1. Introduction
Ischemic stroke is a serious and time-sensitive neurological condition that results from the obstruction of blood supply to brain tissue, accounting for approximately 85% of all stroke cases globally [
1,
2,
3]. It leads to a cascade of metabolic failure and neuronal injury due to reduced cerebral perfusion and oxygen deprivation [
4,
5]. Stroke-related morbidity and mortality are especially concerning in low- and middle-income countries (LMICs), where resource limitations delay diagnosis and limit access to advanced neuroimaging [
6,
7].
Cerebral perfusion parameters, CBF and CBV, are critical indicators of tissue viability in ischemic stroke and are used to distinguish reversible ischemia from irreversible infarction [
8,
9,
10]. Accurate measurement of these parameters aids in identifying viable penumbral tissue (the area of the brain at risk but not yet infarcted) and tailoring timely therapeutic interventions [
11,
12]. While CT perfusion (CTP) and dynamic susceptibility contrast MRI (DSC-MRI) remain the most common modalities used for perfusion imaging, they have limitations in terms of radiation exposure and reliance on susceptibility effects [
13,
14].
An emerging alternative is DCE-MRI, which leverages T1-weighted imaging and time-resolved contrast kinetics to generate semi-quantitative estimations of perfusion metrics [
15,
16,
17]. DCE-MRI offers enhanced signal-to-noise ratio, does not rely on susceptibility artefacts, and allows post-processing of pharmacokinetic data, making it a promising tool in stroke research and management [
18,
19]. Several studies have highlighted the potential of DCE-MRI to evaluate perfusion dynamics across major vascular territories of the brain in stroke patients [
20,
21,
22].
However, a review of existing literature reveals critical research gaps: (1) most perfusion studies are focused on DSC-MRI or CT perfusion techniques [
13,
14,
23]; (2) few investigations have employed DCE-MRI to evaluate CBF and CBV in ischemic stroke, particularly in low and middle-income countries (LMICs) [
15,
18,
24]; and (3) the effects of regional asymmetry (left vs. right hemisphere) and demographic variables such as age and gender on CBF and CBV remain underexplored using DCE-MRI [
20,
25,
26].
While several prior studies have reported that CBF and CBV vary across vascular territories, with differences between right and left hemispheres of MCA, ACA, and PCA [
17,
27,
28], these findings have relied mainly on CT or DSC-MRI modalities. Although a limited number of studies have begun to explore age- and sex-related perfusion differences using DCE-MRI [
3,
21,
22]. Consequently, a significant gap persists in studies using DCE-MRI to investigate perfusion asymmetries and demographic influences, especially in low- and middle-income countries (LMICs), such as Pakistan. This study addresses this gap by providing regional and demographic perfusion analysis in a Pakistani ischemic stroke (AIS) cohort using DCE-MRI.
This study aims to address the gaps identified in the existing literature by using DCE-MRI to: (1) quantify and compare CBF and CBV in contralateral cerebral arterial regions (right vs. left) of the MCA, ACA, and PCA; (2) evaluate regional perfusion variations across different cerebral arterial territories, irrespective of the side of the hemisphere; (3) investigate the correlation between CBF and CBV; and (4) assess the combined influence of demographic variables, including age and gender, on CBF in patients with ischemic stroke.
To the best of our knowledge, this study represents the first DCE-MRI-based evaluation of cerebral perfusion in the ischemic stroke patient population from Pakistan. It provides baseline demographic-specific data on CBF and CBV, which may enhance stroke imaging accuracy, inform clinical decision-making in resource-limited settings, and support the development of personalized therapeutic strategies.
2. Materials and Methods
2.1. Study Design and DCE-MRI Protocol
This cross-sectional, single-center observational study was conducted at the Department of Radiology, The University of Lahore Teaching Hospital, Lahore, Punjab, Pakistan, from July 2024 to January 2025. The primary objective was to assess cerebral perfusion dynamics, including CBF and CBV, in ischemic stroke, using a dynamic contrast-enhanced DCE-MRI protocol.
All imaging was performed within 48 hours of stroke onset, capturing the hyperacute and acute phases of ischemia. Patients were diagnosed through clinical neurological examination and documentation provided by the referring physician. The DCE-MRI protocol, using time-resolved T1-weighted sequences and intravenous contrast, was employed to evaluate regional perfusion in anatomically defined arterial territories of the brain, including the MCA, ACA, and PCA.
Although DCE-MRI is more commonly used in clinical contexts such as tumor imaging or vascular assessments, it was chosen for this study due to its institutional accessibility and its ability to generate temporal enhancement curves, from which semi-quantitative estimates of CBF and CBV were derived. These values were extracted through contrast enhancement dynamics and basic pharmacokinetic modelling applied to defined regions of interest (ROIs). The study also explored the influence of demographic factors, such as age and gender, on CBF variations in ischemic stroke patients.
2.2. Study Cohort and Inclusion/Exclusion Criteria
A convenience sample of ischemic stroke patients was recruited from the Department of Neuroradiology during the study period. Eligible participants were aged between 20 and 80 years, had experienced a clinically diagnosed ischemic stroke within 48 hours of symptom onset, and provided written informed consent before participation. Stroke diagnosis was established based on clinical presentation and referral documentation from the attending neurologist.
The age range for inclusion was set between 20 and 80 years to focus on adults with ischemic stroke while excluding the potential confounding effects of age-related comorbidities. Although older patients are often included in stroke studies, individuals over the age of 80 were excluded in this study to minimize the potential impact of age-related comorbidities, such as severe cardiovascular diseases, cognitive decline, and other systemic conditions, which are more prevalent in this age group and could confound the interpretation of cerebral perfusion dynamics. The sample size of 55 participants was selected based on the resources available at the study site, the institutional capacity for patient recruitment, and the practical considerations of conducting the research within the given timeline. Although power analysis was not performed, the sample size was determined to be adequate for the study's objectives, based on clinical guidelines, prior studies, and resource constraints.
Exclusion criteria included the presence of any contraindication to MRI, such as metallic implants, severe claustrophobia, or inability to receive gadolinium-based contrast agents (GBCAs). Participants with an estimated glomerular filtration rate (eGFR) below 30 mL/min/1.73 m², known contrast-induced hypersensitivity reactions, or any major concurrent neurological disorders (such as multiple sclerosis, intracranial tumors, or a history of stroke with persistent neurological deficits) were excluded, as these conditions could confound the interpretation of cerebral perfusion data. Additionally, individuals who were pregnant or breastfeeding were excluded due to the potential risks of contrast administration to the fetus or infant.
The study was conducted in accordance with the Declaration of Helsinki. This study received ethical approval from the Research Ethics Committee, Faculty of Allied Health Sciences, The University of Lahore, Punjab , Pakistan (Ref No: REC-UOL-680-06-2024), on June 15th, 2024 for involving humans. Informed consent was obtained from all participants, and confidentiality was maintained regarding personal and medical information.
2.3. Outcome Variables
- (a)
Dependent Variables: The primary dependent variables in this study were CBF, measured in mL/100 g/min, and CBV, measured in mL/100 g. These perfusion parameters were quantified using DCE-MRI-derived perfusion maps.
- (b)
Independent Variables: The independent variables were the anatomically defined regions of interest (ROIs) corresponding to major cerebral arterial territories, including the MCA, ACA, and PCA, evaluated bilaterally. These ROIs were identified based on standard anatomical landmarks and applied consistently across all subjects.
2.4. Data Collection Tools and Procedures
Data collection was conducted using a Toshiba Vantage Galan 3.0 Tesla MRI scanner, equipped with a phased array head coil for high-resolution cerebral imaging. The imaging protocol included a pre-contrast T1-weighted sequence, a DCE T1-weighted sequence, and, optionally, a post-contrast T1-weighted sequence for anatomical referencing.
For the DCE-MRI sequence, imaging parameters were optimized to enhance the visualization of contrast perfusion across cerebral territories, with a flip angle of 30°, a repetition time (TR) of 3.5 ms, an echo time (TE) of 2.0 ms, and a field of view (FOV) of 220 mm. Additional acquisition parameters included a slice thickness of 5 mm, a matrix size of 256 × 256, and a dynamic series capturing approximately 60 to 80 time points over 4 to 5 minutes. The selected imaging parameters were optimized to maximize the resolution and sensitivity of the DCE-MRI sequence for assessing regional perfusion in ischemic stroke. A 5 mm slice thickness was chosen to balance spatial resolution with signal-to-noise ratio, allowing for precise visualization of perfusion deficits while minimizing scan time. A flip angle of 30° was selected to optimize tissue contrast without compromising signal intensity. The repetition time (TR) and echo time (TE) were chosen to optimize the contrast enhancement dynamics and temporal resolution for perfusion imaging. The chosen field of view (FOV) and matrix size ensure that all key cerebral regions are adequately covered while maintaining high-quality imaging for accurate perfusion measurements.
The contrast agent used was Omnivist (Gadopentetate Dimeglumine), administered intravenously via the antecubital vein at a dose of 0.2 mL/kg body weight. The agent was delivered at a rate of 3 mL/s using a MEDRAD Spectris Solaris EP power injector, followed by a saline flush.
The collected imaging data were used to obtain values for CBF and CBV within defined arterial regions, including the MCA, ACA, and PCA. These values were evaluated across subjects, taking into account demographic and anatomical factors.
2.5. Statistical Methods and Analysis
All statistical analyses were conducted using IBM SPSS Statistics for Windows, Version 29.0 (IBM Corp., Armonk, NY, USA). Descriptive statistics, including means, medians, standard deviations, and ranges, were calculated to summarize participants' demographic characteristics and perfusion metrics (CBF and CBV).
To assess whether significant differences in perfusion existed between contralateral arterial territories in the left and right hemispheres (i.e., right vs. left MCA, ACA, and PCA), paired-samples t-tests were conducted separately for each pair of arteries.
To analyze whether CBF values varied significantly between different arterial regions overall (irrespective of side), a one-way analysis of variance (ANOVA) was used. This allowed for the comparison of CBF across multiple independent cerebral regions (such as, MCA, ACA, PCA) to detect regional perfusion differences in the brain.
To evaluate the relationship between CBF and CBV, both Pearson's correlation coefficient (for normally distributed data) and Spearman's rank correlation coefficient (for non-parametric data) were computed. These statistical analyses were performed to assess the strength and direction of the association between the two perfusion parameters, providing insights into how closely CBF and CBV are related and the nature of their relationship, whether they move in tandem (positive correlation) or inversely (negative correlation). Using both tests ensured a comprehensive assessment of the relationship, accounting for both linear associations (Pearson's correlation) and monotonic associations (Spearman's correlation).
Finally, a multiple linear regression analysis was conducted to explore the combined effect of age and gender on CBF, with these demographic factors treated as independent variables and CBF as the dependent variable. This analysis enabled the evaluation of how variability in CBF could be attributed to age and gender differences.
A two-tailed p-value < 0.05 was considered statistically significant for all tests. These analyses were intended to identify patterns, associations, and potential influencing factors related to cerebral perfusion in patients with acute ischemic stroke.
3. Results
3.1. Ischemic Stroke Patients' Demographics and Clinical Presentation
A total of 55 patients diagnosed with acute ischemic stroke were included in this study. The demographic details of the participants, including age distribution, are summarized in
Table 1. The overall mean age of the participants was 52.0 years, with a mean age of 51.57 years for male patients and 52.75 years for female patients. The median age was 55.0 years overall, with 56.0 years for males and 55.5 years for females. The age range of participants was 20 to 80 years, with a standard deviation of ±18.30 years for the overall age. The standard deviations were ±18.08 years for males and ±17.40 years for females.
The variability in age allows us to assess how age influences CBF and CBV in ischemic stroke patients, providing insights into potential age-related perfusion changes and their impact on ischemic stroke patients.
Clinical symptomatology observed at the time of presentation is detailed in
Table 2. The most frequently reported symptom was sudden weakness on one side of the body, observed in 10 patients, consistent with the typical motor deficits associated with acute ischemic stroke. Vision problems were reported by 7 patients, followed by confusion in 6 patients, and difficulty speaking, facial drooping, and nausea, each of which occurred in 5 patients. Additionally, severe headache and loss of balance were reported by 4 patients each, while dizziness, loss of coordination, and speech difficulties were reported by 3 patients each.
These findings reflect the diverse neurological manifestations of ischemic stroke, which are crucial for understanding how symptomatology may correlate with perfusion variations in different cerebral arterial territories.
3.2. Cerebral Blood Flow (CBF) and Cerebral Blood Volume (CBV) Measurements
The descriptive statistics for CBF and CBV among the 55 ischemic stroke patients are summarized in
Table 3. The mean CBF was 25.39 mL/100 g/min, with a median value of 25 mL/100 g/min. The recorded values ranged from 20 to 32 mL/100 g/min, with a standard deviation (SD) of ±3.15, indicating a moderate degree of variability in cerebral perfusion across the cohort.
The moderate degree of variability in CBF is common in ischemic stroke, as perfusion in certain regions may fluctuate due to factors such as collateral circulation, tissue viability, and the severity of ischemic injury. This variability in perfusion metrics is crucial for assessing stroke severity, as higher CBF and CBV values are typically associated with better tissue viability and less severe ischemic injury. Conversely, significantly lower CBF and CBV values could indicate areas of irreversible tissue damage, which has important implications for stroke prognosis and recovery.
These findings provide a quantitative baseline of central tendency and variability in cerebral perfusion among patients with ischemic stroke. The measured CBF and CBV values are within the expected physiological ranges for ischemic stroke patients, providing a baseline for subsequent comparative and correlation analyses across arterial territories and demographic subgroups.
3.3. Regional Cerebral Perfusion Patterns
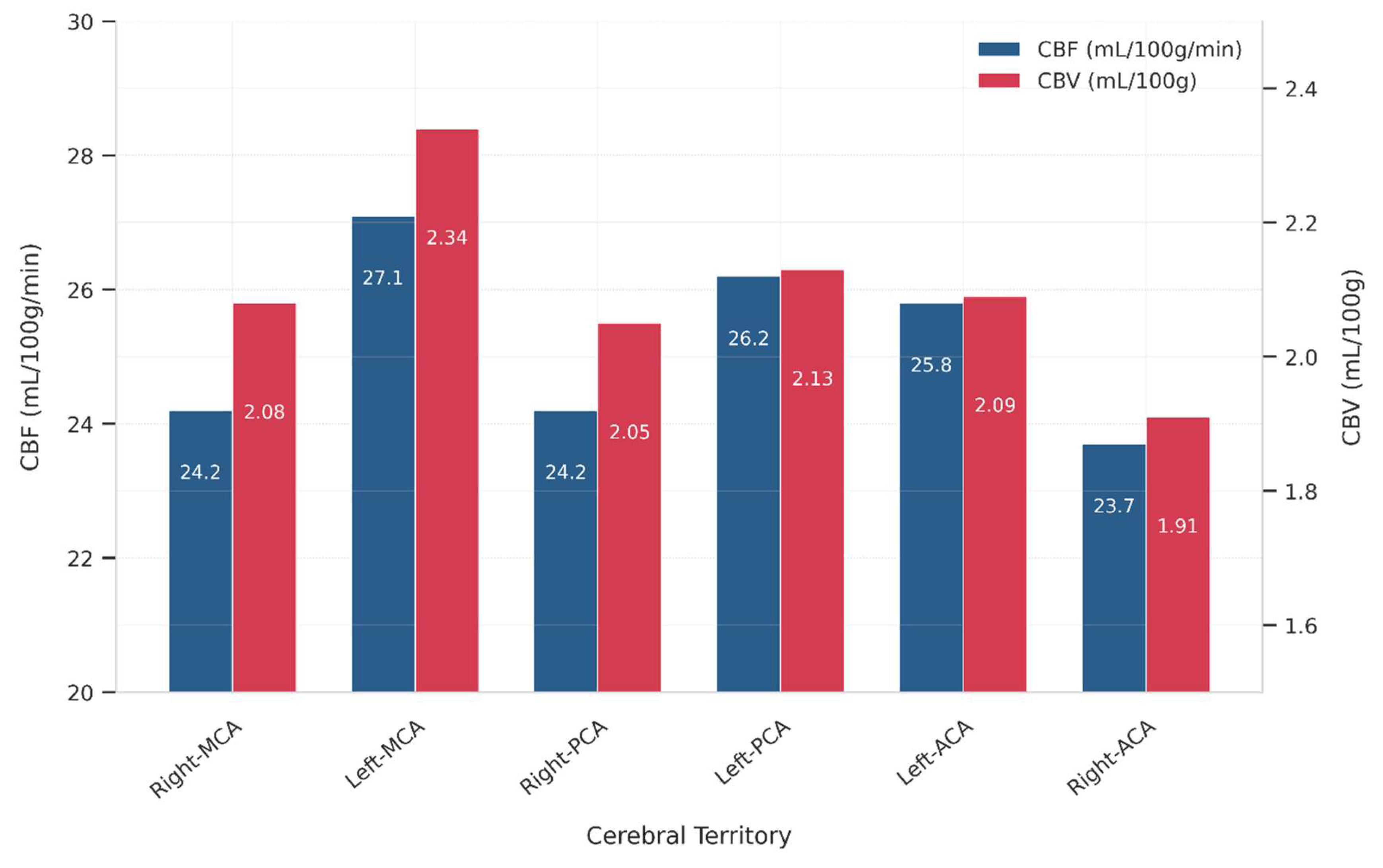
The range of CBF and CBV across major cerebral arterial territories is presented in
Table 4 and visualized as the mean values of CBF and CBV in
Figure 1. The data demonstrate measurable perfusion variability between the left and right hemispheres, as well as distinct vascular territories.
In the MCA region, CBF ranged from 20 to 29 mL/100 g/min on the right side and 22 to 32 mL/100 g/min on the left, while CBV values ranged between 1.8–2.4 mL/100 g (right MCA) and 2.0–2.7 mL/100 g (left MCA). In the PCA territories, the right PCA exhibited a CBF range of 21–30 mL/100 g/min and CBV of 1.7–2.5 mL/100 g, while the left PCA showed slightly lower CBF values (24–28 mL/100 g/min) and CBV values between 2.0–2.4 mL/100 g. For the ACA, the right hemisphere ranged from 20–27 mL/100 g/min (CBF) and 1.7–2.1 mL/100 g (CBV), while the left side demonstrated higher perfusion, with CBF ranging from 23–29 mL/100 g/min and CBV from 1.9–2.4 mL/100 g.
These results indicate a left-sided dominance in both CBF and CBV, particularly in the MCA and ACA regions. This suggests asymmetrical perfusion patterns, which may correlate with stroke lateralization and hemispheric hemodynamic differences. These findings could inform treatment strategies for ischemic stroke, with potential implications for targeting different hemispheres.
3.4. Statistical Test Results: Perfusion and Demographic Relationships
The statistical analysis demonstrated significant differences in perfusion parameters across cerebral territories, as summarized in
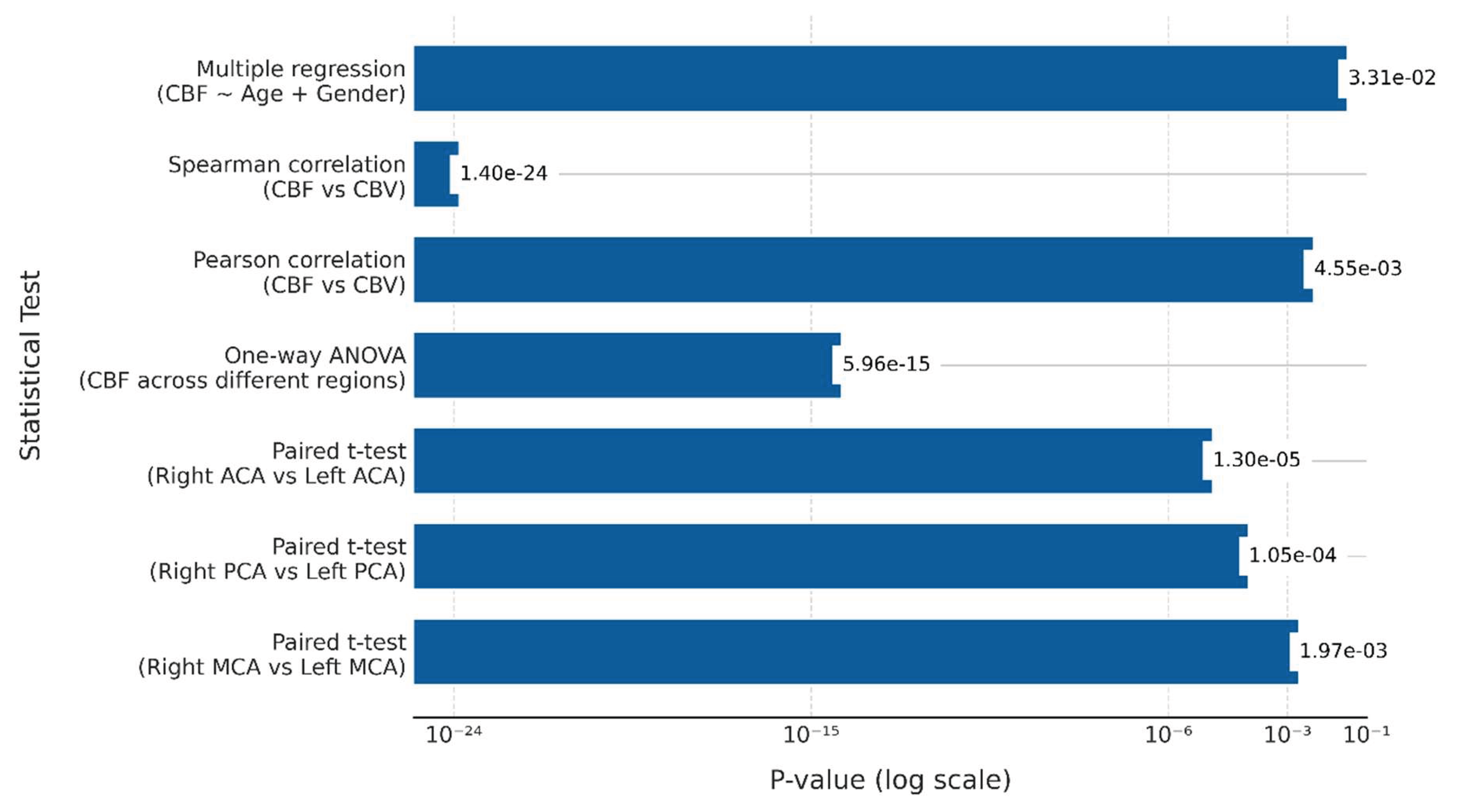
Table 5. Paired t-tests were conducted between contralateral regions (i.e., left and right MCA, ACA, and PCA) to evaluate potential lateralization of cerebral perfusion during ischemic stroke. Significant differences in CBF between contralateral cerebral arterial regions were observed. Specifically, the t-statistic for the right versus left MCA was -7.204 (p = 0.00197), for the right versus left PCA, it was -15.339 (p = 0.000105), and for the right versus left ACA, it was -25.820 (p = 0.000013). These results highlight the lateralization of CBF across different hemispheres in ischemic stroke patients.
One-way ANOVA was used to evaluate whether CBF values varied significantly across different arterial regions irrespective of the side of the hemisphere, and significant variability was found in CBF measurements across brain regions (F-statistic: 93.847, p = 5.96e-15). This test was chosen to compare multiple brain regions (MCA, ACA, PCA) within a single analysis, allowing for a broader understanding of perfusion patterns across different vascular territories.
Pearson and Spearman correlations revealed a strong positive relationship between CBF and CBV (Pearson's correlation coefficient: 0.976, p = 0.00455; Spearman's correlation coefficient: 0.928, p = 1.40e-24). These findings suggest that as CBF increases, CBV also tends to increase. This relationship can be explained by the dynamic coupling of blood flow and volume during ischemic events, a key characteristic in understanding the pathophysiology of stroke. This coupling provides important clinical insights, aiding early assessment of tissue viability, predicting stroke severity, and guiding treatment strategies.
Multiple Regression Analysis was performed to evaluate the combined effect of age and gender on CBF. The results revealed that age and gender together accounted for 96.7% of the variability in CBF, as indicated by the R-squared value of 0.967. This strong explanatory power suggests that these demographic factors significantly influence cerebral perfusion in ischemic stroke patients. The F-statistic of 0.0331 further supports the statistical significance of the model, confirming that the inclusion of age and gender in the regression analysis provides valuable insights into CBF variation. This result highlights the importance of considering demographic factors when assessing cerebral perfusion. It underscores the role of age and gender as significant determinants of CBF in the context of ischemic stroke. These results are consistent with the statistical findings presented in
Figure 2, where the p-values highlight the significance of the observed relationships and differences in ischemic stroke patients.
4. Discussion
This study aimed to assess cerebral perfusion dynamics, specifically CBF and CBV, in ischemic stroke patients using DCE-MRI. Our findings revealed significant regional variations in CBF and CBV across major cerebral arteries, highlighting a pronounced asymmetry between the left and right hemispheres. These results confirm the presence of lateralized perfusion patterns in ischemic stroke, a phenomenon that may be vital for understanding stroke progression and therapeutic interventions. These results align with previous research, reinforcing the concept of lateralized perfusion patterns in ischemic stroke. Additionally, a strong positive correlation was identified between CBF and CBV, emphasizing the interdependence of these two perfusion parameters in stroke evaluation.
Age and gender both had significant effects on CBF in our study cohort. These findings offer valuable insights into the regional perfusion characteristics of ischemic stroke and highlight the potential of DCE-MRI as a reliable diagnostic tool, especially in settings with limited access to advanced imaging technologies.
4.1. Key Findings and Their Interpretation
Our study revealed notable regional differences in CBF and CBV across major cerebral arteries, specifically the MCA, ACA, and PCA regions. Higher perfusion values were observed in the left hemisphere, particularly in the MCA and ACA regions, suggesting left-sided dominance in cerebral perfusion. This finding aligns with established research indicating lateralized perfusion abnormalities in ischemic stroke.
The strong correlation between CBF and CBV (Pearson's r = 0.976, Spearman's r = 0.928) emphasizes the interdependence of these two parameters in assessing stroke severity. CBF and CBV provide complementary information about tissue viability and the extent of ischemic damage, reinforcing their importance in stroke assessment. This relationship reflects the dynamic coupling of blood flow and volume during ischemic events, a phenomenon that is central to understanding stroke pathophysiology. Clinically, this coupling can inform early assessments of tissue viability, enabling clinicians to predict stroke severity and formulate effective treatment strategies.
Regarding age, our findings revealed that age significantly contributes to variability in CBF. While some studies suggest a linear decrease in CBF with advancing age, our results indicate a more complex relationship between age and cerebral perfusion. This suggests that various factors, including underlying comorbidities, may influence the effects of aging on vascular health. Further investigation into these dynamics is necessary to understand better how age-related changes affect perfusion. Such insights could lead to more personalized treatment strategies for elderly patients with stroke.
Interestingly, our study observed that gender also had a significant effect on CBF, which contrasts with some studies suggesting minimal gender-based vascular differences. This finding highlights the importance of considering gender as a factor in cerebral perfusion dynamics, particularly in ischemic stroke. Discrepancies between studies may arise from variations in patient cohorts, methodology, or the specific focus of the studies. Future research with larger, more diverse populations will be essential to understand better the role of gender in cerebral perfusion and stroke pathophysiology.
4.2. Comparison with Existing Literature
4.2.1. Use of DCE-MRI for Perfusion Imaging
Our study, as well as that of Sasannia et al. [
14], demonstrated the clinical utility of DCE-MRI for assessing CBV and CBF in ischemic stroke. Sasannia et al. [
14] highlighted that DCE-MRI can detect early perfusion changes, even before conventional imaging modalities, such as DWI and ASL, become apparent. Our study aligns with these findings, reinforcing the growing body of evidence that DCE-MRI is a highly sensitive and reliable modality for evaluating stroke severity and ischemic tissue viability. This early detection capability is critical in guiding timely interventions and improving patient outcomes.
In addition, the study by Lu et al. [
29], explored the application of both DCE-MRI and Dynamic Susceptibility Contrast MRI (DSC-MRI) for evaluating vascular and hemodynamic features in acute ischemic stroke. Their findings align with our research, underscoring the importance of advanced imaging techniques in assessing stroke severity and informing clinical decision-making. Lu et al. [
29] also highlighted the superiority of DCE-MRI in detecting subtle perfusion abnormalities, a key observation that resonates with our identification of regional perfusion deficits in ischemic stroke. Both studies emphasize the clinical utility of DCE-MRI in elucidating the complex pathophysiology of stroke, as this imaging modality offers valuable insights into the brain's vascular dynamics and facilitates the identification of at-risk brain regions.
4.2.2. Regional Perfusion Variations
Our findings align with those of Valente et al. [
30], who observed regional perfusion abnormalities across the MCA, ACA, and PCA territories in ischemic stroke patients. Using CT vascular territory mapping, Valente et al. [
30] identified collateral circulation in patients with large vessel occlusion (LVO), finding significant perfusion deficits in the MCA, ACA, and PCA regions. This is consistent with our study's observation of asymmetries in CBF and CBV between hemispheres, particularly between the left and right hemispheres. These findings underscore the clinical relevance of regional perfusion differences in stroke assessment, highlighting the value of detailed perfusion imaging in understanding the pathophysiology of stroke.
Furthermore, Furlanis et al. [
31] reinforce the utility of advanced perfusion imaging, particularly CT perfusion (CTP), in detecting acute posterior circulation stroke (PCS) and identifying associated clinical factors. Their work contributes to the growing body of evidence supporting the clinical importance of perfusion metrics in assessing ischemic stroke, emphasizing the need for personalized treatment strategies based on perfusion abnormalities. Our research echoes these findings, further validating the role of advanced imaging in stroke management.
The study by Strinitz et al. [
32] provides additional support for the clinical significance of CBV, showing that higher relative cerebral blood volume (rCBV) in the affected brain region is associated with better long-term functional outcomes in acute ischemic stroke. Their retrospective cohort study demonstrated that elevated rCBV, even after adjusting for baseline stroke severity and collateral circulation, correlates with improved prognosis. This aligns with our observation that regional variations in CBV and CBF are strongly linked to functional outcomes, reinforcing the importance of these biomarkers in determining stroke severity and guiding treatment decisions.
4.2.3. Perfusion Metrics and Their Relationship to Brain Volume
In line with Kikuchi et al. [
33], our study suggests that regional perfusion variations are key indicators of brain tissue viability and stroke severity. Kikuchi et al. [
33] reported that perfusion measurements correlate strongly with brain volume, particularly in areas such as the frontal and temporal lobes, which are crucial for cognitive function. Our findings further support this, as variations in CBV and CBF in specific brain regions were associated with functional outcomes, underscoring the importance of perfusion metrics in assessing the health of brain tissue post-stroke.
4.2.4. Age and Gender Effects on CBF in Ischemic Stroke
This study revealed that both age and gender significantly influence CBF in ischemic stroke patients, although the specific patterns or age groups most affected were not explored in depth. Our findings suggest that age contributes significantly to variations in CBF, supporting the notion that age-related vascular changes may be a key factor influencing perfusion in ischemic stroke. This is consistent with previous studies by Shao et al. [
34] and Hu et al. [
35], which highlighted age-related perfusion changes. However, the exact age group most affected by these changes remains complex, as our study observed a nuanced relationship between age and CBF, rather than a simple linear decrease.
In terms of gender, our study found that gender is a significant factor affecting CBF, which contrasts with some studies, such as Shao et al. [
34], which suggest that males typically exhibit lower CBF values, particularly due to age-related vascular decline. The lack of significant findings in some studies could be due to methodological differences, sample sizes, or the focus on ischemic stroke in our cohort. Studies with broader stroke populations, like Shao et al. [
34], may have observed different gender-related effects on CBF, whereas our research concentrated specifically on ischemic stroke patients.
4.3. Implications for Future Research
Future research should address the limitations of this study, such as the small sample size and exclusion of certain groups. Larger, more diverse cohorts across multiple centers are needed to validate these findings and improve generalizability. Further investigation into the specific effects of age and gender on CBF is required, particularly regarding which age groups are most affected and how gender influences perfusion patterns. Additionally, exploring the impact of comorbidities on cerebral perfusion in ischemic stroke patients will provide deeper insights into stroke pathophysiology and help refine treatment strategies.
5. Conclusions
In this study, we assessed cerebral perfusion dynamics in ischemic stroke patients using DCE-MRI, revealing significant regional variations in CBF and CBV, with a notable left-sided dominance in certain cerebral arterial territories. This finding supports established research demonstrating lateralized perfusion patterns in ischemic stroke. Additionally, a strong positive correlation was observed between CBF and CBV, highlighting their interdependence as essential parameters for assessing stroke severity and tissue viability.
Both age and gender were found to influence CBF in ischemic stroke patients significantly. However, the specific patterns of how these factors affect CBF were not explored in detail. These results underscore the importance of considering age and gender when assessing cerebral perfusion, paving the way for future research that could provide a more nuanced understanding of their effects and ultimately inform personalized stroke management strategies.
The clinical implications of this study are significant. Our results underscore the potential of DCE-MRI as a reliable diagnostic tool for assessing regional perfusion, especially in settings with limited access to advanced imaging technologies. By addressing gaps in regional and demographic perfusion analysis, our study provides valuable insights into stroke imaging, offering directions for more targeted and personalized treatment strategies.
However, the study has limitations, including the small sample size and the exclusion of specific patient groups, which may affect the generalizability of the results. Future research should focus on larger, more diverse populations and explore the full role of age, gender, and comorbidities in cerebral perfusion. This will refine stroke management, improve patient outcomes, and enhance the accuracy of stroke prediction models, ultimately providing more personalized treatment interventions for ischemic stroke patients.
Author Contributions
Conceptualization, B.B. and S.M.; methodology, B.B. and B.A.; software, B.K.; validation, B.B., B.K. and S.M.; formal analysis, B.A. and B.K.; investigation, B.B., B.A. and S.M.; resources, B.B. and S.M.; data curation, B.B. and B.A.; writing—original draft preparation, B.B. and B.A.; writing—review and editing, B.B., B.A. and S.M.; visualization, B.B.; supervision, S.M. and B.K.; project administration, B.B. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
The study was conducted in accordance with the Declaration of Helsinki. This study received ethical approval from the Research Ethics Committee, Faculty of Allied Health Sciences, University of Lahore (Ref No: REC-UOL-680-06-2024), on June 15th, 2024 for involving humans.
Informed Consent Statement
Informed consent was obtained from all the individuals involved in the study. Also, written informed consent has been obtained from the patient(s) to publish this paper.
Data Availability Statement
The datasets used and/or analysed during the current study are available from the corresponding author on reasonable request.
Acknowledgments
None to declare.
Conflicts of Interest
The authors declare no conflicts of interest.
Abbreviations
The following abbreviations are used in this manuscript:
| CBF |
Cerebral Blood Flow |
| CBV |
Cerebral Blood Volume |
| DCE-MRI |
Dynamic Contrast-Enhanced Magnetic Resonance Imaging |
| MCA |
Middle Cerebral Artery |
| ACA |
Anterior Cerebral Artery |
| PCA |
Posterior Cerebral Artery |
| CTP |
Computed Tomography Perfusion |
| DSC-MRI |
Dynamic Susceptibility Contrast Magnetic Resonance Imaging |
| LMICs |
Low- and Middle-Income Countries |
| ANOVA |
Analysis of Variance |
| SD |
Standard Deviation |
| eGFR |
Estimated Glomerular Filtration Rate |
| ROIs |
Regions of Interest |
References
- Liu, S.; et al. Global, regional, and national trends in ischaemic stroke burden and risk factors among adults aged 20 + years (1990-2021): a systematic analysis of data from the Global Burden of Disease study 2021 with projections into 2050. Front Public Health 2025, 13, 1567275. [Google Scholar] [CrossRef] [PubMed]
- Feigin, V.L.; et al. World Stroke Organization: Global Stroke Fact Sheet 2025. Int J Stroke 2025, 20, 132–144. [Google Scholar] [CrossRef]
- Maguida, G.; Shuaib, A. Collateral Circulation in Ischemic Stroke: An Updated Review. J Stroke 2023, 25, 179–198. [Google Scholar] [CrossRef]
- Kakkar; P. ; Kakkar; T.; Patankar, T.; Saha, S. Current approaches and advances in the imaging of stroke. Dis Model Mech 2021, 14, dmm048785. [Google Scholar] [CrossRef]
- Maida; C. D.; Norrito; R.L.; Rizzica; S.; Mazzola; M.; Scarantino, E.R.; Tuttolomondo, A. Molecular Pathogenesis of Ischemic and Hemorrhagic Strokes: Background and Therapeutic Approaches. International Journal of Molecular Sciences 2024, 25. [Google Scholar] [CrossRef]
- Feigin; V. L.; et al. World Stroke Organization: Global Stroke Fact Sheet 2025. Int J Stroke 2025, 20, 132–144. [Google Scholar] [CrossRef]
- Aguirre; et al. Stroke management and outcomes in low-income and lower-middle-income countries: a meta-analysis of 8535 patients. Mar. 2023. [CrossRef]
- Fang; H. ; He; G.; Cheng; Y.; Liang, F.; Y. Zhu. Advances in cerebral perfusion imaging techniques in acute ischemic stroke. J Clin Ultrasound 2022, 50, 1202–1211. [Google Scholar] [CrossRef] [PubMed]
- Lakhani; D.A.; et al. Perfusion-Based Relative Cerebral Blood Volume Is Associated With Functional Dependence in Large-Vessel Occlusion Ischemic Stroke. Journal of the American Heart Association, vol. 13, no. 23, p. e034242, Dec. 2024. [CrossRef]
- Sarraj; Pujara, D. ; Campbell, B. Current State of Evidence for Neuroimaging Paradigms in Management of Acute Ischemic Stroke. Annals of Neurology 2024, 95, 1017–1034, Apr. [Google Scholar] [CrossRef]
- Liu, M.M.; et al. Quantification of Collateral Supply with Local-AIF Dynamic Susceptibility Contrast MRI Predicts Infarct Growth. ArXiv, p. arXiv:2406.04026v1, Jun. 2024.
- Maguida, G.; Shuaib, A. Collateral Circulation in Ischemic Stroke: An Updated Review. J Stroke 2023, 25, 179–198. [Google Scholar] [CrossRef]
- C. Yu, W. Lu, J. Qiu, F. Wang, J. Li, and L. Wang, “Alterations of the Whole Cerebral Blood Flow in Patients With Different Total Cerebral Small Vessel Disease Burden,” Front. Aging Neurosci., vol. 12, Jun. 2020. [CrossRef]
- Sasannia; et al. Blood-brain barrier breakdown in brain ischemia: Insights from MRI perfusion imaging. Neurotherapeutics 2025, 22, e00516. [Google Scholar] [CrossRef] [PubMed]
- Mosteiro; et al. Understanding the Importance of Blood-Brain Barrier Alterations in Brain Arteriovenous Malformations and Implications for Treatment: A Dynamic Contrast-Enhanced-MRI–Based Prospective Study. Neurosurgery 2025, 96, 811. [Google Scholar] [CrossRef]
- Yu, X.; Ji, C.; Shao, A. Neurovascular Unit Dysfunction and Neurodegenerative Disorders. Front Neurosci 2020, 14, 334. [Google Scholar] [CrossRef]
- Hallett; W.A.; Gambarota; G.; Newbould; R.; Lally, P.; Matthews, P.M. MRI and PET Imaging: Clinical Applications. in Encyclopedia of Biophysics, Springer, Berlin, Heidelberg, 2021, pp. 1–8. [CrossRef]
- Chaudhary; S.; Chao; H.H.; Agarwal; K.; Gupta, S.; Li, C.-S.R. Imaging Markers of Neuroinflammation in Aging and Alzheimer’s Disease and Related Dementia: A Comprehensive Review. Neurosci Biobehav Rev, p. 106270, Jun. 2025. [CrossRef]
- Franx; B. A.; et al. Dynamics of cerebral blood volume during and after middle cerebral artery occlusion in rats - Comparison between ultrafast ultrasound and dynamic susceptibility contrast-enhanced MRI measurements. J Cereb Blood Flow Metab 2024, 44, 333–344. [Google Scholar] [CrossRef] [PubMed]
- Tseng, C.-H.; et al. Improved reliability of perfusion estimation in dynamic susceptibility contrast MRI by using the arterial input function from dynamic contrast enhanced MRI”. [CrossRef]
- Kim; K. J.; Park; M.; Joo; B.; Ahn, S.J.; Suh, S.H. Dynamic Contrast-Enhanced MRI and Its Applications in Various Central Nervous System Diseases. Investigative Magnetic Resonance Imaging 2022, 26, 256–264. [Google Scholar] [CrossRef]
- Lu, J.; et al. Imaging Acute Stroke: From One-Size-Fit-All to Biomarkers. Front. Neurol. 2021; 12. [Google Scholar] [CrossRef]
- Cao, A.; et al. Quantitative perfusion maps using a novelty spatiotemporal convolutional neural network. Dec. arXiv, 2023; arXiv:arXiv:2312.05279. [Google Scholar] [CrossRef]
- Fan, J.-L.; et al. Integrative cerebral blood flow regulation in ischemic stroke. J Cereb Blood Flow Metab 2022, 42, 387–403. [CrossRef]
- Włodarczyk; L. ; Cichon; N.; Saluk-Bijak; J.; Bijak; M.; Majos, A.; Miller, E. Neuroimaging Techniques as Potential Tools for Assessment of Angiogenesis and Neuroplasticity Processes after Stroke and Their Clinical Implications for Rehabilitation and Stroke Recovery Prognosis. JCM 2022, 11, 2473. [Google Scholar] [CrossRef]
- C.; Lu; W.; Qiu; J.; Wang; F.; Li, J.; Wang, L. Alterations of the Whole Cerebral Blood Flow in Patients With Different Total Cerebral Small Vessel Disease Burden. Front. Aging Neurosci., vol. 12, p. 175, Jun. 2020. [CrossRef]
- Heye; K. ; Culling; R.D.; Hernández; M.D.C.V.; Thrippleton, M.J.; Wardlaw, J.M. Assessment of blood-brain barrier disruption using dynamic contrast-enhanced MRI. A systematic review. Neuroimage Clin 2014, 6, 262–274. [Google Scholar] [CrossRef]
- Secchinato; K. F.; da Silva; P.H.R.; Rodrigues; G.R.; Ferreira; A.P.A.C.; Pontes-Neto, O.M.; Leoni, R.F. Impaired Cerebral Hemodynamics in Asymptomatic Carotid Artery Stenosis Assessed by Resting-State Functional MRI. Journal of Vascular Diseases 2025, 4. [Google Scholar] [CrossRef]
- Lu, J.; et al. Imaging Acute Stroke: From One-Size-Fit-All to Biomarkers. Front. Neurol. 2021; 12. [Google Scholar] [CrossRef]
- Valente; M. ; Bivard; A.; Cheung; A.; Manning, N.W.; Parsons, M.W. CT vascular territory mapping: a novel method to identify large vessel occlusion collateral. Neuroradiology 2023, 65, 113–119, Jan. [Google Scholar] [CrossRef]
- “Effectiveness of CT perfusion in posterior circulation stroke: evaluation of perfusion abnormalities and associated clinical signs | Journal of Neurology.” Accessed: Jul. 02, 2025. [Online]. Available: https://link.springer.com/article/10.1007/s00415-025-12933-4.
- Strinitz; et al. High relative cerebral blood volume is associated with good long term clinical outcomes in acute ischemic stroke: a retrospective cohort study. BMC Neurology 2024, 24, 294. [Google Scholar] [CrossRef]
- Kikuchi; et al. Brain volume measured by synthetic magnetic resonance imaging in adult moyamoya disease correlates with cerebral blood flow and brain function. Sci Rep 2024, 14, 5468. [Google Scholar] [CrossRef] [PubMed]
- X Shao; et al. Age-related decline in blood-brain barrier function is more pronounced in males than females in parietal and temporal regions. eLife, vol. 13, p. RP96155. 9615; P5. [CrossRef]
- Hu, Y.; Zhang, K.; Liu, R. The effect of post-labeling delay on cerebral blood flow is influenced by age and sex: a study based on arterial spin-labeling magnetic resonance imaging. Quant Imaging Med Surg 2024, 14, 4388–4402. [Google Scholar] [CrossRef] [PubMed]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).