Submitted:
27 May 2025
Posted:
28 May 2025
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Aerogels
2.1. Polymer Aerogels
2.2. Biopolymer Aerogels
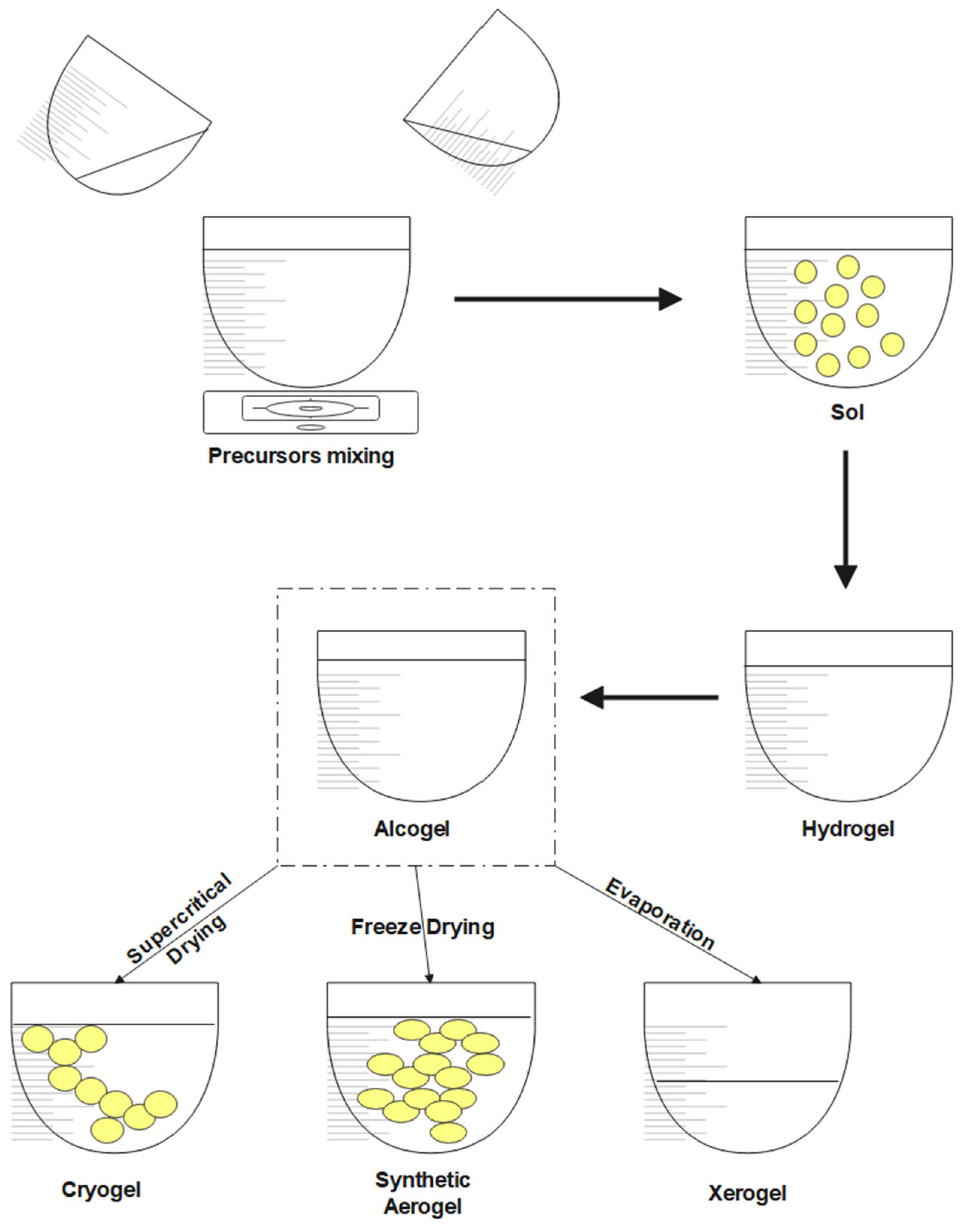
2.3. Synthetic Aerogels
3. Classification of Synthetic Aerogels
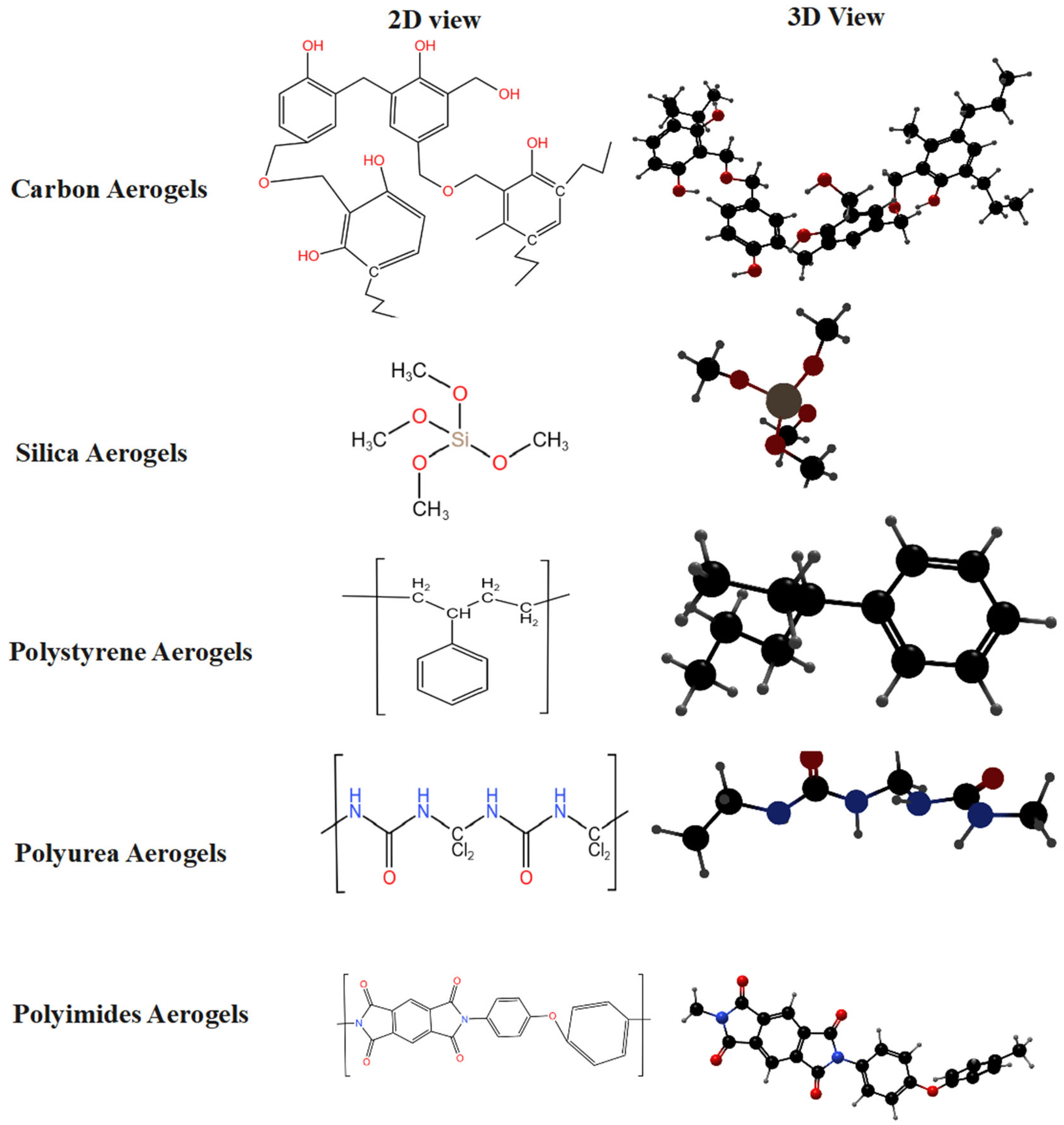
3.1. Silica Aerogels
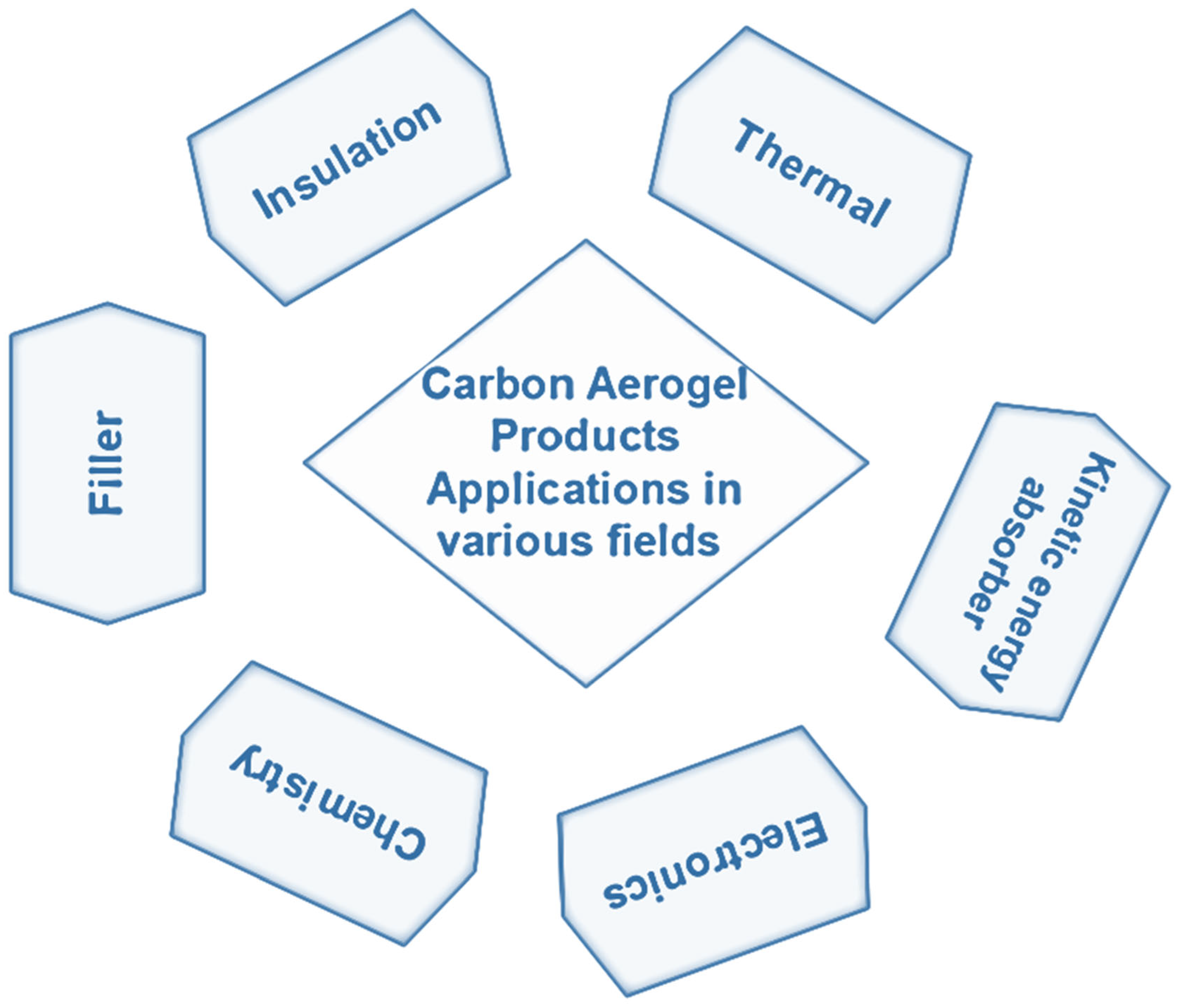
3.2. Carbon Aerogel
3.3. Polyimide Aerogel
3.4. Polyurea Aerogel
3.5. Polystyrene Aerogel
3.6. Synthetic Aerogel Composites
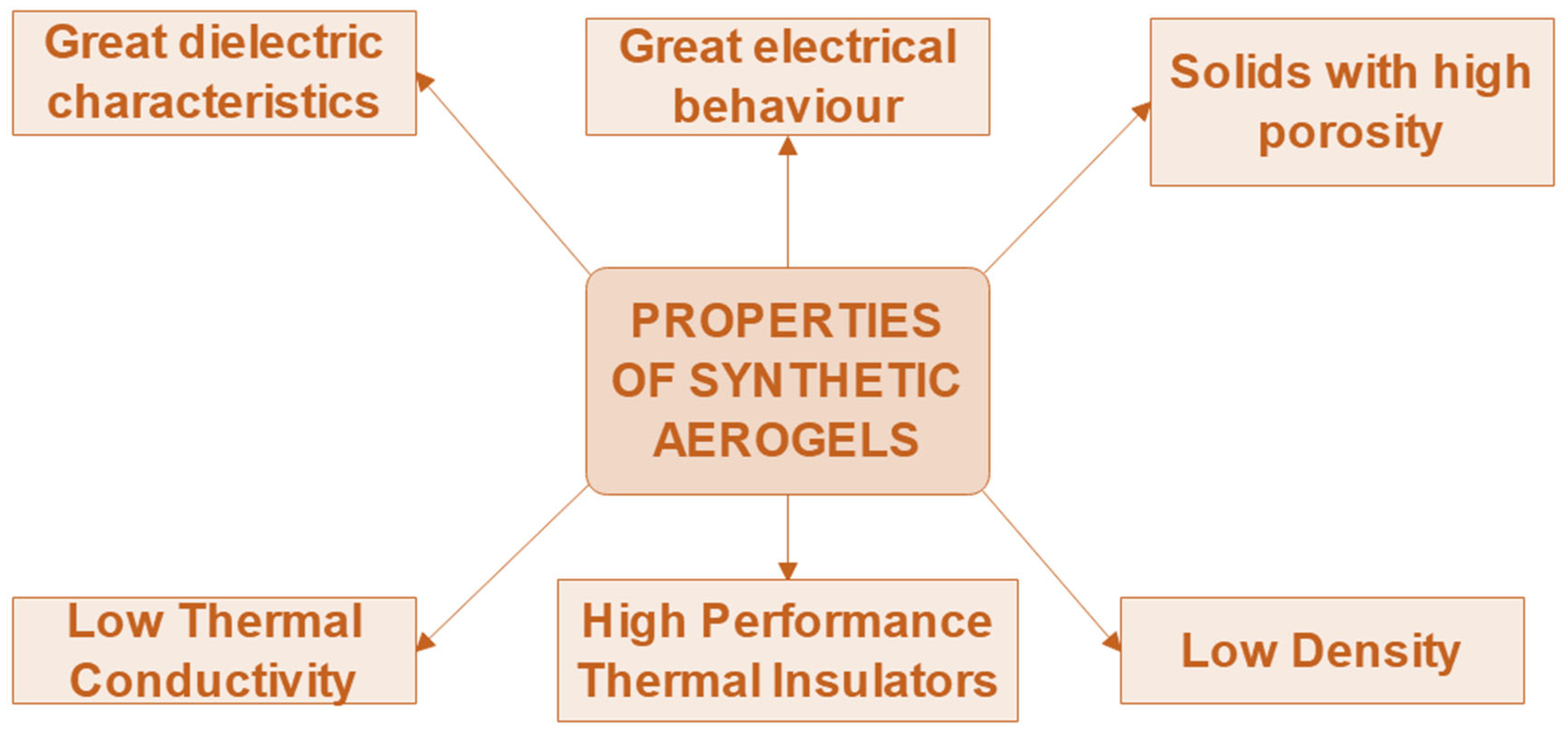
4. Properties of Synthetic Aerogels
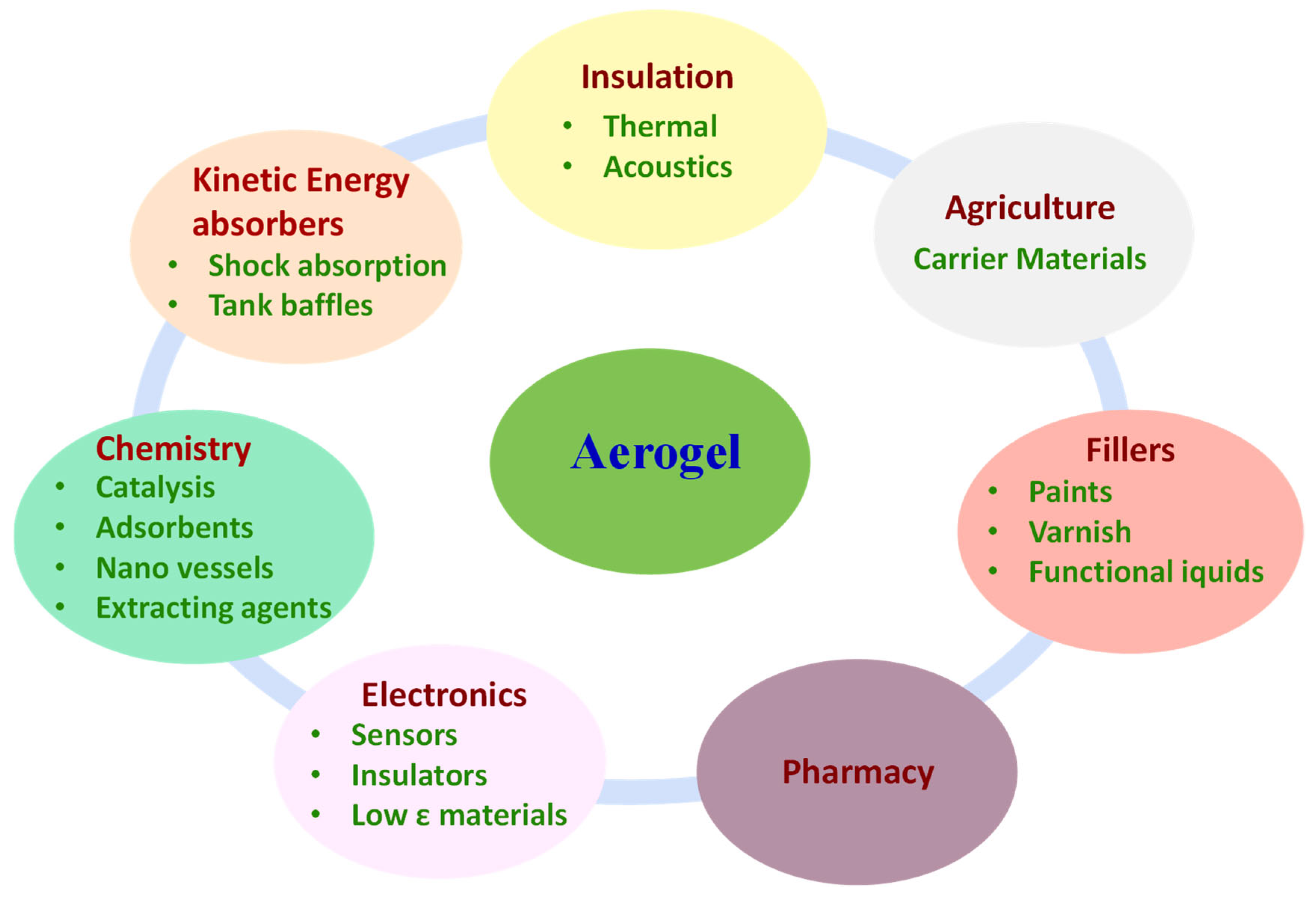
5. Applications of Synthetic Aerogels
6. Characterization Techniques of Synthetic Aerogels
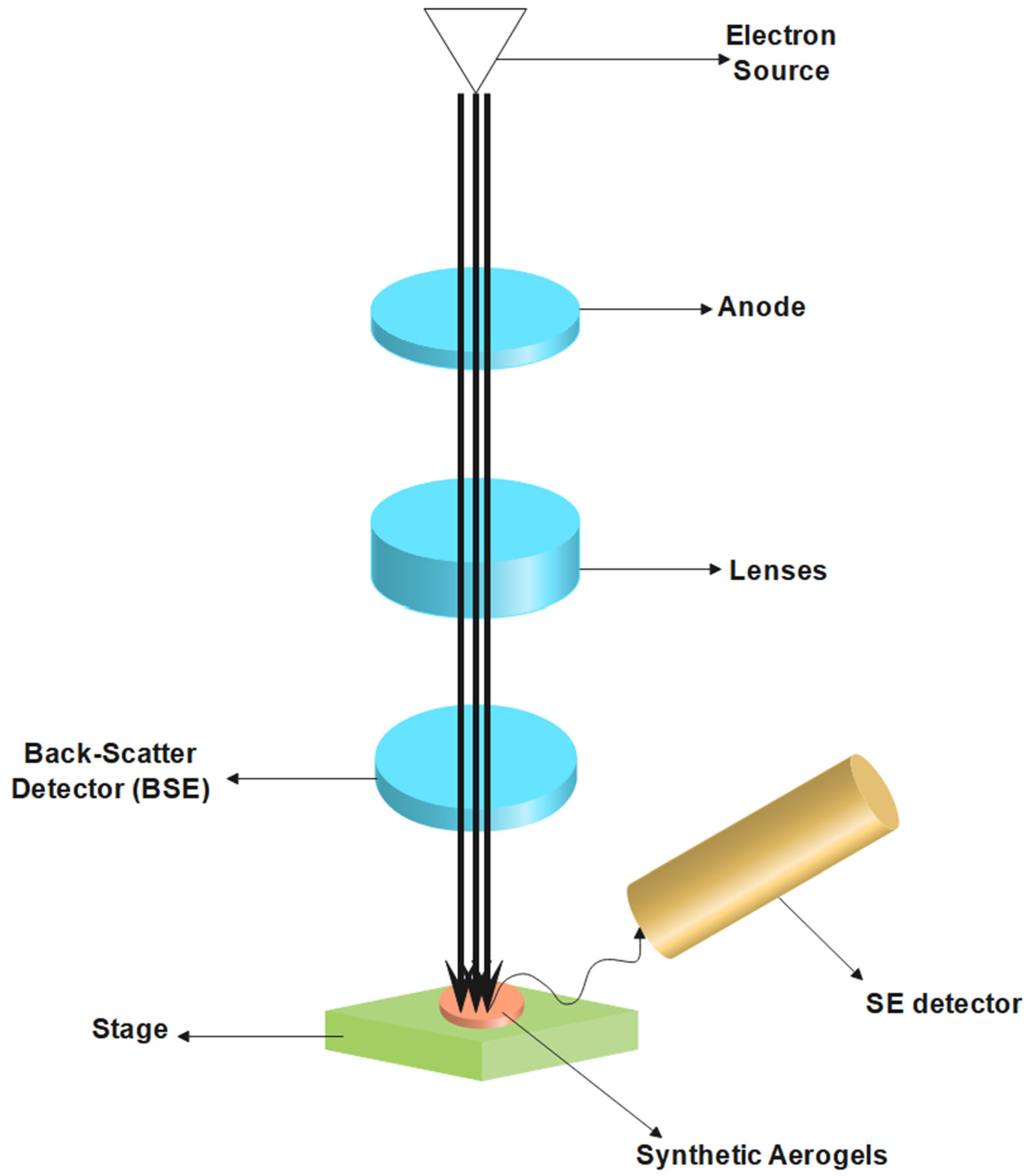
6.1. Electron Microscopy
6.2. Fourier-Transformed Infrared Spectroscopy (FTIR)
6.3. X-Ray Diffraction Patterns
6.4. Raman Spectroscopy
6.5. Other Characterization Techniques for Synthetic Aerogels
7. Current Perspectives
8. Conclusions
- Silica Aerogels have low thermal conductivity and photoluminescence super hydrophobicity and have applications in vast number of fields like drug carriers, thermal insulation, space technology and Knudsen pumps.
- Carbon Aerogels has promising properties with high oil/organic solvent absorption capacity and very good hydrophobicity. Such materials also have showcased incredible properties in rechargeable batteries, supercapacitors and broadband non reflective materials.
- Polyimides Aerogels are one of the most important and crucial aerogels as they also overcome the weak mechanical properties which aerogels usually and in addition showcase superior hydrophobicity, great flexibility and low thermal conductivity with applications in solar collectors, sophisticated optical elements, thermal insulation and fire resistance.
- Polyurea and Polystyrene both have high hydrophobic characteristics and high sorption capacity and low thermal conductivities. The properties of Polyurea aerogels help in the tissue scaffolds and shape memory aerogels whereas polystyrene have their applications in oil-water separation gas storage.
Acknowledgements
References
- Manisalidis I, Stavropoulou E, Stavropoulos A, Bezirtzoglou E. Environmental and Health Impacts of Air Pollution: A Review. Frontiers in Public Health. 2020;8. [CrossRef]
- Rao MG, Bharathi P, Akila R. A COMPREHENSIVE REVIEW ON BIOPOLYMERS. Scientific Reviews and Chemical Communications. 2014;4:61-8. Available online: https://selling.farafile.ir/content/demo/201904/aaa78a1c-dc2e-46c3-9d77-ef6b966c6669.pdf.
- Mustafa NS, Omer MAA, Garlnabi ME, Ismail HA, Ch C. Reviewing of general polymer types, properties and application in medical field. Int J Sci Res (IJSR). 2016;5:212e21.
- Crompton T. Polymer microstructure. Practical Polymer Analysis: Springer; 1993. p. 437-505. [CrossRef]
- Tomar S, Singh L, Sharma V. INTERNATIONAL RESEARCH JOURNAL OF PHARMACY. [CrossRef]
- Pathak VM, Navneet. Review on the current status of polymer degradation: a microbial approach. Bioresources and Bioprocessing. 2017;4:15. [CrossRef]
- George A, Sanjay MR, Srisuk R, Parameswaranpillai J, Siengchin S. A comprehensive review on chemical properties and applications of biopolymers and their composites. International Journal of Biological Macromolecules. 2020;154:329-38. [CrossRef]
- Aaliya B, Sunooj KV, Lackner M. Biopolymer composites: a review. International Journal of Biobased Plastics. 2021;3:40-84. [CrossRef]
- Dassanayake RS, Acharya S, Abidi N. Biopolymer-based materials from polysaccharides: Properties, processing, characterization and sorption applications. Advanced sorption process applications. 2018:1-24. Available online: https://books.google.co.in/books?hl=en&lr=&id=xRf8DwAAQBAJ&oi=fnd&pg=PA3&dq=Dassanayake+RS,+Acharya+S,+Abidi+N.+Biopolymer-based+materials+from+polysaccharides:+Properties,+processing,+characterization+and+sorption+applications.+Advanced+sorption+process+applications.
- Du A, Zhou B, Zhang Z, Shen J. A Special Material or a New State of Matter: A Review and Reconsideration of the Aerogel. Materials (Basel). 2013;6:941-68. [CrossRef]
- Jones, SM. Aerogel: Space exploration applications. Journal of Sol-Gel Science and Technology. 2006;40:351-7. [CrossRef]
- Kharissova OV, Ibarra Torres CE, González LT, Kharisov BI. All-Carbon Hybrid Aerogels: Synthesis, Properties, and Applications. Industrial & Engineering Chemistry Research. 2019;58:16258-86. [CrossRef]
- Nita LE, Ghilan A, Rusu AG, Neamtu I, Chiriac AP. New Trends in Bio-Based Aerogels. Pharmaceutics. 2020;12. [CrossRef]
- Verma A, Thakur S, Goel G, Raj J, Gupta VK, Roberts D, et al. Bio-based sustainable aerogels: New sensation in CO2 capture. Current Research in Green and Sustainable Chemistry. 2020;3:100027. [CrossRef]
- Kistler, SS. Coherent Expanded Aerogels and Jellies. Nature. 1931;127:741-. [CrossRef]
- Fricke J, Emmerling A. Aerogels. Journal of the American Ceramic Society. 1992;75:2027-35. [CrossRef]
- Cashman JL, Nguyen BN, Dosa B, Meador MAB. Flexible Polyimide Aerogels Derived from the Use of a Neopentyl Spacer in the Backbone. ACS Applied Polymer Materials. 2020;2:2179-89. [CrossRef]
- Williams JC, Nguyen BN, McCorkle L, Scheiman D, Griffin JS, Steiner SA, et al. Highly Porous, Rigid-Rod Polyamide Aerogels with Superior Mechanical Properties and Unusually High Thermal Conductivity. ACS Applied Materials & Interfaces. 2017;9:1801-9. [CrossRef]
- Rewatkar PM, Saeed AM, Majedi Far H, Donthula S, Sotiriou-Leventis C, Leventis N. Polyurethane Aerogels Based on Cyclodextrins: High-Capacity Desiccants Regenerated at Room Temperature by Reducing the Relative Humidity of the Environment. ACS Appl Mater Interfaces. 2019;11:34292-304. [CrossRef]
- Chriti D, Raptopoulos G, Papastergiou M, Paraskevopoulou P. Millimeter-Size Spherical Polyurea Aerogel Beads with Narrow Size Distribution. Gels. 2018;4. [CrossRef]
- Lee K-Y, Jung H-N-R, Mahadik D, Park H-H. Characterization of Mechanical Property Change in Polymer Aerogels Depending on the Ligand Structure of Acrylate Monomer. Journal of the Microelectronics and Packaging Society. 2016;23:15-20. [CrossRef]
- Zhao S, Malfait WJ, Guerrero-Alburquerque N, Koebel MM, Nyström G. Biopolymer Aerogels and Foams: Chemistry, Properties, and Applications. Angewandte Chemie International Edition. 2018;57:7580-608. [CrossRef]
- Chen Y, Zhou L, Chen L, Duan G, Mei C, Huang C, et al. Anisotropic nanocellulose aerogels with ordered structures fabricated by directional freeze-drying for fast liquid transport. Cellulose. 2019;26:6653-67. [CrossRef]
- Mi H-Y, Jing X, Liu Y, Li L, Li H, Peng X-F, et al. Highly Durable Superhydrophobic Polymer Foams Fabricated by Extrusion and Supercritical CO2 Foaming for Selective Oil Absorption. ACS Applied Materials & Interfaces. 2019;11:7479-87. [CrossRef]
- Pirzada T, Ashrafi Z, Xie W, Khan SA. Cellulose Silica Hybrid Nanofiber Aerogels: From Sol–Gel Electrospun Nanofibers to Multifunctional Aerogels. Advanced Functional Materials. 2020;30:1907359. [CrossRef]
- Liu R, Wang J, Du Y, Liao J, Zhang X. Phase-separation induced synthesis of superhydrophobic silica aerogel powders and granules. Journal of Solid State Chemistry. 2019;279:120971. [CrossRef]
- Yahya EB, Jummaat F, Amirul AA, Adnan AS, Olaiya NG, Abdullah CK, et al. A Review on Revolutionary Natural Biopolymer-Based Aerogels for Antibacterial Delivery. Antibiotics. 2020;9:648. [CrossRef]
- Subrahmanyam R, Gurikov P, Meissner I, Smirnova I. Preparation of Biopolymer Aerogels Using Green Solvents. J Vis Exp. 2016:54116. [CrossRef]
- Baetens R, Jelle BP, Gustavsen A. Aerogel insulation for building applications: A state-of-the-art review. Energy and Buildings. 2011;43:761-9. [CrossRef]
- Thapliyal PC, Singh K. Aerogels as Promising Thermal Insulating Materials: An Overview. Journal of Materials. 2014;2014:127049. [CrossRef]
- Hrubesh L, Pekala R. Dielectric properties and electronic applications of aerogels. Sol-Gel Processing and Applications: Springer; 1994. p. 363-7.
- Akimov, YK. Fields of Application of Aerogels (Review). Instruments and Experimental Techniques. 2003;46:287-99. [CrossRef]
- Bostanci L, Sola OC. Mechanical Properties and Thermal Conductivity of Aerogel-Incorporated Alkali-Activated Slag Mortars. Advances in Civil Engineering. 2018;2018:4156248. [CrossRef]
- Ma H, Zheng X, Luo X, Yi Y, Yang F. Simulation and Analysis of Mechanical Properties of Silica Aerogels: From Rationalization to Prediction. Materials. 2018;11:214. [CrossRef]
- Woignier T, Primera J, Alaoui A, Etienne P, Despestis F, Calas-Etienne S. Mechanical Properties and Brittle Behavior of Silica Aerogels. Gels. 2015;1:256-75. [CrossRef]
- Ghodake Sagar S, Londhe Babasaheb C. A Review on Aerogel An Introduction. 2018. Available online: https://d1wqtxts1xzle7.cloudfront.net/56794759/IRJET-V5I3961-libre.pdf?1528977771=&response-content-disposition=inline%3B+filename%3DA_Review_on_Aerogel_An_Introduction.pdf&Expires=1680807528&Signature=HyD1UhnT1AZjcek2ZwnoHBgoOWY4U1E90Zyuu~Cp4rPkqt9KCOmVpJx8Z7OLzUE2sKOkYOC9dSQ-KX~2dG52E5E-sgyZmnQfShVlqRD0rMhm6VMYu6eRtDYewxcxybXiWSg7pGpf9lp1AIn6bOwQoPOcYGLf~~Xl0A1M7Eo5R2xSgLb60376O7rJw7-H3ymde-XkJsWcZh5JXwjUJsWfCcBrBOStBJmVbdsfmVTwUWXoF5i5XnFjedSWG2tlei2odBD-ogAQZ2oncRzDLfrF2rVXkMowlvlslFcgbh1yr6tiXrR2y66LJKBb8jd8IQz5tzaqfUqpO0lMm9mBDKivJQ__&Key-Pair-Id=APKAJLOHF5GGSLRBV4ZA.
- Nazeran N, Moghaddas J. Synthesis and characterization of silica aerogel reinforced rigid polyurethane foam for thermal insulation application. Journal of Non-Crystalline Solids. 2017;461:1-11. [CrossRef]
- Wi S, Berardi U, Loreto SD, Kim S. Microstructure and thermal characterization of aerogel–graphite polyurethane spray-foam composite for high efficiency thermal energy utilization. Journal of Hazardous Materials. 2020;397:122656. [CrossRef]
- Xiao W, Wang P, Song X, Liao B, Yan K, Zhang J-J. Facile Fabrication of Anisotropic Chitosan Aerogel with Hydrophobicity and Thermal Superinsulation for Advanced Thermal Management. ACS Sustainable Chemistry & Engineering. 2021;9:9348-57. [CrossRef]
- Nocentini K, Achard P, Biwole P, Stipetic M. Hygro-thermal properties of silica aerogel blankets dried using microwave heating for building thermal insulation. Energy and Buildings. 2018;158:14-22. [CrossRef]
- Liu Z, Lyu J, Fang D, Zhang X. Nanofibrous Kevlar Aerogel Threads for Thermal Insulation in Harsh Environments. ACS Nano. 2019;13:5703-11. [CrossRef]
- Jia G, Li Z, Liu P, Jing Q. Preparation and characterization of aerogel/expanded perlite composite as building thermal insulation material. Journal of Non-Crystalline Solids. 2018;482:192-202. [CrossRef]
- Berardi U, Zaidi S. Characterization of commercial aerogel-enhanced blankets obtained with supercritical drying and of a new ambient pressure drying blanket. Energy and Buildings. 2019;198:542-52. [CrossRef]
- Liu S, Wu X, Li Y, Cui S, Shen X, Tan G. Hydrophobic in-situ SiO2-TiO2 composite aerogel for heavy oil thermal recovery: Synthesis and high temperature performance. Applied Thermal Engineering. 2021;190:116745. [CrossRef]
- Zhang H, Zhang C, Ji W, Wang X, Li Y, Tao W. Experimental Characterization of the Thermal Conductivity and Microstructure of Opacifier-Fiber-Aerogel Composite. Molecules. 2018;23:2198. [CrossRef]
- Lakatos Á, Csík A, Csarnovics I. Experimental verification of thermal properties of the aerogel blanket. Case Studies in Thermal Engineering. 2021;25:100966. [CrossRef]
- Katti A, Shimpi N, Roy S, Lu H, Fabrizio EF, Dass A, et al. Chemical, Physical, and Mechanical Characterization of Isocyanate Cross-linked Amine-Modified Silica Aerogels. Chemistry of Materials. 2006;18:285-96. [CrossRef]
- Ye X, Chen Z, Ai S, Hou B, Zhang J, Liang X, et al. Novel Three-Dimensional SiC/Melamine-Derived Carbon Foam-Reinforced SiO2 Aerogel Composite with Low Dielectric Loss and High Impedance Matching Ratio. ACS Sustainable Chemistry & Engineering. 2019;7:2774-83. [CrossRef]
- Meador MAB, McMillon E, Sandberg A, Barrios E, Wilmoth NG, Mueller CH, et al. Dielectric and Other Properties of Polyimide Aerogels Containing Fluorinated Blocks. ACS Applied Materials & Interfaces. 2014;6:6062-8. [CrossRef]
- Tabernero A, Baldino L, Misol A, Cardea S, del Valle EMM. Role of rheological properties on physical chitosan aerogels obtained by supercritical drying. Carbohydrate Polymers. 2020;233:115850. [CrossRef]
- Kistler SS, Fischer EA, Freeman IR. Sorption and Surface Area in Silica Aerogel. Journal of the American Chemical Society. 1943;65:1909-19. [CrossRef]
- White JF, Wilson IV. Silica Aerogel as a Flatting Agent for Protective Coatings. Industrial & Engineering Chemistry. 1941;33:1169-73. [CrossRef]
- Rivas Murillo JS, Bachlechner ME, Campo FA, Barbero EJ. Structure and mechanical properties of silica aerogels and xerogels modeled by molecular dynamics simulation. Journal of Non-Crystalline Solids. 2010;356:1325-31. [CrossRef]
- Patil SP, Rege A, Sagardas, Itskov M, Markert B. Mechanics of Nanostructured Porous Silica Aerogel Resulting from Molecular Dynamics Simulations. The Journal of Physical Chemistry B. 2017;121:5660-8. [CrossRef]
- Du Y, Zhang X, Wang J, Liu Z, Zhang K, Ji X, et al. Reaction-Spun Transparent Silica Aerogel Fibers. ACS Nano. 2020;14:11919-28. [CrossRef]
- White, JF. Silica Aerogel Effect of Variables on Its Thermal Conductivity. Industrial & Engineering Chemistry. 1939;31:827-31. [CrossRef]
- Johnson MFL, Ries HE. Effect of Wetting on the Nitrogen Adsorption—Desorption Isotherm of a Silica Aerogel. Journal of the American Chemical Society. 1950;72:4289-. [CrossRef]
- Kistler, SS. The Relation between Heat Conductivity and Structure in Silica Aerogel. The Journal of Physical Chemistry. 1935;39:79-86. [CrossRef]
- Kistler SS, Caldwell AG. Thermal Conductivity of Silica Aërogel. Industrial & Engineering Chemistry. 1934;26:658-62. [CrossRef]
- An L, Petit D, Di Luigi M, Sheng A, Huang Y, Hu Y, et al. Reflective Paint Consisting of Mesoporous Silica Aerogel and Titania Nanoparticles for Thermal Management. ACS Applied Nano Materials. 2021;4:6357-63. [CrossRef]
- Malfait WJ, Jurányi F, Zhao S, Arreguin SA, Koebel MM. Dynamics of Silica Aerogel’s Hydrophobic Groups: A Quasielastic Neutron Scattering Study. The Journal of Physical Chemistry C. 2017;121:20335-44. [CrossRef]
- Kehrle J, Purkait TK, Kaiser S, Raftopoulos KN, Winnacker M, Ludwig T, et al. Superhydrophobic Silicon Nanocrystal–Silica Aerogel Hybrid Materials: Synthesis, Properties, and Sensing Application. Langmuir. 2018;34:4888-96. [CrossRef]
- Aghajamali M, Iqbal M, Purkait TK, Hadidi L, Sinelnikov R, Veinot JGC. Synthesis and Properties of Luminescent Silicon Nanocrystal/Silica Aerogel Hybrid Materials. Chemistry of Materials. 2016;28:3877-86. [CrossRef]
- Rajanna SK, Kumar D, Vinjamur M, Mukhopadhyay M. Silica Aerogel Microparticles from Rice Husk Ash for Drug Delivery. Industrial & Engineering Chemistry Research. 2015;54:949-56. [CrossRef]
- Sui R, Rizkalla AS, Charpentier PA. Synthesis and Formation of Silica Aerogel Particles By a Novel Sol−Gel Route in Supercritical Carbon Dioxide. The Journal of Physical Chemistry B. 2004;108:11886-92. [CrossRef]
- Chao X, Jun S, Bin Z. Ultralow density silica aerogels prepared with PEDS. Journal of Non-Crystalline Solids. 2009;355:492-5. [CrossRef]
- Saquing CD, Cheng T-T, Aindow M, Erkey C. Preparation of Platinum/Carbon Aerogel Nanocomposites Using a Supercritical Deposition Method. The Journal of Physical Chemistry B. 2004;108:7716-22. [CrossRef]
- Worsley MA, Satcher JH, Baumann TF. Synthesis and Characterization of Monolithic Carbon Aerogel Nanocomposites Containing Double-Walled Carbon Nanotubes. Langmuir. 2008;24:9763-6. [CrossRef]
- Zhao B, Huang H, Jiang P, Zhao H, Huang X, Shen P, et al. Flexible Counter Electrodes Based on Mesoporous Carbon Aerogel for High-Performance Dye-Sensitized Solar Cells. The Journal of Physical Chemistry C. 2011;115:22615-21. [CrossRef]
- Yang K-L, Ying T-Y, Yiacoumi S, Tsouris C, Vittoratos ES. Electrosorption of Ions from Aqueous Solutions by Carbon Aerogel: An Electrical Double-Layer Model. Langmuir. 2001;17:1961-9. [CrossRef]
- Dong J, Zeng J, Wang B, Cheng Z, Xu J, Gao W, et al. Mechanically Flexible Carbon Aerogel with Wavy Layers and Springboard Elastic Supporting Structure for Selective Oil/Organic Solvent Recovery. ACS Applied Materials & Interfaces. 2021;13:15910-24. [CrossRef]
- Zhang Y, Musselman IH, Ferraris JP, Balkus KJ. Gas Permeability Properties of Mixed-Matrix Matrimid Membranes Containing a Carbon Aerogel: A Material with Both Micropores and Mesopores. Industrial & Engineering Chemistry Research. 2008;47:2794-802. [CrossRef]
- Zhuo H, Hu Y, Chen Z, Peng X, Lai H, Liu L, et al. Linking Renewable Cellulose Nanocrystal into Lightweight and Highly Elastic Carbon Aerogel. ACS Sustainable Chemistry & Engineering. 2020;8:11921-9. [CrossRef]
- Lai H, Zhuo H, Hu Y, Shi G, Chen Z, Zhong L, et al. Anisotropic Carbon Aerogel from Cellulose Nanofibers Featuring Highly Effective Compression Stress Transfer and Pressure Sensing. ACS Sustainable Chemistry & Engineering. 2021;9:9761-9. [CrossRef]
- Li Y-Q, Samad YA, Polychronopoulou K, Alhassan SM, Liao K. Carbon Aerogel from Winter Melon for Highly Efficient and Recyclable Oils and Organic Solvents Absorption. ACS Sustainable Chemistry & Engineering. 2014;2:1492-7. [CrossRef]
- Hu Y, Zhuo H, Chen Z, Wu K, Luo Q, Liu Q, et al. Superelastic Carbon Aerogel with Ultrahigh and Wide-Range Linear Sensitivity. ACS Applied Materials & Interfaces. 2018;10:40641-50. [CrossRef]
- Zuo L, Zhang Y, Zhang L, Miao Y-E, Fan W, Liu T. Polymer/Carbon-Based Hybrid Aerogels: Preparation, Properties and Applications. Materials. 2015;8. [CrossRef]
- Li X, Dong G, Liu Z, Zhang X. Polyimide Aerogel Fibers with Superior Flame Resistance, Strength, Hydrophobicity, and Flexibility Made via a Universal Sol–Gel Confined Transition Strategy. ACS Nano. 2021;15:4759-68. [CrossRef]
- Meador MAB, Malow EJ, Silva R, Wright S, Quade D, Vivod SL, et al. Mechanically Strong, Flexible Polyimide Aerogels Cross-Linked with Aromatic Triamine. ACS Applied Materials & Interfaces. 2012;4:536-44. [CrossRef]
- Li M, Gan F, Dong J, Fang Y, Zhao X, Zhang Q. Facile Preparation of Continuous and Porous Polyimide Aerogel Fibers for Multifunctional Applications. ACS Applied Materials & Interfaces. 2021;13:10416-27. [CrossRef]
- Zhong Y, Kong Y, Zhang J, Chen Y, Li B, Wu X, et al. Preparation and Characterization of Polyimide Aerogels with a Uniform Nanoporous Framework. Langmuir. 2018;34:10529-36. [CrossRef]
- Zhai C, Jana SC. Tuning Porous Networks in Polyimide Aerogels for Airborne Nanoparticle Filtration. ACS Applied Materials & Interfaces. 2017;9:30074-82. [CrossRef]
- Meador MAB, Wright S, Sandberg A, Nguyen BN, Van Keuls FW, Mueller CH, et al. Low Dielectric Polyimide Aerogels As Substrates for Lightweight Patch Antennas. ACS Applied Materials & Interfaces. 2012;4:6346-53. [CrossRef]
- Guo H, Dewey OS, McCorkle LS, Meador MAB, Pasquali M. Polyimide Aerogels as Lightweight Dielectric Insulators for Carbon Nanotube Cables. ACS Applied Polymer Materials. 2019;1:1680-8. [CrossRef]
- Vivod SL, Meador MAB, Pugh C, Wilkosz M, Calomino K, McCorkle L. Toward Improved Optical Transparency of Polyimide Aerogels. ACS Applied Materials & Interfaces. 2020;12:8622-33. [CrossRef]
- Feng J, Wang X, Jiang Y, Du D, Feng J. Study on Thermal Conductivities of Aromatic Polyimide Aerogels. ACS Applied Materials & Interfaces. 2016;8:12992-6. [CrossRef]
- Hou X, Zhang R, Fang D. Polyimide Aerogel Membranes with Adjustable Transparency and High Flexibility for Highly Efficient Solar Thermal Collection. ACS Sustainable Chemistry & Engineering. 2021;9:7638-48. [CrossRef]
- Jin C, Kulkarni A, Teo N, Jana SC. Fabrication of Pill-Shaped Polyimide Aerogel Particles Using Microfluidic Flows. Industrial & Engineering Chemistry Research. 2021;60:361-70. [CrossRef]
- Teo N, Jana SC. Surfactant-Free Process for the Fabrication of Polyimide Aerogel Microparticles. Langmuir. 2019;35:2303-12. [CrossRef]
- Smith TM, Williams MK, Fesmire JE, Sass JP, Weiser ES. Fire and Engineering Properties of Polyimide-Aerogel Hybrid Foam Composites for Advanced Applications. Fire and Polymers V: American Chemical Society; 2009. p. 148-73. [CrossRef]
- Guo H, Meador MAB, McCorkle L, Quade DJ, Guo J, Hamilton B, et al. Tailoring Properties of Cross-Linked Polyimide Aerogels for Better Moisture Resistance, Flexibility, and Strength. ACS Applied Materials & Interfaces. 2012;4:5422-9. [CrossRef]
- Shen Y, Wang L, Liu F, Liu H, Li D, Liu Q, et al. Solvent Vapor Strengthened Polyimide Nanofiber-Based Aerogels with High Resilience and Controllable Porous Structure. ACS Applied Materials & Interfaces. 2020;12:53104-14. [CrossRef]
- Paraskevopoulou P, Chriti D, Raptopoulos G, Anyfantis GC. Synthetic Polymer Aerogels in Particulate Form. Materials. 2019;12. [CrossRef]
- Leventis N, Chidambareswarapattar C, Bang A, Sotiriou-Leventis C. Cocoon-in-Web-Like Superhydrophobic Aerogels from Hydrophilic Polyurea and Use in Environmental Remediation. ACS Applied Materials & Interfaces. 2014;6:6872-82. [CrossRef]
- Shinko A, Jana SC, Meador MA. Crosslinked polyurea aerogels with controlled porosity. RSC advances. 2015;5:105329-38. [CrossRef]
- Chidambareswarapattar C, McCarver PM, Luo H, Lu H, Sotiriou-Leventis C, Leventis N. Fractal Multiscale Nanoporous Polyurethanes: Flexible to Extremely Rigid Aerogels from Multifunctional Small Molecules. Chemistry of Materials. 2013;25:3205-24. [CrossRef]
- Donthula S, Mandal C, Leventis T, Schisler J, Saeed AM, Sotiriou-Leventis C, et al. Shape Memory Superelastic Poly(isocyanurate-urethane) Aerogels (PIR-PUR) for Deployable Panels and Biomimetic Applications. Chemistry of Materials. 2017;29:4461-77. [CrossRef]
- Yin W, Lu H, Leventis N, Rubenstein DA. Characterization of the Biocompatibility and Mechanical Properties of Polyurea Organic Aerogels with the Vascular System: Potential as a Blood Implantable Material. International Journal of Polymeric Materials and Polymeric Biomaterials. 2013;62:109-18. [CrossRef]
- Bang A, Buback C, Sotiriou-Leventis C, Leventis N. Flexible Aerogels from Hyperbranched Polyurethanes: Probing the Role of Molecular Rigidity with Poly(Urethane Acrylates) Versus Poly(Urethane Norbornenes). Chemistry of Materials. 2014;26:6979-93. [CrossRef]
- Mawhinney K, Jana SC. Design Of Emulsion-Templated Mesoporous–Macroporous Polyurea Gels and Aerogels. ACS Applied Polymer Materials. 2019;1:3115-29. [CrossRef]
- Weigold L, Mohite DP, Mahadik-Khanolkar S, Leventis N, Reichenauer G. Correlation of microstructure and thermal conductivity in nanoporous solids: The case of polyurea aerogels synthesized from an aliphatic tri-isocyanate and water. Journal of Non-Crystalline Solids. 2013;368:105-11. [CrossRef]
- Lee JK, Gould GL, Rhine W. Polyurea based aerogel for a high performance thermal insulation material. Journal of Sol-Gel Science and Technology. 2009;49:209-20. [CrossRef]
- Malakooti S, Churu HG, Lee A, Xu T, Luo H, Xiang N, et al. Sound insulation properties in low-density, mechanically strong and ductile nanoporous polyurea aerogels. Journal of Non-Crystalline Solids. 2017;476:36-45. [CrossRef]
- Leventis N, Sotiriou-Leventis C, Chandrasekaran N, Mulik S, Larimore ZJ, Lu H, et al. Multifunctional Polyurea Aerogels from Isocyanates and Water. A Structure−Property Case Study. Chemistry of Materials. 2010;22:6692-710. [CrossRef]
- Malakooti S, ud Doulah ABMS, Ren Y, Kulkarni VN, Soni RU, Edlabadkar VA, et al. Meta-Aerogels: Auxetic Shape-Memory Polyurethane Aerogels. ACS Applied Polymer Materials. 2021;3:5727-38. [CrossRef]
- Gurman JL, Baier L, Levin BC. Polystyrenes: A review of the literature on the products of thermal decomposition and toxicity. Fire and Materials. 1987;11:109-30. [CrossRef]
- Baugh LS, Schulz DN. Discovery of Syndiotactic Polystyrene: Its Synthesis and Impact. Macromolecules. 2020;53:3627-31. [CrossRef]
- Daniel C, Sannino D, Guerra G. Syndiotactic Polystyrene Aerogels: Adsorption in Amorphous Pores and Absorption in Crystalline Nanocavities. Chemistry of Materials. 2008;20:577-82. [CrossRef]
- Daniel C, Guerra G. Crystalline Organization in Syndiotactic Polystyrene Gels and Aerogels. Macromolecular Symposia. 2005;222:247-52. [CrossRef]
- D’Aniello C, Daniel C, Guerra G. ε Form Gels and Aerogels of Syndiotactic Polystyrene. Macromolecules. 2015;48:1187-93. [CrossRef]
- Daniel C, Giudice S, Guerra G. Syndiotatic Polystyrene Aerogels with β, γ, and ε Crystalline Phases. Chemistry of Materials. 2009;21:1028-34. [CrossRef]
- Wang X, Jana SC. Tailoring of Morphology and Surface Properties of Syndiotactic Polystyrene Aerogels. Langmuir. 2013;29:5589-98. [CrossRef]
- Joseph AM, Nagendra B, Shaiju P, Surendran KP, Gowd EB. Aerogels of hierarchically porous syndiotactic polystyrene with a dielectric constant near to air. Journal of Materials Chemistry C. 2018;6:360-8. [CrossRef]
- Daniel C, Guerra G. Structure and Sorption Properties of Syndiotactic Polystyrene Aerogels. Contemporary Science of Polymeric Materials: American Chemical Society; 2010. p. 131-47. [CrossRef]
- Longo S, Vitillo JG, Daniel C, Guerra G. Monolithic Aerogels Based on Poly(2,6-diphenyl-1,4-phenylene oxide) and Syndiotactic Polystyrene. ACS Applied Materials & Interfaces. 2013;5:5493-9. [CrossRef]
- Kulkarni A, Jana SC. Surfactant-free syndiotactic polystyrene aerogel foams via Pickering emulsion. Polymer. 2021;212:123125. [CrossRef]
- Nemoto N, Nagai K, Ono Y, Tanji K, Tanji T, Nakai M, et al. Polystyrene Based Foam Materials for Cryogenic Targets of Fast Ignition Realization Experiment (FIREX). Fusion Science and Technology. 2006;49:695-700. [CrossRef]
- Krishnan VG, Joseph AM, Kuzhichalil Peethambharan S, Gowd EB. Nanoporous Crystalline Aerogels of Syndiotactic Polystyrene: Polymorphism, Dielectric, Thermal, and Acoustic Properties. Macromolecules. 2021;54:10605-15. [CrossRef]
- Nguyen BN, Meador MAB, Tousley ME, Shonkwiler B, McCorkle L, Scheiman DA, et al. Tailoring Elastic Properties of Silica Aerogels Cross-Linked with Polystyrene. ACS Applied Materials & Interfaces. 2009;1:621-30. [CrossRef]
- Nagavally, RR. Composite materials-history, types, fabrication techniques, advantages, and applications. Int J Mech Prod Eng. 2017;5:82-7. Available online: https://www.digitalxplore.org/up_proc/pdf/240-146959633425-30.pdf.
- Hsissou R, Seghiri R, Benzekri Z, Hilali M, Rafik M, Elharfi A. Polymer composite materials: A comprehensive review. Composite Structures. 2021;262:113640. [CrossRef]
- Zu G, Shen J, Wang W, Zou L, Lian Y, Zhang Z. Silica–Titania Composite Aerogel Photocatalysts by Chemical Liquid Deposition of Titania onto Nanoporous Silica Scaffolds. ACS Applied Materials & Interfaces. 2015;7:5400-9. [CrossRef]
- Zheng Z, Zhao Y, Hu J, Wang H. Flexible, Strong, Multifunctional Graphene Oxide/Silica-Based Composite Aerogels via a Double-Cross-Linked Network Approach. ACS Applied Materials & Interfaces. 2020;12:47854-64. [CrossRef]
- Heiligtag FJ, Kränzlin N, Süess MJ, Niederberger M. Anatase–silica composite aerogels: a nanoparticle-based approach. Journal of Sol-Gel Science and Technology. 2014;70:300-6. [CrossRef]
- Li X, Feng J, Jiang Y, Li L, Feng J. Preparation and properties of PAN-based carbon fiber-reinforced SiCO aerogel composites. Ceramics International. 2019;45:17064-72. [CrossRef]
- Wu H, Chen Y, Chen Q, Ding Y, Zhou X, Gao H. Synthesis of flexible aerogel composites reinforced with electrospun nanofibers and microparticles for thermal insulation. J Nanomaterials. 2013;2013:Article 10. [CrossRef]
- Wu X, Zhong Y, Kong Y, Shao G, Cui S, Wang L, et al. Preparation and characterization of C/Al2 O3 composite aerogel with high compressive strength and low thermal conductivity. Journal of Porous Materials. 2015;22:1235-43. [CrossRef]
- Feng J, Zhang C, Feng J, Jiang Y, Zhao N. Carbon Aerogel Composites Prepared by Ambient Drying and Using Oxidized Polyacrylonitrile Fibers as Reinforcements. ACS Applied Materials & Interfaces. 2011;3:4796-803. [CrossRef]
- Kokhanovskaya OA, Razdyakonova GI, Likholobov VA. Method of Synthesis of Composite Materials of Aerogel Type Polyvinyl Alcohol/Technical Carbon. Procedia Engineering. 2015;113:103-7. [CrossRef]
- Wu W, Wang K, Zhan M-S. Preparation and Performance of Polyimide-Reinforced Clay Aerogel Composites. Industrial & Engineering Chemistry Research. 2012;51:12821-6. [CrossRef]
- Yu Z, Dai T, Yuan S, Zou H, Liu P. Electromagnetic Interference Shielding Performance of Anisotropic Polyimide/Graphene Composite Aerogels. ACS Applied Materials & Interfaces. 2020;12:30990-1001. [CrossRef]
- Y. Song, B. Li, S. Yang, G. Ding, C. Zhang, and X. Xie, “Ultralight boron nitride aerogels via template-assisted chemical vapor deposition,” Sci Rep, vol. 5, no. 1, p. 10337, May 2015. [CrossRef]
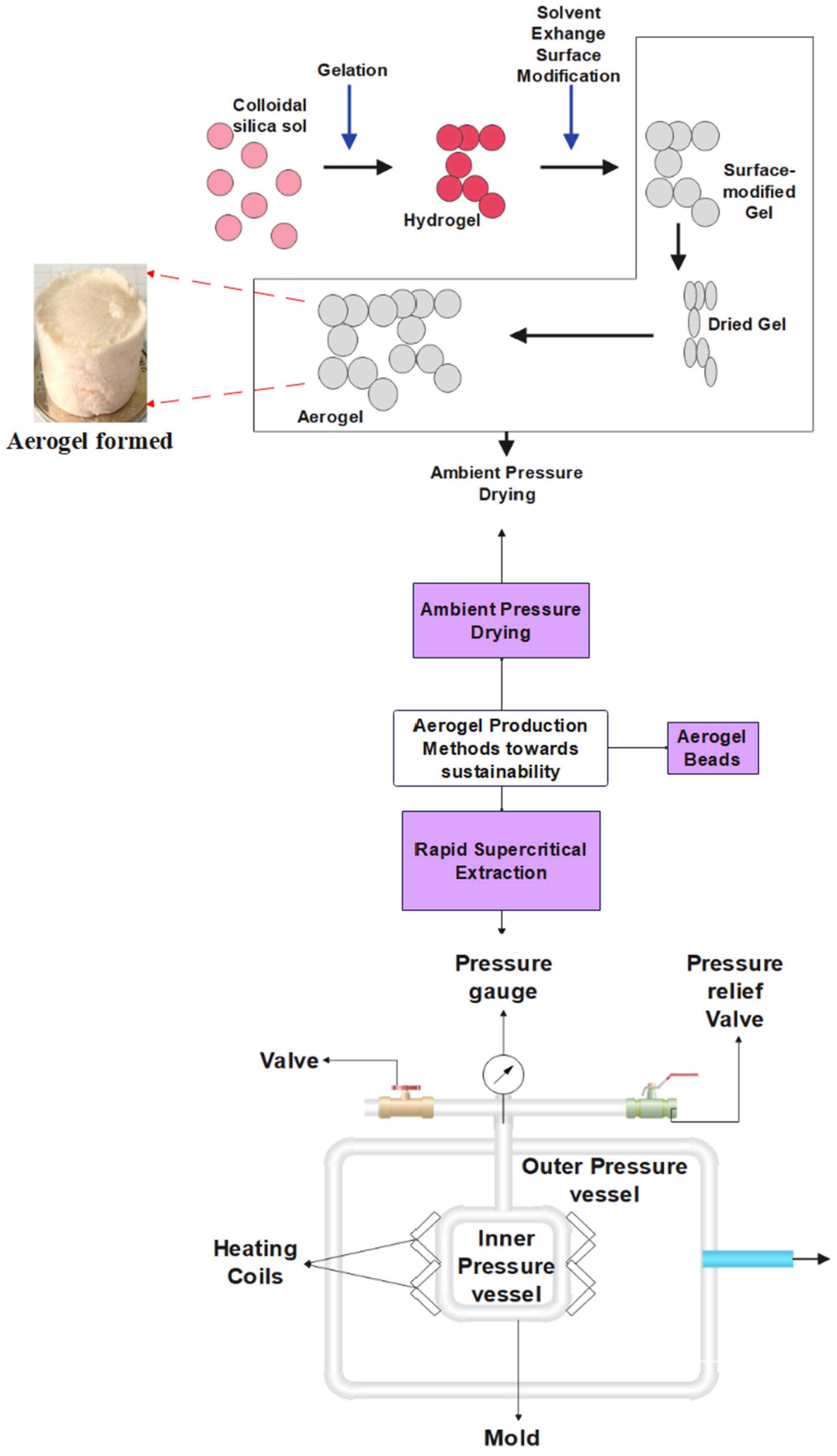

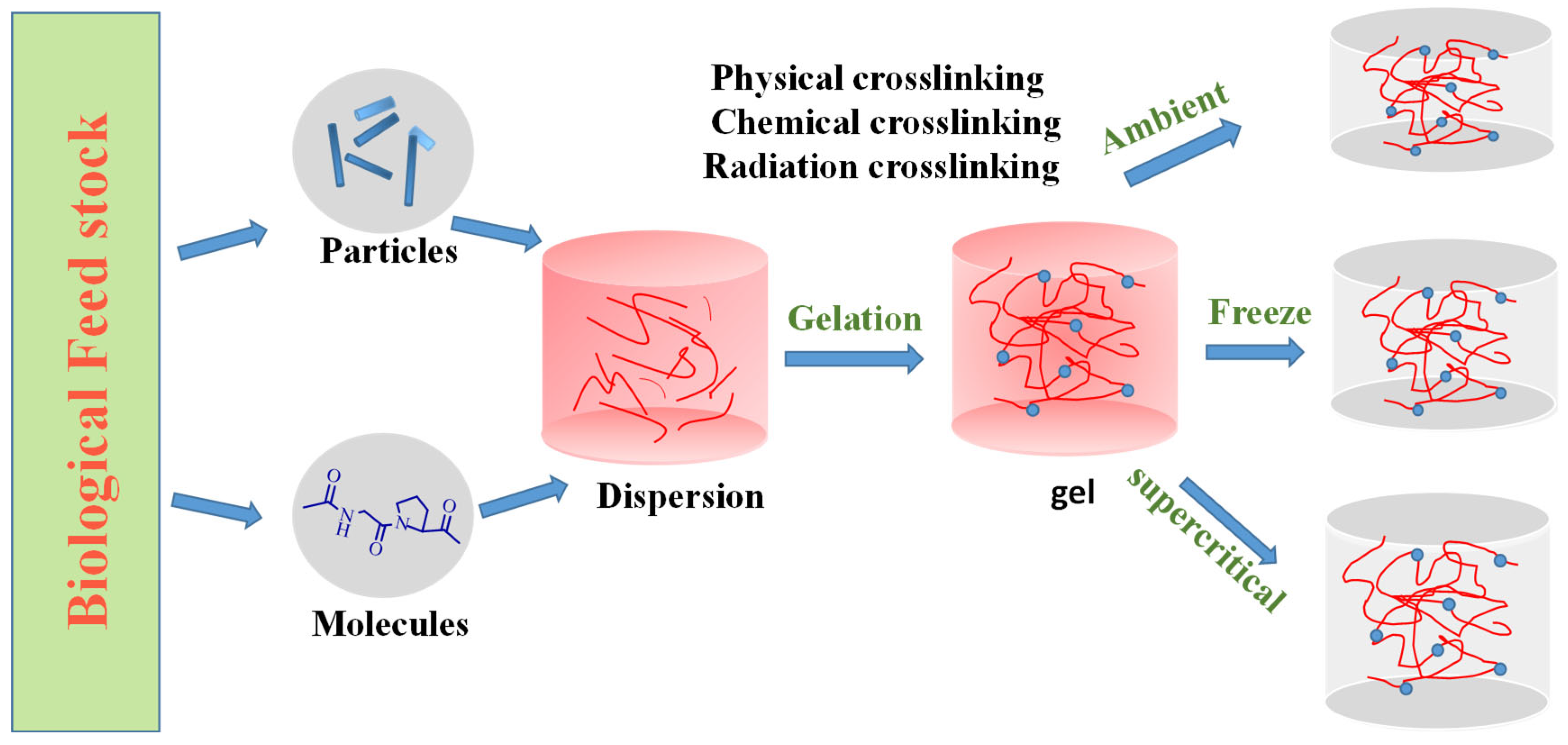
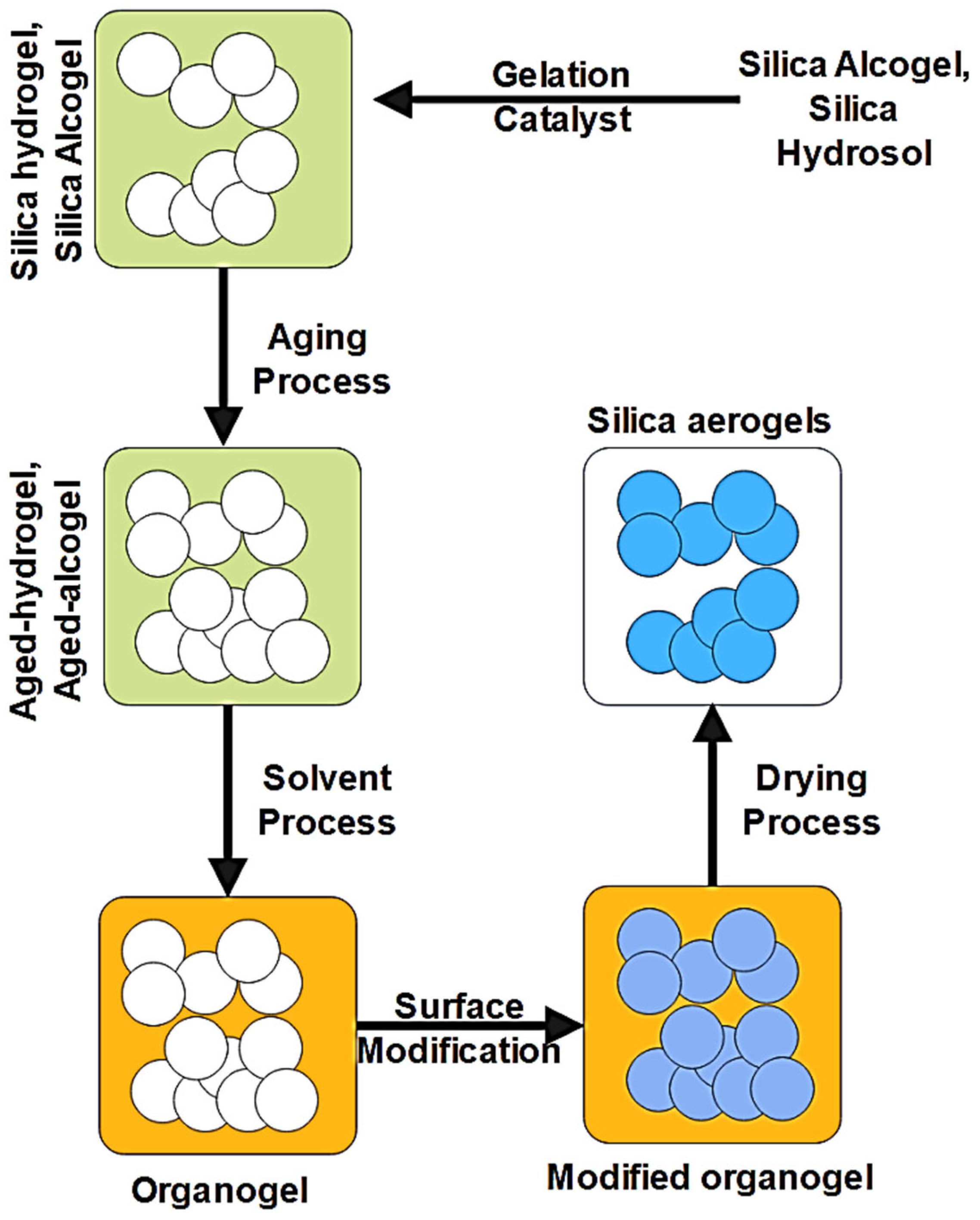
| Aerogels | Manufacturing Technique | Properties | Applications |
|---|---|---|---|
| Silica | Direct expansion of β-cristobalite | 1.Low Thermal conductivity 2.Photoluminescencesuperhydrophobicity |
1. shock absorbers 2. drug carriers 3. Knudsen pumps 4. thermal -superinsulation 5. space technologyautomotive |
| Carbon | Sol-gel polycondensation reaction of resorcinol and formaldehyde | 1.high oil/organic solvent absorption capacity 2.good hydrophobicityelectrically conductive |
1.electrosorption of ions from aqueous solutions 2. rechargeable batteries3.supercapacitors4. broadband nonreflective materials |
| Polyimides | Sol-gel confined transition | 1. good mechanical properties 2. superior hydrophobicity 3. great flexibility low thermal conductivity |
1.solar collectors 2. sophisticatedoptical elements 3. fire resistance 4. Thermal Insulation |
| Polyurea | Sol-gel technique | 1. hydrophobic characteristics 2. Super-elasticity low thermal conductivities |
1. tissue scaffolds 2. shape memory aerogels |
| Polystyrene | 1. Solvent exchange followed by freeze-drying 2. Pickering emulsion |
1. highly hydrophobic 2. oleophilic properties high sorption capacity |
Oil-water separation gas storage |
| Materials | Composite Aerogel | Method of synthesis | Characteristics |
|---|---|---|---|
| Silica-Titania | Chemical liquid deposition of titania onto silica | Photocatalytic property | |
| Graphene oxide-Silica | In-situ-Sol -gel reaction | Stable Piezo- resistive behavior | |
| Silica | Mechanical Property | ||
| anatase–silica | co-gelation of nanoparticles | Better compositional and structural property | |
| C/Al2O3 | sol-gel process | good strength | |
| low thermal conductivity | |||
| Carbon | organic fiber-reinforced organic aerogel | co-pyrolysis | Superior property than pure Carbon aerogel |
| polyimide (PI)/graphene | Unidirectional freezing | Anisotropic conductivity | |
| EMI shielding | |||
| compression performance | |||
| Graphene | graphene/ZIF-8 | two-step reduction procedure and a layer-by-layer assembly method | high CO2 uptake capacity |
| variable mechanical robustness |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





