuk, B.; Anik, I. Endoscopic Approach for Giant Pituitary Adenoma: Clinical Outcomes of 205 Patients and Comparison of Two Proposed Classification Systems for Preoperative Prediction of Extent of Resection. J Neurosurg 2022, 136, 786–800. [Google Scholar] [CrossRef]
- Ceylan, S.; Anik, I.; Koc, K.; Cabuk, B. Extended Endoscopic Transsphenoidal Approach Infrachiasmatic Corridor. Neurosurg Rev 2015, 38, 137–147. [Google Scholar] [CrossRef] [PubMed]
- Caklili, M.; Emengen, A.; Yilmaz, E.; Genc, H.; Cabuk, B.; Anik, I.; Ceylan, S. Endoscopic Endonasal Approach Limitations and Evolutions for Tuberculum Sellae Meningiomas: Data from Single-Center Experience of Sixty Patients. Turk Neurosurg 2023, 33, 272–282. [Google Scholar] [CrossRef]
- Cheng, J.-H.; Nie, D.; Li, B.; Gui, S.-B.; Li, C.-Z.; Zhang, Y.-Z.; Cavallo, L.M.; Zhao, P. Clinical Features, Radiological Profiles, Pathological Features and Surgical Outcomes of Pituicytomas: A Report of 11 Cases and a Pooled Analysis of Individual Patient Data. Mil Med Res 2021, 8, 39. [Google Scholar] [CrossRef]
- Peng, Z.; Wang, Y.; Wang, Y.; Li, Q.; Fang, Y.; Fan, R.; Zhang, H.; Jiang, W. Hemangiopericytoma/Solitary Fibrous Tumor of the Cranial Base: A Case Series and Literature Review. BMC Surg 2022, 22, 289. [Google Scholar] [CrossRef]
- Feng, M.; Carmichael, J.D.; Bonert, V.; Bannykh, S.; Mamelak, A.N. Surgical Management of Pituicytomas: Case Series and Comprehensive Literature Review. Pituitary 2014, 17, 399–413. [Google Scholar] [CrossRef]
- Benveniste, R.J.; Purohit, D.; Byun, H. Pituicytoma Presenting with Spontaneous Hemorrhage. Pituitary 2006, 9, 53–58. [Google Scholar] [CrossRef]
- DiDomenico, J.; Ampie, L.; Choy, W.; Lamano, J.B.; Oyon, D.E.; Kesavabhotla, K.; Bloch, O. Sellar Plasmacytomas Masquerading as Pituitary Adenomas: A Systematic Review. Journal of Clinical Neuroscience 2018, 50, 20–23. [Google Scholar] [CrossRef]
- Joukhadar, R.; Chiu, K. Sellar Plasmacytomas: A Concise Review. Pituitary 2012, 15, 146–149. [Google Scholar] [CrossRef] [PubMed]
- Na’ara, S.; Amit, M.; Gil, Z.; Billan, S. Plasmacytoma of the Skull Base: A Meta-Analysis. J Neurol Surg B Skull Base 2015, 77, 061–065. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, H.; Ahmad, Y.; Ktyman, H.; Alali, M.; Saifo, M. A Challenging Diagnosis of Langerhans’ Cell Histiocytosis with Hypothalamic-Pituitary and Mandibular Involvement: Case Report and Literature Review. Annals of Medicine & Surgery 2024, 86, 4191–4196. [Google Scholar] [CrossRef]
- Wilejto, M.; Abla, O. Langerhans Cell Histiocytosis and Erdheim–Chester Disease. Curr Opin Rheumatol 2012, 24, 90–96. [Google Scholar] [CrossRef]
- Hong, S.; Hasegawa, H.; Shin, M.; Shinya, Y.; Saito, N. Endoscopic Transsphenoidal Resection of Suprasellar Histiocytosis in a Patient with Erdheim-Chester Disease: A Case Report. NMC Case Rep J 2021, 8, 117–122. [Google Scholar] [CrossRef]
- Feng, X.; Zhang, L.; Chen, F.; Yuan, G. Multi-System Langerhans Cell Histiocytosis as a Mimic of IgG4-Related Disease: A Case Report and Literature Review. Front Endocrinol (Lausanne) 2022, 13. [Google Scholar] [CrossRef] [PubMed]
- Rahmani, R.; Sukumaran, M.; Donaldson, A.M.; Akselrod, O.; Lavi, E.; Schwartz, T.H. Parasellar Xanthogranulomas. J Neurosurg 2015, 122, 812–817. [Google Scholar] [CrossRef]
- García-Fernández, A.; Fernández-Rueda, M.; García-González, E.; Mata-Castro, N. Endoscopic Surgical Management of Juvenile Nasopharyngeal Angiofibroma: Correlating Tumour Characteristics, Risk of Hemorrhage, and Recurrence. Auris Nasus Larynx 2024, 51, 940–946. [Google Scholar] [CrossRef]
- Nguyen, H.M.H.; Le, M.T.Q.; Nguyen, H.T.; Tran, H.V.; Tran, L.V. Investigation of Vascularization Patterns in Juvenile Angiofibroma and the Impact of Preoperative Embolization on Surgical Excision. Am J Otolaryngol 2025, 46, 104632. [Google Scholar] [CrossRef] [PubMed]
- Gardner, P.A.; Tormenti, M.J.; Pant, H.; Fernandez-Miranda, J.C.; Snyderman, C.H.; Horowitz, M.B. Carotid Artery Injury During Endoscopic Endonasal Skull Base Surgery. Operative Neurosurgery 2013, 73, ons261–ons270. [Google Scholar] [CrossRef] [PubMed]
- Emengen, A.; Yilmaz, E.; Gokbel, A.; Uzuner, A.; Cakir, O.; Ciftci, E.; Ozsoy, B.; Ergen, A.; Caklili, M.; Cabuk, B.; et al. Management of Major Arterial Injuries: A Critical Complication of Endoscopic Endonasal Surgery. Operative Neurosurgery 2025. [CrossRef] [PubMed]
- He, Y.; Hong, R.; Wang, S.; Wu, J.; Li, W.; Zhang, H.; Xue, K.; Liu, Q.; Gu, Y.; Sun, X.; et al. Preoperative Embolization Followed by Tumor Resection Without Time Interval in Advanced Juvenile Nasopharyngeal Angiofibroma. Cardiovasc Intervent Radiol 2025, 48, 815–822. [Google Scholar] [CrossRef]
- Orr, T.; Lesha, E.; Dugan, J.E.; Cecia, A.; Kramer, A.H.; Blum, D.; Zhang, J.; Klimo, P. Langerhans Cell Histiocytosis of the Sella in a Pediatric Patient: Case Report with Review of the Literature. Child’s Nervous System 2024, 40, 2947–2952. [Google Scholar] [CrossRef]
- Miranda, J.C.F.; Gardner, P.A.; Snyderman, C.H.; Devaney, K.O.; Mendenhall, W.M.; Su, C.; Rinaldo, A.; Eng, F. Clival Chordomas : A Pathological , Surgical , and Radiotherapeutic Review. 2014. [CrossRef]
- Jian, B.J.; Bloch, O.G.; Yang, I.; Han, S.J.; Aranda, D.; Tihan, T.; Parsa, A.T. Adjuvant Radiation Therapy and Chondroid Chordoma Subtype Are Associated with a Lower Tumor Recurrence Rate of Cranial Chordoma. J Neurooncol 2010, 98, 101–108. [Google Scholar] [CrossRef]
- Bae, J.W.; Kim, Y.H.; Kim, S.-K.; Wang, K.-C.; Shin, H.-Y.; Kang, H.J.; Park, S.-H.; Phi, J.H. Langerhans Cell Histiocytosis Causing Acute Optic Neuropathy. Child’s Nervous System 2015, 31, 615–619. [Google Scholar] [CrossRef]
- Kahilogullari, G.; Bayatli, E.; Geyik, M.; Cabuk, B.; Beton, S.; Gunaldi, O.; Tanrıverdi, O.; Cetinalp, N.E.; Tarkan, O.; Yıldırım, A.E.; et al. Endonasal Endoscopic Approach for Sellar Metastatic Pathologies: A National Observation. Br J Neurosurg 2023, 37, 206–212. [Google Scholar] [CrossRef]
- Hong, S.; Atkinson, J.L.; Erickson, D.; Kizilbash, S.H.; Little, J.T.; Routman, D.M.; Van Gompel, J.J. Treatment Outcome of Metastasis to the Pituitary Gland: A Case Series of 21 Patients with Pathological Diagnosis. Neurosurg Focus 2023, 55, E13. [Google Scholar] [CrossRef]
- Dagher, S.A.; Alizada, S.; Al Qudah, H.; Waguespack, S.G.; Shah, K.B.; Eldaya, R.W. Imaging and Clinical Course of Metastatic Pituitary Neuroendocrine Tumors (PitNET): A Single Center Case Series. Neuroradiol J 2025. [CrossRef] [PubMed]
- Ricciuti, R.A.; Paracino, R.; Mancini, F.; De Domenico, P.; Ricciuti, V.; Barbieri, F.R.; Ottaviani, M.M.; Pagano, S.; Marruzzo, D. Clinical and Neuroradiological Red Flags in Differential Diagnosis of Pituitary Metastases and PitNETs (Adenomas): A Surgeon’s Experience and Systematic Literature Review. Neurol Neurochir Pol 2025, 59, 254–271. [Google Scholar] [CrossRef] [PubMed]
- Conger, A.; Zhao, F.; Wang, X.; Eisenberg, A.; Griffiths, C.; Esposito, F.; Carrau, R.L.; Barkhoudarian, G.; Kelly, D.F. Evolution of the Graded Repair of CSF Leaks and Skull Base Defects in Endonasal Endoscopic Tumor Surgery: Trends in Repair Failure and Meningitis Rates in 509 Patients. J Neurosurg 2019, 130, 861–875. [Google Scholar] [CrossRef] [PubMed]
- Riesgo, P.; Mariño, P.; Platero, A.; Tarazona, F.J.; Fajardo, C.; Llácer, J.L.; Rovira, V.; Rodríguez, R.; Flor-Goikoetxea, A.; Piquer, J. Postoperative CSF Leakages after Transsphenoidal Surgery for Pituitary Adenomas: Analysis of a Series of 302 Surgical Procedures. Neurocirugia 2019, 30, 215–221. [Google Scholar] [CrossRef]
- Chen, G.; Li, M.; Xu, W.; Wang, X.; Feng, M.; Wang, R.; Liu, X. Surgical Outcomes of Clival Chordoma Through Endoscopic Endonasal Approach: A Single-Center Experience. Front Endocrinol (Lausanne) 2022, 13. [Google Scholar] [CrossRef] [PubMed]
- Emengen, A.; Yilmaz, E.; Gokbel, A.; Uzuner, A.; Cakir, O.; Ciftci, E.; Ozsoy, B.; Ergen, A.; Caklili, M.; Cabuk, B.; et al. Management of Major Arterial Injuries: A Critical Complication of Endoscopic Endonasal Surgery. Operative Neurosurgery 2025. [CrossRef]
- Zaki, U.; Shakeel, A.S.; Rauf, Y.; Raza, M. Pituicytoma: A Rare Tumor of the Sella. A Case Report and Review of Literature for Diagnosis and Management. Surg Neurol Int 2023, 14, 220. [Google Scholar] [CrossRef]
- Peng, Z.; Wang, Y.; Wang, Y.; Li, Q.; Fang, Y.; Fan, R.; Zhang, H.; Jiang, W. Hemangiopericytoma/Solitary Fibrous Tumor of the Cranial Base: A Case Series and Literature Review. BMC Surg 2022, 22, 289. [Google Scholar] [CrossRef]
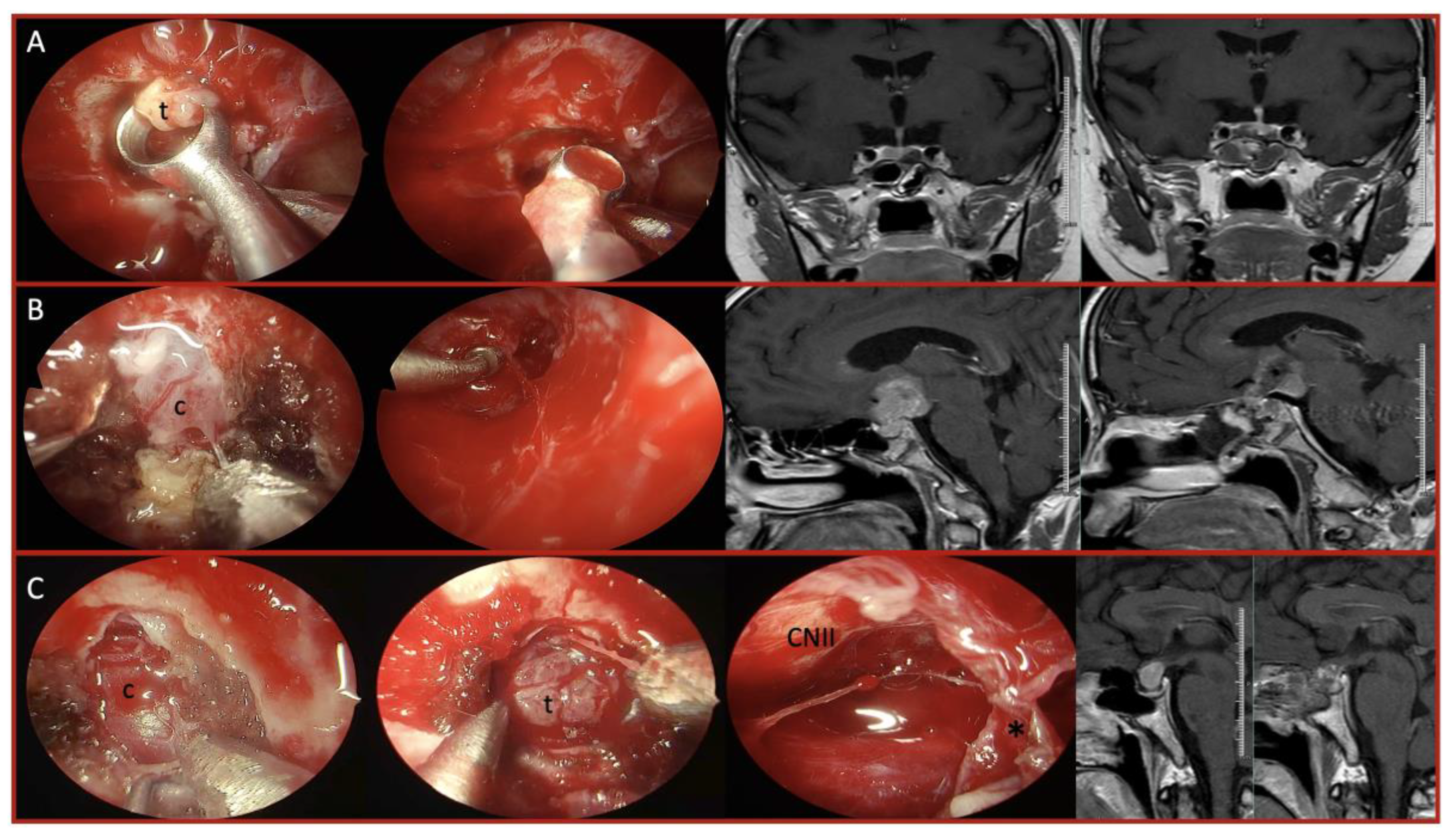
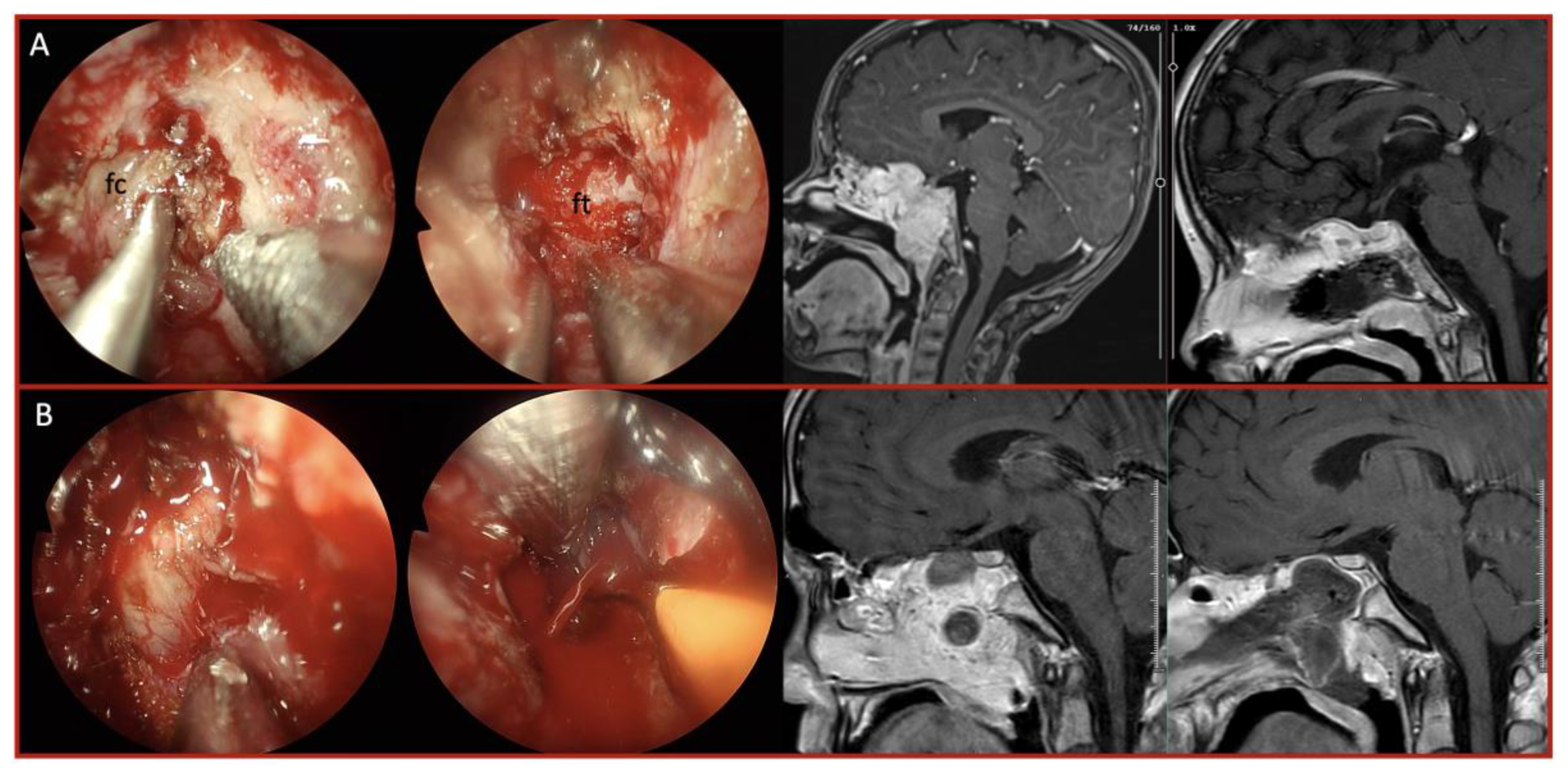
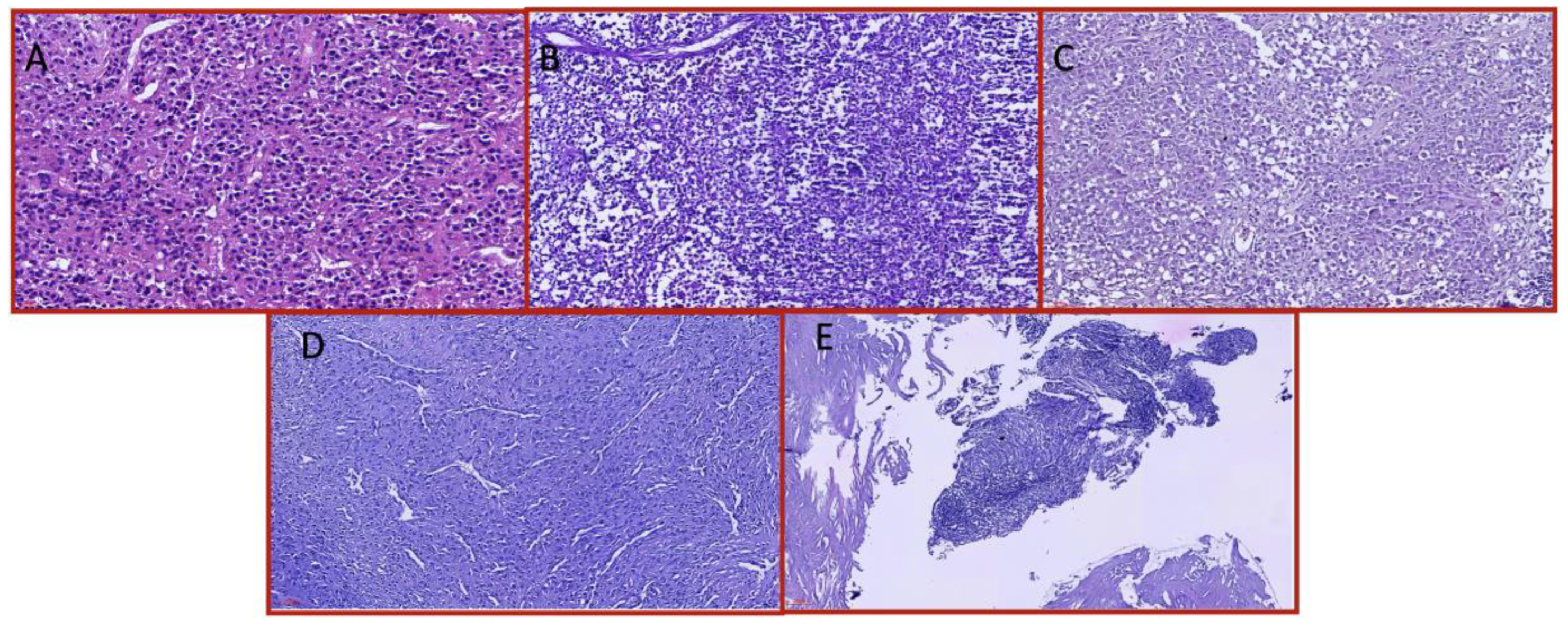
| Characteristic | Total (n=41) | Fibrotic/Adherent Tumors | Hypervascular Tumors |
|---|---|---|---|
| Mean age (years) | 34.7 ± SD | — | — |
| Sex (M/F) | 24 / 17 | — | — |
| Most common symptoms | Headache (51%), visual deficit (46%), hormonal dysfunction (32%) | DI, cranial nerve deficits | Epistaxis,nasal obstruction |
| Misdiagnosis rate | 48% Nonfunctioning pituitary adenoma |
Frequently misdiagnosed as PA and craniopharyngioma | Frequently misdiagnosed as PA and meningioma |
| Most frequent misdiagnosis | Nonfunctioning pituitary adenoma | Craniopharyngioma | Pituitary adenoma |
| Lesion location | Sellar/Parasellar dominant | Sellar/suprasellar | Clival, sphenoid sinus and nasal passage extensions |
| Preoperative imaging findings | Heterogeneous | Solid, low contrast enhancement | Intense contrast enhancement |
| Tumor Type | No. of Cases | Consistency / Vascularity | Extent of Resection | Adjuvant Therapy / Recurrence |
|---|---|---|---|---|
| Pituicytoma | 6 | Firm, highly vascular capsule | GTR/STR (5/1) |
No recurrence, no adjuvant |
| Plasmacytoma | 5 | Soft, moderately vascular | GTR/STR (3/2) |
RT + chemo; 2 recurrences |
| Xanthogranuloma | 5 | Fibrotic, adherent | GTR | No recurrence |
| Granular cell tumor | 4 | Soft intense | GTR | Gamma Knife (case-based) |
| Giant cell tumor | 3 | Highly vascular | GTR Pre-operative embolization |
Denosumab Fully controlled |
| Germinoma | 2 | Soft, Vascular capsule | STR | Chemo + RT; 1 recurrence |
| HPC | 2 | Hypervascular | STR | RT; 1 recurrence |
| LCH | 2 | Firm, fibrotic | GTR/STR (1/1) |
Systemic chemo; stable |
| Malignant epithelial | 2 | Adherent, fibrotic | STR | 1 Recurrence |
| Spindle cell oncocytoma | 2 | Firm, vascular | GTR/ STR 1/1 |
1 Gamma Knife |
| Others (each 1) | 10 | Varied | Varied | Case-specific |
| Variable | Total (n=41) | Fibrotic/Adherent Tumors | Hypervascular Tumors |
|---|---|---|---|
| Extent of Resection: GTR / STR | 28 (68%) / 13 (32%) | STR(8 patients) | STR(5patient) |
| Intraoperative bleeding (moderate-severe) | 14 (34.1%) | Rare | Frequent |
| Blood transfusion needed | 3 | 0 | 3 |
| CSF leak | 3 (7%) | 2 | 1 |
| New cranial nerve deficit | 1 (2.4%) | 1 | 0 |
| Transient DI | 4 (9.7%) | 3 | 1 |
| Permanent DI | 1 (2.4%) | 1 (LCH) | — |
| Recurrence rate | 7 (17%) | 4 | 3 |
| Adjuvant therapy needed | 16(39%) | Systemic therapy (LCH, lymphoma) | RT/Gamma Knife for HPC, plasmacytoma |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





