Submitted:
21 July 2025
Posted:
22 July 2025
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Experimental Methods
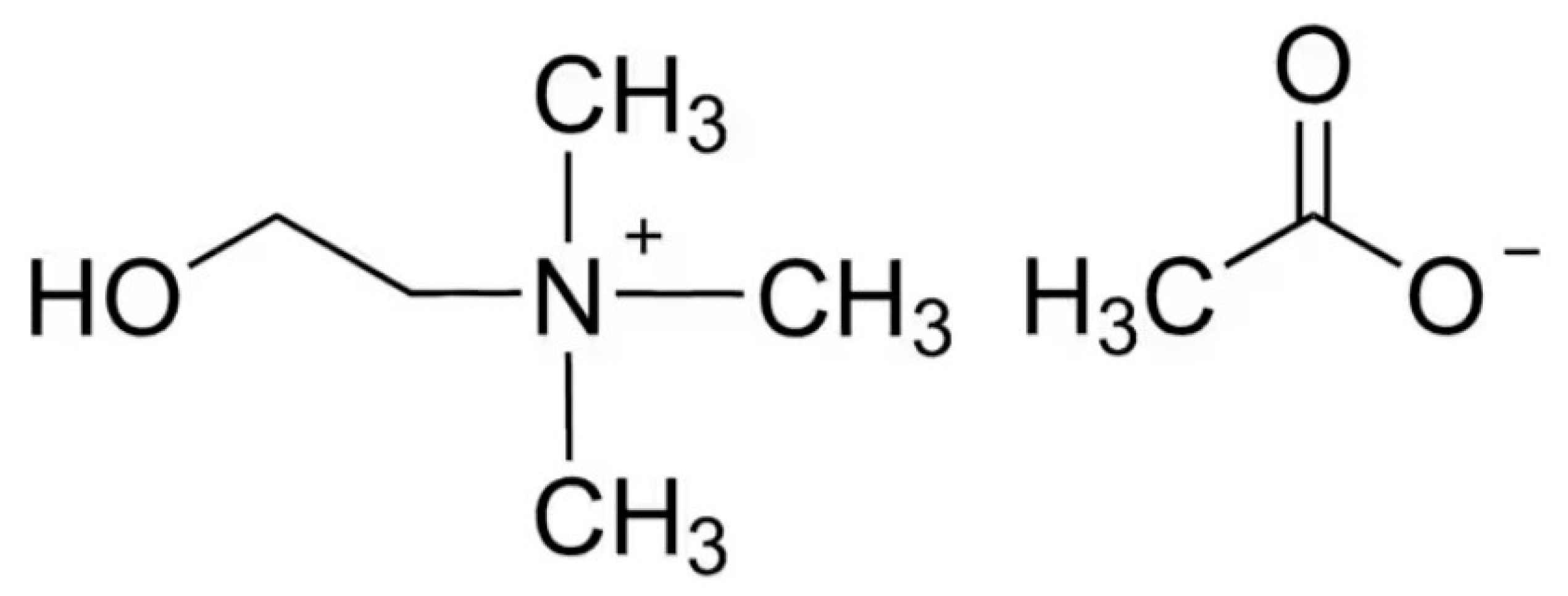
2.1. Chemicals
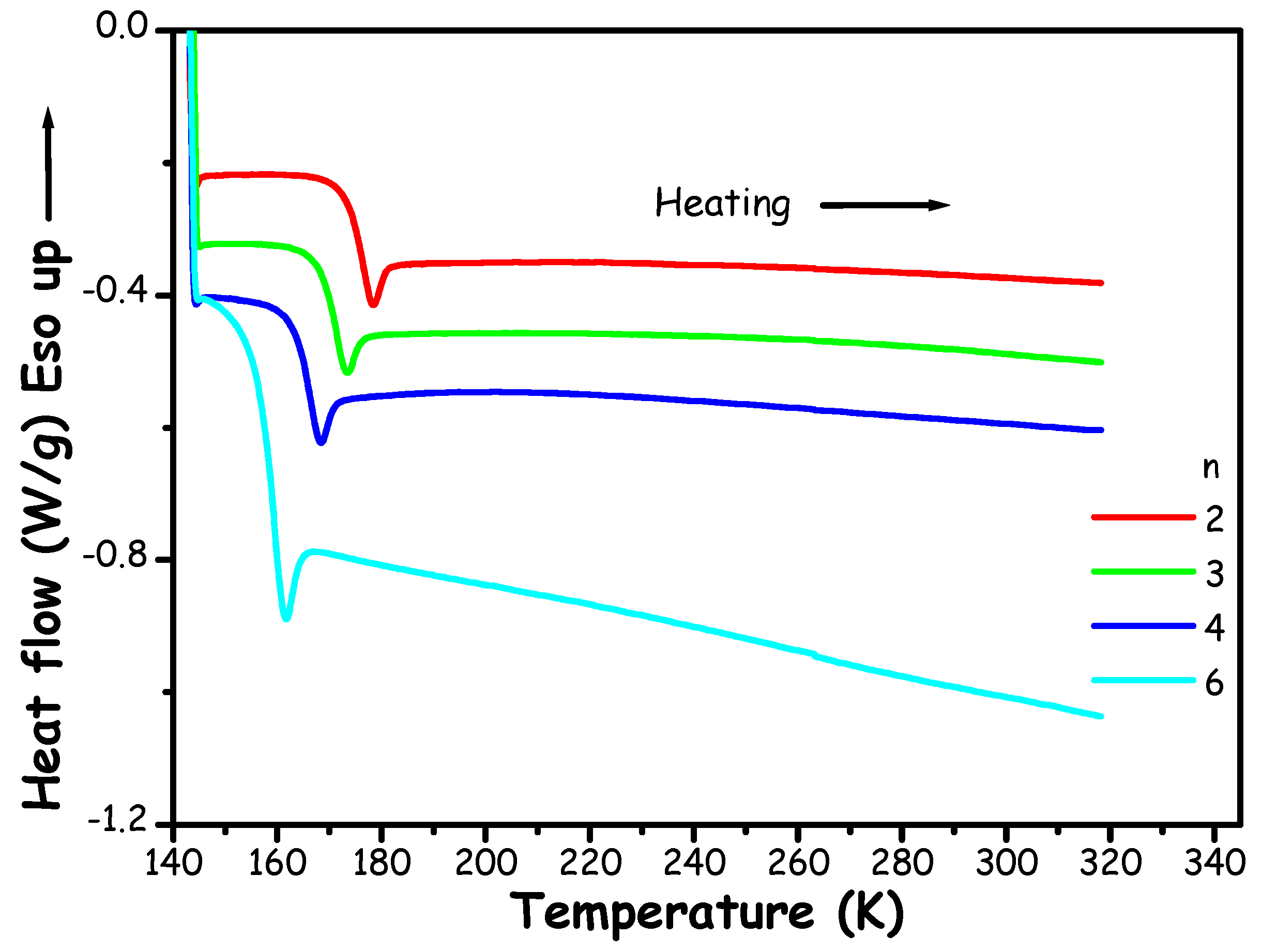
2.2. DSC Measurements
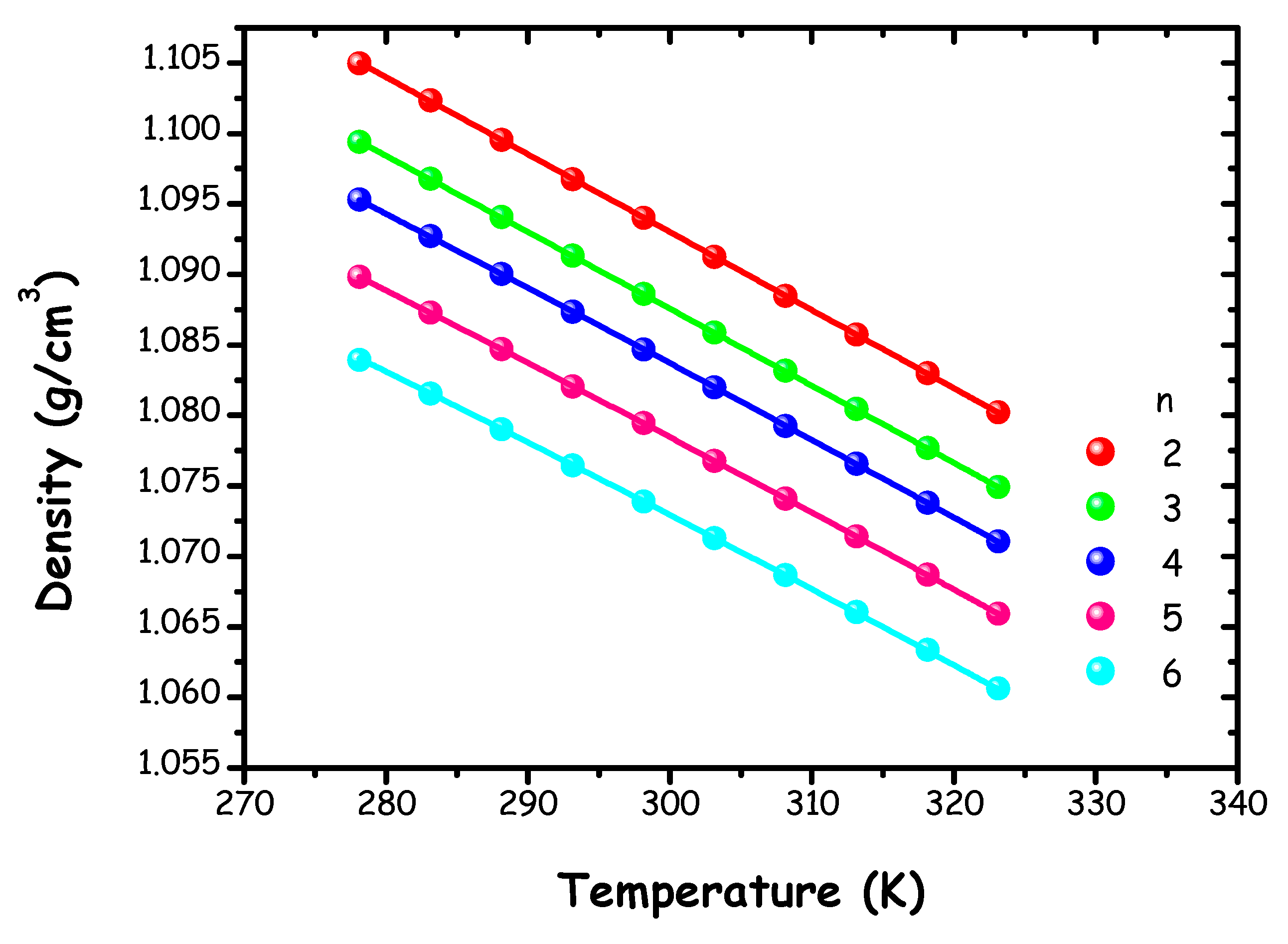
2.3. Density Measurements
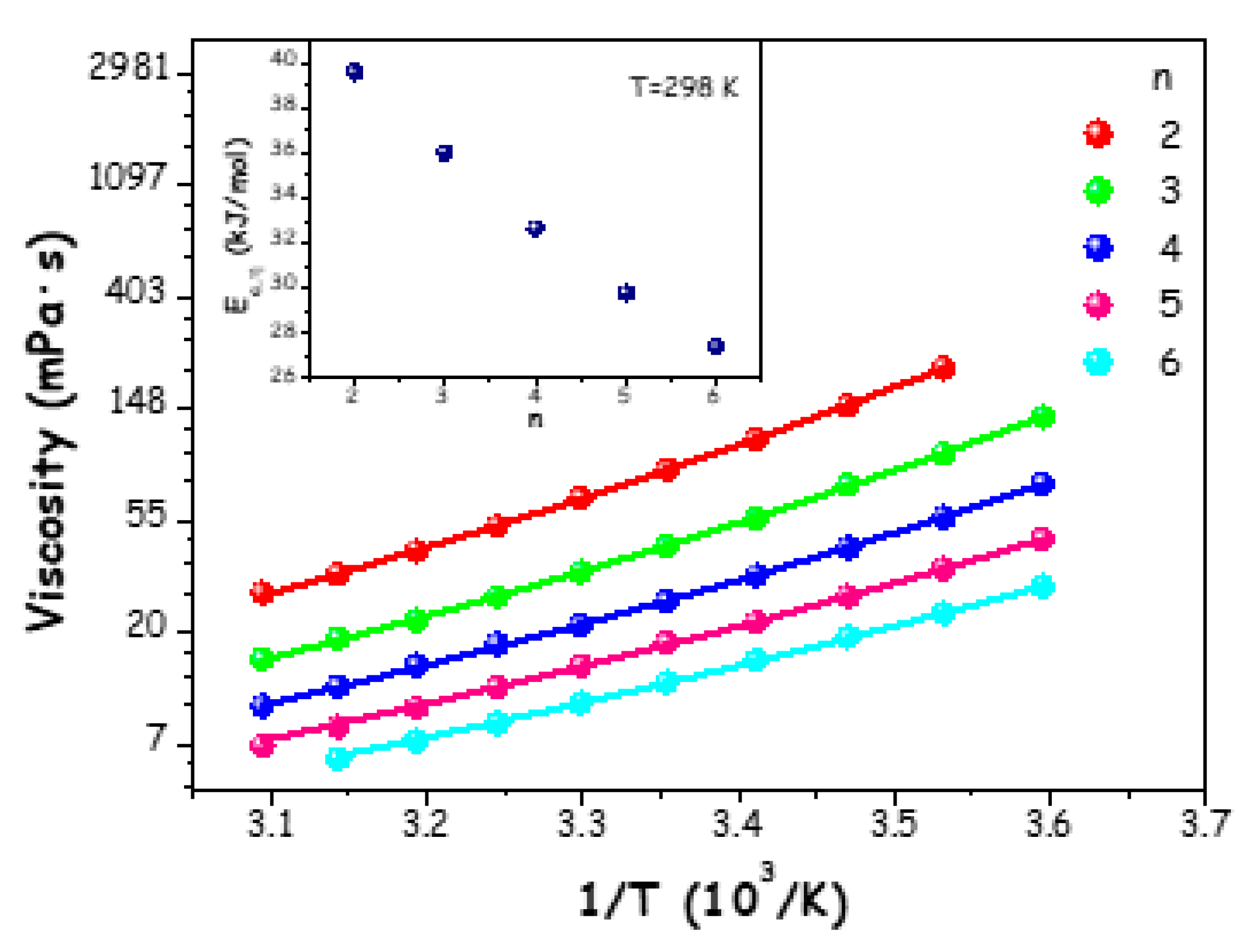
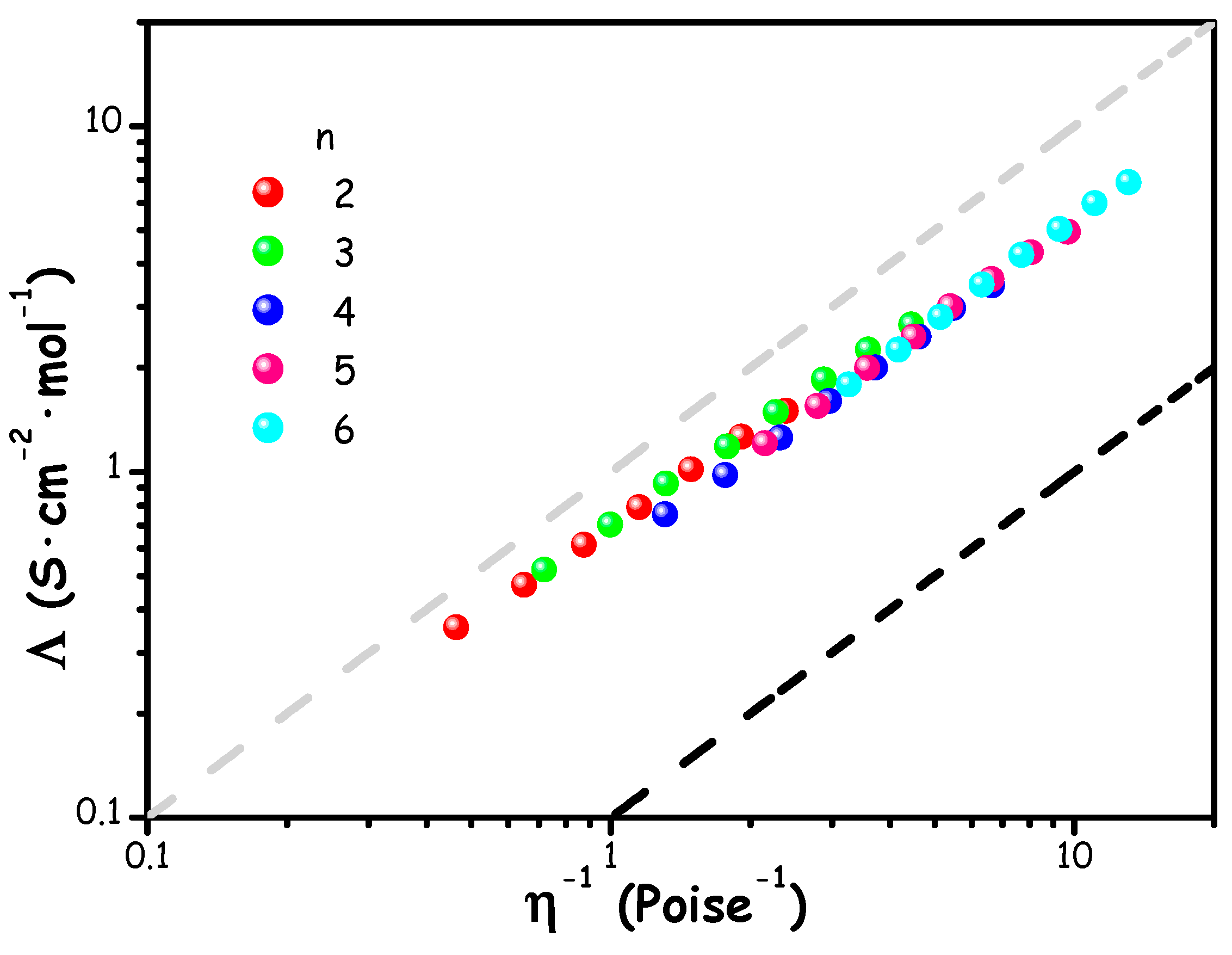
2.4. Viscosity Measurements
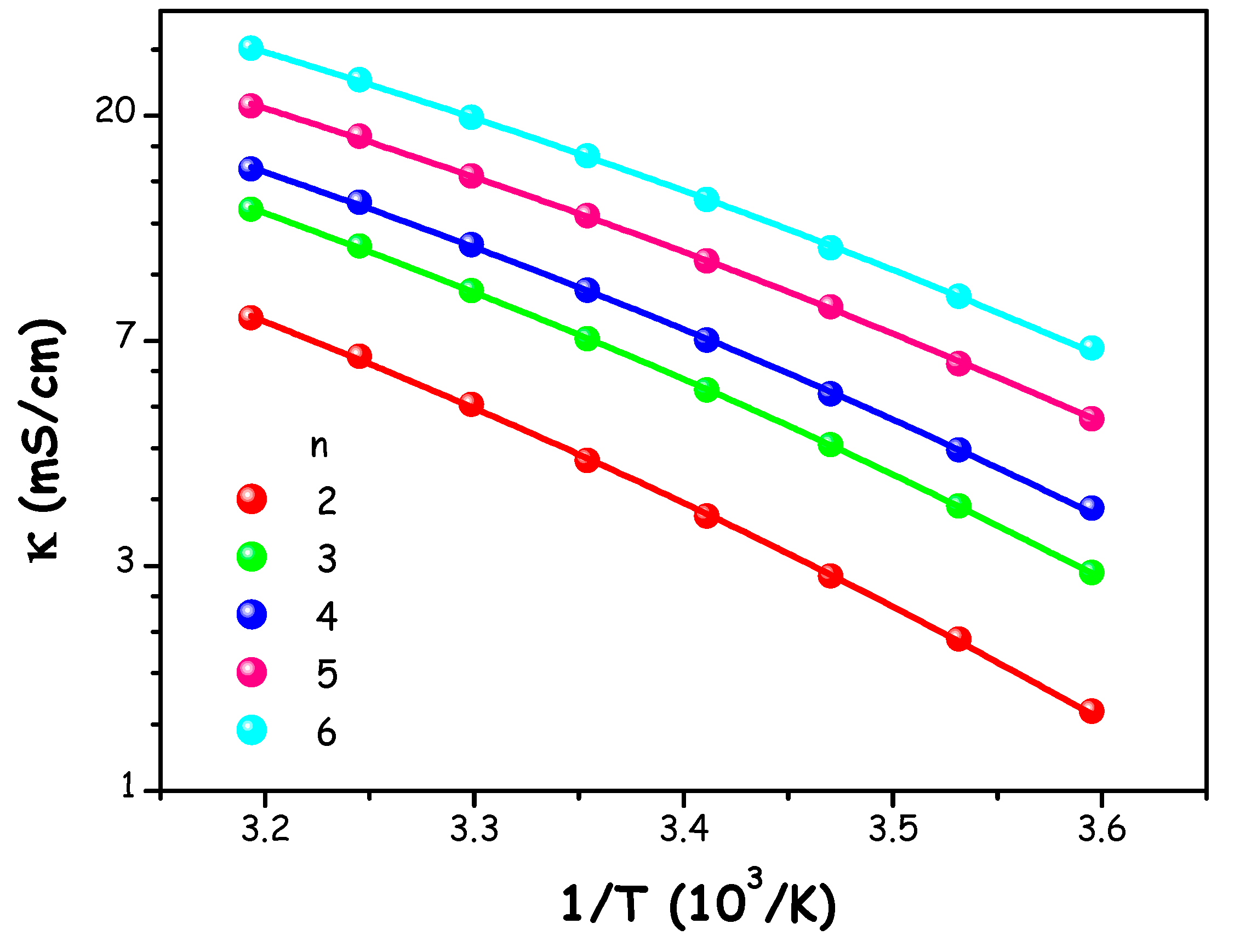
2.5. Electrical Conductivity Measurements
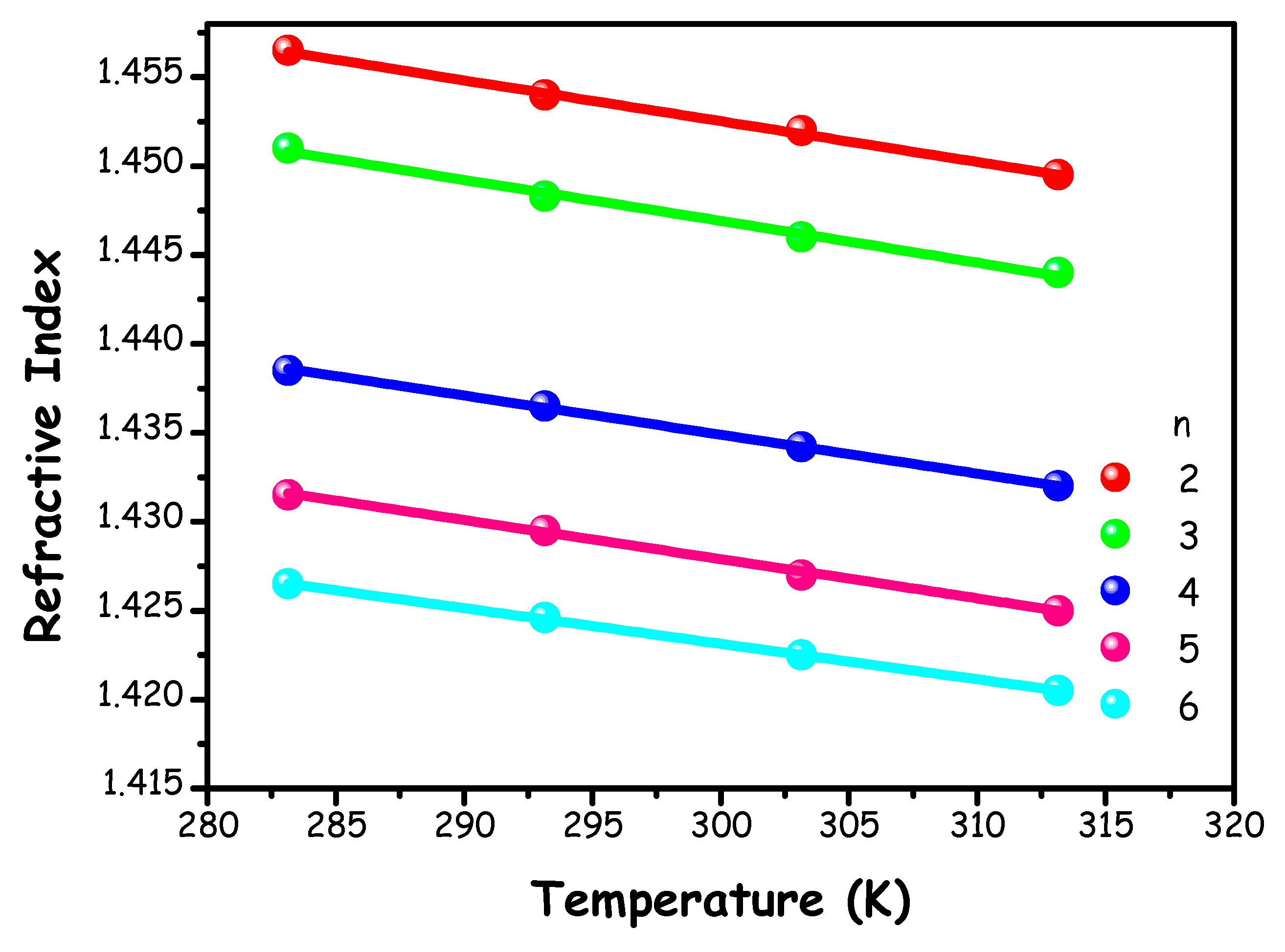
2.6. Refractive Index Measurements
2.7. X-Ray Scattering Experiments
2.7.1. Small Angle X-Ray Sscattering
2.7.2. Wide Angle X-Ray Scattering
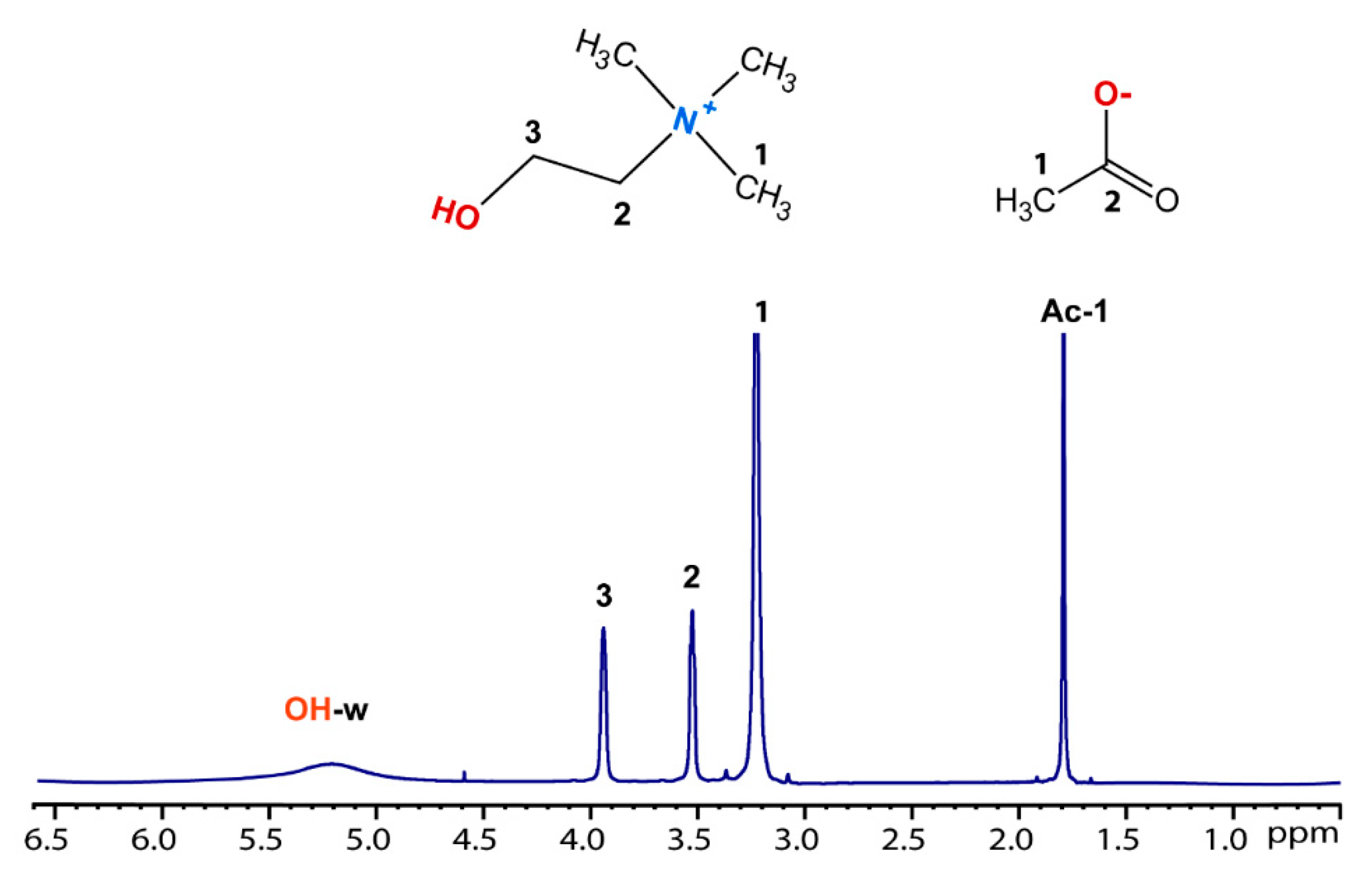
2.8. NMR Measurements
2.9. Computational Details
3. Results and Discussion

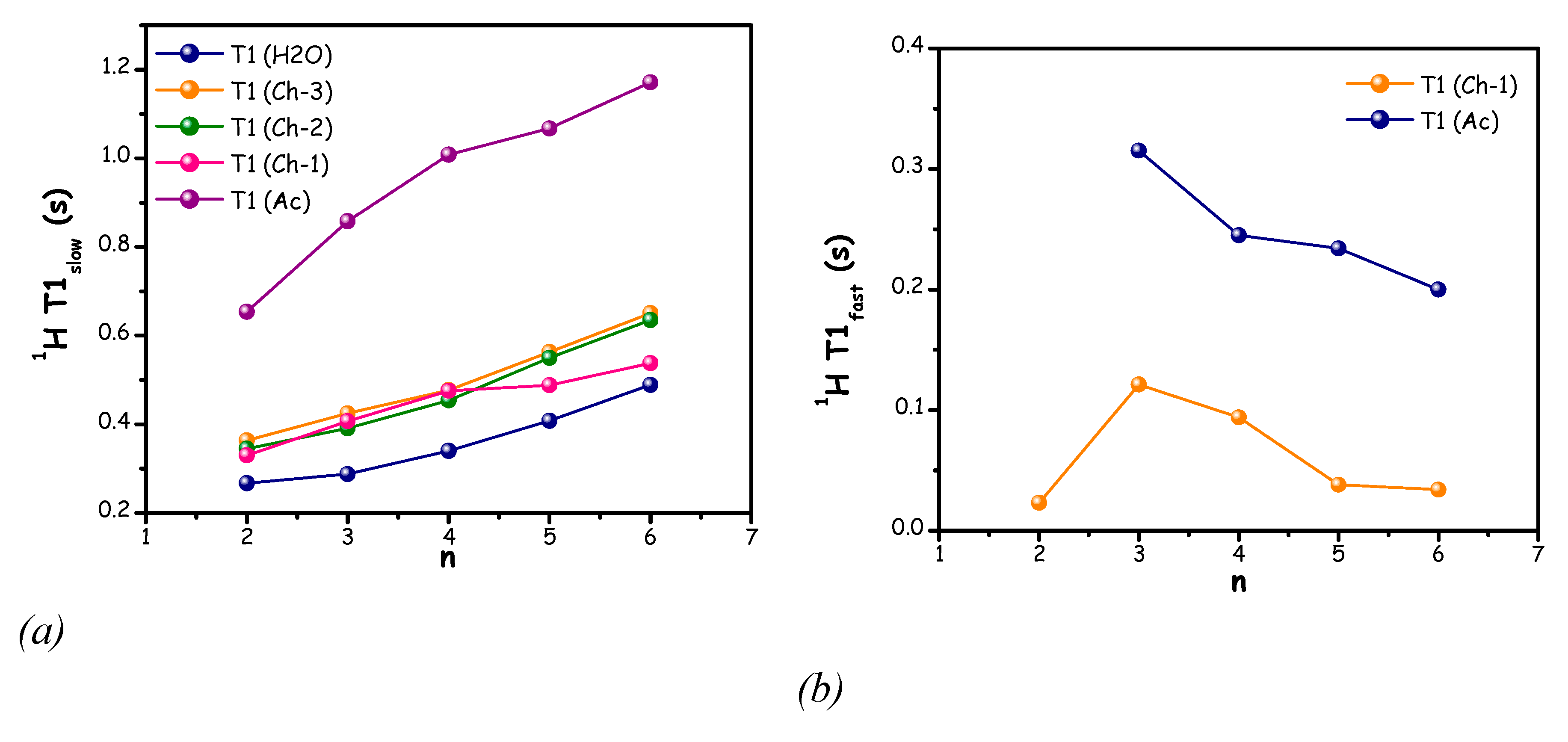
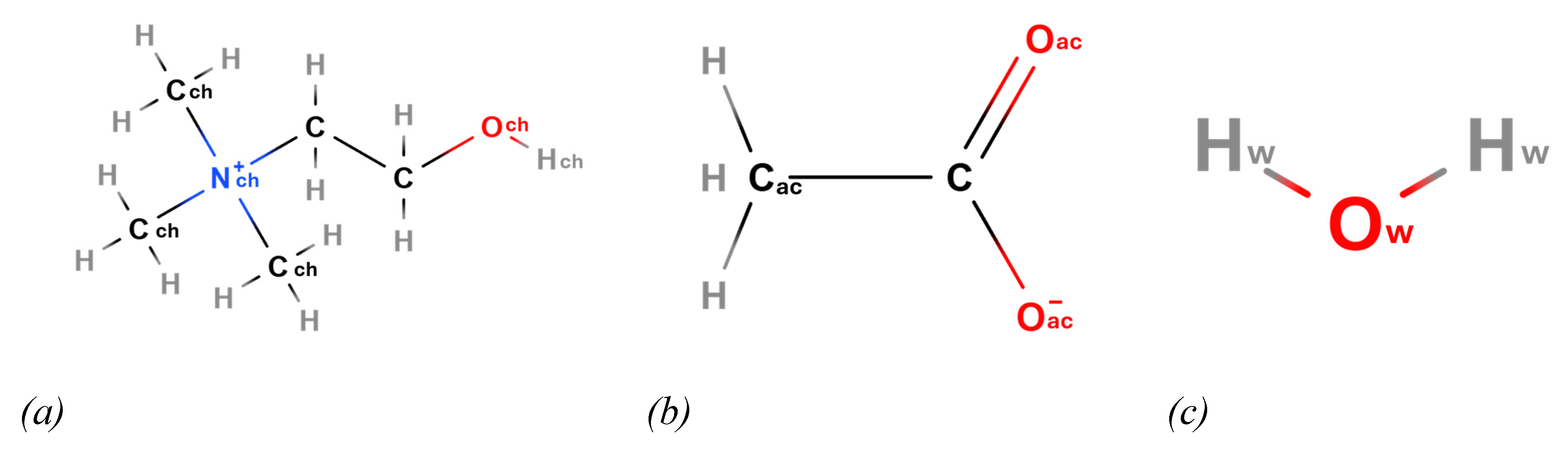
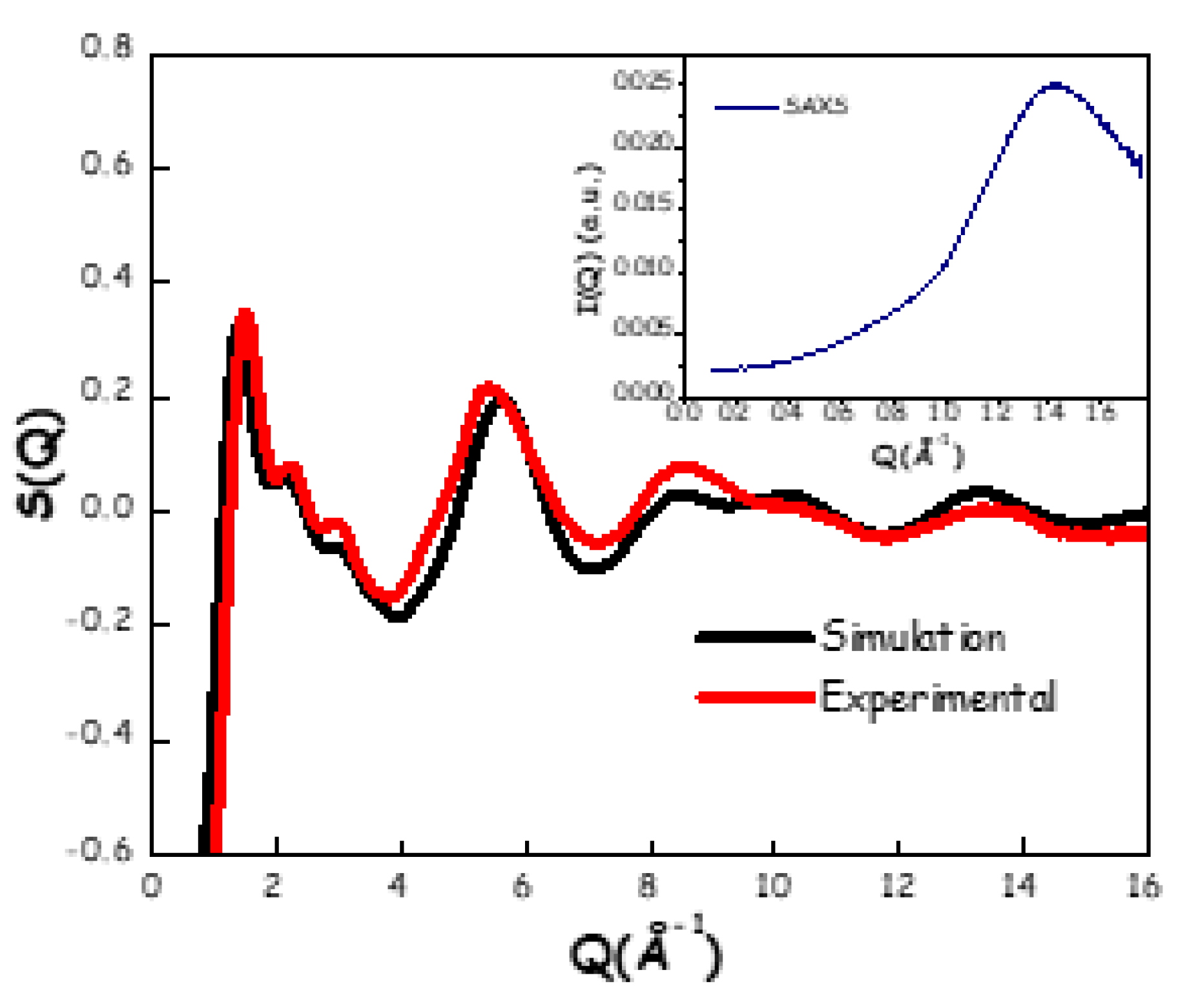
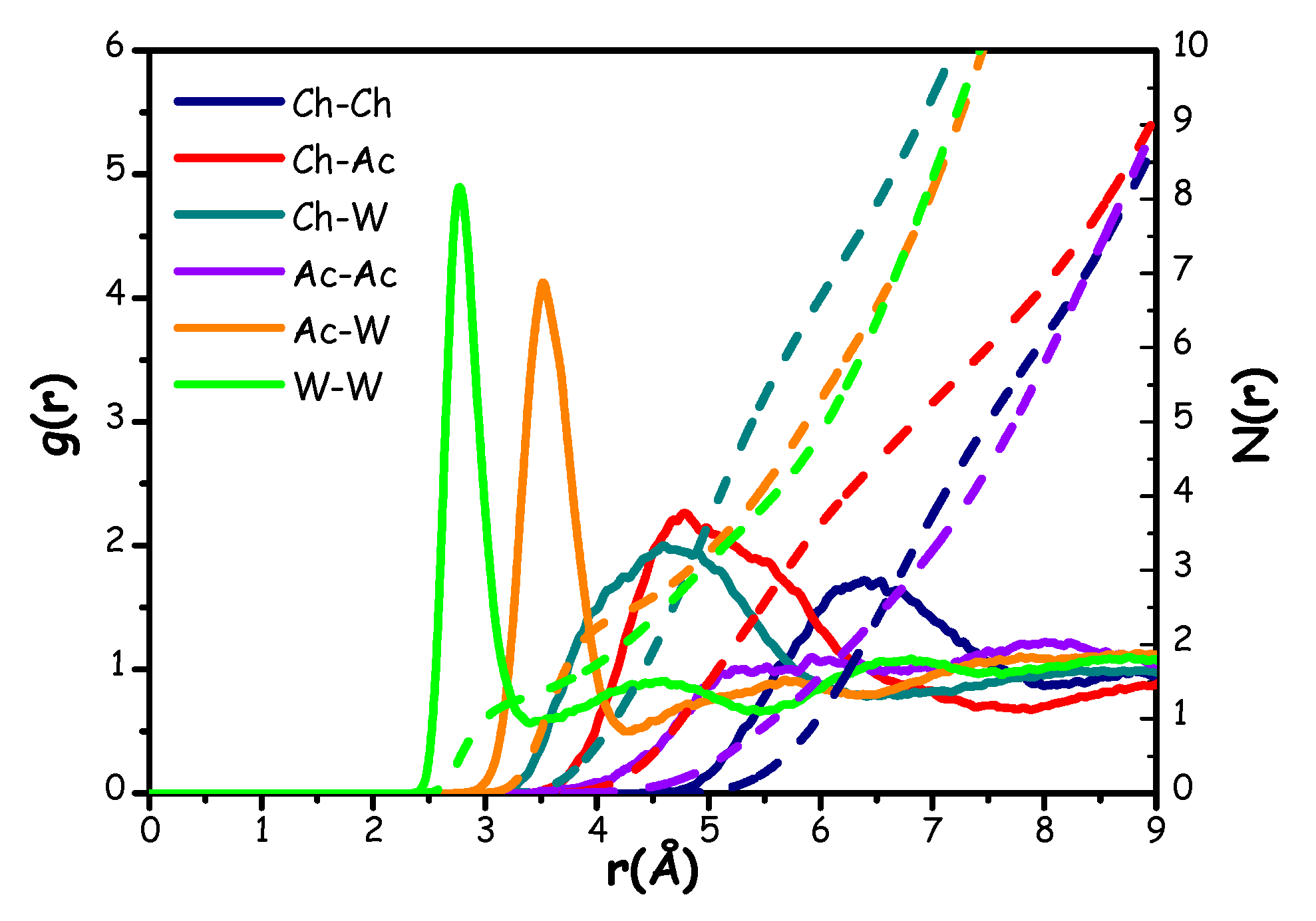
| Correlation | Peak position (Å) | Integration distance (Å) | N(r) |
| Ch-Ch | 6.4 | 8.0 | 6.2 |
| Ch-Ac | 4.9 | 7.0 | 5.3 |
| Ch-W | 4.6 | 6.3 | 7.5 |
| W-Ch | 4.6 | 6.3 | 3.7 |
| Ac-Ac | 8.0 | 9.0 | 9.1 |
| Ac-W | 3.5 | 4.3 | 2.5 |
| W-Ac | 3.5 | 4.3 | 1.2 |
| W-W | 2.8 | 3.5 | 1.4 |
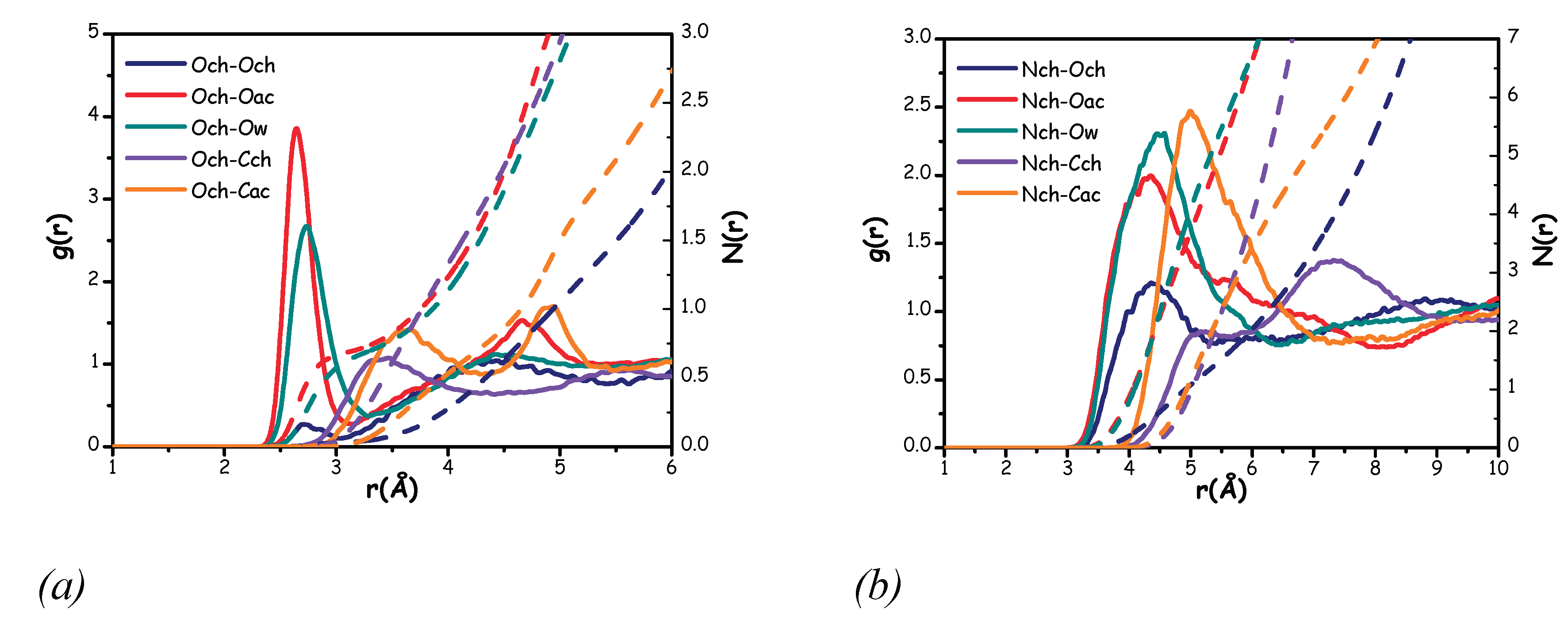
| Correlation | Peak position (Å) | Integration distance (Å) | N(r) |
| Och-Oac | 2.6 | 3.5 | 0.83 |
| Och-Ow | 2.7 | 3.5 | 0.77 |
| Och-Cch | 3.4 | 4.3 | 1.70 |
| Och-Cac | 3.6 | 4.3 | 0.74 |
| Nch-Och | 4.4 | 5.5 | 0.63 |
| Nch-Oac | 4.3 | 5.5 | 2.20 |
| Nch-Ow | 4.5 | 5.5 | 2.30 |
| Nch-Cch | 5.1 | 5.5 | 1.03 |
| Nch-Cac | 5.0 | 7.5 | 2.50 |
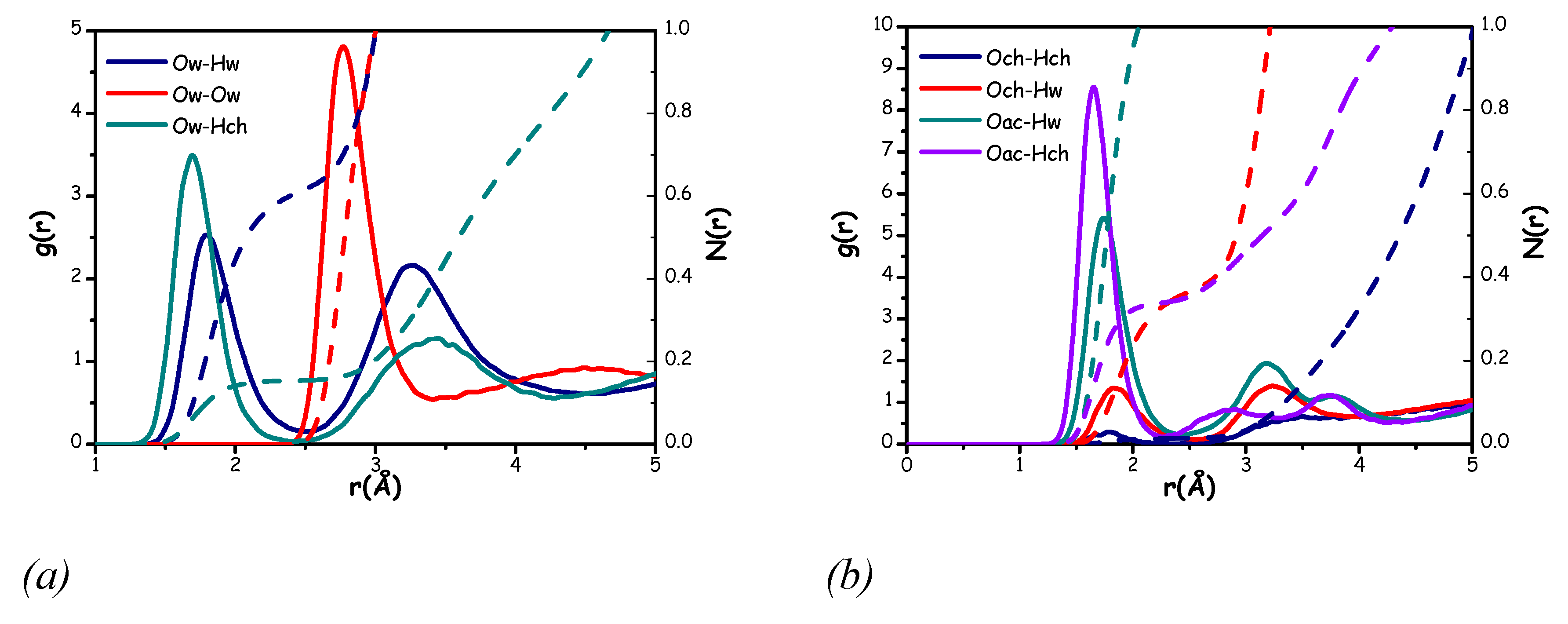
| Correlation | Peak position (Å) | Integration distance (Å) | N(r) |
| Ow-Hw | 1.8 | 2.5 | 0.62 |
| Ow-Ow | 2.8 | 3.5 | 1.4 |
| Ow-Hch | 1.7 | 2.5 | 0.16 |
| Hw-Och | 1.9 | 2.5 | 0.10 |
| Hch-Ow | 1.7 | 2.5 | 0.31 |
| Och-Hw | 1.9 | 2.5 | 0.37 |
| Oac-Hw | 1.7 | 2.5 | 1.16 |
| Oac-Hch | 1.7 | 2.5 | 0.36 |
| Hw-Oac | 1.7 | 2.5 | 0.58 |
| Hch-Oac | 1.7 | 2.5 | 0.71 |
| Och-Hch | 1.8 | 2.5 | 0.02 |
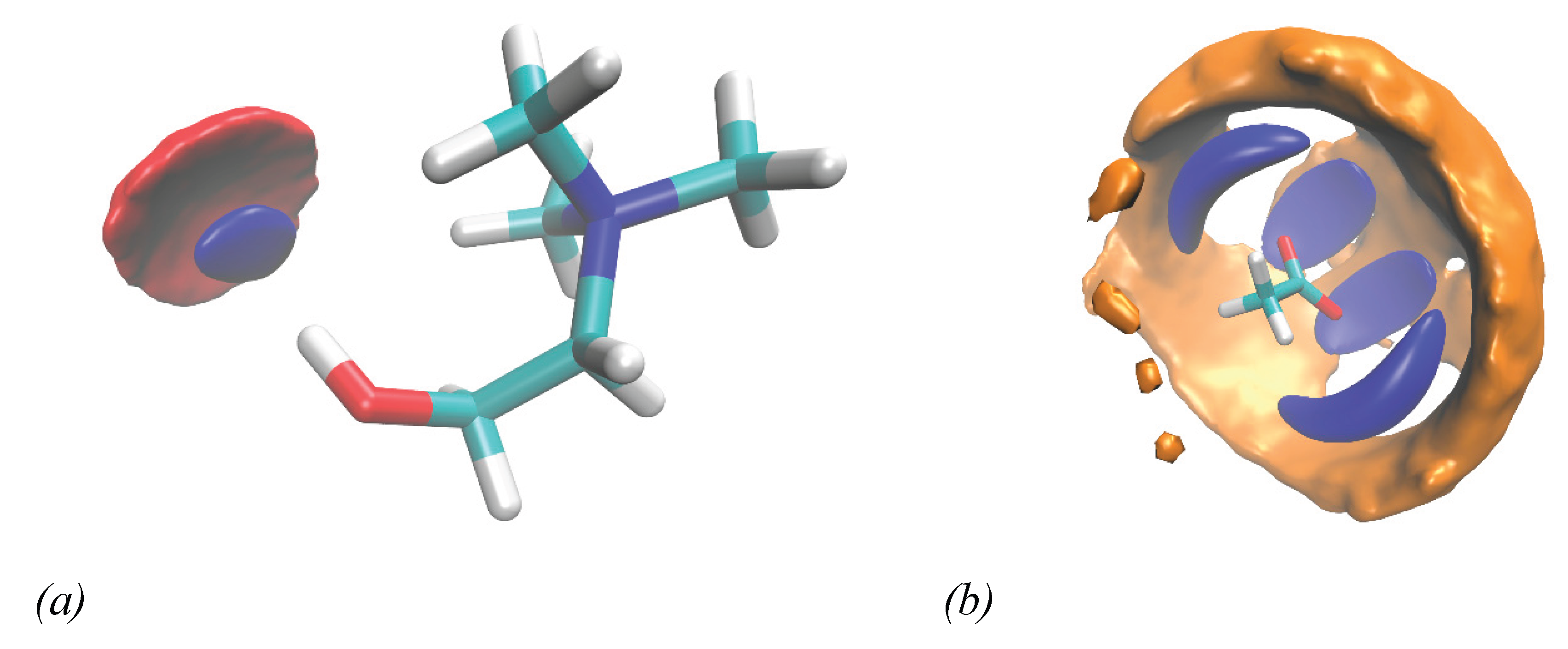
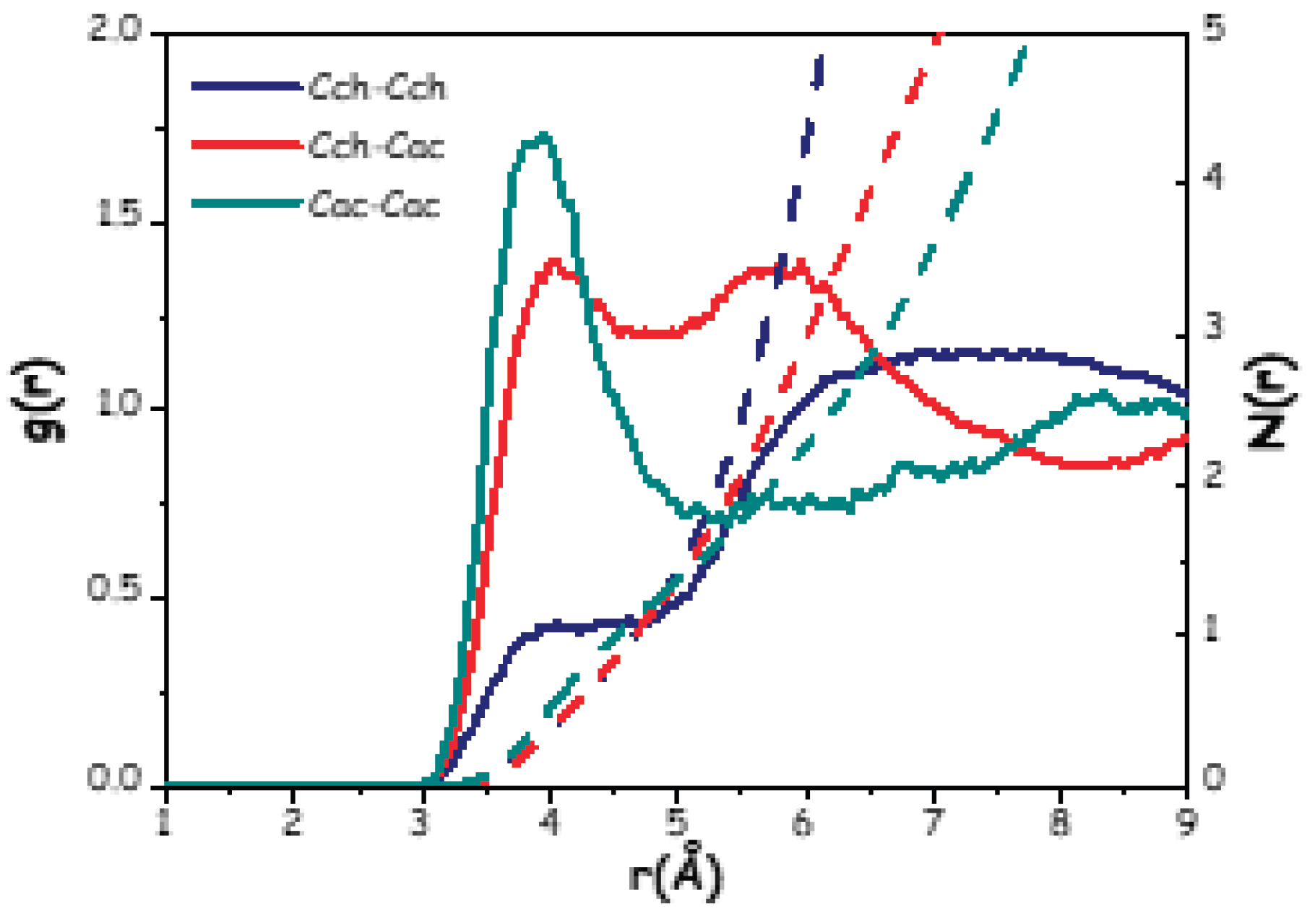
4. Conclusions
Supplementary Materials
Author Contributions
Acknowledgments
References
- E.L. Smith, A.P. Abbott, K.S. Ryder, Deep Eutectic Solvents (DESs) and Their Applications, Chem. Rev. 114 (2014) 11060–11082. [CrossRef]
- A.P. Abbott, Deep eutectic solvents and their application in electrochemistry, Curr. Opin. Green Sustain. Chem. 36 (2022) 100649. [CrossRef]
- A.P. Abbott, G. Capper, D.L. Davies, R.K. Rasheed, V. Tambyrajah, Novel solvent properties of choline chloride/urea mixtures, Chemical Communications (2003) 70–71. [CrossRef]
- M.A.R. Martins, S.P. Pinho, J.A.P. Coutinho, Insights into the Nature of Eutectic and Deep Eutectic Mixtures, J. Solution Chem. 48 (2019) 962–982. [CrossRef]
- van den Bruinhorst, M. Costa Gomes, Is there depth to eutectic solvents?, Curr. Opin. Green Sustain. Chem. 37 (2022) Article-100659. [CrossRef]
- D.O. Abranches, J.A.P. Coutinho, Annual Review of Chemical and Biomolecular Engineering Everything You Wanted to Know about Deep Eutectic Solvents but Were Afraid to Be Told, Annu. Rev. Chem. Biomol. Eng. 2023 14 (2023) 141–163. [CrossRef]
- Z. Wang, X. Zhao, Y. Chen, C. Wei, J. Jiang, A review of designable deep eutectic solvents for green fabrication of advanced functional materials, RSC Sustainability 3 (2025) 738–756. [CrossRef]
- D.O. Abranches, J.A.P. Coutinho, Type V deep eutectic solvents: Design and applications, Curr. Opin. Green Sustain. Chem. 35 (2022) Article 100612. [CrossRef]
- M. Gilmore, L.M. Moura, A.H. Turner, M. Swadźba-Kwaśny, S.K. Callear, J.A. McCune, O.A. Scherman, J.D. Holbrey, A comparison of choline:urea and choline:oxalic acid deep eutectic solvents at 338 K, Journal of Chemical Physics 148 (2018) Article 193823. [CrossRef]
- Z. Maugeri, P. de María, Novel choline-chloride-based deep-eutectic-solvents with renewable hydrogen bond donors: levulinic acid and sugar-based polyols, RSC Adv. 2 (2012) 421–425. [CrossRef]
- C. Fanali, S. Della Posta, L. Dugo, A. Gentili, L. Mondello, L. De Gara, Choline-chloride and betaine-based deep eutectic solvents for green extraction of nutraceutical compounds from spent coffee ground, J. Pharm. Biomed. Anal. 189 (2020) 113421. [CrossRef]
- N.F. Gajardo-Parra, M.J. Lubben, J.M. Winnert, Á. Leiva, J.F. Brennecke, R.I. Canales, Physicochemical properties of choline chloride-based deep eutectic solvents and excess properties of their pseudo-binary mixtures with 1-butanol, J. Chem. Thermodyn. 133 (2019) 272–284. [CrossRef]
- A.R. Harifi-Mood, R. Buchner, Density, viscosity, and conductivity of choline chloride + ethylene glycol as a deep eutectic solvent and its binary mixtures with dimethyl sulfoxide, J. Mol. Liq. 225 (2017) 689–695. [CrossRef]
- S. Di Muzio, O. Russina, D. Mastrippolito, P. Benassi, L. Rossi, A. Paolone, F. Ramondo, Mixtures of choline chloride and tetrabutylammonium bromide with imidazole as examples of deep eutectic solvents: their structure by theoretical and experimental investigation, J. Mol. Liq. 352 (2022) Article 118427. [CrossRef]
- K. Radošević, M. Cvjetko Bubalo, V. Gaurina Srček, D. Grgas, T. Landeka Dragičević, I. Radojčić Redovniković, Evaluation of toxicity and biodegradability of choline chloride based deep eutectic solvents, Ecotoxicol. Environ. Saf. 112 (2015) 46–53. [CrossRef]
- E. Mangiacapre, F. Castiglione, M. D’Aristotile, V. Di Lisio, A. Triolo, O. Russina, Choline chloride-water mixtures as new generation of green solvents: A comprehensive physico-chemical study, J. Mol. Liq. 383 (2023) Article 122120. [CrossRef]
- Triolo, F. Lo Celso, M. Brehm, V. Di Lisio, O. Russina, Liquid structure of a choline chloride-water natural deep eutectic solvent: A molecular dynamics characterization, J. Mol. Liq. 331 (2021) 115750. [CrossRef]
- Asakawa, M. Kohara, C. Sasaki, C. Asada, Y. Nakamura, Comparison of choline acetate ionic liquid pretreatment with various pretreatments for enhancing the enzymatic saccharification of sugarcane bagasse, Ind. Crops Prod. 71 (2015) 147–152. [CrossRef]
- D. Połomski, P. Garbacz, K. Czerwinski, M. Chotkowski, Synthesis and physicochemical properties of the mixtures based on choline acetate or choline chloride, J. Mol. Liq. 327 (2021) Article 114820. [CrossRef]
- Triolo, M.E. Di Pietro, A. Mele, F. Lo Celso, M. Brehm, V. Di Lisio, A. Martinelli, P. Chater, O. Russina, Liquid structure and dynamics in the choline acetate:urea 1:2 deep eutectic solvent, Journal of Chemical Physics 154 (2021) Article 244501. [CrossRef]
- H. Zhao, G.A. Baker, S. Holmes, New eutectic ionic liquids for lipase activation and enzymatic preparation of biodiesel, Org. Biomol. Chem. 9 (2011) 1908–1916. [CrossRef]
- J. Hoppe, R. Drozd, E. Byzia, M. Smiglak, Deep eutectic solvents based on choline cation - Physicochemical properties and influence on enzymatic reaction with β-galactosidase, Int. J. Biol. Macromol. 136 (2019) 296–304. [CrossRef]
- Y. Ma, G. Vernet, N. Zhang, S. Kara, Exploring DES-Water Mixtures for Improved ADH-CHMO Fusion Enzyme Performance in Linear Cascades, ChemCatChem 17 (2025) e202401792. [CrossRef]
- Z.L. Huang, B.P. Wu, Q. Wen, T.X. Yang, Z. Yang, Deep eutectic solvents can be viable enzyme activators and stabilizers, Journal of Chemical Technology and Biotechnology 89 (2014) 1975–1981. [CrossRef]
- B.P. Wu, Q. Wen, H. Xu, Z. Yang, Insights into the impact of deep eutectic solvents on horseradish peroxidase: Activity, stability and structure, J. Mol. Catal. B Enzym. 101 (2014) 101–107. [CrossRef]
- G. Colombo Dugoni, A. Mezzetta, L. Guazzelli, C. Chiappe, M. Ferro, A. Mele, Purification of Kraft cellulose under mild conditions using choline acetate based deep eutectic solvents, Green. Chem. 22 (2020) 8680–8691. [CrossRef]
- M.L. Segatto, L. Schnarr, O. Olsson, K. Kümmerer, V.G. Zuin, Ionic liquids vs. ethanol as extraction media of algicidal compounds from mango processing waste, Front. Chem. 10 (2022) 986987. [CrossRef]
- Bansal, D. Chauhan, K.K. Bhasin, G.R. Chaudhary, The study of molecular interactions of aqueous solutions of Choline Acetate at different temperatures, J. Mol. Liq. 286 (2019) 110878. [CrossRef]
- M. Eckert, W. Peters, J.F. Drillet, Fast microwave-assisted hydrothermal synthesis of pure layered δ-MnO2 for multivalent ion intercalation, Materials 11 (2018) 2399. [CrossRef]
- M. Sakthivel, S.P. Batchu, A.A. Shah, K. Kim, W. Peters, J.F. Drillet, An electrically rechargeable Zinc/Air cell with an aqueous choline acetate electrolyte, Materials 13 (2020) 1–21. [CrossRef]
- E. Veroutis, S. Merz, R.-A. Eichel, J. Granwehr, Solvation and Ion-Pairing Effects of Choline Acetate Electrolyte in Protic and Aprotic Solvents Studied by NMR Titrations, ChemPhysChem 23 (2022) e202100602. [CrossRef]
- S. Miao, H.J. Jiang, S. Imberti, R. Atkin, G. Warr, Aqueous choline amino acid deep eutectic solvents, Journal of Chemical Physics 154 (2021) 214504. [CrossRef]
- Triolo, F. Lo Celso, O. Russina, Liquid structure of a water-based, hydrophobic and natural deep eutectic solvent: The case of thymol-water. A Molecular Dynamics study, J. Mol. Liq. 372 (2023) 121151. [CrossRef]
- M. Basham, J. Filik, M.T. Wharmby, P.C.Y. Chang, B. El Kassaby, M. Gerring, J. Aishima, K. Levik, B.C.A. Pulford, I. Sikharulidze, D. Sneddon, M. Webber, S.S. Dhesi, F. Maccherozzi, O. Svensson, S. Brockhauser, G. Náray, A.W. Ashton, Data Analysis WorkbeNch (DAWN), J. Synchrotron Radiat. 22 (2015) 853–858. [CrossRef]
- A.K. Soper, GudrunN and GudrunX: programs for correcting raw neutron and X-ray diffraction data to differential scattering cross section, 2011. http://epubs.stfc.ac.uk.
- J. Hutter, M. Iannuzzi, F. Schiffmann, J. Vandevondele, Cp2k: Atomistic simulations of condensed matter systems, Wiley Interdiscip. Rev. Comput. Mol. Sci. 4 (2014) 15–25. [CrossRef]
- T.D. Kühne, M. Iannuzzi, M. Del Ben, V. V. Rybkin, P. Seewald, F. Stein, T. Laino, R.Z. Khaliullin, O. Schütt, F. Schiffmann, D. Golze, J. Wilhelm, S. Chulkov, M.H. Bani-Hashemian, V. Weber, U. Borštnik, M. Taillefumier, A.S. Jakobovits, A. Lazzaro, H. Pabst, T. Müller, R. Schade, M. Guidon, S. Andermatt, N. Holmberg, G.K. Schenter, A. Hehn, A. Bussy, F. Belleflamme, G. Tabacchi, A. Glöß, M. Lass, I. Bethune, C.J. Mundy, C. Plessl, M. Watkins, J. VandeVondele, M. Krack, J. Hutter, CP2K: An electronic structure and molecular dynamics software package -Quickstep: Efficient and accurate electronic structure calculations, Journal of Chemical Physics 152 (2020) Article 194103. [CrossRef]
- J. Vandevondele, M. Krack, F. Mohamed, M. Parrinello, T. Chassaing, J. Hutter, Quickstep: Fast and accurate density functional calculations using a mixed Gaussian and plane waves approach, Comput. Phys. Commun. 167 (2005) 103–128. [CrossRef]
- J. VandeVondele, J. Hutter, An efficient orbital transformation method for electronic structure calculations, Journal of Chemical Physics 118 (2003) 4365–4369. [CrossRef]
- P. Hohenberg, W. Kohn, Inhomogeneous Electron Gas, Physical Review 136 (1964) B864–B871. [CrossRef]
- W. Kohn, L.J. Sham, Self-Consistent Equations Including Exchange and Correlation Effects, Physical Review 140 (1965) A1133–A1138. [CrossRef]
- Lee, W. Yang, R.G. Parr, Development of the Colle-Salvetti correlation-energy formula into a functional of the electron density, Phys. Rev. B 37 (1988) 785–789. [CrossRef]
- A.D. Becke, Density-functional exchange-energy approximation with correct asymptotic behavior, Phys. Rev. A (Coll Park) 38 (1988) 3098–3100. [CrossRef]
- S. Grimme, S. Ehrlich, L. Goerigk, Effect of the damping function in dispersion corrected density functional theory, J. Comput. Chem. 32 (2011) 1456–1465. [CrossRef]
- S. Grimme, J. Antony, S. Ehrlich, H. Krieg, A consistent and accurate ab initio parametrization of density functional dispersion correction (DFT-D) for the 94 elements H-Pu, Journal of Chemical Physics 132 (2010) Article 154104. [CrossRef]
- D.G.A. Smith, L.A. Burns, K. Patkowski, C.D. Sherrill, Revised Damping Parameters for the D3 Dispersion Correction to Density Functional Theory, Journal of Physical Chemistry Letters 7 (2016) 2197–2203. [CrossRef]
- J. VandeVondele, J. Hutter, Gaussian basis sets for accurate calculations on molecular systems in gas and condensed phases, Journal of Chemical Physics 127 (2007) Article 114105. [CrossRef]
- Hartwigsen, S. Goedecker, J. Hutter, Relativistic separable dual-space Gaussian pseudopotentials from H to Rn, Phys. Rev. B 58 (1998) 3641–3662. [CrossRef]
- S. Goedecker, M. Teter, J. Hutter, Separable dual-space Gaussian pseudopotentials, Phys. Rev. B 54 (1996) 1703–1710. [CrossRef]
- S. Nosé, A unified formulation of the constant temperature molecular dynamics methods, J. Chem. Phys. 81 (1984) 511–519. [CrossRef]
- G.J. Martyna, M.L. Klein, M. Tuckerman, Nosé-Hoover chains: The canonical ensemble via continuous dynamics, J. Chem. Phys. 97 (1992) 2635–2643. [CrossRef]
- L. Martinez, R. Andrade, E.G. Birgin, J.M. Martínez, PACKMOL: A package for building initial configurations for molecular dynamics simulations, J. Comput. Chem. 30 (2009) 2157–2164. [CrossRef]
- S. Plimpton, Fast Parallel Algorithms for Short-Range Molecular Dynamics, J. Comput. Phys. 117 (1995) 1–19. [CrossRef]
- H.W. Horn, W.C. Swope, J.W. Pitera, J.D. Madura, T.J. Dick, G.L. Hura, T. Head-Gordon, Development of an improved four-site water model for biomolecular simulations: TIP4P-Ew, Journal of Chemical Physics 120 (2004) 9665–9678. [CrossRef]
- B. Doherty, O. Acevedo, OPLS Force Field for Choline Chloride-Based Deep Eutectic Solvents, Journal of Physical Chemistry B 122 (2018) 9982–9993. [CrossRef]
- H.C. Andersen, Rattle: A “velocity” version of the shake algorithm for molecular dynamics calculations, J. Comput. Phys. 52 (1983) 24–34. [CrossRef]
- J.-P. Ryckaert, G. Ciccotti, H.J.C. Berendsen, Numerical integration of the cartesian equations of motion of a system with constraints: molecular dynamics of n-alkanes, J. Comput. Phys. 23 (1977) 327–341. [CrossRef]
- H.J.C. Berendsen, J.P.M. Postma, W.F. Van Gunsteren, A. Dinola, J.R. Haak, Molecular dynamics with coupling to an external bath, J. Chem. Phys. 81 (1984) 3684–3690. [CrossRef]
- T. Schneider, E. Stoll, Molecular-dynamics study of a three-dimensional one-component model for distortive phase transitions, Phys. Rev. B 17 (1978) 1302–1322. [CrossRef]
- B. DÜNWEG, W. PAUL, BROWNIAN DYNAMICS SIMULATIONS WITHOUT GAUSSIAN RANDOM NUMBERS, International Journal of Modern Physics C 02 (1991) 817–827. [CrossRef]
- M. Brehm, B. Kirchner, TRAVIS - A free analyzer and visualizer for monte carlo and molecular dynamics trajectories, J. Chem. Inf. Model 51 (2011) 2007–2023. [CrossRef]
- M. Brehm, M. Thomas, S. Gehrke, B. Kirchner, TRAVIS—A free analyzer for trajectories from molecular simulation, Journal of Chemical Physics 152 (2020) Article 164105. [CrossRef]
- O. Hollõczki, M. Macchiagodena, H. Weber, M. Thomas, M. Brehm, A. Stark, O. Russina, A. Triolo, B. Kirchner, Triphilic Ionic-Liquid Mixtures: Fluorinated and Non-fluorinated Aprotic Ionic-Liquid Mixtures, ChemPhysChem 16 (2015) 3325–3333. [CrossRef]
- H. Zhao, G.A. Baker, S. Holmes, New eutectic ionic liquids for lipase activation and enzymatic preparation of biodiesel, Org. Biomol. Chem. 9 (2011) 1908–1916. [CrossRef]
- Y. Xie, H. Dong, S. Zhang, X. Lu, X. Ji, Effect of water on the density, viscosity, and CO2 solubility in choline chloride/urea, J. Chem. Eng. Data 59 (2014) 3344–3352. [CrossRef]
- V. Agieienko, R. Buchner, Variation of Density, Viscosity, and Electrical Conductivity of the Deep Eutectic Solvent Reline, Composed of Choline Chloride and Urea at a Molar Ratio of 1:2, Mixed with Dimethylsulfoxide as a Cosolvent, J. Chem. Eng. Data 65 (2020) 1900–1910. [CrossRef]
- R.B. Leron, M.H. Li, High-pressure density measurements for choline chloride: Urea deep eutectic solvent and its aqueous mixtures at T = (298.15 to 323.15) K and up to 50 MPa, Journal of Chemical Thermodynamics 54 (2012) 293–301. [CrossRef]
- Andreani, C. Corsaro, D. Mallamace, G. Romanelli, R. Senesi, F. Mallamace, The onset of the tetrabonded structure in liquid water, Sci. China Phys. Mech. Astron. 62 (2019) Article 107008. [CrossRef]
- H. Jin, B. O’Hare, J. Dong, S. Arzhantsev, G.A. Baker, J.F. Wishart, A.J. Benesi, M. Maroncelli, Physical properties of ionic liquids consisting of the 1-butyl-3- methylimidazolium cation with various anions and the bis(trifluoromethylsulfonyl)imide anion with various cations, Journal of Physical Chemistry B 112 (2008) 81–92. [CrossRef]
- V. Piacentini, C. Simari, E. Mangiacapre, A. Pierini, A. Gentile, S. Marchionna, I. Nicotera, S. Brutti, E. Bodo, Aprotic Electrolytes Beyond Organic Carbonates: Transport Properties of LiTFSI Solutions in S-Based Solvents, ChemSusChem 18 (2024) e202402273. [CrossRef]
- S.-Y. Lee, K. Ueno, C.A. Angell, Lithium Salt Solutions in Mixed Sulfone and Sulfone-Carbonate Solvents: A Walden Plot Analysis of the Maximally Conductive Compositions, The Journal of Physical Chemistry C 116 (2012) 23915–23920. [CrossRef]
- M. Dahbi, F. Ghamouss, F. Tran-Van, D. Lemordant, M. Anouti, Comparative study of EC/DMC LiTFSI and LiPF6 electrolytes for electrochemical storage, J. Power Sources 196 (2011) 9743–9750. [CrossRef]
- Triolo, F. Lo Celso, C. Ottaviani, P. Ji, G.B. Appetecchi, F. Leonelli, D.S. Keeble, O. Russina, Structural features of selected protic ionic liquids based on a super-strong base, Phys. Chem. Chem. Phys. 21 (2019) 25369–25378. [CrossRef]
- G. De Araujo Lima E Souza, M.E. Di Pietro, F. Castiglione, P.H. Marques Mezencio, P. Fazzio Martins Martinez, A. Mariani, H.M. Schütz, S. Passerini, M. Middendorf, M. Schönhoff, A. Triolo, G.B. Appetecchi, A. Mele, Implications of Anion Structure on Physicochemical Properties of DBU-Based Protic Ionic Liquids, Journal of Physical Chemistry B 126 (2022) 7006–7014. [CrossRef]
- O. Russina, R. Caminiti, A. Triolo, S. Rajamani, B. Melai, A. Bertoli, C. Chiappe, Physico-chemical properties and nanoscale morphology in N-alkyl-N-methylmorpholinium dicyanamide room temperature ionic liquids, J. Mol. Liq. 187 (2013) 252–259. [CrossRef]
- O. Russina, A. Triolo, L. Gontrani, R. Caminiti, Mesoscopic Structural Heterogeneities in Room-Temperature Ionic Liquids, J. Phys. Chem. Lett. 3 (2012) 27–33. [CrossRef]
- W. Chen, X. Bai, Z. Xue, H. Mou, J. Chen, Z. Liu, T. Mu, The formation and physicochemical properties of PEGylated deep eutectic solvents, New J. Chem. 43 (2019) 8804–8810. [CrossRef]
- M. Hayyan, T. Aissaoui, M.A. Hashim, M.A. AlSaadi, A. Hayyan, Triethylene glycol based deep eutectic solvents and their physical properties, J. Taiwan Inst. Chem. Eng. 50 (2015) 24–30. [CrossRef]
- Y. Cui, C. Li, J. Yin, S. Li, Y. Jia, M. Bao, Design, synthesis and properties of acidic deep eutectic solvents based on choline chloride, J. Mol. Liq. 236 (2017) 338–343. [CrossRef]
- Y. Wang, W. Chen, Q. Zhao, G. Jin, Z. Xue, Y. Wang, T. Mu, Ionicity of deep eutectic solvents by Walden plot and pulsed field gradient nuclear magnetic resonance (PFG-NMR), Physical Chemistry Chemical Physics 22 (2020) 25760–25768. [CrossRef]
- D.R. MacFarlane, M. Forsyth, E.I. Izgorodina, A.P. Abbott, G. Annat, K. Fraser, On the concept of ionicity in ionic liquids, Physical Chemistry Chemical Physics 11 (2009) 4962–4967. [CrossRef]
- C.A. Angell, N. Byrne, J.-P. Belieres, Parallel Developments in Aprotic and Protic Ionic Liquids: Physical Chemistry and Applications, Acc. Chem. Res. 40 (2007) 1228–1236. [CrossRef]
- R. Nanda, K. Damodaran, A review of NMR methods used in the study of the structure and dynamics of ionic liquids, Magnetic Resonance in Chemistry 56 (2018) 62–72. [CrossRef]
- M. Zubkov, G.R. Dennis, T. Stait-Gardner, A.M. Torres, S.A. Willis, G. Zheng, W.S. Price, Physical characterization using diffusion NMR spectroscopy, Magnetic Resonance in Chemistry 55 (2017) 414–424. [CrossRef]
- Lepore, G. Ciancaleoni, D.R. Perinelli, G. Bonacucina, S. Gabrielli, G. de Simone, R. Gabbianelli, L. Bordoni, M. Tiecco, Cluster aggregation of water-based deep eutectic solvents in water and evaluation of their cytotoxicity, J. Mol. Liq. 415 (2024) 126427. [CrossRef]
- C. Allegretti, P. D’Arrigo, F.G. Gatti, L.A.M. Rossato, E. Ruffini, Dependence of 1H-NMR T1 relaxation time of trimethylglycine betaine deep eutectic solvents on the molar composition and on the presence of water, RSC Adv. 13 (2023) 3004–3007. [CrossRef]
- O.S. Hammond, D.T. Bowron, K.J. Edler, Liquid structure of the choline chloride-urea deep eutectic solvent (reline) from neutron diffraction and atomistic modelling, Green Chemistry 18 (2016) 2736–2744. [CrossRef]
- A.H. Turner, J.D. Holbrey, Investigation of glycerol hydrogen-bonding networks in choline chloride/glycerol eutectic-forming liquids using neutron diffraction, Phys. Chem. Chem. Phys. 21 (2019) 21782–21789. [CrossRef]
- Y. Zhang, D. Poe, L. Heroux, H. Squire, B.W. Doherty, Z. Long, M. Dadmun, B. Gurkan, M.E. Tuckerman, E.J. Maginn, Liquid Structure and Transport Properties of the Deep Eutectic Solvent Ethaline, Journal of Physical Chemistry B 124 (2020) 5251–5264. [CrossRef]
- S. Kaur, S. Sharma, H.K. Kashyap, Bulk and interfacial structures of reline deep eutectic solvent: A molecular dynamics study, J. Chem. Phys. 147 (2017) 194507. [CrossRef]
- O.S. Hammond, D.T. Bowron, K.J. Edler, The Effect of Water upon Deep Eutectic Solvent Nanostructure: An Unusual Transition from Ionic Mixture to Aqueous Solution, Angewandte Chemie - International Edition 56 (2017) 9782–9785. [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





