Submitted:
01 April 2025
Posted:
02 April 2025
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Materials and Methods
2.1. Raw Materials
2.2. Characterization Techniques
2.3. Mechanical and Durability Testing
2.4. Statistical Analysis
2.5. Physical and Chemical Characterization of Raw Materials
2.6. X-ray Diffraction (XRD) Analysis of Raw Materials
2.7. Particle Size Distribution of Raw Materials
2.8. Soil Plasticity and Thermal Analysis of Gypsum and Soil
2.9. Preparation and Testing of Soil-Cement Formulations
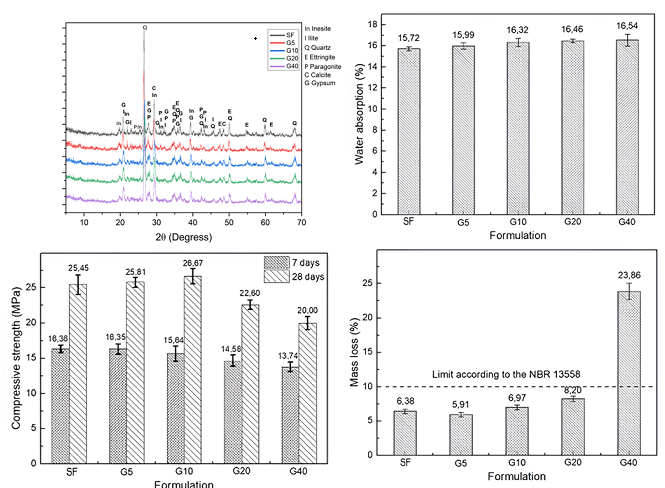
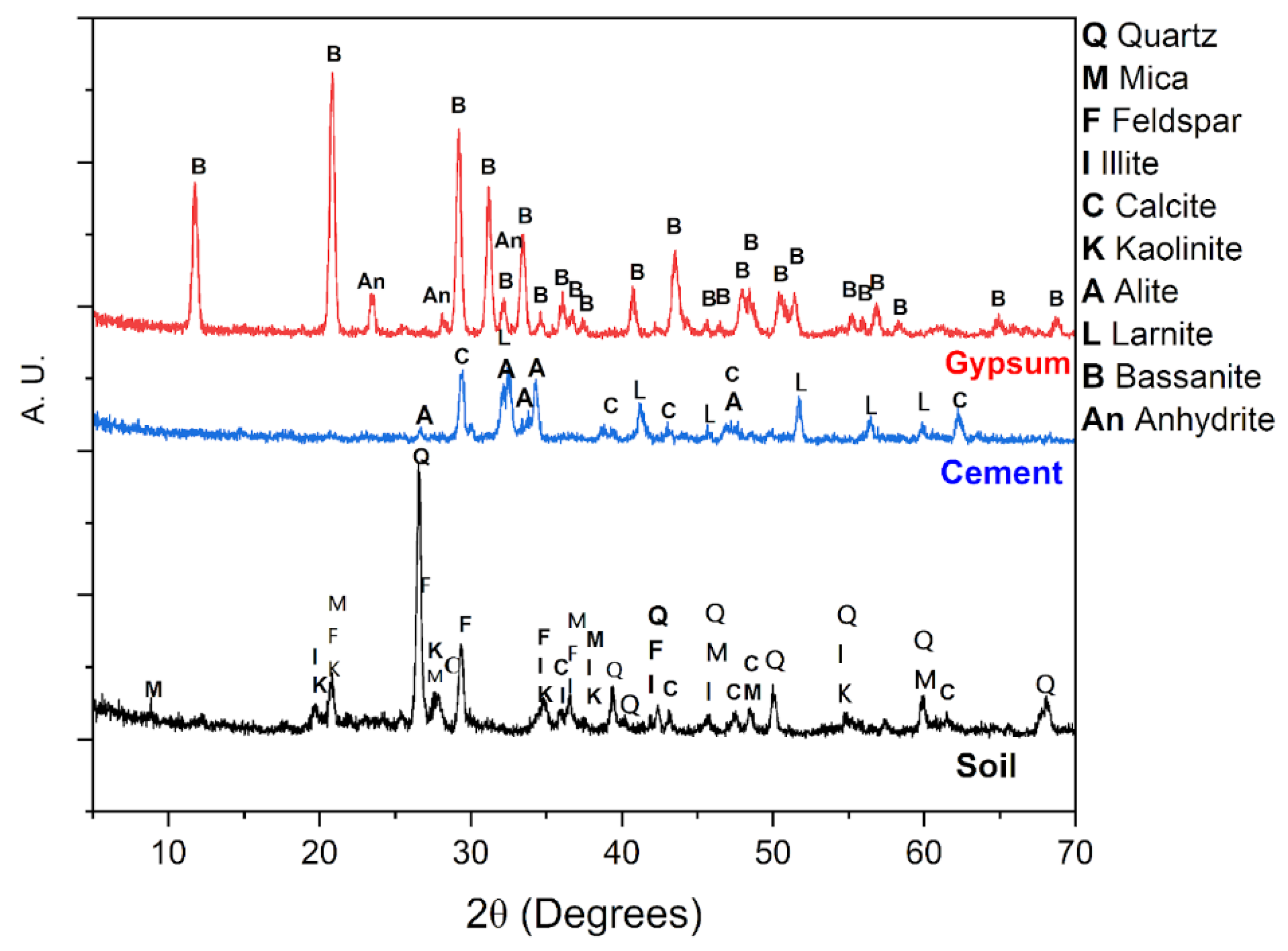
3. Results
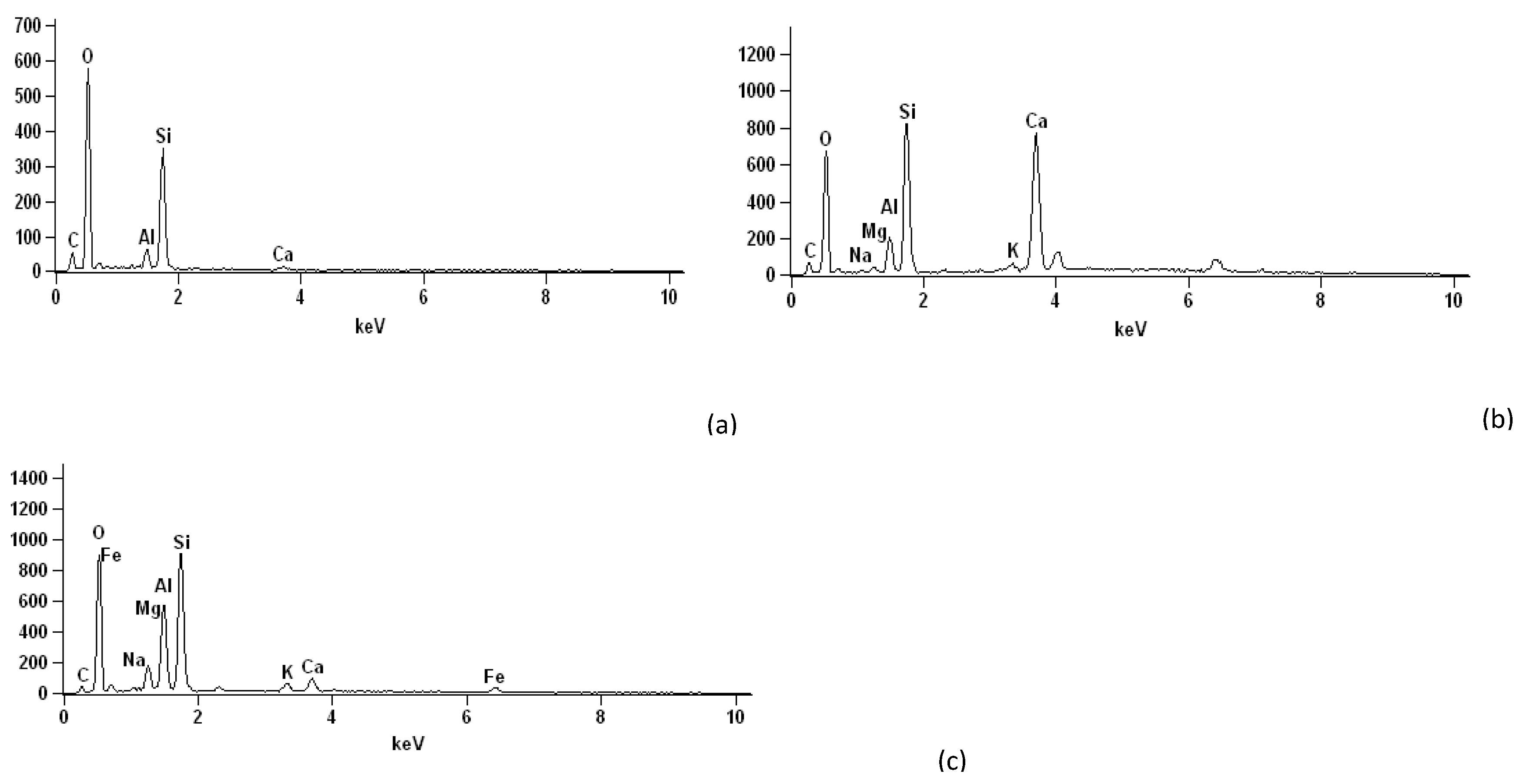
5. Conclusions
Acknowledgments
References
- Mariyam, S.; et al. A framework to support localized solid waste management decision making: Evidence from Qatar. Environ. Dev. 2024, 50, 100986. [Google Scholar] [CrossRef]
- Beccarello, M.; Di Foggia, G. Sustainable Development Goals data-driven local policy: Focus on SDG 11 and SDG 12. Adm. Sci. 2022, 12(4), 167. [Google Scholar] [CrossRef]
- Vilela, A. P.; et al. Technological properties of soil-cement bricks produced with iron ore mining waste. Constr. Build. Mater. 2020, 262, 120883. [Google Scholar] [CrossRef]
- Silva, M. V. V. da; et al. Técnicas de estabilização e reforço sustentável do solo. Rev. FT 2024, 29, 29–30. [Google Scholar] [CrossRef]
- Xiong, W.; et al. Reuse of engineering waste soil and recycled fine aggregate to manufacture eco-friendly unfired clay bricks: Experimental assessment, data-driven modeling and environmental friendliness evaluation. Case Stud. Constr. Mater. 2023, 19, e02608. [Google Scholar] [CrossRef]
- Hashim, A. A.; et al. Improving the mechanical, corrosion resistance, microstructural and environmental performance of recycled aggregate concrete using ceramic waste powder as an alternative to cement. Ceramics 2025, 8. [Google Scholar] [CrossRef]
- Rocha Silva, B.; et al. Soil cement brick production process: Literature review. MOJ Civil Engineering 2023, 7(1), 19–26. [Google Scholar] [CrossRef]
- Leão, A. S.; et al. Is the soil-cement brick an ecological brick? An analysis of the life cycle environmental and energy performance of masonry walls. Sustainability 2022, 14. [Google Scholar] [CrossRef]
- Ângelo, F. A.; Simões, G. F. Tijolos ecoeficientes de barro cru com resíduos sólidos e efluente industrial utilizando tecnologias não convencionais. Ambiente Construído 2023, 23, 1–15. [Google Scholar] [CrossRef]
- Castro, E. D. de; et al. Analysis of the coffee peel application over the soil-cement bricks properties. Coffee Science 2019, 14(1), 12. [Google Scholar] [CrossRef]
- Azevedo, A. R. G.; et al. Assessing the potential of sludge generated by the pulp and paper industry in assembling locking blocks. Journal of Building Engineering 2019, 23, 334–340. [Google Scholar] [CrossRef]
- Araújo, V. D.; et al. Incorporation of tannery waste and sugarcane bagasse ash in soil–cement bricks. Revista AIDIS de Ingeniería y Ciencias Ambientales 2022, 15, 154. [Google Scholar] [CrossRef]
- Ahmed, A.; Ugai, K.; Kamei, T. Laboratory and field evaluations of recycled gypsum as a stabilizer agent in embankment construction. Soils and Foundations 2011, 51(6), 975–990. [Google Scholar] [CrossRef]
- Azevedo, A. C.; et al. Adhesion of gypsum plaster coatings: Experimental evaluation. In Building Pathology and Rehabilitation (pp. 41–66); Springer: 2021. [CrossRef]
- Camarini, G.; Pimentel, L. L.; De Sá, N. H. R. Assessment of the material loss in walls renderings with β-hemihydrate paste. Applied Mechanics and Materials 2011, 71–78, 1242–1245. [Google Scholar] [CrossRef]
- Jiménez-Rivero, A.; García-Navarro, J. Exploring factors influencing post-consumer gypsum recycling and landfilling in the European Union. Resources, Conservation and Recycling 2017, 116, 116–123. [Google Scholar] [CrossRef]
- Antunes, M. L. P.; et al. Utilization of gypsum from construction and demolition waste in Portland cement mortar. Cerâmics 2019, 65 (Suppl 1), 1–6. [Google Scholar] [CrossRef]
- ASTM International. ASTM D6913: Standard test methods for particle-size distribution (gradation) of soils using sieve analysis. West Conshohocken, PA: ASTM International, 2017.
- Departamento Nacional de Estradas de Rodagem (DNER). DNER-ME 093/94: Solos – Determinação da Densidade Real. Rio de Janeiro: DNER, 1994.
- ASTM International. ASTM D4318-18: Standard Test Methods for Liquid Limit, Plastic Limit, and Plasticity Index of Soils. West Conshohocken, PA: ASTM International, 2018.
- ASTM International. ASTM C191: Standard test methods for time of setting of hydraulic cement by Vicat needle. West Conshohocken, PA: ASTM International, 2023.
- ASTM International. ASTM C188: Standard test method for density of hydraulic cement. West Conshohocken, PA: ASTM International, 2022.
- Associação Brasileira de Normas Técnicas. NBR 16372:2015 – Cimento Portland e outros materiais em pó – Determinação da finura pelo método de permeabilidade ao ar (método de Blaine). ABNT, 2015.
- ASTM International. ASTM C109/C109M: Standard test method for compressive strength of hydraulic cement mortars. West Conshohocken, PA: ASTM International, 2023.
- Krejsová, J.; et al. New insight into the phase changes of gypsum. Materials and Structures 2024, 57. [Google Scholar] [CrossRef]
- Associação Brasileira de Normas Técnicas. NBR 8492: Tijolo de solo-cimento — Análise dimensional, determinação da resistência à compressão e da absorção de água — Método de ensaio. ABNT, 2012.
- Associação Brasileira de Normas Técnicas. NBR 13554: Solo-cimento – Ensaio de durabilidade por molhagem e secagem – Método de ensaio. ABNT, 2012.
- Vieira, S. Análise de variância ANOVA (1ª ed.). Editora Atlas, 2006.
- Mitchell, J. K. , & Soga, K. Fundamentals of soil behavior, 3rd ed.; John Wiley & Sons: Hoboken, NJ, USA, 2005. [Google Scholar]
- Chernyshova, N.; et al. Enhancement of fresh properties and performances of the eco-friendly gypsum-cement composite (EGCC). Construction and Building Materials 2020, 260, 120462. [Google Scholar] [CrossRef]
- Alves, M. N. de L. X. de F.; Barros, S. V. A.; de Oliveira, F. N. Physical and chemical characterization of soil for use in ecological bricks. In Uniting Knowledge Integrated Scientific Research For Global Development, Vol. 2; Seven Editora: São Paulo, 2024.
- Costa Gonçalves, L. F. Da; Balestra, C. E. T.; Ramirez Gil, M. A. Evaluation of mechanical, physical and chemical properties of ecological modular soil-alkali activated bricks without Portland cement. Environmental Development 2023, 48, 100932. [Google Scholar] [CrossRef]
- Alcântara, A. C.; Beltrão, M. S.; Oliveira, H. A.; Gimenez, I. F.; Barreto, L. S. Characterization of ceramic tiles prepared from two clays from Sergipe-Brazil. Appl. Clay Sci. 2008, 39, 160–165. [Google Scholar] [CrossRef]
- Boussen, S.; Sghaqier, D.; Chaabani, F.; Jamoussi, B.; Bennour, A. Characteristics and industrial application of the Lower Cretaceous clay deposits (Bouhedma Formation), southeast Tunisia: Potential use for the manufacturing of ceramic tiles and bricks. Applied Clay Science 2016, 123, 210–221. [Google Scholar] [CrossRef]
- Celik, H. Technological characterization and industrial application of two Turkish clays for the ceramic industry. Applied Clay Science 2010, 50, 245–254. [Google Scholar] [CrossRef]
- Egole, C. P. , Medupin, R. O., Nzebuka, G. C., Nnodum, N. A., Ochieze, U. P., Eterigho-Ikelegbe, O., Wilson, U. N., & Yoro, K. O. Quartz and feldspar-blended clay composites for thermal and structural applications. Results in Materials 2024, 23, 100584. [Google Scholar] [CrossRef]
- Sridharan, A. , Sudhakar, M. R., Ramanath, K. P., & Nagaraj, H. B. The role of iron hydroxide in the engineering behaviour of tropical soils. Geotechnics in the African Environment 2022, 7–14. [Google Scholar] [CrossRef]
- Harrison, T. , Jones, M. R., & Lawrence, D. The production of low energy cements. In Lea’s Chemistry of Cement and Concrete (p. 341–361). Elsevier, 2019.
- Reigl, S.; et al. Toward more sustainable hydraulic binders: Controlling calcium sulfate phase selection via specific additives. ACS Sustainable Chemistry & Engineering 2023, 11, 8450–8461. [Google Scholar] [CrossRef]
- Buryanov, A. F.; et al. Research on the influence of gypsum and anhydrite stone impurities on the properties of the binder. In Lecture Notes in Civil Engineering (pp. 138–146). Springer, 2021. [CrossRef]
- Stawski, T. M. et al. Nucleation pathway of calcium sulfate hemihydrate (bassanite) from solution: Implications for calcium sulfates on mars. The Journal of Physical Chemistry C, 2020, 124(15), 8411–8422. [CrossRef]
- Carvalho, J. C. de et al. (Eds.). Solos não saturados no contexto geotécnico. ABMS, 2023.
- Ashour, T. , Korjenic, A., & Korjenic, S. Equilibrium moisture content of earth bricks biocomposites stabilized with cement and gypsum. Cement and Concrete Composites, 2015, 59, 18–25. [Google Scholar] [CrossRef]
- Associação Brasileira de Normas Técnicas. NBR 10833: Fabricação de tijolo e bloco de solo-cimento com utilização de prensa manual ou hidráulica - Procedimento. Rio de Janeiro: ABNT, 2012.
- Rosa, J. M. de S. S., Silva, R. G. P. da, & Lafayette, K. P. V. Sustainable alternative for the production of soil cement bricks. Journal of Management and Sustainability 2023, 13, 45. [Google Scholar] [CrossRef]
- Metzker, S. L. O.; et al. Soil-cement bricks development using polymeric waste. Environmental Science and Pollution Research 2022, 29, 21034–21048. [Google Scholar] [CrossRef]
- Bian, X.; et al. Plasticity role in strength behavior of cement-phosphogypsum stabilized soils. Journal of Rock Mechanics and Geotechnical Engineering 2022, 14, 1977–1988. [Google Scholar] [CrossRef]
- Krause, F.; et al. Reactivity of gypsum-based materials subjected to thermal load: Investigation of reaction mechanisms. Materials 2020, 13, 1427. [Google Scholar] [CrossRef]
- Ritterbach, L. , & Becker, P. Temperature and humidity dependent formation of CaSO4·xH2O (x = 0...2) phases. Global and Planetary Change, 2020, 187, 103132. [Google Scholar] [CrossRef]
- Kyono, A.; et al. Structural evolution of gypsum (CaSO4·2H2O) during thermal dehydration. Journal of Mineralogical and Petrological Sciences 2022, 117. [Google Scholar] [CrossRef]
- Azimi, G. , & Papangelakis, V. G. Mechanism and kinetics of gypsum–anhydrite transformation in aqueous electrolyte solutions. Hydrometallurgy. [CrossRef]
- Voigt, W. , & Freyer, D. Solubility of anhydrite and gypsum at temperatures below 100 °C and the gypsum-anhydrite transition temperature in aqueous solutions: A re-assessment. Frontiers in Nuclear Engineering. [CrossRef]
- Al Disi, Z. A.; et al. Microbially influenced formation of anhydrite at low temperature. The Science of the Total Environment, 2023, 902, 165820. [Google Scholar] [CrossRef] [PubMed]
- Engbrecht, D. C. , & Hirschfeld, D. A. Thermal analysis of calcium sulfate dihydrate sources used to manufacture gypsum wallboard. Thermochimica Acta, 2016, 639, 173–185. [Google Scholar] [CrossRef]
- Lira, M. C. de A., & Neiva, L. S. Investigative study of the potentialities of noble applications for the Brazilian calcium sulfate α-hemihydrate. Pesquisa e Ensino em Ciências Exatas e da Natureza, 2020, 4, 01. [Google Scholar] [CrossRef]
- Vipulanandan, C. , & Mohammed, A. XRD and TGA, swelling and compacted properties of polymer treated sulfate contaminated CL soil. Journal of Testing and Evaluation 2016, 44, 2270–2284. [Google Scholar] [CrossRef]
- Kongkajun, N. , Laitila, E. A., Ineure, P., Prakaypan, W., Cherdhirunkorn, B., & Chakartnarodom, P. Soil-cement bricks produced from local clay brick waste and soft sludge from fiber cement production. Case Studies in Construction Materials, 2020, 13, e00448. [Google Scholar] [CrossRef]
- Leonel, R. F. , Folgueras, M. V., Dalla Valentina, L. V. O., Prim, S. R., Prates, G. A., & Caraschi, J. C. Characterization of soil-cement bricks with incorporation of used foundry sand. Cerâmica 2017, 63, 329–335. [Google Scholar] [CrossRef]
- Edike, U. E. , Ameh, O. J., & Dada, M. O. Performance of polymer bricks produced with plastic waste. Innovative Infrastructure Solutions 2023, 8. [Google Scholar] [CrossRef]
- Kumar, R., Patel, K., & Singh, B. New coal char-based bricks: Effects of curing temperature, humidity, pressing pressure, and addition of superplasticizer on physical, mechanical, and thermal properties. OSTI.gov. 2023. Disponível em: https://www.osti.gov/pages/biblio/2279157.
- Zhao, X., Wang, Y., & Li, J. Hydration characteristics of hybrid cements containing granulated blast furnace slag and fly ash at elevated curing temperatures. Frontiers in Materials. 2022. Disponível em: https://www.frontiersin.org/journals/materials/articles/10.3389/fmats.2022.982568/full.
- Hu, W.; et al. Effects of wetting–drying cycles on the macro and micro properties of the cement-stabilized soil with curing agent. Buildings, 2024, 14. [Google Scholar] [CrossRef]
- Associação Brasileira de Normas Técnicas. 2012. NBR 12025: Solo-cimento – Ensaio de compressão simples de corpos de prova cilíndricos – Método de ensaio. ABNT.
- Associação Brasileira de Normas Técnicas. 2007. NBR 5739: Cimento Portland – Determinação da resistência à compressão. ABNT.
- Associação Brasileira de Normas Técnicas. 2012. NBR 10836: Bloco de solo-cimento sem função estrutural – Análise dimensional, determinação da resistência à compressão e da absorção de água – Método de ensaio. ABNT.
- Macioski, G. , Soto, N. T. A., Medeiros, M. H. F. de, Hoppe Filho, J., Araújo, M. S. de, & Cerri, J. A. 2021. Portlandite consumption by red ceramic waste due to alkali activation reaction. Ambiente Construído 2021, 21, 7–21. [Google Scholar] [CrossRef]
- Wild, S. , & Khatib, J. M. 1997. Portlandite consumption in metakaolin cement pastes and mortars. Cement and Concrete Research 1997, 27, 137–146. [Google Scholar] [CrossRef]
- Kleib, J., Amar, M., Aouad, G., Bourbon, X., Benzerzour, M., & Abriak, N.-E. 2022. The use of Callovo-Oxfordian Argillite as a raw material for Portland cement clinker production. Buildings, 12(9), 1421. [CrossRef]
- Al-Tabbaa, A., Yi, Y. L., Liska, M., & Unluer, C. 2013. Initial investigation into the carbonation of MgO for soil stabilisation. Cement and Concrete Research, 53, 1–9. [CrossRef]
- Moon, D. H., Lee, J.-R., Grubb, D. G., & Park, J.-H. 2010. An assessment of Portland cement, cement kiln dust and Class C fly ash for the immobilization of Zn in contaminated soils. Environmental Earth Sciences, 61(8), 1745–1750. [CrossRef]
- Park, S.-S. et al. 2015. A study on soil cementation and calcite precipitation with clay as a medium. Journal of the Korean Geotechnical Society, 31(12), 17–27. [CrossRef]
- Siqueira, F. B., & Holanda, J. N. F. 2013. Reuse of grits waste for the production of soil–cement bricks. Journal of Environmental Management, 131, 1–6. [CrossRef]
- Amaral, M. C. et al. 2013. Soil-cement bricks incorporated with eggshell waste. Proceedings of the Institution of Civil Engineers - Waste and Resource Management, 166(3), 137–141. [CrossRef]
- Holmes, N., Tyrer, M., & Kelliher, D. 2022. Employing discrete solid phases to represent C-S-H solid solutions in the Cemdata07 thermodynamic database to model cement hydration using the PHREEQC geochemical software. Applied Sciences, 12(19), 10039. [CrossRef]
- Dong, P. et al. 2022. Liquid cell transmission electron microscopy reveals C-S-H growth mechanism during Portland cement hydration. Materialia, 22, 101387. [CrossRef]
- Associação Brasileira de Normas Técnicas. 1994. NBR 10834: Tijolos solo-cimento - especificações. ABNT: Rio de Janeiro, Brasil.
- Dulal, P.; X, Y.; Z, W. Engineering properties of cement-stabilized compressed earth bricks. J. Build. Eng. 2023, 77, 107453. [Google Scholar] [CrossRef]
- Thennarasan Latha, A.; Murugesan, B.; Thomas, B. S. Compressed stabilized earth block incorporating municipal solid waste incinerator bottom ash as a partial replacement for fine aggregates. Buildings 2023, 13, 1114. [Google Scholar] [CrossRef]
- Corrêa-Silva, M.; X, Y.; Z, W. Constitutive behaviour of a clay stabilised with alkali-activated cement based on blast furnace slag. Sustainability 2022, 14, 13736. [Google Scholar] [CrossRef]
- Zak, P.; X, Y.; Z, W. The influence of natural reinforcement fibers, gypsum and cement on compressive strength of earth bricks materials. Constr. Build. Mater. 2016, 106, 179–188. [Google Scholar] [CrossRef]
- Maichin, P.; X, Y.; Z, W. Stabilized high clay content lateritic soil using cement-FGD gypsum mixtures for road subbase applications. Materials 2021, 14, 1858. [Google Scholar] [CrossRef]
- Cai, H.; X, Y.; Z, W. Strength development of cemented soil cured in water-air conditions at varied temperatures: Experimental investigation and model characterization. J. Mater. Civ. Eng. 2023, 35, 3. [Google Scholar] [CrossRef]
- Associação Brasileira de Normas Técnicas. 2012. NBR 13553: Materiais para emprego em parede monolítica de solo-cimento sem função estrutural – Requisitos. ABNT: Rio de Janeiro, Brasil.
- Höglund, L.O. Some notes of ettringite formation in cementitious materials; Influence of hydration and thermodynamic constraints for durability. Cem. Concr. Res. 1992, 22, 217–228. [Google Scholar] [CrossRef]
- Gu, Y.; X, Y.; Z, W. Pore size analyses of cement paste exposed to external sulfate attack and delayed ettringite formation. Cem. Concr. Res. 2019, 123, 105766. [Google Scholar] [CrossRef]
- Afflerbach, S.; Pritzel, C.; Hartwich, P.; Killian, M. S.; Krumm, W. Effects of thermal treatment on the mechanical properties, microstructure and phase composition of an ettringite-rich cement. CEMENT 2023, 11, 100058. [Google Scholar] [CrossRef]
- in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content.
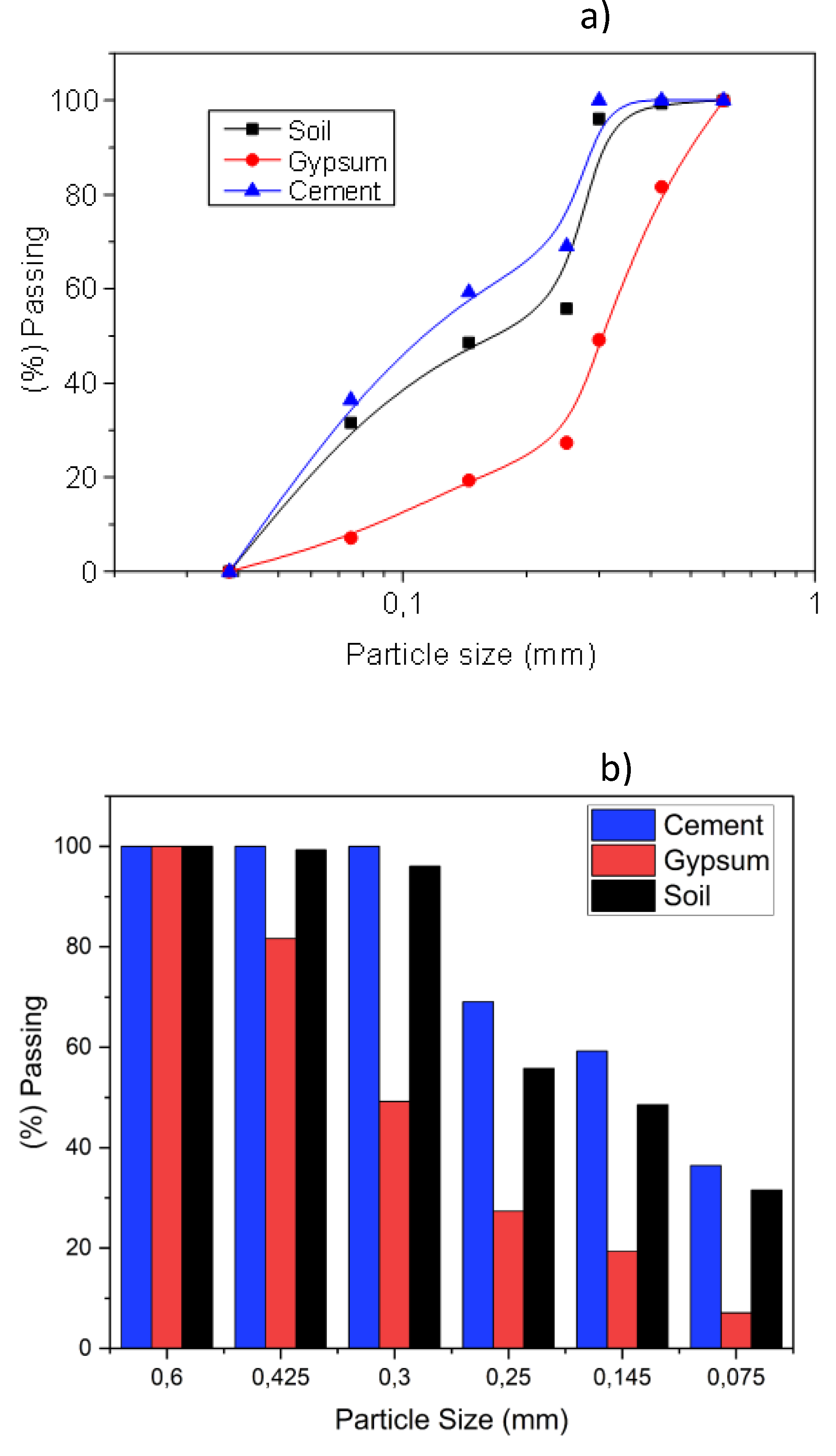
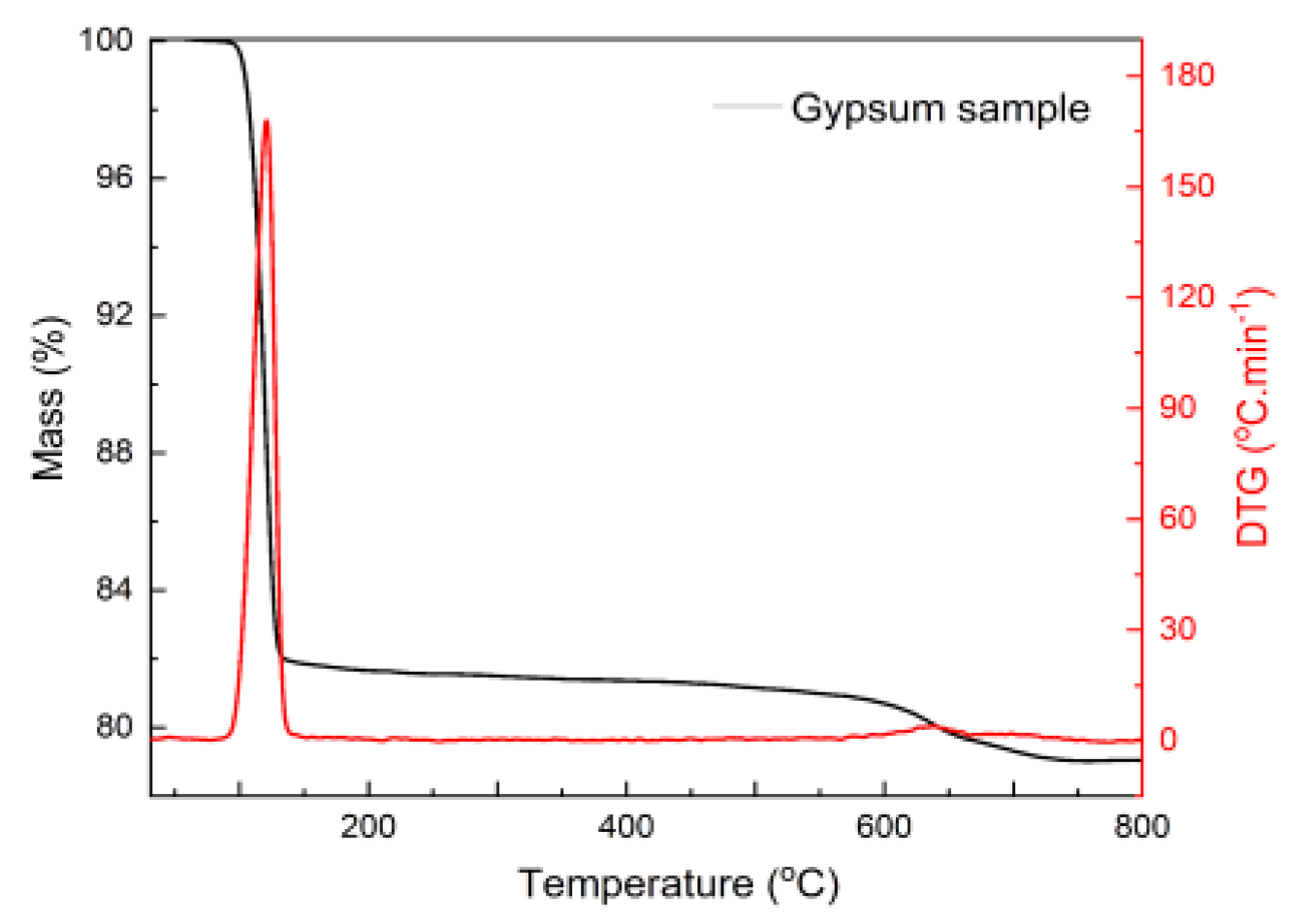
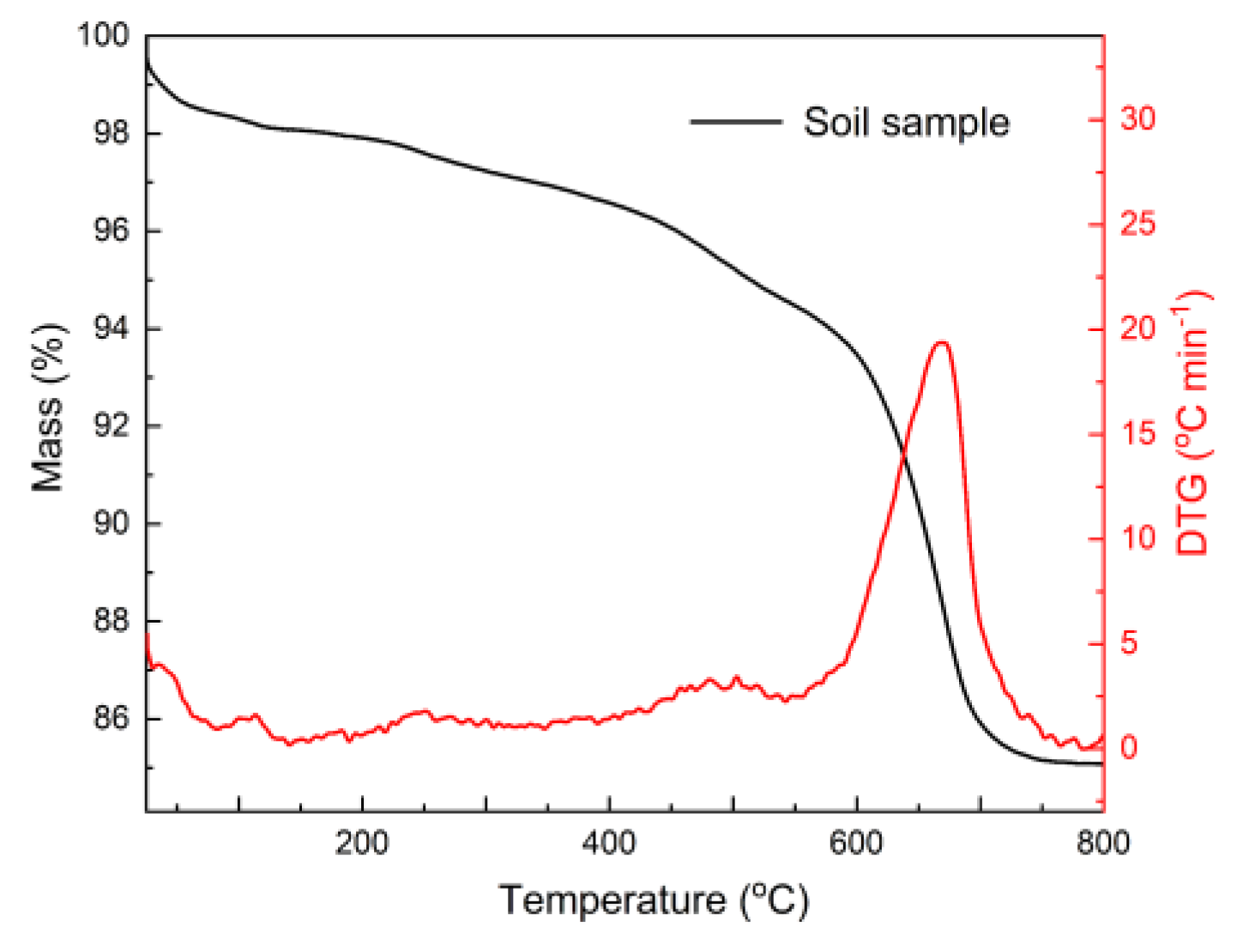
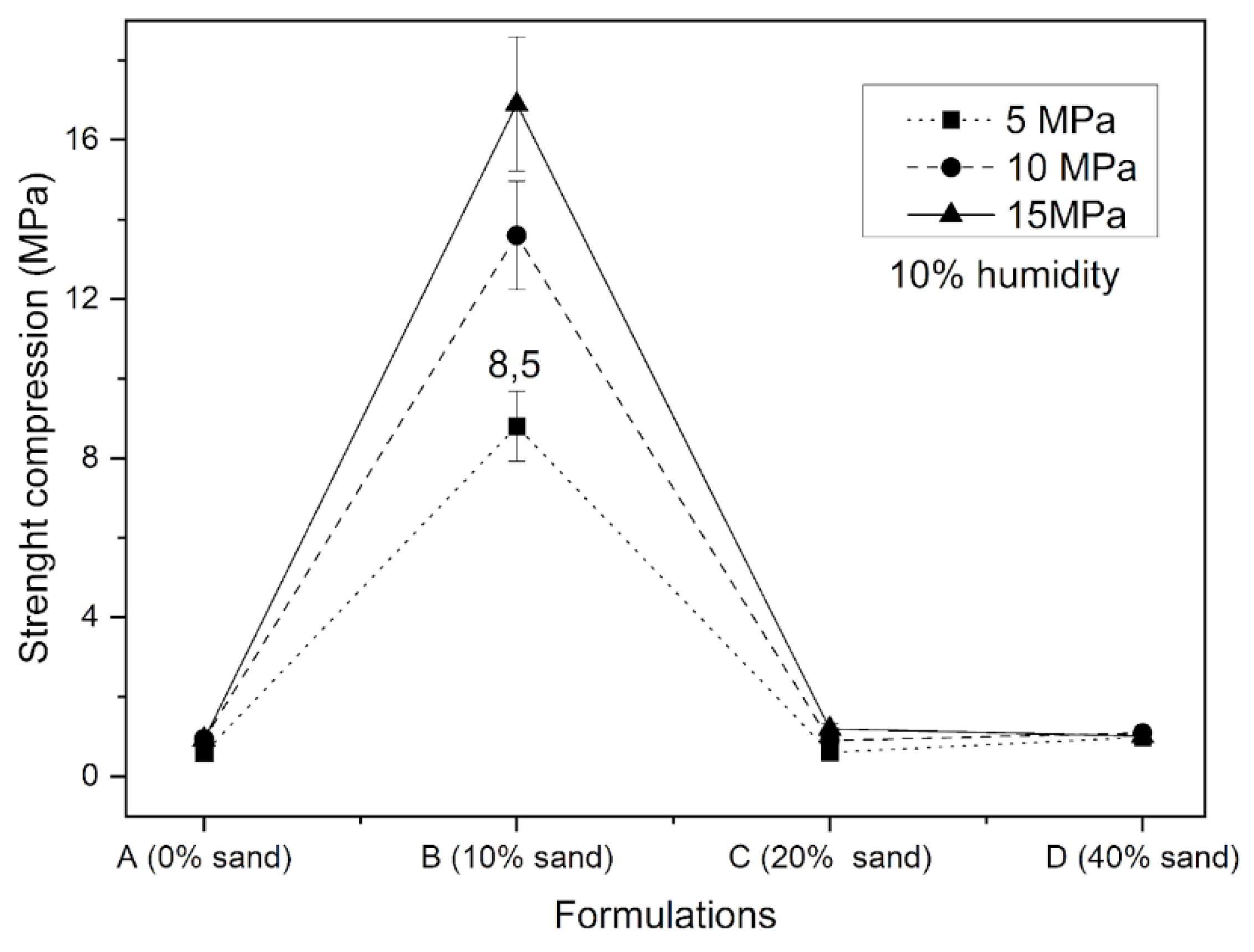
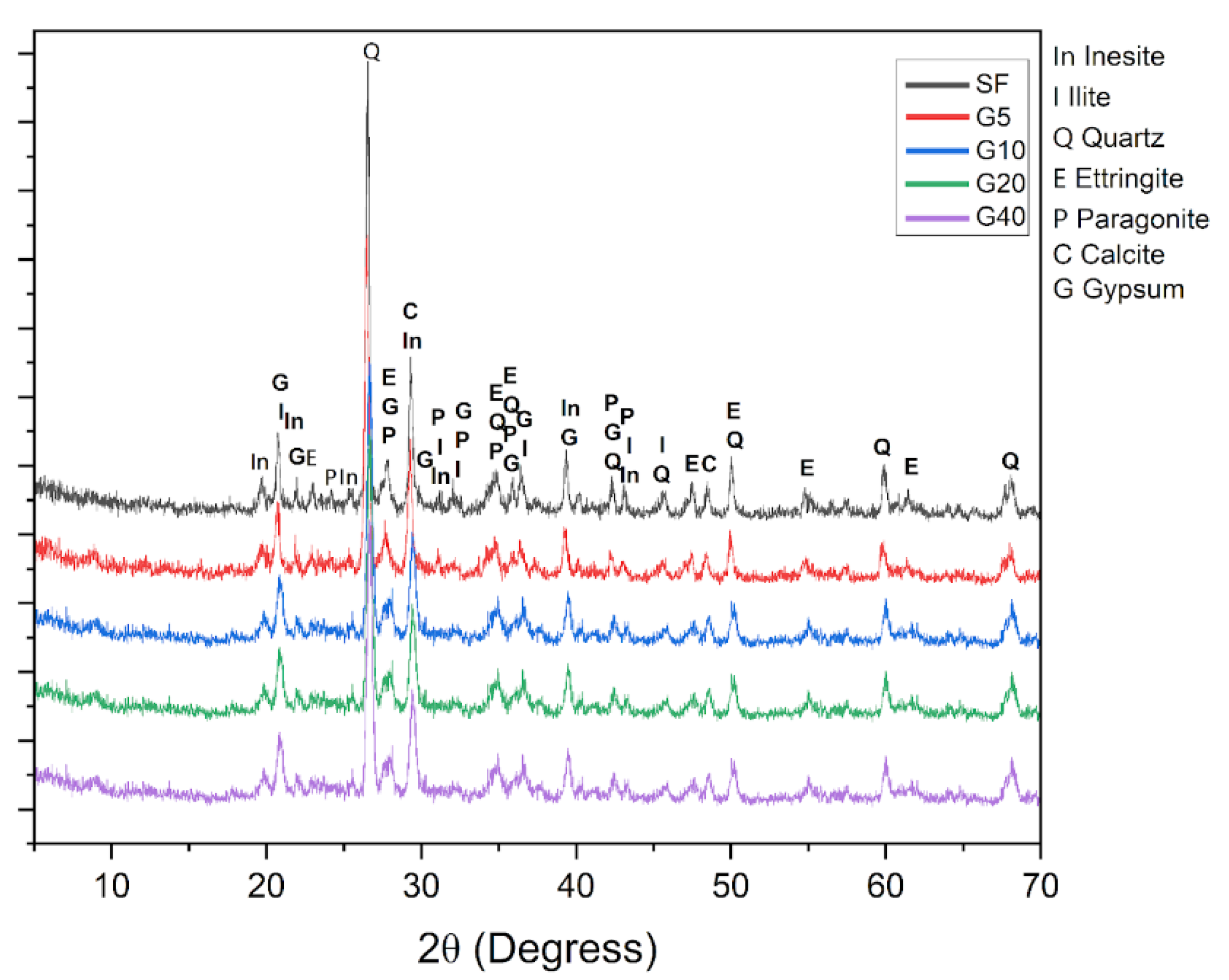
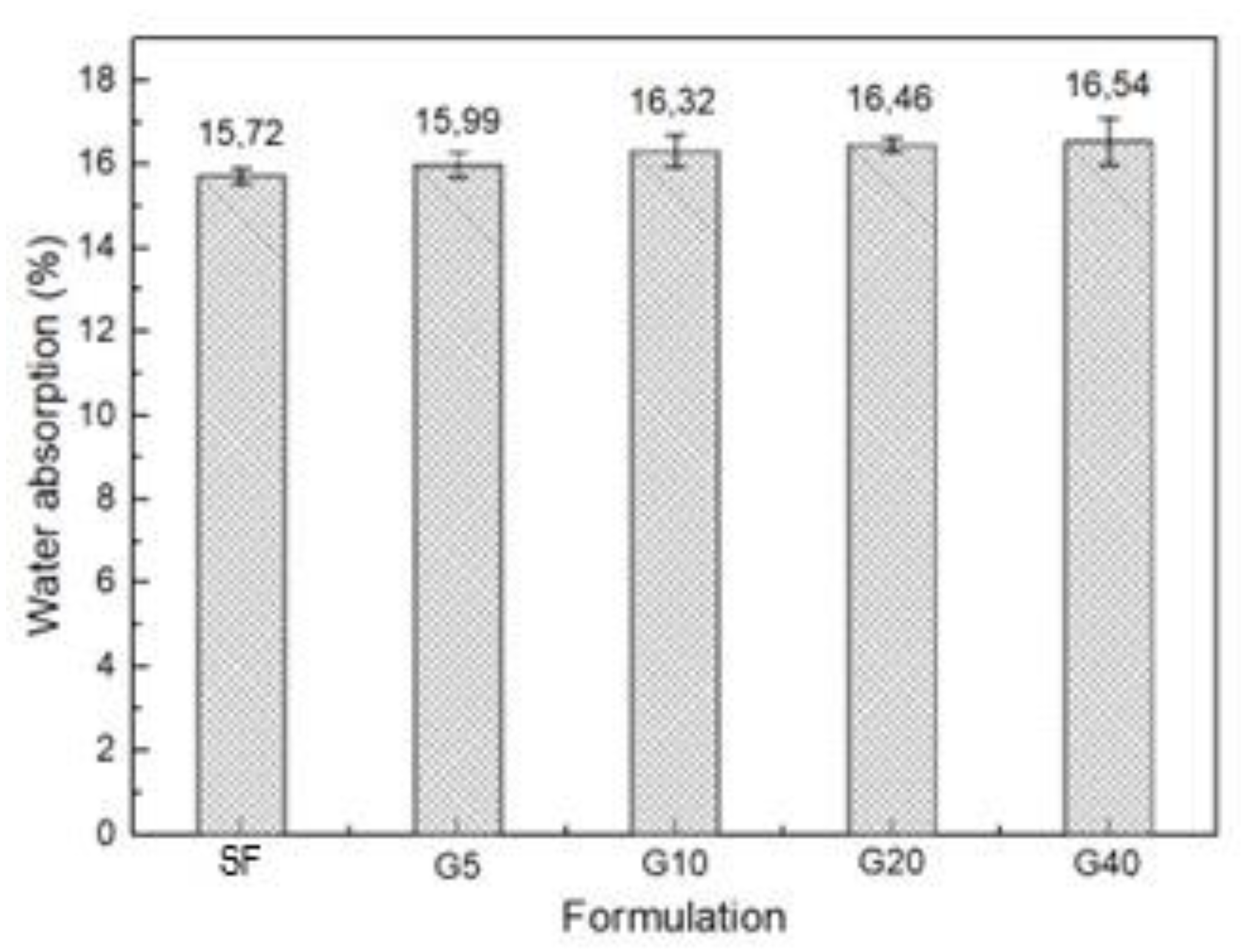
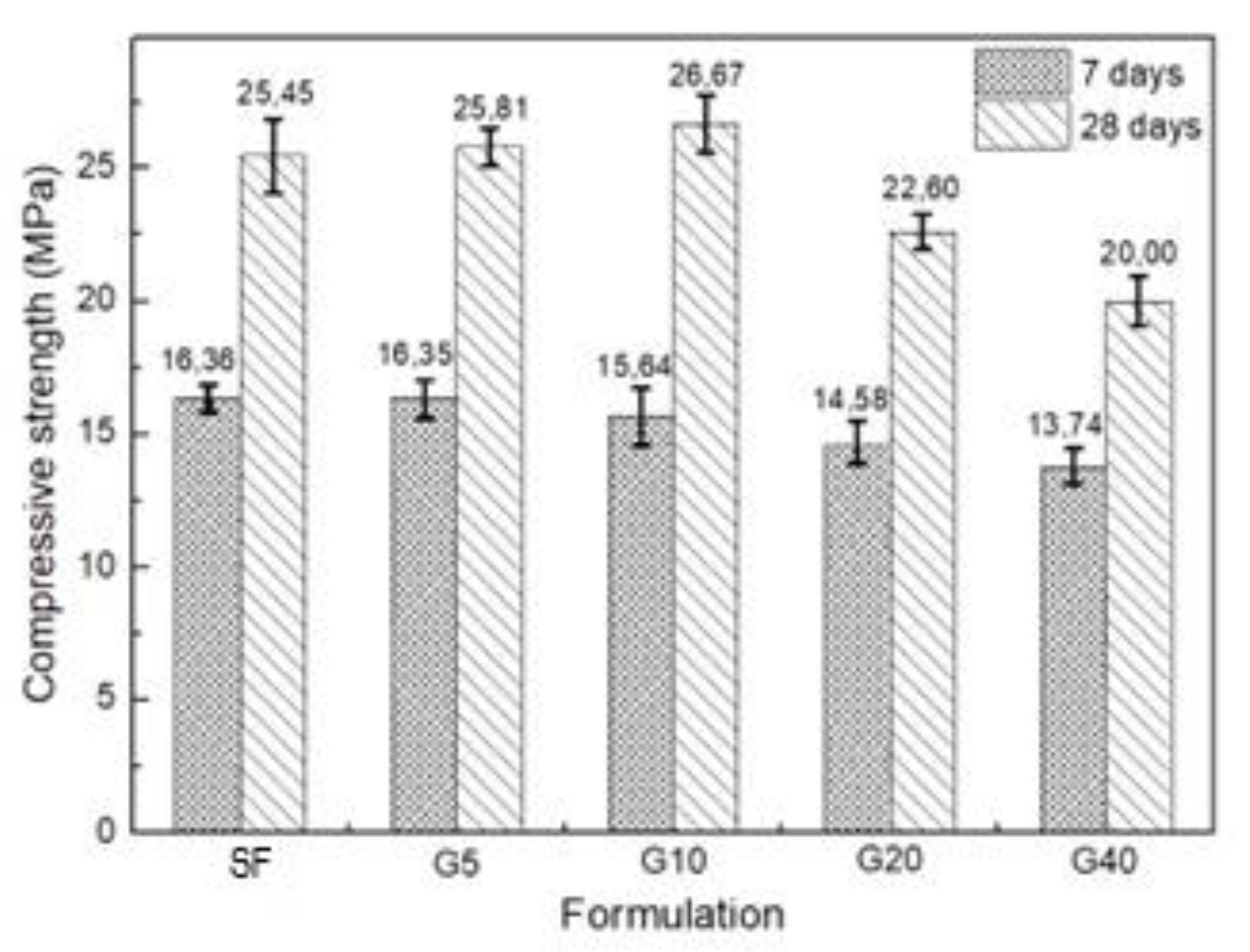
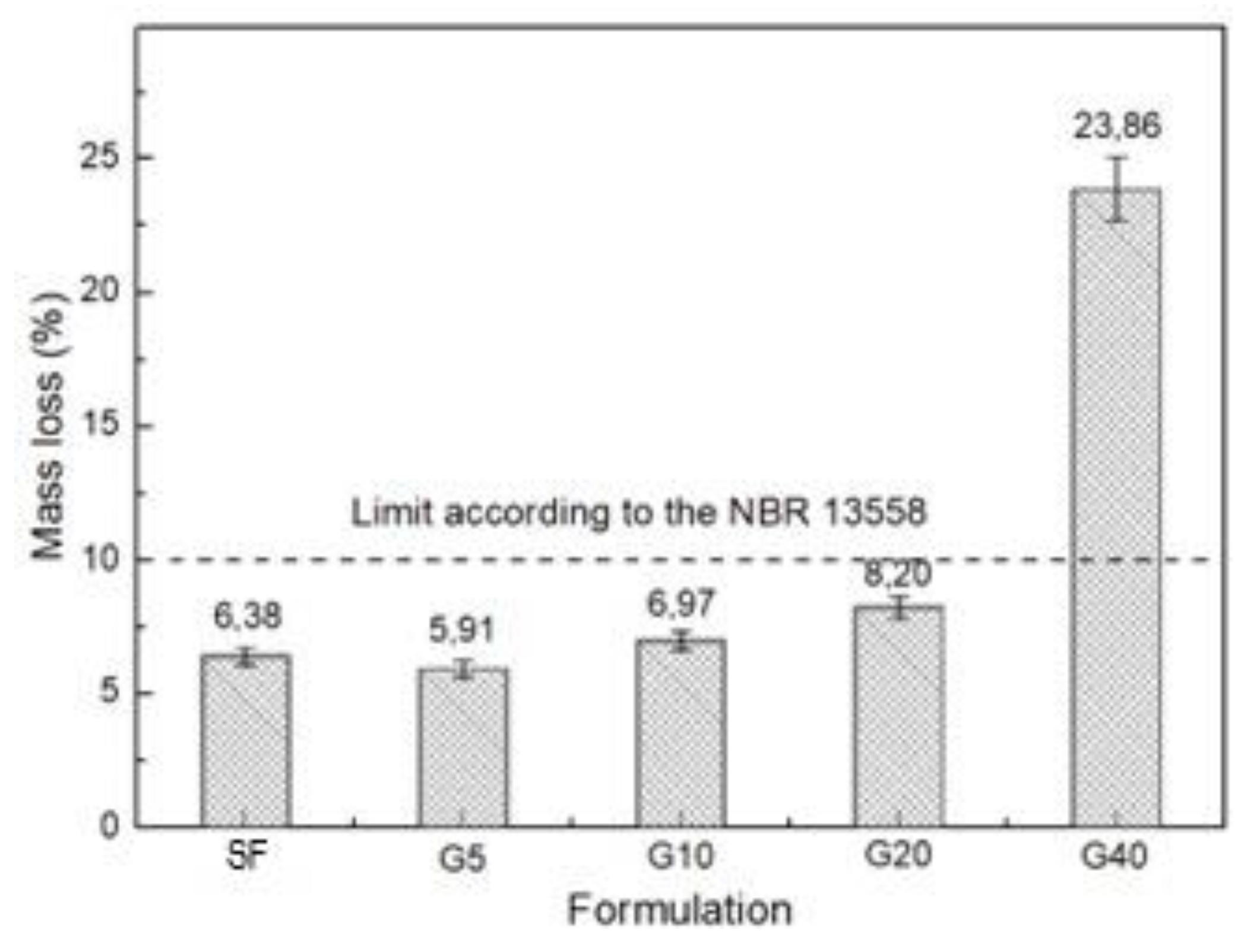
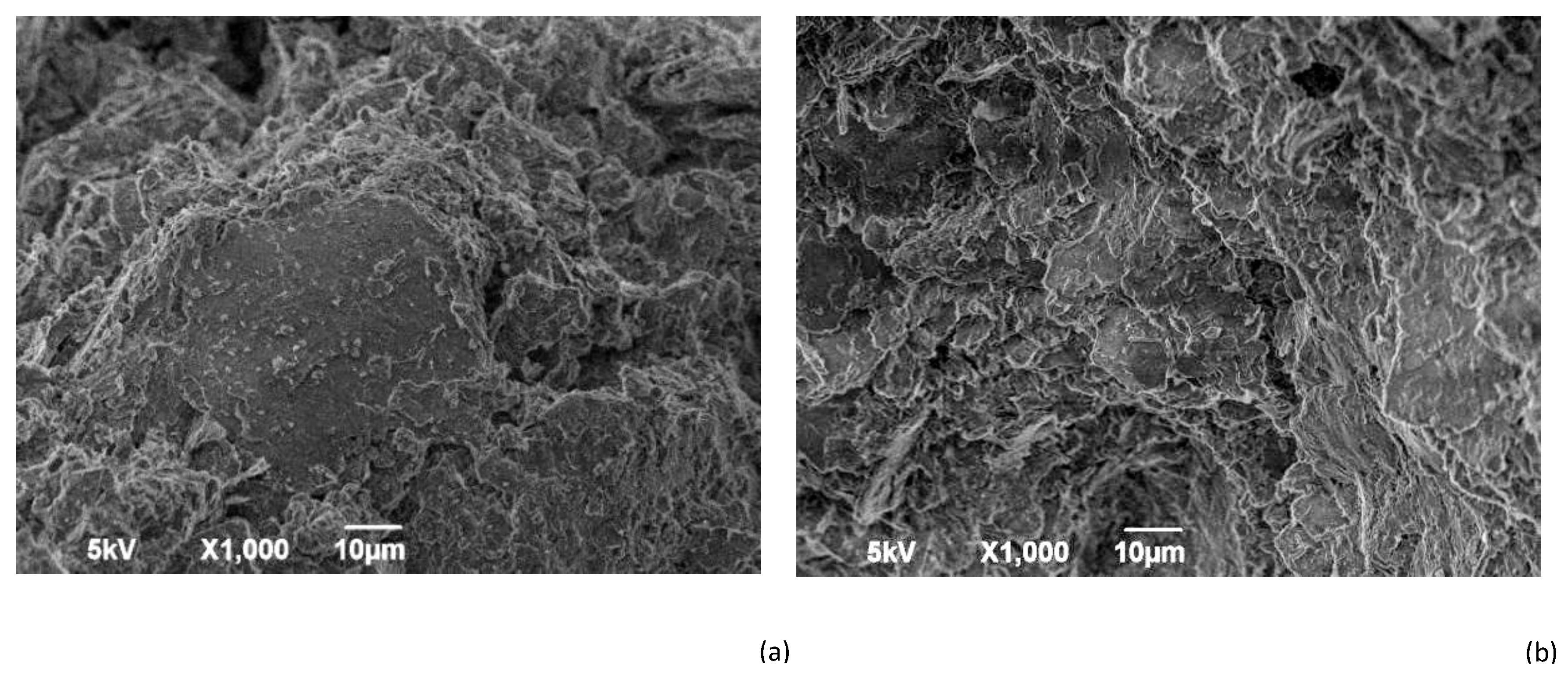
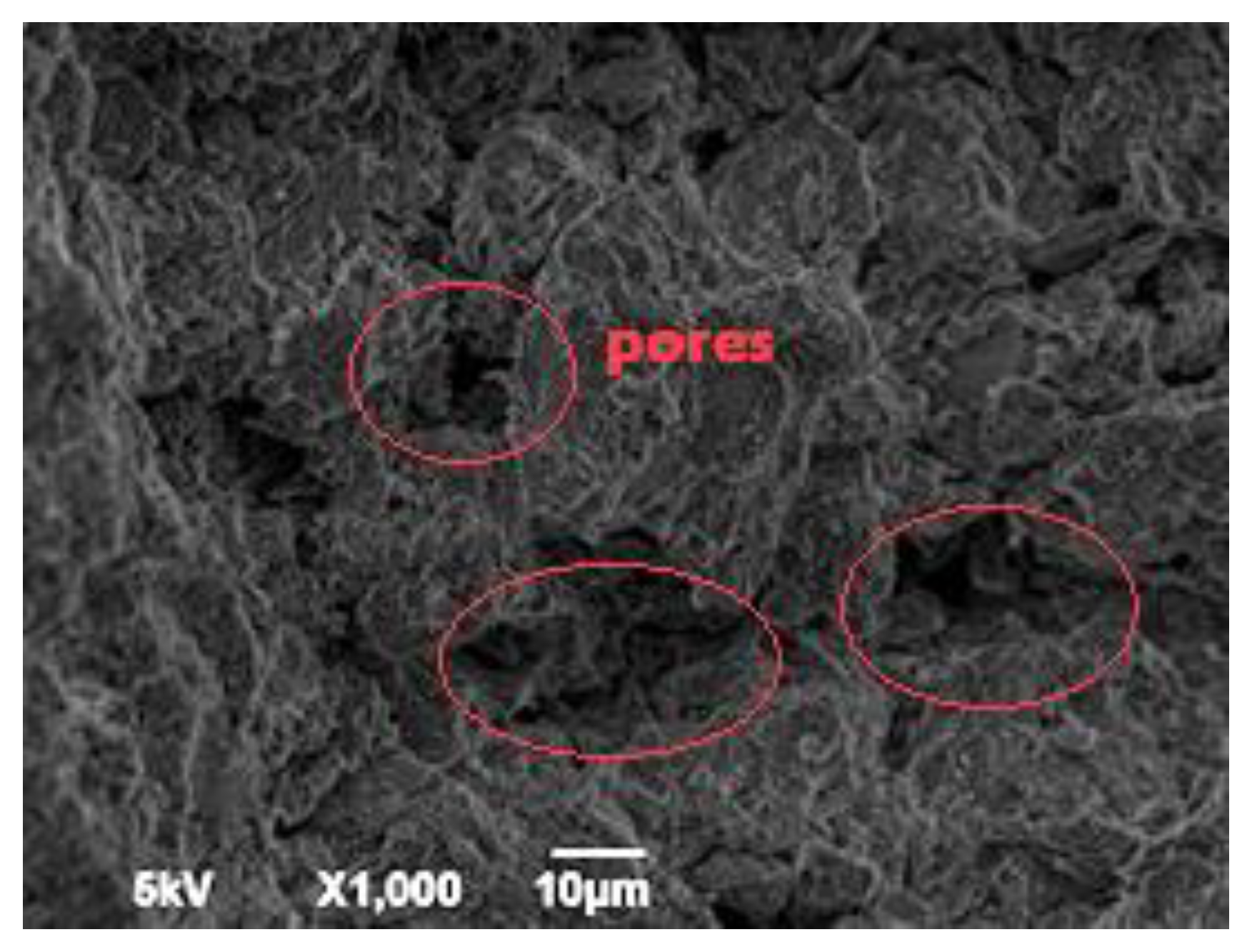
| Specific Gravity (g.cm3) | Bulk Density (g.cm3) | Specific Surface Area (cm2/g) | |
| Soil | 2,71 ± 0,02 | 1,15 ± 0,01 | 4243 ± 100 |
| Gypsum | 2,25 ± 0,02 | 1,10 ± 0,02 | 5500 ± 150 |
| Cement | 3,10 ± 0,02 | 1,40 ± 0,02 | 4400 ± 120 |
| Sand | 2,63 ± 0,01 | 1,65 ± 0,01 | - |
| Component | Soil | Cement | Sand | Gypsum |
| CaO | 22.97 | 80.95 | 1.54 | 68.01 |
| SiO2 | 39.39 | 8.46 | 89.79 | 0.63 |
| Fe2O3 | 18.45 | 5.95 | 1.721 | - |
| SO3 | 0.11 | 2.06 | 0.32 | 29.97 |
| K2O | 6.95 | 1.60 | 0.66 | 0.30 |
| Al2O3 | 9.40 | - | 1.82 | - |
| TiO2 | 2.09 | 0.37 | 3.56 | - |
| Loss on ignition | 0.64 | 0.61 | 0.59 | 1.09 |
| Compositions | Soil | Cement | Sand | Gypsum |
| SF | 80 | 10 | 10 | - |
| G5 | 80 | 9.5 | 10 | 0.5 |
| G10 | 80 | 9 | 10 | 1 |
| G20 | 80 | 8 | 10 | 2 |
| G40 | 80 | 6 | 10 | 4 |
| Mineral Phase | Concentration Range (%) |
|---|---|
| Quartz | 24,7 - 38,0 |
| Paragonite | 18,4 - 22,8 |
| Inesite | 10,1 - 15,8 |
| Ettringite | 8,0 - 10,0 |
| Calcite | 12,5 - 16,2 |
| Gypsum | 0,9 - 2,5 |
| Ilite | 16,9 - 20,1 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





