The HB thermodynamic formalism allows us to define a probability density see (
10) which is related to the microcanonical one but it is "projected" on the configuration space. Instead of prescribing the interaction between the elementary units of the system, all the information about the system is encoded in its potential energy
depending on multiple parameters and the energy
. So we are using a formalism, which loosely speaking is in between statistical mechanics and catastrophe theory, and a probability density, which is not an exponential family. Nevertheless we still have, see (
16) and (
32) that for multidimensional HB thermodynamics –compare with (
38)–
We want to investigate whether the divergence of elements of the Fisher matrix for particular values of parameters can detect qualitative changes in the dynamics in mechanical systems described by potential energy .
5.1. Harmonic Potential Energy
Let us consider the simplest potential energy with no external parameters
with
. This energy represents
d independent one-dimensional harmonic oscillators. Then
and
, the ball of radius
in
. Let us compute
so that
where
is the Gamma function and the entropy function are
By direct computation we get the temperature
and the specific heat can be computed from
a result which holds also in Boltzmann Gibbs statistical mechanics (see [
19]). The one dimensional Fisher matrix, also called Fisher information is, see (
33)
where the probability density (
29) is
and
thereby obtaining (note that the average value exists for
)
We see that diverges for , that is, when the system energy e tends to the minimum of the potential energy u.
Remark. This quadratic potential energy system is the prototype of the description of a mechanical system in the vicinity of a minimum of the potential energy. By a translation of the reference frame we can suppose that the minimum is in
and that
so that using a Taylor expansion we have
and
where
and
are the smallest and greatest (real) eigenvalues of the symmetric matrix
. So we can conclude that the Fisher information diverges when the energy
e approaches the minimum of the potential energy from above. This can be interpreted by recalling that the Fisher information measures the change in the "shape" of the probability distribution
with respect to a change in the parameter
e; when the energy becomes equal to the minimum of the potential energy, the probability density becomes a Dirac delta concentrated at the minimum
. We can say that the divergence of the Fisher information
at
corresponds to the qualitative "phase transition" between a point orbit and a set of
d closed orbits for the
d oscillators when
.
5.2. The Elastic Chain
The following example shows that for a
n-dimensional mechanical systems with convex potential energy, HB thermodynamics gives a sound description of the system’s behavior (see also [
7]).
Let us consider a chain of
point particles of mass
m linked by linearly elastic springs and constrained to move on the real axis. Suppose that particle 0 is fixed in the origin and that the coordinate of the
n-th particle is
so that the length
of the chain is a controlled parameter
corresponding to the equilibrium length of the
n springs. Let the elongation of the spring
be written as
. The length constraint is
Due to the length constraint the chain potential energy of the resulting
dimensional system can be written as
where
. Therefore the region
is
i.e.
where
is the ball in
of radius
a,
and
L is the
dimensional hyperplane
. We compute the entropy
by setting
and (here
)
By standard computation on radial function we get
Let us compute the temperature of this elongated chain using
which coincides with the kinetic energy
per degree of freedom. Let us compute the pressure
P corresponding to the controlled parameter
which is the elongation on the elastic chain using
. We have
which corresponds to our physical intuition of the reaction force exerted by the chain on its controlled end. The specific heat
is
Remark. Note that the elastic chain model fails to define a statistical model because conditions 1) and 2) in Definition 1 are not met. Indeed concerning condition 2) we have
so the two score vectors
and
are not independent functions on
. Therefore we can not compute the Fisher matrix for this mechanical system. If we consider only the energy parameter
e, the system is similar to the previous example 1.
5.3. Two-Body System
In this example we consider an isolated system of two points of equal mass
m subject to gravitational forces. If
,
,
denote the positions of the two points, the potential energy is
, where
can be considered as a parameter. A system of points interacting via gravity requires a specific description in Statistical Mechanics due to the long-range nature of the force which prevents these systems to display the extensive character of the total energy. Also, it is known that gravitating systems exhibit the phenomenon of negative specific heat [
24], a possibility which is excluded in the canonical description, see (
36). Therefore, the correct statistical ensemble to adopt is the microcanonical one, see [
25]. In [
25] the above two body system is modified to construct a toy model which exhibits all the features of a many-body self-gravitating system. This is obtained by imposing a short range cutoff on the particles inter-distance
, which behaves as hard spheres of radius
, and a long range cutoff
. This modified system display a phase transition between phases of positive and negative specific heat when the system energy is varied. We want to discuss this mechanical system using HB thermodynamics. See also [
5] for a different analysis of a two body system with HB thermodynamics. Therefore we compute the probability density (
10) and the Fisher information for this system and discuss its ability to describe phase transitions. Using (12) we have for
,
and from (
10) with
,
,
To compute the last integral it is useful to perform the change of variables
where
is the inter-particle vector. We have
and
where
therefore, by using the change of variable and Fubini theorem, we can write
The integral in
is infinite unless we assume that the particle
x is confined in a bounded region of volume
V. Therefore we can write
and, since we deal with a function radial in
s we have that
is finite and
The probability density (
10) is thus factorized into the product of two densities
showing that the positions of the two particles are independent random variables. Therefore we can forget about the
particle and perform all the computations in the
s variable.
If we let the energy tend to zero, so that the inter-particle distance
s can be arbitrarily large, we make
Z in (
40), hence the microcanonical entropy of the system
, infinite. The HB or volume entropy is
and it is easy to see that
is infinite if we let the inter-particle distance
s go to zero, so the entropy
S is infinite when
stend to zero or to infinity, while
is infinite only in the latter case.
Therefore, it is necessary to assume, as in [
25], that there are two cutoffs
, usually with
, so that the two body system is confined in a bounded region of space. Moreover the system displays two energy scales
Also, it is stipulated in [
25] that if the energy is in the range
, the integration in the
s variable is performed between extrema
a and
, while for
the integration is between fixed extrema
a and
R. Due to the presence of the cutoff
a, the system displays a negative specific heat phase (see again [
25]).
We can now compute the temperature
T, the microcanonical temperature
and the pressure
P using (
21) for our system in the two energy ranges and then juxtapose the plots that have continuous junction at
. See Appendix 1 for computations. We also compute the Fisher information
from (
33). See
Figure 1 for the plot of
T,
and
.
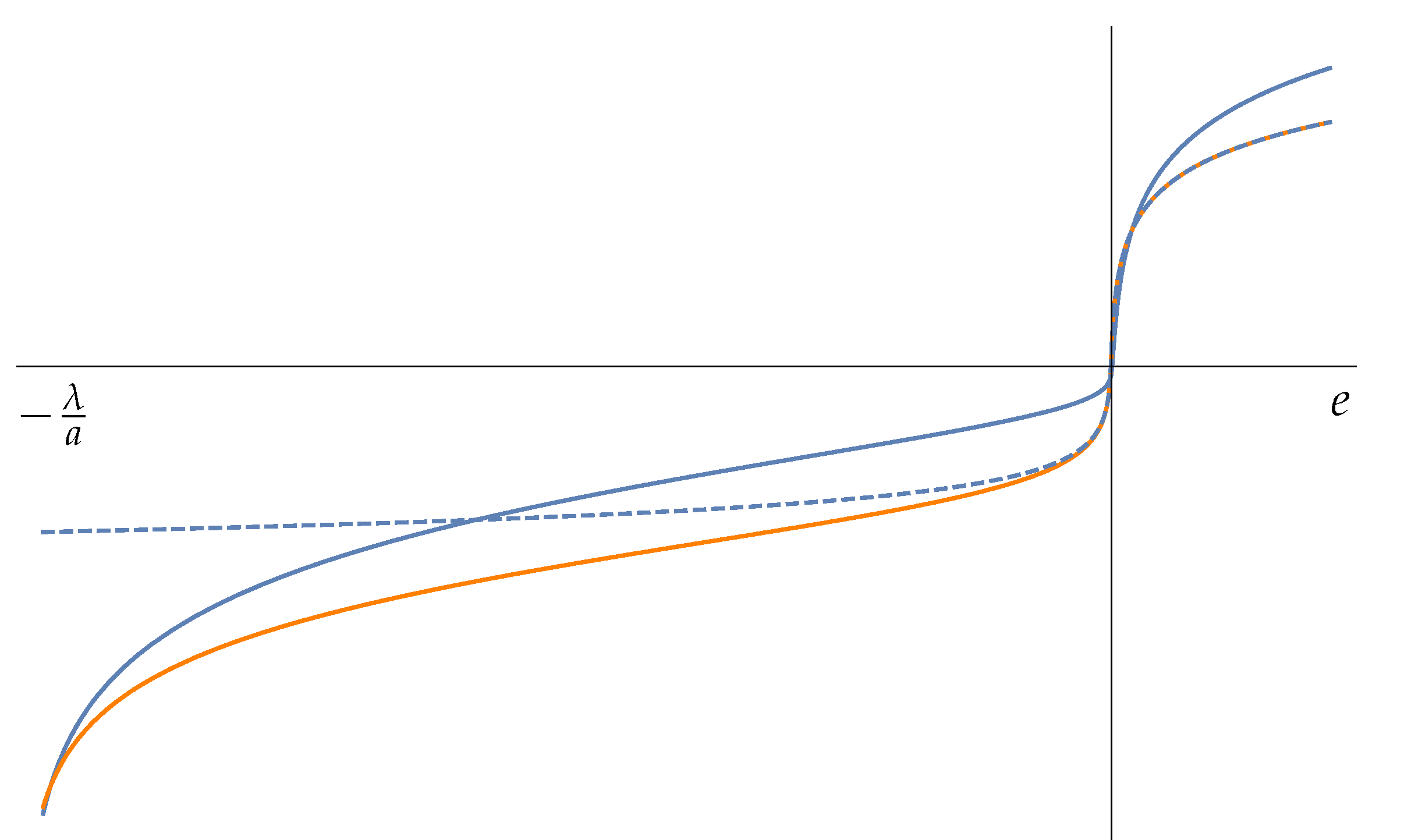
The temperature
(blue curve of
Figure 1) presents a gentle maximum for negative energy (point B) and a sharp minimum (point C) for a positive value of the energy. We see that for
the temperature is zero as in the previous example 1. The temperature curve of this simple mechanical system displays the same features of more complex and realistic models of gravitating many particle systems: a phase (A to B) of positive specific heat followed by a phase (B to C) of
negative specific heat and again a phase of positive specific heat (after C). The microcanonical temperature
(orange curve) has a similar behavior of
T. The points where the two temperature curves cross each other are the points where the specific heat
diverges; see (
24). See [
25] for a physical interpretation of these phases.
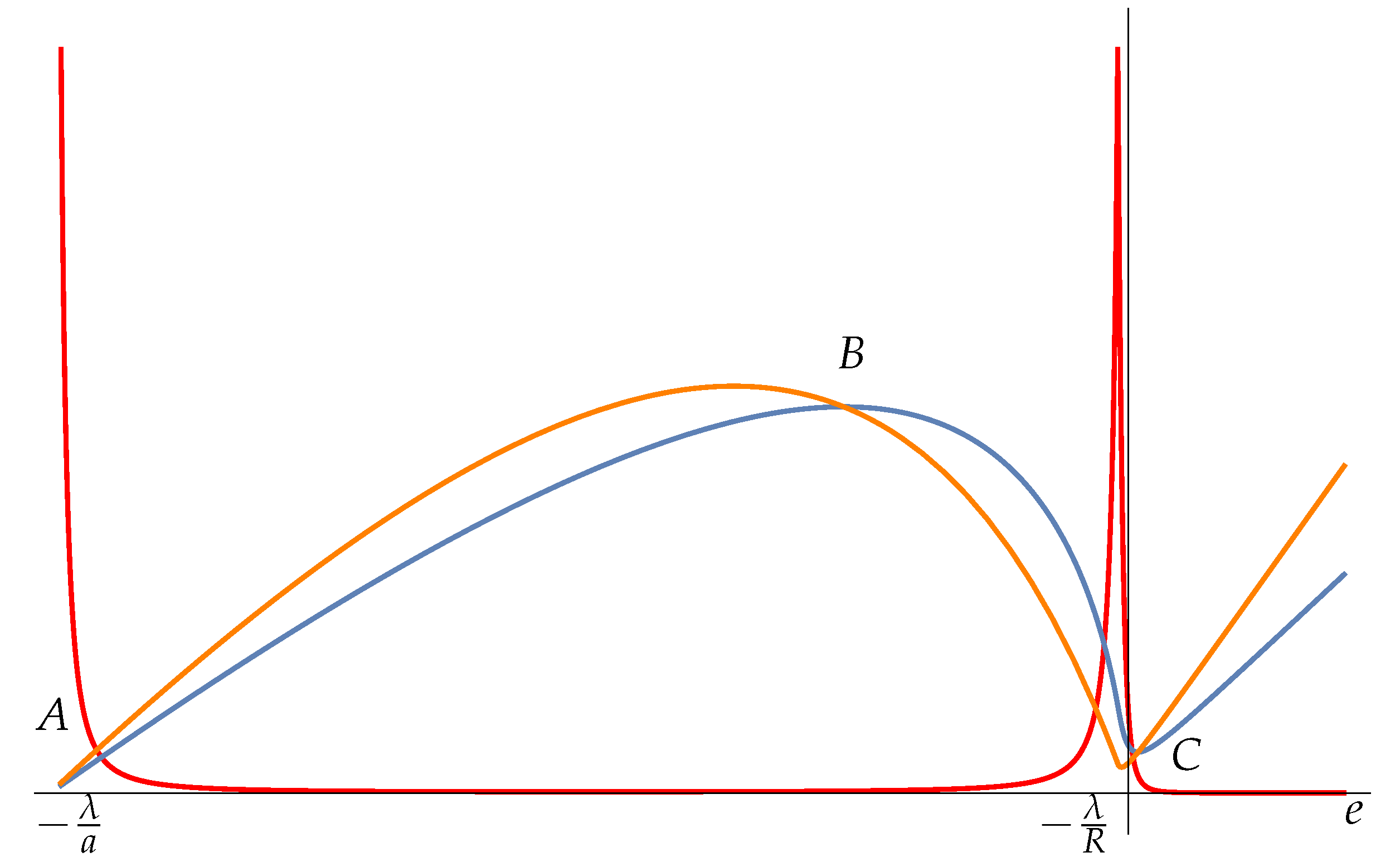
We see that the Fisher information
(red curve of
Figure 1) diverges for
and has a peak for
. The divergence in
is located at the minimum of the potential energy (due to the cutoff at
) and it is similar to the one found in the previous example 1. Here we are concerned with the assessment of the ability of Fisher information to locate the phase transitions at B and C. It seems that the phase transition in B is not detected, and the one in C is not exactly located. This is due to the presence of the cutoffs
(which are essential for the existence of the negative specific heat phase). As far as the phase transition in
B is concerned, the shape of the curve of temperature
T does not change with
a and in the limit
entropy
S becomes infinite. On the other hand, the microcanonical entropy
is defined for
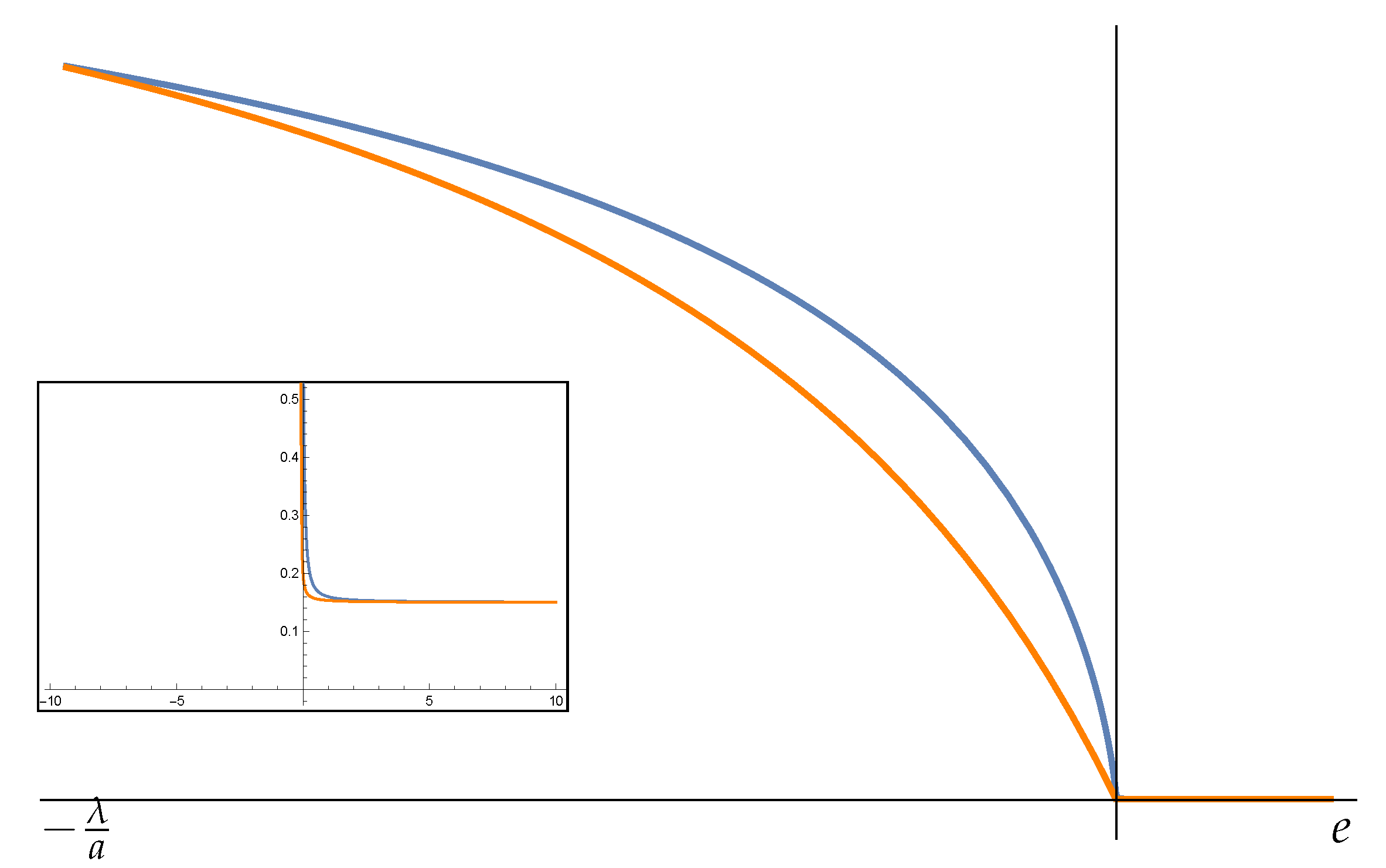
and the curve of the microcanonical temperature computed for
(dashed line of
Figure 2) does not show the phase A to B of positive specific heat. Therefore, for
, the phase transition in B is removed in the microcanonical description of the system, while the remaining one is detected by the divergence of Fisher information.
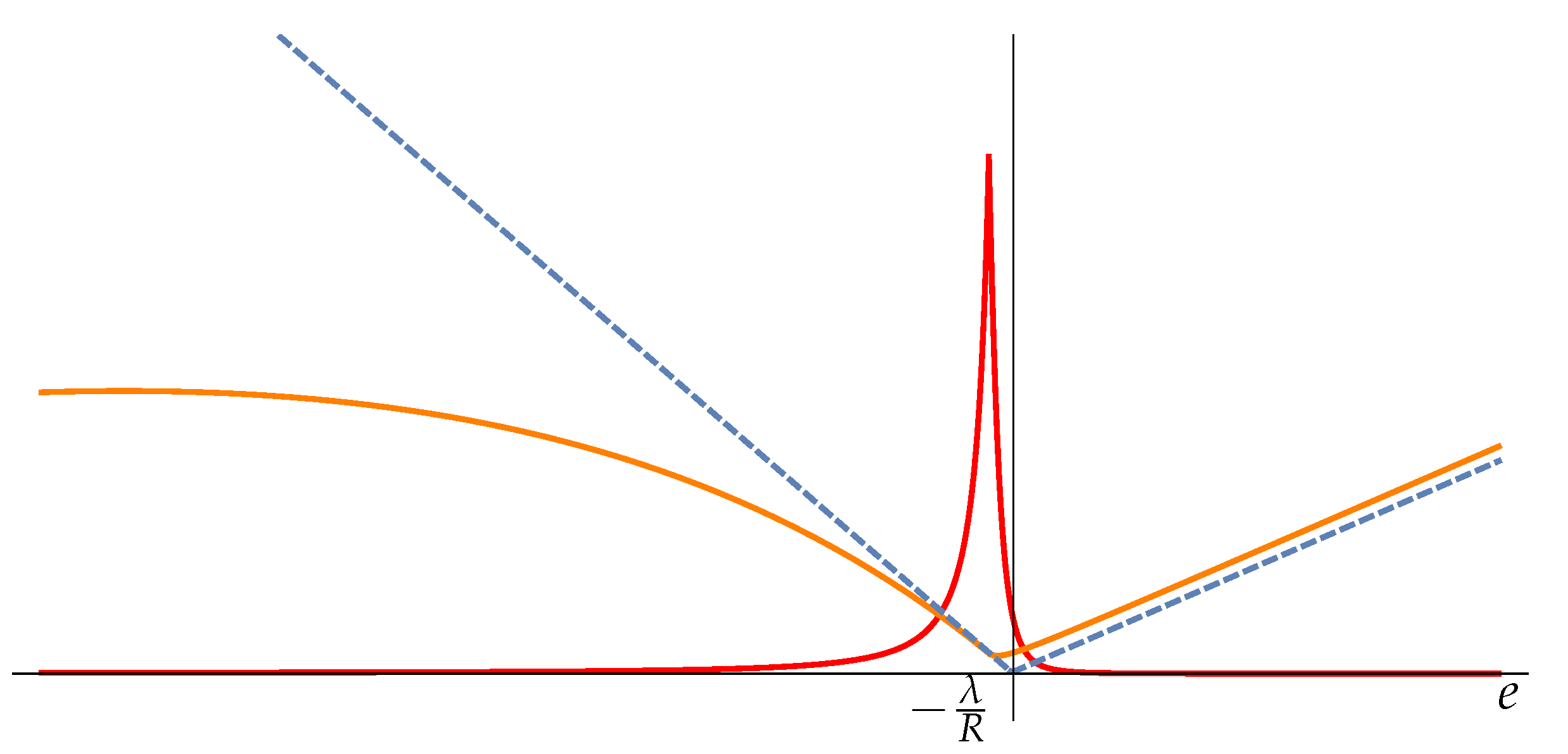
We will show that if the bounds are removed, letting
and
the minimum C and the peak in Fisher information
coincide at
. In fact, the energy corresponding to point C can be determined using the condition
. By computing
at the leading order in
we deduce that the minimum in C in
Figure 1 is located at
The peak of Fisher information is located at
and it height is
In the limit both and tends to 0. So we can say that the divergence of Fisher information correctly detects the phase transition from negative to positive specific heat located at .