Submitted:
20 January 2025
Posted:
21 January 2025
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Materials and Methods
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Kimmel RM, Murphy RXJ, Chowdary RP. Optimal management of inguinal vascular graft infections. Ann Plast Surg 1994; 32:623 – 629. [CrossRef]
- Zetrenne E, McIntosh BC, McRae MH, et al. Prosthetic vascular graft infection: a multi-center review of surgical management. Yale J Biol Med 2007; 80: 113 – 121.
- Herrera FA, Kohanzadeh S, Nasseri Y, et al. Management of vascular graft infections with soft tissue flap coverage: improving limb salvage rates – a veterans affairs experience. Am Surg 2009; 75: 877 – 881. [CrossRef]
- Giles KA, Hamdan AD, Pomposelli FB, et al. Body mass index: surgical site infections and mortality after lower extremity bypass from the National Surgical Quality Improvement Program 2005–2007. Ann Vasc Surg. 2010 Jan; 24(1):48–56. [CrossRef]
- Greenblatt DY, Rajamanickam V, Mell MW. Predictors of surgical site infection after open lower extremity revascularization. J Vasc Surg. 2011 Aug; 54(2):433–9. [CrossRef]
- Vogel TR, Dombrovskiy VY, Carson JL, et al. Infectious complications after elective vascular surgical procedures. J Vasc Surg. 2010 Jan; 51(1):122–9. [CrossRef]
- Wilson, SE. New alternatives in management of the infected vascular prosthesis. Surg Infect. 2001;2(2):171-7. [CrossRef]
- Williams M, Milling M, Shandall A. Vascularized muscular flaps and arterial graft infection in the groin. Eur J Vasc Surg. 2003;25:390-95.
- Mertens RA, O'Hara PJ, Hertzer NR, et al. Surgical management of infrainguinal arterial prosthetic graft infections: review of a thirty-five-year experience. J Vasc Surg. 1995;21(5):782-91. [CrossRef]
- Henke PK, Bergamini TM, Rose SM, et al. Current options in prosthetic vascular graft infection. Am Surg. 1998;64(1):39-46.
- Morasch MD, Sam AD 2nd, Kibbe MR, et al. Early results with use of gracilis muscle flap coverage of infected groin wounds after vascular surgery. J Vasc Surg. 2004 Jun; 39(6): 1277–83.
- Kwaan JH, Connolly JE. Successful management of prosthetic graft infection with continuous povidone-iodine irrigation. Arch Surg. 1981;116(5):716-20. [CrossRef]
- Calligaro KD, Veith FJ, Gupta SK et al. A modified method for management of prosthetic graft infections involving an anastomosis to the common femoral artery. J Vasc Surg. 1990; 11: 485-492.
- Mixter RC, Turnipseed WD, Smith DJ, et al. Rotational muscle flaps: a new technique for covering infected vascular grafts. J Vasc Surg 1989;9:472–8.
- Meland NB, Arnold PG, Pairolero PC. Infected vascular prosthesis management with muscle transposition. Plast Reconstr Surg. 1994 Apr;93(5):1005-11.
- Edwards WH Jr, Martin RS 3rd, Jenkins JM, et al. Primary graft infections. J Vasc Surg. 1987 Sep; 6(3):235–9.
- Dosluoglu HH, Loghmanee C, Lall P, et al. Management of early (<30 day) vascular groin infections using vacuum-assisted closure alone without muscle flap coverage in a consecutive patient series. J Vasc Surg. 2010 May; 51(5):1160–6. [CrossRef]
- Landry GJ, Carlson JR, Liem TK, et al. The sartorius muscle flap: an important adjunct for complicated femoral wounds involving vascular grafts. Am J Surg. 2009 May; 197(5):655–9.
- Mayer D, Hasse B, Koelliker J, et al. Long-term results of vascular graft and artery preserving treatment with negative pressure wound therapy in Szilagyi grade III infections justify a paradigm shift. Ann Surg. 2011 Nov;254(5):754-59.
- Monsen C, Wann-Hansson C, Wictorsson C, et al. Vacuum-assisted wound closure versus alginate for the treatment of deep perivascular wound infections in the groin after vascular surgery. J Vasc Surg. 2014 Jan;59(1):145-51. [CrossRef]
- Hisata Y, Hashizume K, Tanigawa K, et al. Vacuum-assisted closure therapy for salvaging a methicillin-resistant Staphylococcus aureus-infected prosthetic graft. Asian J Surg. 2014 Jan;37(1):46-8. [CrossRef]
- Massara M, Cascio A, Benedetto F, et al. Septic skin lesions: an uncommon manifestation of peripheral prosthetic vascular graft infection. Acta medica mediterranea 2014; 30: 651-654.
- Massara M, De Caridi G, Barberi G, et al. Peripheral septic arterial embolism of non cardiac source: A systematic review of literature. Reviews in Vascular Medicine. Dec 2014,Vol.2(4):127135. [CrossRef]
- Piano, G. Infections in lower extremity vascular grafts. Surg Clin North Am 1995;74:799–809. [CrossRef]
- Edwards MJ, Richardson JD, Klamer TW. Management of aortic prosthetic infections. Am J Surg 1988;155:327–30. [CrossRef]
- Reilly LM, Ehrenfeld WK, Goldstone J, et al. Gastrointestinal tract involvement by prosthetic graft infection. The significance of gastrointestinal hemorrhage. Ann Surg 1985; 202:342–8. [CrossRef]
- O’Connor S, Andrew P, Batt M, et al. A systematic review and meta-analysis of treatments for aortic graft infection. J Vasc Surg 2006; 44: 38 – 45. [CrossRef]
- Neufang A, Doemland M. Clinical results with the denaturated human umbilical vein (HUV) and the ovine collagen prothesis (Omniflow) as small caliber prosthetic vascular replacement. Gefäßchirurgie 2010; 15: 83 – 89.
- Amman W, Tiesenhausen K, Fruwirth J, et al. Aneurysms in biosynthetic grafts. Z Herz Thorax Gefäßchirurgie 2000; 14: 113 – 116.
- Koch G, Gutschi S, Pascher O, et al. Analysis of 274 Omniflow Vascular Prosthesis implanted over an eight-year period. Aust N Z J Surg. 1997; 67: 637 – 639.
- Töpel I1, Betz T, Uhl C, et al. Use of biosynthetic prosthesis (Omniflow II®) to replace infected infrainguinal prosthetic grafts--first results. Vasa. 2012 May;41(3):215-20.
- Chaudhry A, Shetty R, Crimmin S, et al. Bio-synthetic Graft Repair of Mycotic Aneurysm of the Common Femoral Artery. EJVES Extra 2009; 18: 1 – 2. [CrossRef]
- Ozelci A, Yetkin U, Akyuz M, et al. A clinical application of the Omniflow II collagen- polymer vascular prosthesis in a polytetrafluoroethylene graft infection case. Int J Th or Cardiovasc Surg 2009.
- De Caridi G, Serra R, Massara M, et al. VAC therapy for the treatment of complex wounds after cardio-thoracic surgery. Int Wound J. 2014 Sep 17. [CrossRef]
- De Caridi G, Massara M, Greco M, et al. VAC therapy to promote wound healing after surgical revascularisation for critical lower limb ischaemia. Int Wound J. 2014 May 28. [CrossRef]
- Svensson S, Monsen C, Kölbel T, et al. Predictors for outcome after vacuum assisted closure therapy of peri-vascular surgical site infections in the groin. Eur J Vasc Endovasc 2008;36:84e9. [CrossRef]
- Pinocy J, Albes JM, Wicke C, et al. Treatment of periprosthetic soft tissue infection of the groin following vascular surgical procedures by means of polyvinyl alcohol-vacuum sponge system. Wound Repair Regen 2003;11:104e9. [CrossRef]
- Dee, A. The successful management of a dehisced surgical wound with TNP following femoropopliteal bypass. J Wound Care 2007;16:42e4. [CrossRef]
- Hisata Y, Hashizume K, Tanigawa K, et al. Vacuum-assisted closure therapy for salvaging a methicillin-resistant Staphylococcus aureus-infected prosthetic graft. Asian J Surg. 2014 Jan;37(1):46-8. [CrossRef]
- Acosta S, Monsen C. Outcome after VAC® therapy for infected bypass grafts in the lower limb. Eur J Vasc Endovasc Surg. 2012 Sep;44(3):294-9.
- Siracuse JJ, Nandivada P, Giles KA, et al. Prosthetic graft infections involving the femoral artery. J Vasc Surg. 2013 Mar;57(3):700-5. [CrossRef]
- Massara M, De Caridi G, Serra R, et al. The role of procalcitonin as a marker of diabetic foot ulcer infection. Int Wound J. 2015 Oct 28. [CrossRef]
- Cherry KJ Jr, Roland CF, Pairolero PC, et al. Infected femorodistal bypass: is graft removal mandatory? J Vasc Surg. 1992 Feb;15(2):295-303.
| Patient# Sex, age |
Type of bypass | Type of graft | Time, type and location of infection | Organism. Site of bacteriological sampling |
Surgical treatment | Antibiotic treatment | Outcome |
|---|---|---|---|---|---|---|---|
| 1, M 82 y |
Right axillo-femoral bypass |
Dacron | Late infection. Abscess along the abdominal wall |
Staphylococcus epidermidis. Prosthesis |
Bypass excision. New bypass in a clean field |
Tigecycline, gentamicin | Limb salvage |
| 2, F 72 y |
Right ilio-tibial bypass (redo-bypass) | PTFE | Early infection. Abscess and surgical wound dehiscence with pus discharge |
MSSA. Prosthesis, dehiscent groin wound |
Bypass excision. Impossible to revascularize |
Ceftriaxone, amikacin | Amputation |
| 3, M 78 y |
Left ilio-peroneal bypass (redo-bypass) | PTFE | Early infection. Leg abscess with surgical wound dehiscence |
Pseudomonas aeruginosa. Prosthesis, dehiscent leg wound. Negative blood culture |
Surgical debridement of the dehiscent wound. VAC therapy. Surgical closure | Piperacillin-tazobactam | Limb salvage |
| 4, F 81 y |
Right femoro-tibial bypass (redo-bypass) |
PTFE | Early infection. Graft occlusion |
Pseudomonas aeruginosa. Prosthesis, dehiscent groin wound |
Bypass excision. Impossible to revascularize |
Amikacin | Amputation |
| 5, M 61 y |
Left femoro-tibial bypass (redo-bypass) |
PTFE | Early infection. Proximal false aneurysm |
Sterile. Prosthesis |
Proximal bypass excision and replacemnt with Omniflow II | Ceftazidime, gentamicin | Limb salvage |
| 6, F 79 y |
Left femoro-tibial bypass (redo-bypass) |
PTFE | Early infection. Bleeding |
Sterile. Prosthesis. |
Bypass excision. Impossible to revascularize |
Ceftazidime | Amputation |
| 7, M 77 y |
Right femoro-popliteal bypass | PTFE | Late infection. Groin suture dehiscence with persistent pus discharge |
MRSA (dehiscent groin wound); Pseudomonas aeruginosa (prosthesis) |
Bypass excision and replacement in GSV | Imipenem, ciprofloxacin | Limb salvage |
| 8, M 76 y |
Left ilio-femoral bypass | PTFE | Early infection. Abscess |
Sterile (periprosthetic material) | Surgical site debridement and pus evacuation. VAC therapy. Muscle flap transposition |
Ceftriaxone | Limb salvage |
| 9,M 78 y |
Right femoro-popliteal bypass | PTFE | Late infection. Acute ischemia |
Staphylococcus lugdunensis. Prosthesis |
Bypass excision and femoro-peroneal bypass in GSV | Amoxicillin clavulanic acid, Gentamicin | Limb Salvage |
| 10, M 78 y |
Femoro-tibial bypass (redo-bypass) | PTFE | Early infection. Bleeding |
MRSA. Prosthesis and dehiscent groin wound |
Bypass excision. Impossible to revascularize |
Teicoplanin, vancomycin | Amputation. Death during follow-up |
| 11, M 75 |
Femoro-femoral crossover bypass | PTFE | Late infection. Graft occlusion |
Sterile (prosthesis) | Bypass excision. Revascularization not required |
Vancomycin, rifampicin | Limb salvage |
| 12, M 69 y |
Right femoro-tibial bypass (redo- bypass) |
PTFE | Early infection. Surgical wound dehiscence with persistent discharge |
Sterile (prosthesis) | Bypass excision. Impossible to revascularize |
Ceftazidime | Amputation |
| 13, F 78 y |
Left femoro-popliteal bypass | PTFE | Early infection. Bleeding |
Enterococcus faecalis. Prosthesis and dehiscent groin wound |
Bypass excision. Femoro-peroneal bypass in GSV |
Piperacillin-tazobactam, amikacin | Limb salvage |
| 14, M 67 y |
Right femoro-popliteal bypass | PTFE | Late infection. Proximal false aneurysm |
Pseudomonas aeruginosa. Prosthesis, dehiscent groin wound |
Proximal bypass excision and replacement in Omniflow II with muscle flap transposition. | Ampicillin sulbactam | Limb salvage |
| 15, M 67 y |
Left axillo-femoral bypass (Dacron) |
Dacron | Late infection. Seroma |
MRSA. Prosthesis |
Bypass excision and femoral artery patch in GSV. Not required revascularization | Teicoplanin, vancomycin | Limb salvage |
| 16, M 82 y |
Right axillo-femoral bypass (Dacron) | Dacron | Late infection. Seroma |
Escherichia coli (periprosthetic fluid), Pseudomonas aeruginosa (prosthesis) | Bypass excision and replacement in a clean field | Amikacin, colistimethate sodium | Limb salvage. Death during follow-up |
| 17, F 68 y |
Right femoro-popliteal bypass | PTFE | Early infection. Acute ischemia |
MRSA (blood culture). Pseudomonas aeruginosa and Staphylococcus aureus (prosthesis) |
Bypass excision. Impossible to revascularize | Meropenem, colistimethate sodium | Amputation. Death during hospital stay for sepsis |
| 18, F 65 y |
Left femoro-popliteal bypass | PTFE | Late infection. Proximal false aneurysm |
Proteus mirabilis (dehiscent groin wound). MSSA (dehiscent thigh wound) |
Proximal bypass excision and replacement in Omniflow II. Muscle flap transposition. Graft occlusion |
Ertapenem | Amputation after new bypass occlusion. Death during follow-up |
| 19,F 71 y |
Right femoro-popliteal bypass | PTFE | Early infection. Acute ischemia and peripheral septic arterial embolism on the skin |
Staphylococcus warneri (thrombus and prosthesis) | Bypass excision and femoro-peroneal bypass in GSV | Teicoplanin | Limb salvage |
| 20, M 65 y |
Femoro-femoral crossover bypass | PTFE | Early infection. Proximal false aneurysm |
MSSA. Prosthesis |
Bypass excision and axillo-bifemoral bypass | Rifampicin, trimethoprim/sulfamethoxazole, daptomycin | Limb salvage |
| 21, F 48 y |
Left Ilio-tibial bypass (redo-bypass) |
PTFE | Early infection. Surgical wound dehiscence with persistent discharge |
Pseudomonas aeruginosa. Prosthesis |
Bypass excision and replacement in cephalic vein. Graft occlusion |
Amikacin | Amputation after new bypass occlusion |
| 22, F 83 y |
Right axillo-femoral bypass in Dacron | Dacron | Late infection. Groin surgical wound dehiscence with persistent discharge |
MSSA. Dehiscent groin wound |
Surgical debridement of the dehiscent groin wound. VAC therapy. Surgical closure of the wound |
Meropenem | Limb salvage |
| 23, M 80 y |
Right femoro-popliteal bypass | PTFE | Early infection. Groin surgical wound dehiscence with persistent discharge |
Escherichia coli, Pseudomonas aeruginosa. Dehiscent groin wound |
Surgical debridement of the dehiscent groin wound. VAC therapy. Surgical closure of the dehiscent wound |
Amikacin, colistimethate sodium | Limb salvage |
| 24, F 77 y |
Left ilio-femoral bypass | PTFE | Early infection. Surgical wound dehiscence with persistent discharge |
MRSA | Surgical debridement, VAC therapy. Muscle flap transposition. |
Teicoplanin, vancomycin | Limb salvage |
| 25, M 83 y |
Left femoro-popliteal bypass | PTFE | Early infection. Surgical wound dehiscence with persistent discharge |
MRSA | Surgical debridement of the dehiscent groin wound and VAC therapy. Surgical closure of the dehiscent wound | Teicoplanin, vancomycin | Limb salvage. Death during follow-up |
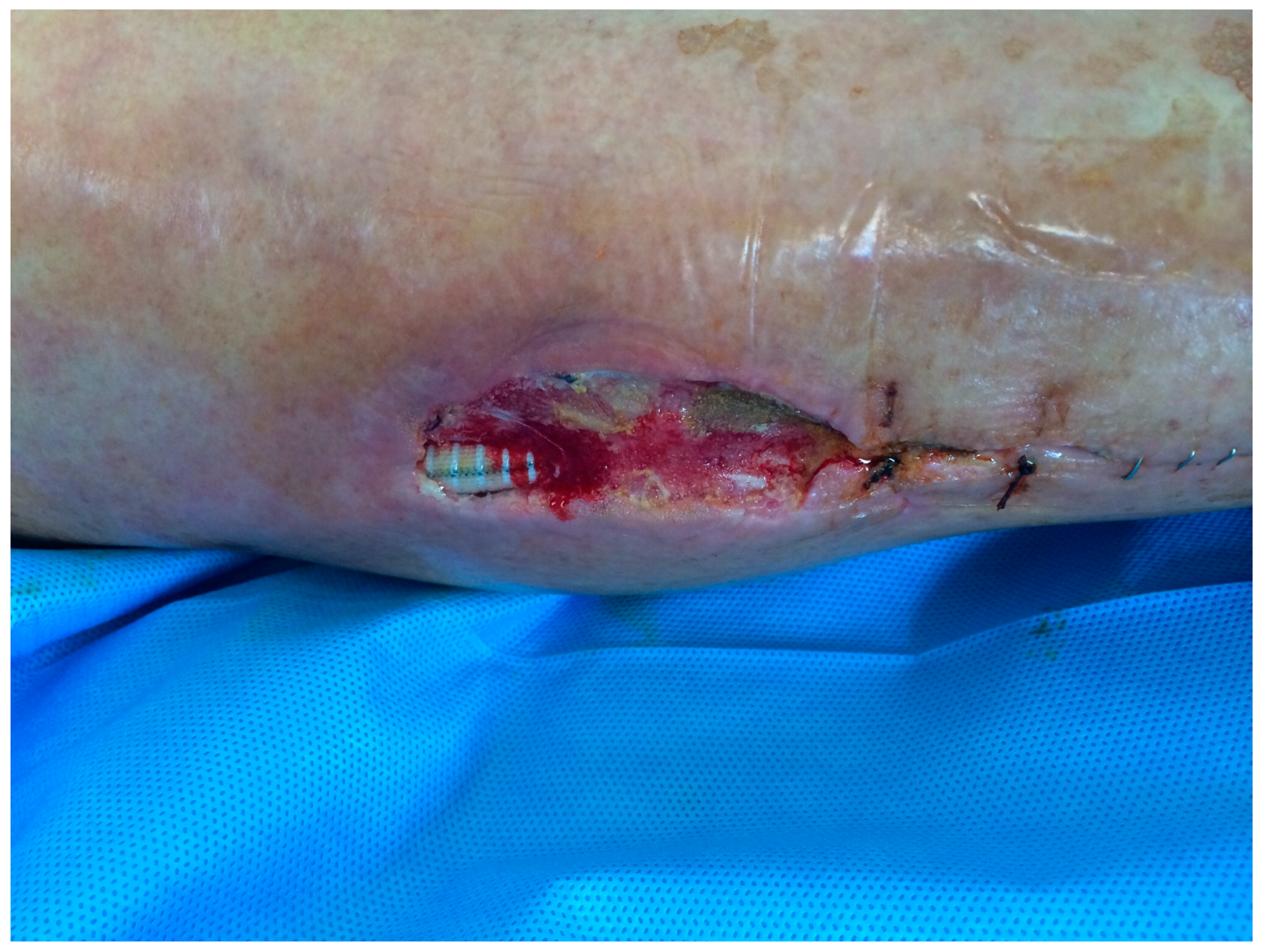
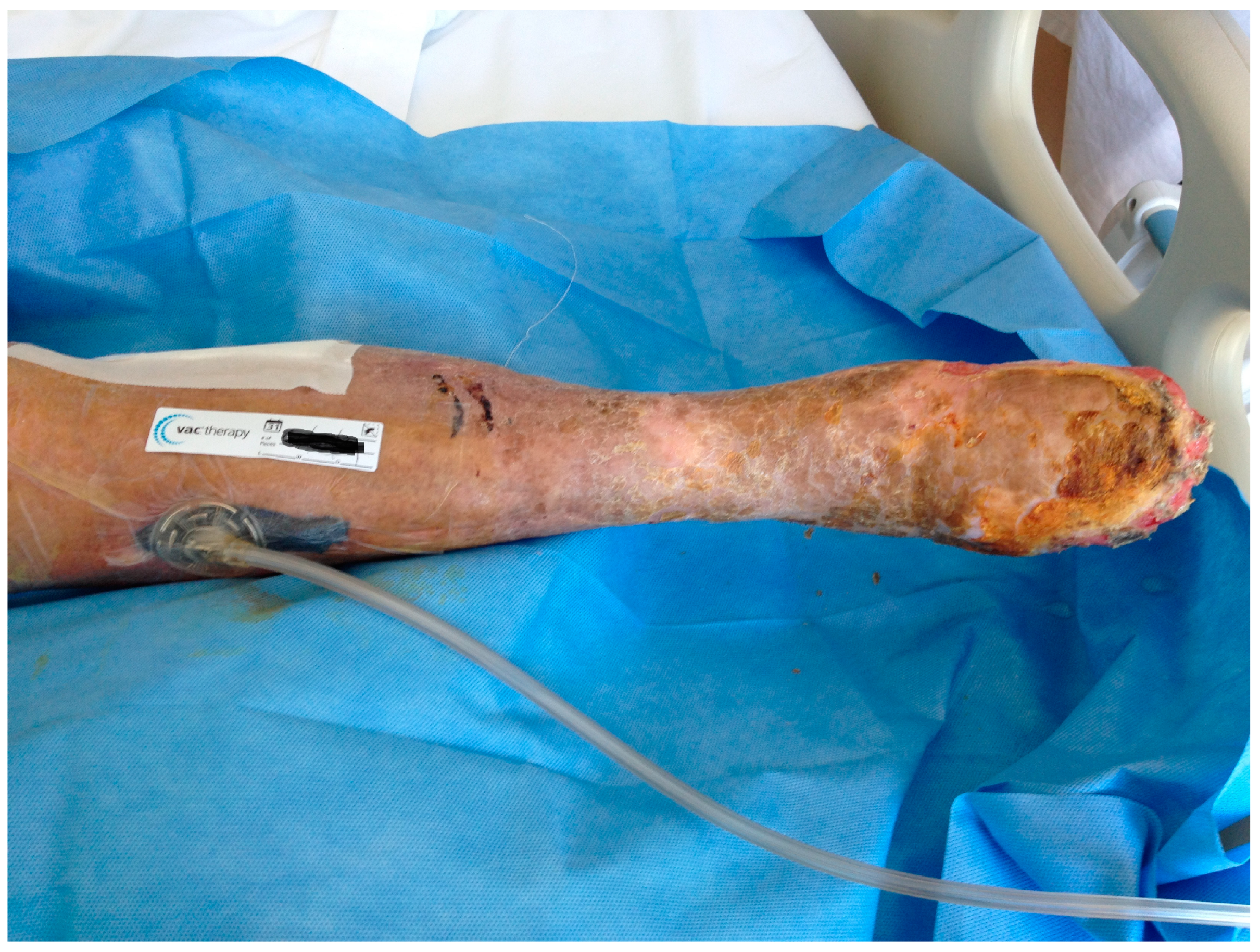
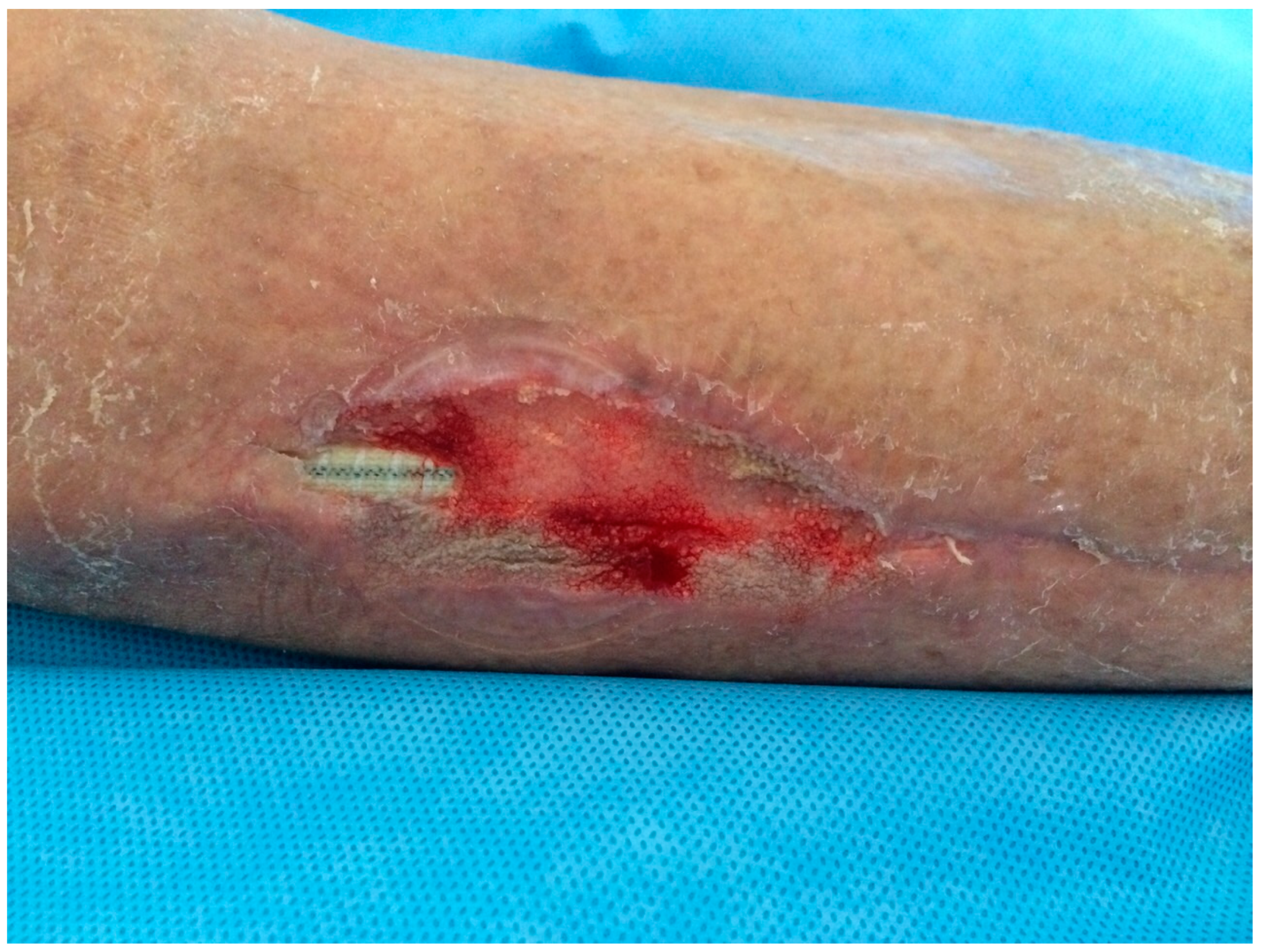
| 26, F 79 y (Fig. 1,2,3) |
Right femoro- peroneal bypass (redo-bypass) |
PTFE | Early infection. Dehiscence of the distal surgical wound with prosthesis exposure |
Pseudomonas aeruginosa. Periprosthetic material |
Surgical debridements and VAC-therapy | Piperacillin-tazobactam | Limb salvage |
| 27, M 73 y |
Femoro-femoral crossover bypass | PTFE | Late infection. Proximal false aneurysm |
Sterile Periprosthetic material |
Surgical repair | Ceftriaxone | Limb salvage |
| 28, M 76 y |
Left femoro-tibial bypass (redo-bypass) |
PTFE | Early infection. Dehiscence of the proximal surgical wound with prosthesis exposure |
Pseudomonas aeruginosa. Periprosthetic fluid |
Surgical debridements and VAC-therapy | Piperacillin-tazobactam | Limb salvage |
| 29, M 74 y |
Right femoro-popliteal bypass | PTFE | Late infection. Proxiaml false aneurysm |
MSSA. Prosthesis |
Bypass excision and replacement with a femoro-peroneal bypass in GSV | Rifampicin, trimethoprim/sulfamethoxazole, daptomycin | Limb salvage |
| Vascular bypass and type of prosthesis implanted | In our Institution (N, %) |
In other Institutions |
|---|---|---|
| Axillo-femoral (Dacron) | 2 (4.9) | 2 |
| Femoro-femoral crossover (PTFE) | 1 (2.3) | 2 |
| Ilio-femoral (PTFE) | 1 (3.7) | 1 |
| Femoro-popliteal (PTFE) | 2 (4.9) | 8 |
| Redo-bypass after failure of multiple attempts of revascularization (PTFE): - Ilio-tibial - Femoro-tibial |
10 (22.7) 3 7 |
|
| TOT = 29 | 16 (8.2) / 196 | 13 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).





