1. Introduction
Cervical cancer (CC) remains a significant global health challenge, ranking as the fourth most common cancer among women. According to GLOBOCAN 2024 data [
1], approximately 660,000 new cases and 350,000 deaths were recorded worldwide, with 90% of cases occurring in low- and middle-income countries due to limited healthcare access, early screening, and HPV vaccination coverage.
In Europe, cervical cancer poses a substantial public health issue, with over 66,000 new cases and 30,000 deaths annually. Disparities in healthcare infrastructure, public health funding, and cultural factors influence the effectiveness of screening and vaccination programs. Romania has one of the highest cervical cancer burdens in Europe, with 3,368 new cases and 1,793 deaths annually. Approximately 11,278 women are living with cervical cancer, highlighting the urgency of enhancing early detection, care access, and HPV vaccination efforts [
2]. These statistics underscore the critical need for preventive measures, including widespread HPV vaccination and effective screening programs, to reduce the burden of cervical cancer in Romania and across Europe [
3].
Due to advancements in treatment and screening, CC is often diagnosed earlier, resulting in improved survival rates, with a 5-year relative survival rate of 91.5% in early stages [
4]. Around 67.2% of women diagnosed with cervical cancer survive for five or more years after diagnosis. In Europe, the net survival rate is approximately 64%. It is important to note that early detection of cervical cancer significantly improves survival rates, as early-stage diagnoses offer a better prognosis [
5].
HPV infection accounts for 90–100% of cervical cancer cases, particularly in women under 35 years [
6]. Treatment strategies, including surgery, radiation, and chemotherapy, depend on the disease stage and may involve combinations of these modalities [
7]. Radiation therapy, often paired with chemotherapy, is the standard for advanced cases [
8], while drugs like Cisplatin and Paclitaxel demonstrate consistent efficacy [
9].
Survivors often face physical and psychosocial challenges, with chemoradiotherapy causing side effects like bladder and bowel dysfunction, which can greatly influence health-related quality of life (HRQoL). Patient-centered care and QOL assessment tools, endorsed by the WHO, are essential for improving treatment outcomes [
10,
11].
This aim of this study is to evaluate the impact of chemoradiotherapy on the QOL of cervical cancer survivors, focusing on physical, social, and emotional well-being to address the broader effects of treatment.
2. Materials and Methods
This study was conducted as a prospective observational study involving women diagnosed with cervical cancer, which received treatment par chemoradiation. The data was collected in accordance with ethical guidelines to ensure patient confidentiality. Patients were selected based on eligibility for chemoradiotherapy, with ECOG 0-2, stage I-IVA.
Data was collected through assessments conducted at baseline (pre-treatment), during treatment, and at a follow-up point after treatment completion to measure changes in QoL over time. Parameters assessed included demographic information (age, education level, and residence), histopathological subtype, disease stage at diagnosis, radiotherapy dose, chemotherapy type, number of brachytherapy insertions and adverse events during treatment with corresponding grades.
The quality of life (QoL) was assessed using the Functional Assessment of Cancer Therapy-General (FACT-G) questionnaire, which evaluates multiple scales of well-being: Physical Well-Being (PWB), Social Well-Being (SWB), Emotional Well-Being (EWB) and Functional Well-Being (FWB). Scores on these scales provide insight into the broader impacts of treatment beyond clinical outcomes. Baseline QoL was compared to post-treatment scores to assess the impact of chemoradiation on patients' quality of life. [
12]
The primary treatment for cervical cancer patients in this cohort was chemoradiation. Radiotherapy protocols varied according to cancer type and intent of treatment. The target volumes included the tumor bed and the pelvic and lower para-aortic lymph nodes. Patients with biopsy-proven cervical cancer underwent definitive chemoradiotherapy with a mean EBRT dose of 52.2 Gy (range: 45–59.4 Gy) to the primary tumor and pelvic lymph nodes. In selected cases with total doses >50 Gy, a tumor boost was delivered either as a simultaneous integrated boost (SIB) up to 55 Gy or sequentially up to 59.4 Gy, based on initial MRI or PET-CT imaging. Image-guided brachytherapy (CT-based) was administered in 3 to 5 sessions, with a mean dose of 6.5 Gy per fraction (range: 5.5–7.5 Gy)
Descriptive statistics, including means, standard deviations, and frequency distributions, were calculated for demographic and clinical characteristics using Microsoft Excel, R Studio with ggplot2 and tidyverse packages.[
13,
14,
15,
16] Comparisons between pre- and post-treatment QoL scores were conducted using t-tests for continuous variables, one-way/two-way ANOVA for multiple comparisons with post-hoc test Tukey HSD, and chi-square tests were used to assess associations between categorical variables. Statistical significance was set at p<0.05.
To evaluate the impact of treatment-related toxicities on patient-reported quality of life, we conducted a series of linear mixed-effects models using the lmer function from the lme4 package in R.[
17] The primary outcome was the total score on the FACT-G questionnaire, measured before treatment and at two treatment timepoints (during and after treatment). Each model included one binary toxicity variable (e.g., nausea, dysuria, diarrhea, hematological adverse events) as a fixed effect, along with timepoint and their interaction, and a random intercept for patient ID to account for within-subject correlation. The main effect of each toxicity represents the difference in FACT-G score at T1 between patients with and without that specific symptom. The interaction term (timepoint × toxicity) tested whether the change in FACT-G score from T1 to T2 differed by toxicity status. All models were fit using restricted maximum likelihood (REML). Effect estimates and 95% confidence intervals were extracted and visualized to compare the relative impact of each toxicity on baseline quality of life and its trajectory over time.
3. Results
The study analyzed a total of 111 patients diagnosed with cervical cancer, ranging in age between 29 and 82 years old, with a mean of 58 years. Most patients were diagnosed with squamous cell carcinoma (n=81), followed by adenocarcinoma (n=27) and 3 patients with neuroendocrine carcinoma.
3.1. Patients' Characteristics
As presented in
Table 1, most patients were married at the time of treatment, lived in urban areas, and had middle levels of income. Fifty percent of patients had stage III cervical cancer, and 34% had stage II. The most used chemotherapy agent was cisplatin, administered to 92% of patients. There were 5 grade 3 toxicities and 4 grade 4 toxicities. The most common toxicities were nausea, diarrhea, and emesis. Eight patients had grade 1 ototoxicity and 4 had grade 1 renal toxicity.
3.2. Interpretation of FACT-G Score
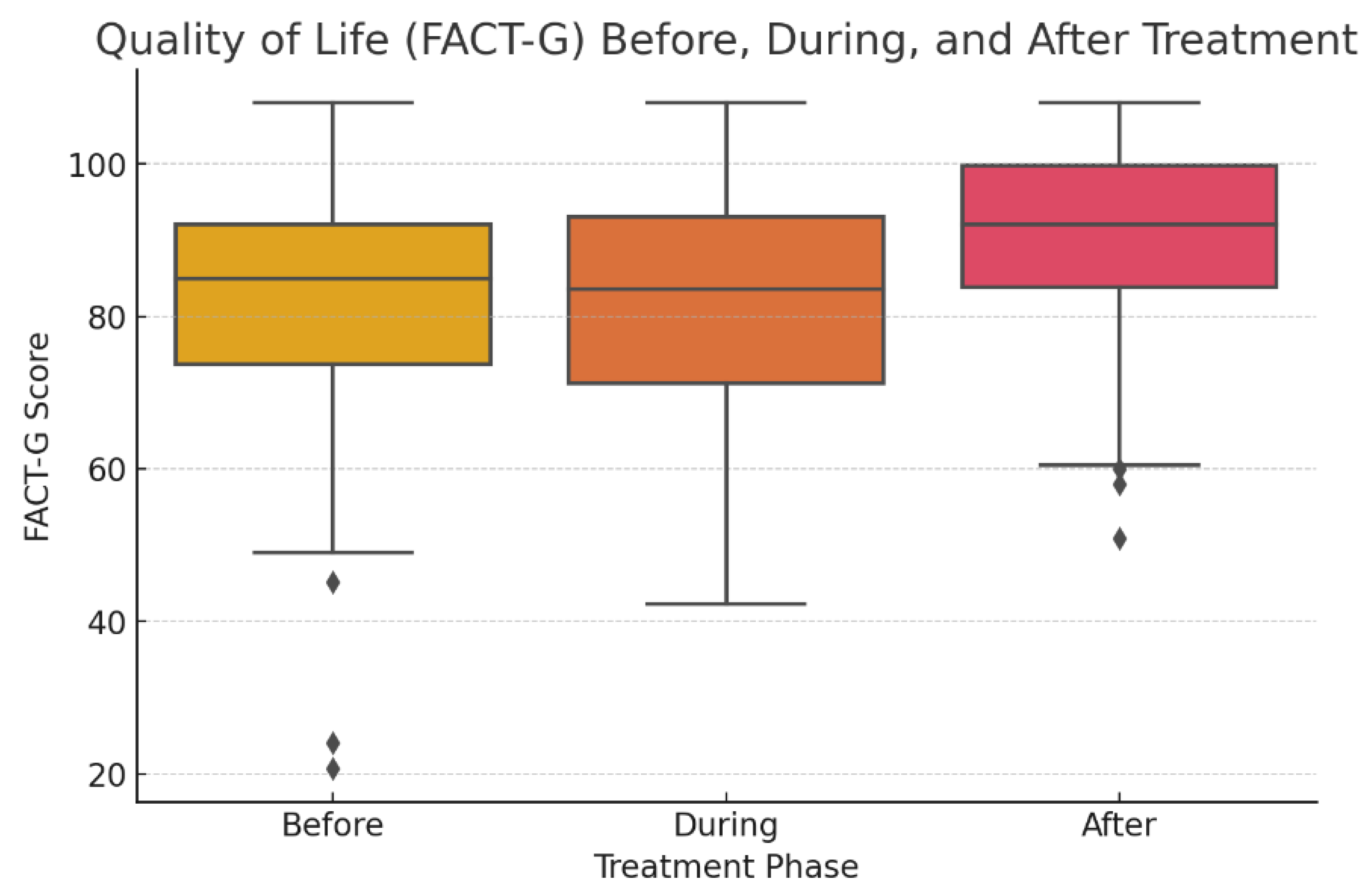
The median general quality of life score (FACT-G) before treatment was 85 (IQR 73-92), during treatment 84 (71-93), and after treatment 92 (84-100). The scores are summarized in
Table 2 along with the subdomains. The FACT-G score increased significantly after treatment (F-statistic = 13.05, p-value <0.001). This difference was significant between the score before and after treatment (Tukey’s HSD p <0.001) and for during and after treatment (p<0.001,
Figure 1).
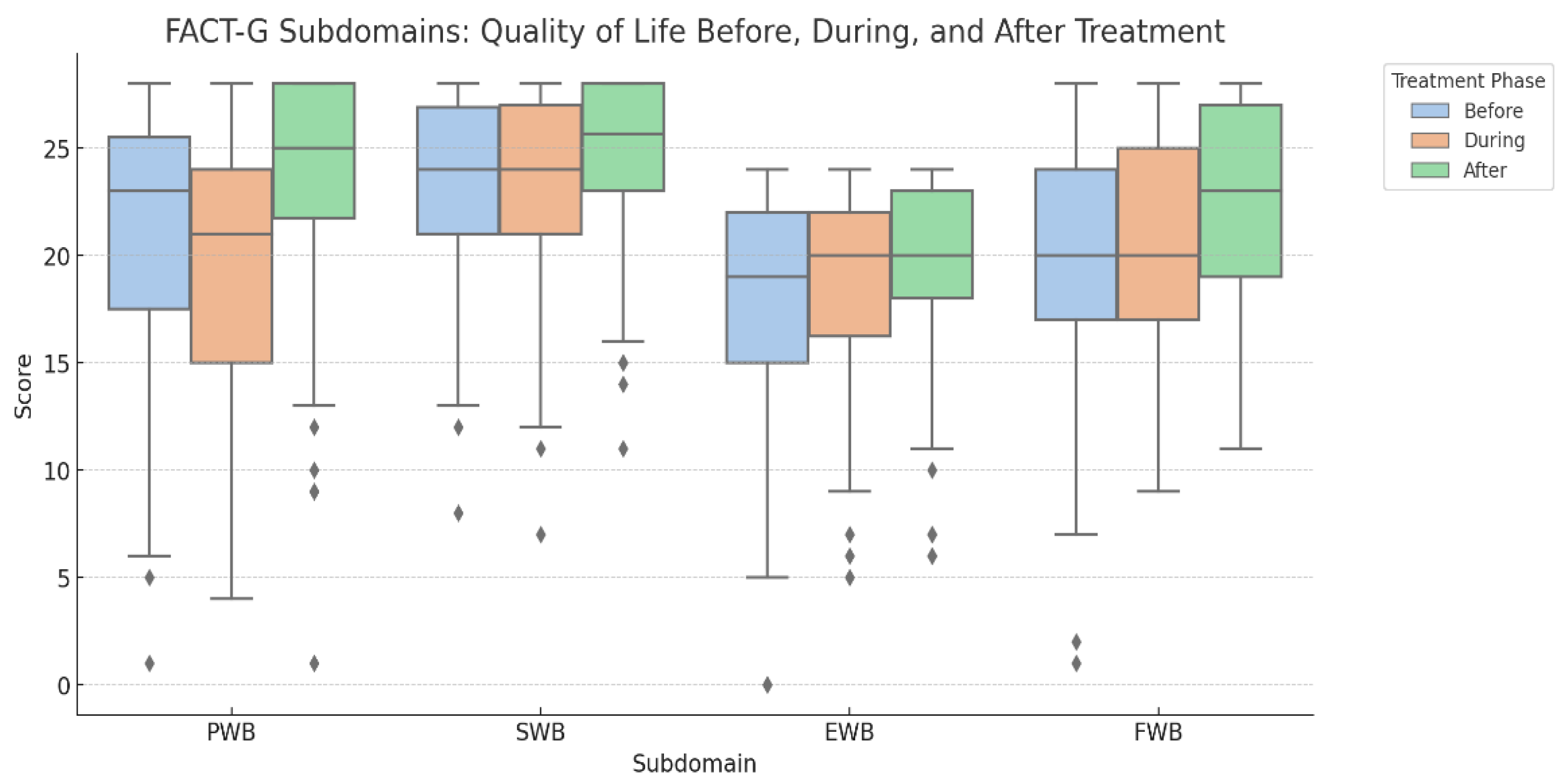
The effect size for overall FACT-G improvement was moderate, with Cohen’s d = 0.61 for before vs. after treatment and d = 0.63 for during vs. after treatment. Overall, the score increases for each subdomain after treatment with slightly worse score during treatment, as seen in
Figure 2.
3.3. On Each Subdomain We Observed
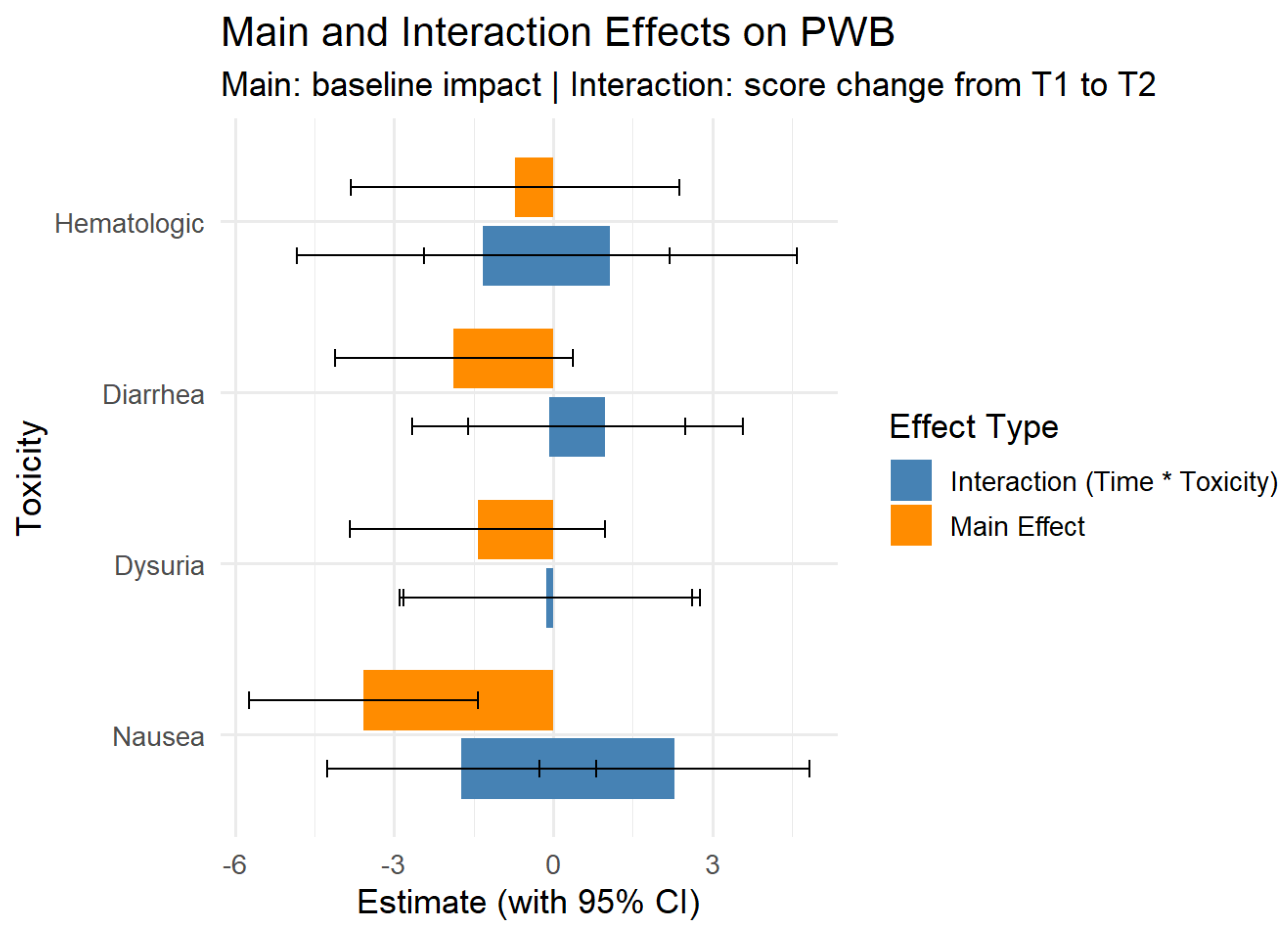
physical well-being (PWB) median score increased after treatment compared to before and during treatment (25 points vs. 23 and 21, p = 0.0017). The effect size for PWB was moderate to large (Cohen’s d = 0.48 before vs. after, d = 0.75 during vs. after). Social well-being (SWB) was slightly better after treatment than before, although not statistically significant (p=0.068), with a small effect size (Cohen’s d = 0.31). Emotional well-being (EWB) slightly decreased during treatment and returned to the initial value after treatment (19 points during, 20 points before and after treatment), with a small effect size (Cohen’s d = 0.20 during vs. after, d = 0.44 before vs. after). Functional well-being (FWB) improved significantly after treatment (23 points after vs. 20 during and before, p <0.001), with a moderate effect size (Cohen’s d = 0.50 before vs. after, d = 0.45 during vs. after).
3.4. Influence of Sociodemographic Factors
Patients from urban areas had a baseline FACT-G score with 8.3 points higher than patients in rural areas (p < 0.001), while single status vs married status showed a difference of 6.59 points in the baseline scores (p = 0.004, 95% CI [ 0.26-12.93]). Patients with stage 3-4 had lower score than patients with stage 1-2 with a difference of 4.43 points (p = 0.046, 95% CI [7.5 – 1.38]). For EWB scores, only the area associated a difference in the baseline score (2.19, p < 0.001, 95% CI [0.93-3.03]). For PWB, education was associated with a better score in patients with middle education vs elementary education (4 points difference, p = 0.0014, 95% CI [1.5-7.4]).
Sociodemographic factors significantly influenced baseline quality of life: patients living in urban areas, those with early-stage cancer, and married individuals reported higher overall FACT-G scores.
Physical Well-Being was positively associated with education, living environment, and stage. Functional Well-Being was strongly influenced by area of residence and Emotional Well-Being was modestly affected by living environment. Social Well-Being showed no significant sociodemographic associations.
3.5. Effects of Chemotherapy and Toxicities on Quality of Life
Chemotherapy type did not influence the general score (p = 0.11) nor did the number of cycles.
There was a main effect of time with better FACT-G score from baseline to after treatment. There was a main effect of cancer at baseline and mid treatment, patients with stage I-II having a better QOL than patients with stage III-IV (difference 4.43 points, p=0.0046). The interaction between stage and time was not statistically significant.
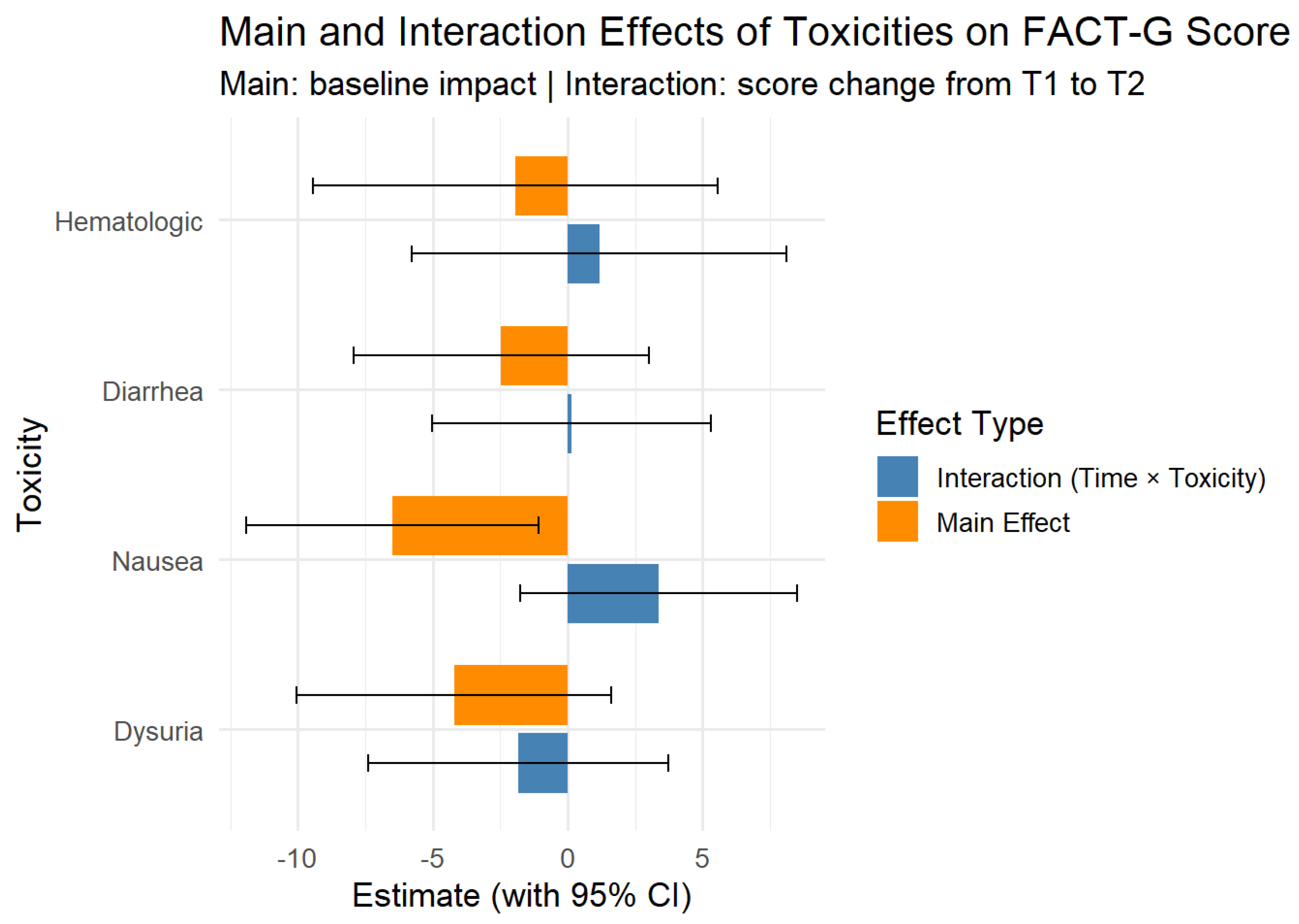
Regarding the toxicity effects on scores (toxicity levels as seen in
Table 3), only nausea was statistically significant, patients with nausea had lower FACT-G score during the treatment compared to those without nausea (β = -6.49, 95% CI [-11.91, - 1.07],
Figure 3). Patients with nausea also had lower PWB scores during treatment than patients without (β= -3.58, 95% CI [-5.74, -1.41]). The other toxicities showed associated lower scores, but they weren’t statistically significant on any of the subdomains
(Figure 4).
Linear mixed-effects models revealed that among treatment-related toxicities, only nausea had a statistically significant impact on quality of life, being associated with lower Physical Well-Being (β = −3.58, 95% CI [−5.74, −1.41]). Other toxicities, including hematologic effects and dysuria, did not significantly influence any subdomains of the FACT-G score. No significant interaction effects were found.
4. Discussion
4.1. Impact of Cervical Cancer Chemoradiation on Quality of Life
Cervical cancer (CC) treatments, particularly chemoradiation, profoundly influence patients’ quality of life (QoL). This study aimed to evaluate the effects of chemoradiation on the physical, social, emotional, and functional well-being of women with cervical cancer. The findings underscore the need to balance treatment efficacy with the broader implications for patients' QoL. Evaluating health-related quality of life (HRQoL) is critical to enhancing treatment outcomes and prioritizing overall health in medical decision-making [
18,
19]. Tailored treatment plans considering clinical and sociodemographic factors are essential to improving QoL for cervical cancer patients [
20].
4.2. Physical and Functional Impact of Chemoradiation
While chemoradiation is an effective treatment for cervical cancer, it often causes significant adverse effects that negatively affect QoL [
21,
22,
23]. This study identified several treatment-related toxicities, including dysuria, nausea, vomiting, fatigue, pain, gastrointestinal symptoms, and hematologic complications. These physical challenges often led to declines in functional well-being, impairing daily activities and social interactions. These findings align with previous research highlighting the negative physical effects of chemoradiation and emphasizing the importance of minimizing these side effects to improve patient outcomes [
23].
4.3. Influence of Demographics and Disease Characteristics
The study revealed that QoL outcomes varied according to demographic and disease-related factors. Older age and advanced disease stages were linked to substantial declines in physical and functional well-being, while higher education levels were associated with better social well-being. These results align with earlier findings demonstrating the impact of socioeconomic and demographic factors—such as cancer stage, treatment type, and social support—on treatment outcomes and recovery [
4].
4.4. Treatment Modalities and QoL Outcomes
This study’s findings align with prior research on the impact of chemoradiation. Systematic reviews have shown that physical functioning often declines significantly, with global QoL reaching its lowest point five years post-treatment [
21]. However, studies also emphasize the protective effects of spiritual health and social support, suggesting targeted therapies to address social and emotional challenges [
4].
Our study focused on acute and medium-term QoL changes, contrasting with earlier research that noted persistent QoL declines up to ten years post-treatment [
24]. Expanding future analyses to include long-term follow-ups may provide deeper insights into recovery patterns [
21]. Larger studies have documented the influence of healthcare access and socioeconomic factors on treatment outcomes [
24,
25,
26].
4.5. Importance of QoL Assessments in Routine Care
Integrating QoL assessments into routine care for cervical cancer patients undergoing chemoradiation is essential. Identifying patients at risk of significant QoL declines enables timely interventions, such as managing adverse events and educating patients about potential treatment challenges. Supportive care strategies, including counseling and rehabilitation, can help alleviate the physical, emotional, and social burdens associated with treatment.
4.6. Advancements in Radiotherapy
Radiotherapy remains a cornerstone for managing locally advanced cervical cancer, particularly when combined with brachytherapy [
27]. Modern approaches, such as intensity-modulated radiation therapy (IMRT), have demonstrated reduced gastrointestinal and genitourinary toxicity compared to traditional methods, enhancing patients’ treatment experiences [
28]. However, side effects, such as diarrhea, nausea, vomiting, dysuria, and peripheral neuropathy, remain common [
29,
30]. Studies, including those by Yucel et al., have shown that while global health status and appetite loss scores decline during radiation therapy, they significantly improve within six months post-treatment [
30].
4.7. Socio-Demographic Factors and QoL
Sociodemographic variables such as education, income, and employment status significantly influence QoL and survival. Higher education levels are associated with improved social well-being and survival rates, possibly due to better health literacy and navigation of healthcare systems. Conversely, lower income and unemployment are linked to poorer survival outcomes. Advanced disease stages at diagnosis further exacerbate QoL and survival disparities [
31].
4.8. Combined Treatment Approaches and QoL Measurement
Combining treatments, such as concurrent chemoradiotherapy (CCRT), offers better overall QoL outcomes compared to single therapies. Studies using the EORTC QLQ-C30 and QLQ-CX24 questionnaires have shown that patients undergoing combined external beam radiation therapy (EBRT) and brachytherapy report better functional outcomes. However, EBRT alone is associated with more side effects [
19]. Additionally, cancer stage significantly affects QoL across various domains, underscoring the importance of early diagnosis and stage-appropriate treatment [
32].
4.9. Emotional and Psychological Well-Being
Emotional well-being often remains a challenge even after physical, social, and functional improvements post-treatment. Persistent psychological issues, such as anxiety, depression, and concerns about recurrence, affect patients regardless of the treatment modality [
29,
33]. For instance, women undergoing CCRT frequently report negative impacts on body image, sexual enjoyment, and self-perception [
34]. Emotional distress is further influenced by marital status, with single and widowed patients experiencing greater loneliness and less emotional support [
23]. Depression, often linked to functional limitations and disrupted daily routines, also contributes to poor emotional well-being [
35,
36].
4.10. Long-Term Adaptations and Trends in QoL
Longitudinal studies reveal that treatment-related symptoms peak during therapy, while functional, social, and emotional domains often recover to pre-treatment levels by the end of treatment [
23,
37]. However, persistent psychological and physiological stress caused by cancer and its treatment may continue to impair interrelated areas of health over the long term [
38]. Some patients adapt to physical limitations, reporting perceived improvements in QoL despite ongoing physical challenges [
39].
4.11. Study Limitations
This study has several limitations. While real-time data collection allows for accurate assessments, there is a possibility that participants who completed the QoL evaluations differ from those who opted out. Additionally, factors such as individual resilience, social support, and coexisting health conditions may not have been fully captured.
The Functional Assessment of Cancer Therapy-General (FACT-G) questionnaire, while validated, may not adequately address issues specific to cervical cancer treatment, particularly gynecologic health-related challenges. Side effects such as changes in sexual health and gastrointestinal symptoms may be underrepresented, potentially leading to an incomplete understanding of the treatment’s impact on daily life.
The demographic homogeneity of the sample also limits the generalizability of findings. Most participants were from similar healthcare settings, which may not fully reflect the experiences of patients from diverse backgrounds or healthcare environments.
4.12. Future Directions
Future research should aim to include larger, more diverse participant groups to improve the generalizability of findings. QoL tools tailored to address the unique challenges of cervical cancer patients would enhance the understanding of treatment impacts. Longitudinal studies assessing QoL at multiple intervals post-treatment could provide valuable insights into recovery trajectories and long-term outcomes. Exploring additional factors, such as mental health and social support systems, would further contribute to a comprehensive understanding of QoL in this population.
5. Conclusions
This study shows that women with cervical cancer benefit greatly from chemoradiation in terms of their physical, mental, social, and functional well-being, among other aspects of their quality of life (QoL). Quality of life improved significantly after treatment, particularly in physical and functional well-being. Nausea was the only toxicity significantly associated with lower scores, especially in the physical domain.
The most pronounced improvements were observed in physical and functional well-being, particularly among participants who began treatment with lower initial scores. Emotional well-being also improved substantially, while social well-being showed more variability, likely influenced by external social factors beyond medical treatment.
With decreased variability and fewer outliers across all variables, the consistency of QoL outcomes increased after therapy, indicating that the majority of patients benefited similarly. All domains showed statistically significant improvements (p < 0.05), with physical and functional well-being showing the biggest influence (p < 0.001).
These findings highlight the dual impact of chemoradiation: it effectively controls disease progression while simultaneously posing challenges to QoL. It is crucial to address these issues with all-encompassing treatment that incorporates social, emotional, and physical assistance. To maximize the benefits of treatment, healthcare providers should consider implementing QoL-focused interventions, such as counseling, social support programs, and rehabilitative care.
Clinicians can improve clinical results and the entire treatment experience by taking a more personalized approach to patient care, especially for patients who are dealing with socioeconomic or illness-related issues.
Author Contributions
Conceptualization, Maria-Alexandra Barbu and Mihai-Teodor Georgescu; Data curation, Maria-Alexandra Barbu, Miruna Ghigeanu, Sarah Bahaa-Eddin , Alexandru Michire, Alexandra Hanu, Gentiana Eremia, Ianina Draganescu and Alina Birca; Formal analysis, Miruna Ghigeanu, Alexandru Michire and Gentiana Eremia; Investigation, Miruna Ghigeanu, Sarah Bahaa-Eddin , Gentiana Eremia, Ianina Draganescu and Alina Birca; Methodology, Maria-Alexandra Barbu, Miruna Ghigeanu, Alexandru Michire and Mihai-Teodor Georgescu; Project administration, Maria-Alexandra Barbu and Mihai-Teodor Georgescu; Resources, Maria-Alexandra Barbu, Miruna Ghigeanu, Alexandra Hanu and Gentiana Eremia; Software, Alexandru Michire; Supervision, Maria-Alexandra Barbu, Alexandru Michire and Mihai-Teodor Georgescu; Validation, Maria-Alexandra Barbu; Visualization, Maria-Alexandra Barbu and Mihai-Teodor Georgescu; Writing – original draft, Miruna Ghigeanu, Sarah Bahaa-Eddin , Alexandru Michire, Alexandra Hanu, Gentiana Eremia, Ianina Draganescu and Alina Birca; Writing – review & editing, Maria-Alexandra Barbu, Miruna Ghigeanu, Alexandru Michire, Gentiana Eremia and Mihai-Teodor Georgescu.
Institutional Review Board Statement
The study was conducted in accordance with the Declaration of Helsinki, and approved by the Ethics Committee of “Prof. Dr. Alexandru Trestioreanu” Institute of Oncology, Bucharest (Approval No 9100/25.06.2024).
Informed Consent Statement
Informed consent was obtained from all subjects involved in the study.
Data Availability Statement
The raw data supporting the conclusions of this article will be made available by the authors on request.
Conflicts of Interest
The authors declare no conflicts of interest.
Abbreviations
The following abbreviations are used in this manuscript:
| AEs |
Adverse Events |
| BT |
Brachytherapy |
| CC |
Cervical Cancer |
| CCRT |
Concurrent Chemoradiotherapy |
| EBRT |
External Beam Radiation Therapy |
| EWB |
Emotional Well-Being |
| FACT-G |
Functional Assessment of Cancer Therapy-General |
| FWB |
Functional Well-Being |
| HPV |
Human Papilloma Virus |
| HRQoL |
Health-related quality of life |
| IMRT |
Intensity-Modulated Radiation Therapy |
| PWB |
Physical Well-Being |
| SWB |
Social Well-Being |
| QoL |
Quality of Life |
| |
|
References
- Cancer (IARC), T.I.A. for R. Available online: https://gco.iarc.fr/ (accessed on May 6, 2025).
- Globocan, Romania 2022, Factsheet Available online:. Available online: https://gco.iarc.who.int/media/globocan/factsheets/populations/642-romania-fact-sheet.pdf (accessed on Jan 6, 2025).
- Cervical cancer Available online:. Available online: https://www.who.int/news-room/fact-sheets/detail/cervical-cancer (accessed on May 6, 2025).
- Khalil, J.; Bellefqih, S.; Sahli, N.; Afif, M.; Elkacemi, H.; Elmajjaoui, S.; Kebdani, T.; Benjaafar, N. Impact of cervical cancer on quality of life: beyond the short term (Results from a single institution). Gynecol. Oncol. Res. Pract. 2015, 2, 7. [Google Scholar] [CrossRef] [PubMed]
- Soares*, L.; Dantas, S.A. Cervical Cancer and Quality of Life: Systematic Review. Clin. J. Obstet. Gynecol. 2024, 7, 017–024. [Google Scholar] [CrossRef]
- Hull, R.; Mbele, M.; Makhafola, T.; Hicks, C.; Wang, S.-M.; Reis, R.M.; Mehrotra, R.; Mkhize-Kwitshana, Z.; Kibiki, G.; Bates, D.O.; et al. Cervical cancer in low and middle-income countries. Oncol. Lett. 2020, 20, 2058–2074. [Google Scholar] [CrossRef] [PubMed]
- Stalmeijer, R.E.; Mcnaughton, N.; Van Mook, W.N.K.A. Using focus groups in medical education research: AMEE Guide No. 91. Med. Teach. 2014, 36, 923–939. [Google Scholar] [CrossRef] [PubMed]
- Correia, R.A.; Bonfim, C.V. do; Ferreira, D.K. da S.; Furtado, B.M.A.S.M.; Costa, H.V.V. da; Feitosa, K.M.A.; Santos, S.L. dos Qualidade de vida após o tratamento do câncer do colo do útero. Esc. Anna Nery 2018, 22, e20180130. [Google Scholar] [CrossRef]
- Johnson, C.A.; James, D.; Marzan, A.; Armaos, M. Cervical Cancer: An Overview of Pathophysiology and Management. Semin. Oncol. Nurs. 2019, 35, 166–174. [Google Scholar] [CrossRef]
- Alsulami, A.; Orabi, A.; Timraz, S. Saudi women’s perspectives on postpartum depression. Front. Glob. Womens Health 2024, 5. [Google Scholar] [CrossRef]
- Dowling, M.; Eicher, M.; Drury, A. Experiences of cancer care in COVID-19: A longitudinal qualitative study. Eur. J. Oncol. Nurs. 2022, 61, 102228. [Google Scholar] [CrossRef]
- FACIT.org Functional Assessment of Cancer Therapy – General (FACT-G), Version 4 1993.
- Microsoft Corporation Microsoft Excel 2023.
- RStudio Team RStudio: Integrated Development Environment for R 2023.
- Wickham, H. ggplot2: Elegant Graphics for Data Analysis; Springer: New York, NY, 2009; ISBN 978-0-387-98140-6. [Google Scholar]
- Wickham, H.; Vaughan, D.; Girlich, M.; Ushey, K.; Software, P. ; PBC tidyr: Tidy Messy Data 2024.
- Bates, D.; Mächler, M.; Bolker, B.; Walker, S. Fitting Linear Mixed-Effects Models Using lme4. J. Stat. Softw. 2015, 67, 1–48. [Google Scholar] [CrossRef]
- Rutherford, C.; Mercieca-Bebber, R.; Tait, M.; Mileshkin, L.; King, M.T. Quality of Life in Women with Cervical Cancer. In Uterine Cervical Cancer. In Uterine Cervical Cancer: Clinical and Therapeutic Perspectives; Farghaly, S.A., Ed.; Springer International Publishing: Cham, 2019; ISBN 978-3-030-02701-8. [Google Scholar]
- Terrell, J.E.; Ronis, D.L.; Fowler, K.E.; Bradford, C.R.; Chepeha, D.B.; Prince, M.E.; Teknos, T.N.; Wolf, G.T.; Duffy, S.A. Clinical Predictors of Quality of Life in Patients With Head and Neck Cancer. Arch. Otolaryngol. Neck Surg. 2004, 130, 401–408. [Google Scholar] [CrossRef]
- Chen, H.; Zhou, L.; Fong, D.; Cun, Y.; Yang, Z.; Wan, C. Quality of life and its related-influencing factors in patients with cervical cancer based on the scale QLICP-CE(V2.0). BMC Womens Health 2024, 24, 277. [Google Scholar] [CrossRef] [PubMed]
- Zhao, M.; Luo, L.; zhang, C.; zhang, J.; Yuan, J.; Gu, R.; Ding, S. Healthy-related quality of life in patients with cervical cancer in Southwest China: a cross-sectional study. BMC Health Serv. Res. 2021, 21, 841. [Google Scholar] [CrossRef] [PubMed]
- Kyei, K.A.; Yakanu, F.; Donkor, A.; Kitson-Mills, D.; Opoku, S.Y.; Yarney, J.; Tagoe, S.N.; Addo, M.K.; Anarfi, K.K.; Abakuri, E.; et al. Quality of life among cervical cancer patients undergoing radiotherapy. Pan Afr. Med. J. 2020, 35, 125. [Google Scholar] [CrossRef]
- Pasek, M.; Suchocka, L.; Osuch-Pęcak, G.; Muzykiewicz, K.; Iwańska, E.; Kaducakowa, H.; Goździalska, A.; Goździalska, M. Longitudinal Health-Related Quality of Life Study among Cervical Cancer Patients Treated with Radiotherapy. J. Clin. Med. 2021, 10, 226. [Google Scholar] [CrossRef]
- Conway, J.L.; Felder, S.; Tang, J.; Lukovic, J.; Han, K.; Liu, Z.; Milosevic, M.; Fyles, A.; Croke, J. Long-term patient-reported distress in locally advanced cervical cancer patients treated with definitive chemoradiation. Clin. Transl. Radiat. Oncol. 2020, 23, 1–8. [Google Scholar] [CrossRef]
- Cea García, J.; Márquez Maraver, F.; Rodríguez Jiménez, I.; Ríos-Pena, L.; Rubio Rodríguez, M.C. A Prospective Study Assessing Sexual Function and Body Image Among Cervical Cancer Survivors: Exploring Treatment Modality Differences. J. Obstet. Gynecol. Cancer Res. 2024, 9, 185–200. [Google Scholar] [CrossRef]
- Pfaendler, K.S.; Wenzel, L.; Mechanic, M.B.; Penner, K.R. Cervical cancer survivorship: Long-term quality of life and social support. Clin. Ther. 2015, 37, 39–48. [Google Scholar] [CrossRef]
- Mayadev, J.S.; Ke, G.; Mahantshetty, U.; Pereira, M.D.; Tarnawski, R.; Toita, T. Global challenges of radiotherapy for the treatment of locally advanced cervical cancer. Int. J. Gynecol. Cancer Off. J. Int. Gynecol. Cancer Soc. 2022, 32, 436–445. [Google Scholar] [CrossRef]
- Klopp, A.H.; Yeung, A.R.; Deshmukh, S.; Gil, K.M.; Wenzel, L.; Westin, S.N.; Gifford, K.; Gaffney, D.K.; Small, W.; Thompson, S.; et al. Patient-Reported Toxicity During Pelvic Intensity-Modulated Radiation Therapy: NRG Oncology-RTOG 1203. J. Clin. Oncol. Off. J. Am. Soc. Clin. Oncol. 2018, 36, 2538–2544. [Google Scholar] [CrossRef]
- Korfage, I.J.; Essink-Bot, M.-L.; Mols, F.; van de Poll-Franse, L.; Kruitwagen, R.; van Ballegooijen, M. Health-related quality of life in cervical cancer survivors: a population-based survey. Int. J. Radiat. Oncol. Biol. Phys. 2009, 73, 1501–1509. [Google Scholar] [CrossRef]
- Yucel, B.; Akkaş, E.A.; Okur, Y.; Eren, A.A.; Eren, M.F.; Karapinar, H.; Babacan, N.A.; Kiliçkap, S. The impact of radiotherapy on quality of life for cancer patients: a longitudinal study. Support. Care Cancer Off. J. Multinatl. Assoc. Support. Care Cancer 2014, 22, 2479–2487. [Google Scholar] [CrossRef]
- Cetina-Pérez, L.; Luvián-Morales, J.; Delgadillo-González, M.; Castro-Eguiluz, D.; Galicia-Carmona, T.; Rely, K.; Vaca González, R.; Lugo-Martínez, G.; García-Barrientos, N.; Nateras, A. Sociodemographic characteristics and their association with survival in women with cervical cancer. BMC Cancer 2024, 24, 161. [Google Scholar] [CrossRef] [PubMed]
- Mvunta, D.H.; August, F.; Dharsee, N.; Mvunta, M.H.; Wangwe, P.; Ngarina, M.; Simba, B.M.; Kidanto, H. Quality of life among cervical cancer patients following completion of chemoradiotherapy at Ocean Road Cancer Institute (ORCI) in Tanzania. BMC Womens Health 2022, 22, 426. [Google Scholar] [CrossRef] [PubMed]
- Millet, N.; Moss, E.L.; Munir, F.; Rogers, E.; McDermott, H.J. A qualitative exploration of physical and psychosocial well-being in the short and long term after treatments for cervical cancer. Eur. J. Cancer Care (Engl.) 2022, 31, e13560. [Google Scholar] [CrossRef]
- Stuopelytė, R.; Žukienė, G.; Breivienė, R.; Rudaitis, V.; Bartkevičienė, D. Quality of Life in Cervical Cancer Survivors Treated with Concurrent Chemoradiotherapy. Med. Kaunas Lith. 2023, 59, 777. [Google Scholar] [CrossRef]
- Kirchheiner, K.; Nout, R.A.; Czajka-Pepl, A.; Ponocny-Seliger, E.; Sturdza, A.E.; Dimopoulos, J.C.; Dörr, W.; Pötter, R. Health related quality of life and patient reported symptoms before and during definitive radio(chemo)therapy using image-guided adaptive brachytherapy for locally advanced cervical cancer and early recovery - a mono-institutional prospective study. Gynecol. Oncol. 2015, 136, 415–423. [Google Scholar] [CrossRef]
- Ye, S.; Yang, J.; Cao, D.; Lang, J.; Shen, K. A systematic review of quality of life and sexual function of patients with cervical cancer after treatment. Int. J. Gynecol. Cancer Off. J. Int. Gynecol. Cancer Soc. 2014, 24, 1146–1157. [Google Scholar] [CrossRef]
- Barnaś, E.; Skręt-Magierło, J.; Skręt, A.; Bidziński, M. The quality of life of women treated for cervical cancer. Eur. J. Oncol. Nurs. Off. J. Eur. Oncol. Nurs. Soc. 2012, 16, 59–63. [Google Scholar] [CrossRef]
- Wenzel, L.; Osann, K.; Hsieh, S.; Tucker, J.A.; Monk, B.J.; Nelson, E.L. Psychosocial telephone counseling for survivors of cervical cancer: results of a randomized biobehavioral trial. J. Clin. Oncol. Off. J. Am. Soc. Clin. Oncol. 2015, 33, 1171–1179. [Google Scholar] [CrossRef]
- Lutgendorf, S.K.; Anderson, B.; Ullrich, P.; Johnsen, E.L.; Buller, R.E.; Sood, A.K.; Sorosky, J.I.; Ritchie, J. Quality of life and mood in women with gynecologic cancer: a one year prospective study. Cancer 2002, 94, 131–140. [Google Scholar] [CrossRef]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).