Submitted:
27 December 2024
Posted:
30 December 2024
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Theory
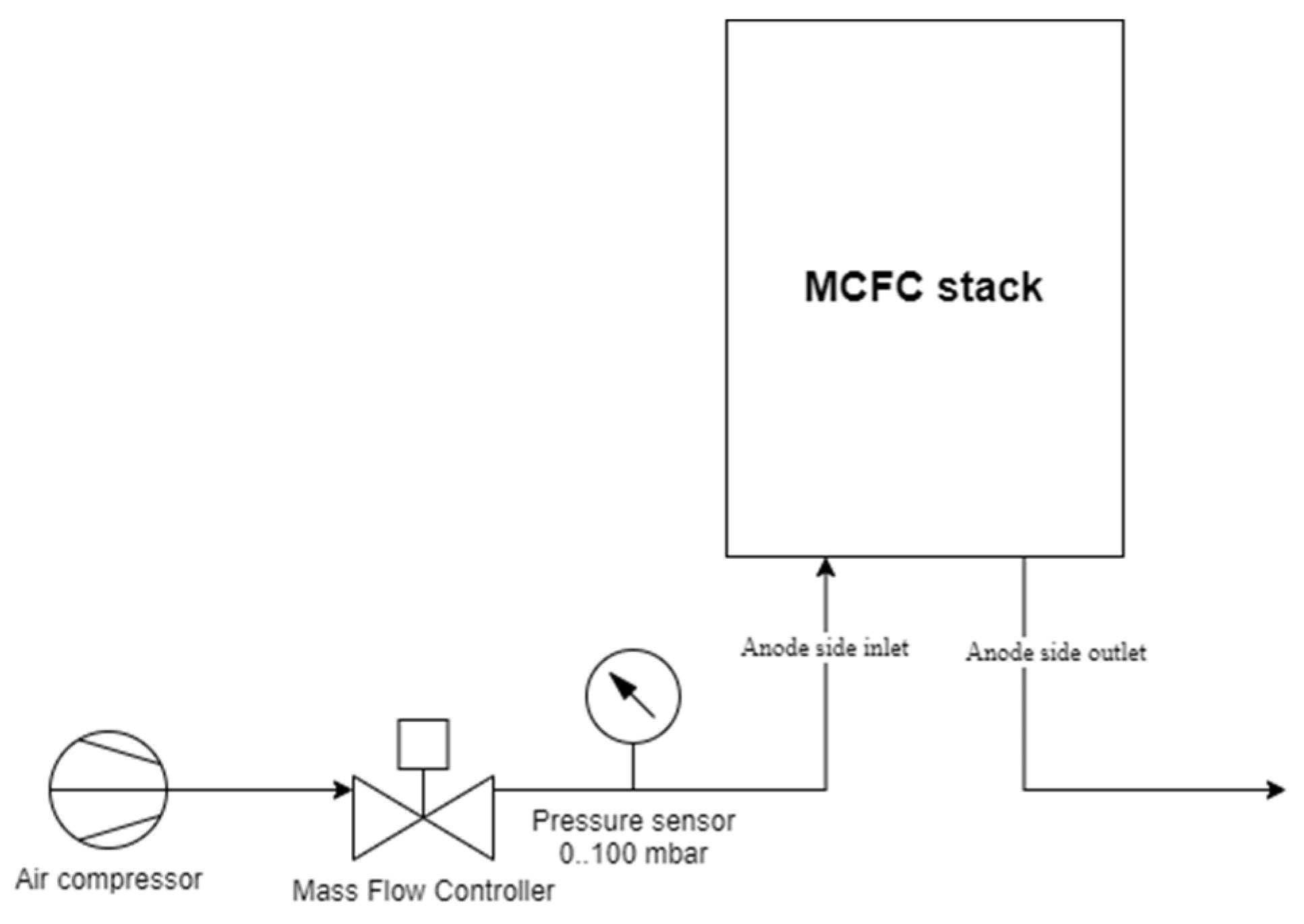
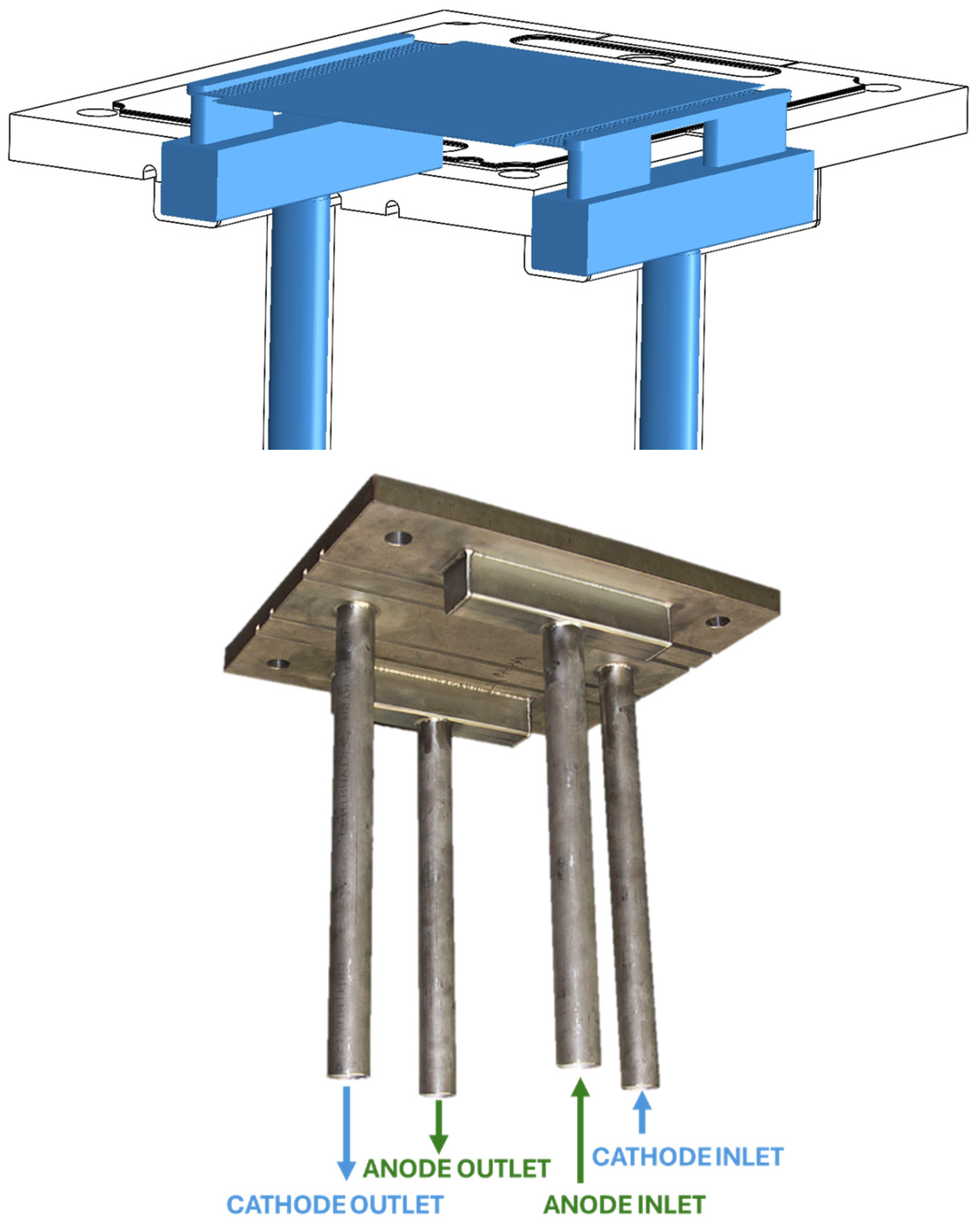
2.1. Experimental Setup
2.2. Numerical Model
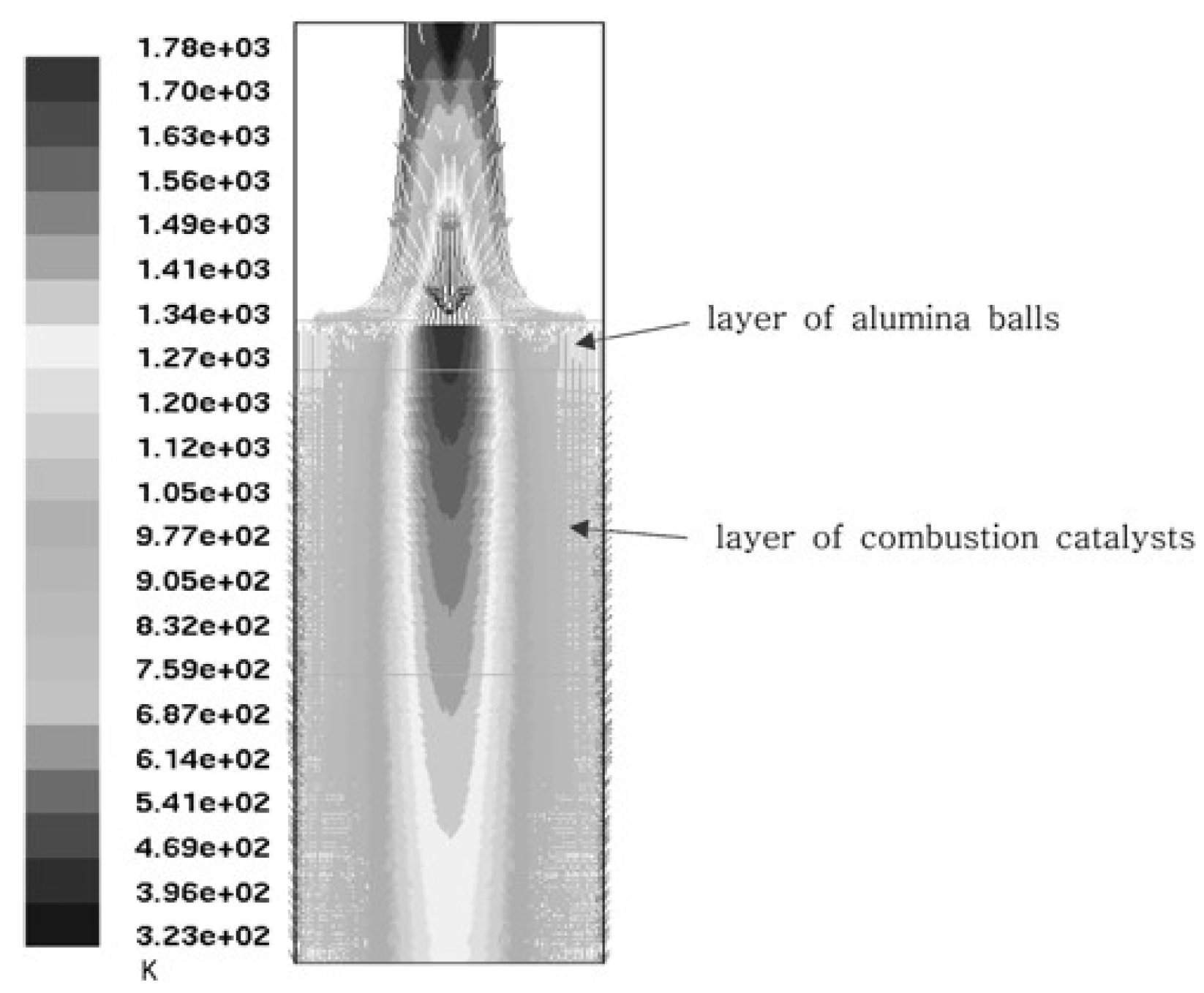
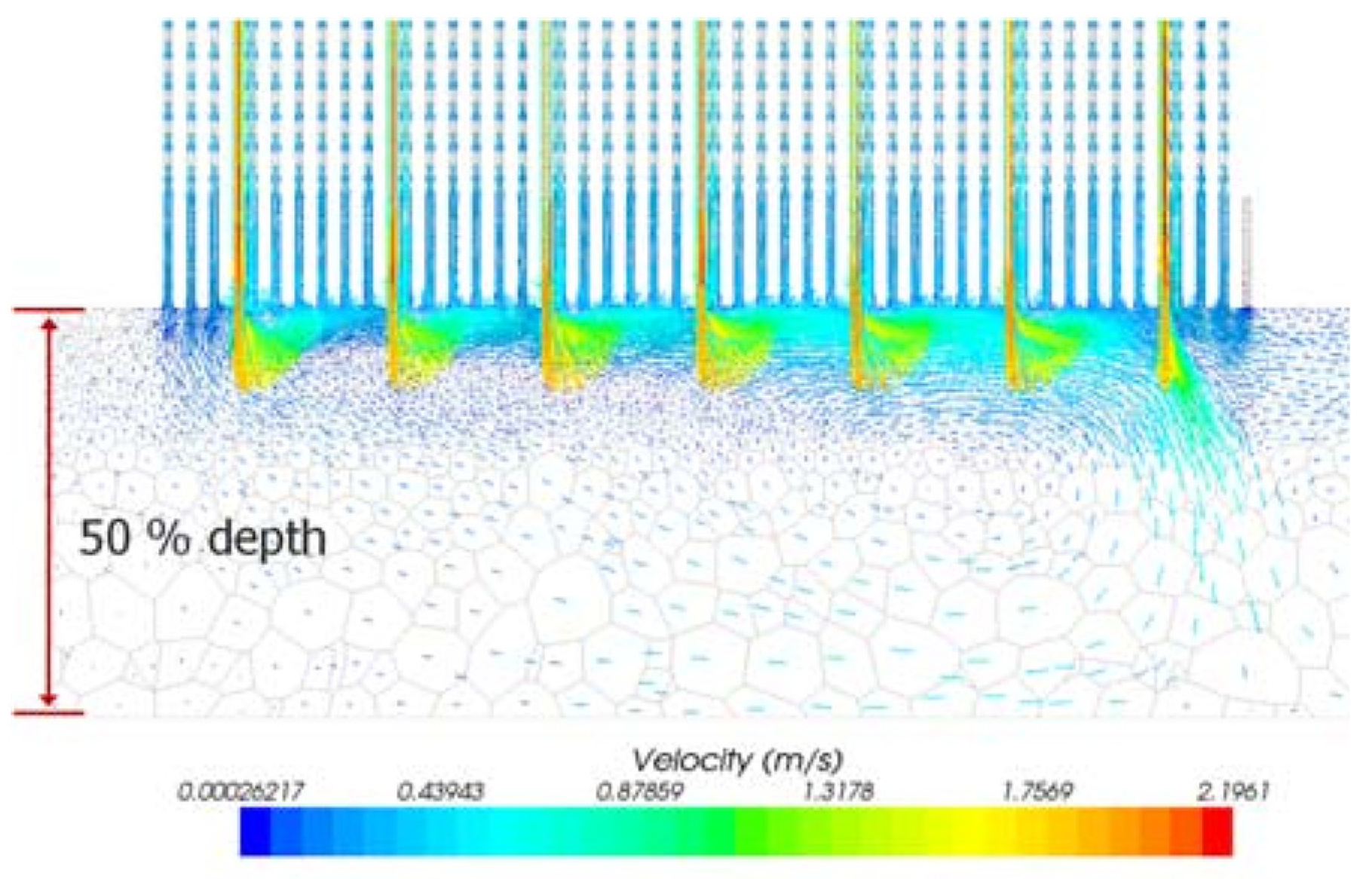
3. Numerical Analysis of the Flow Distribution Inside the Electrode Channel
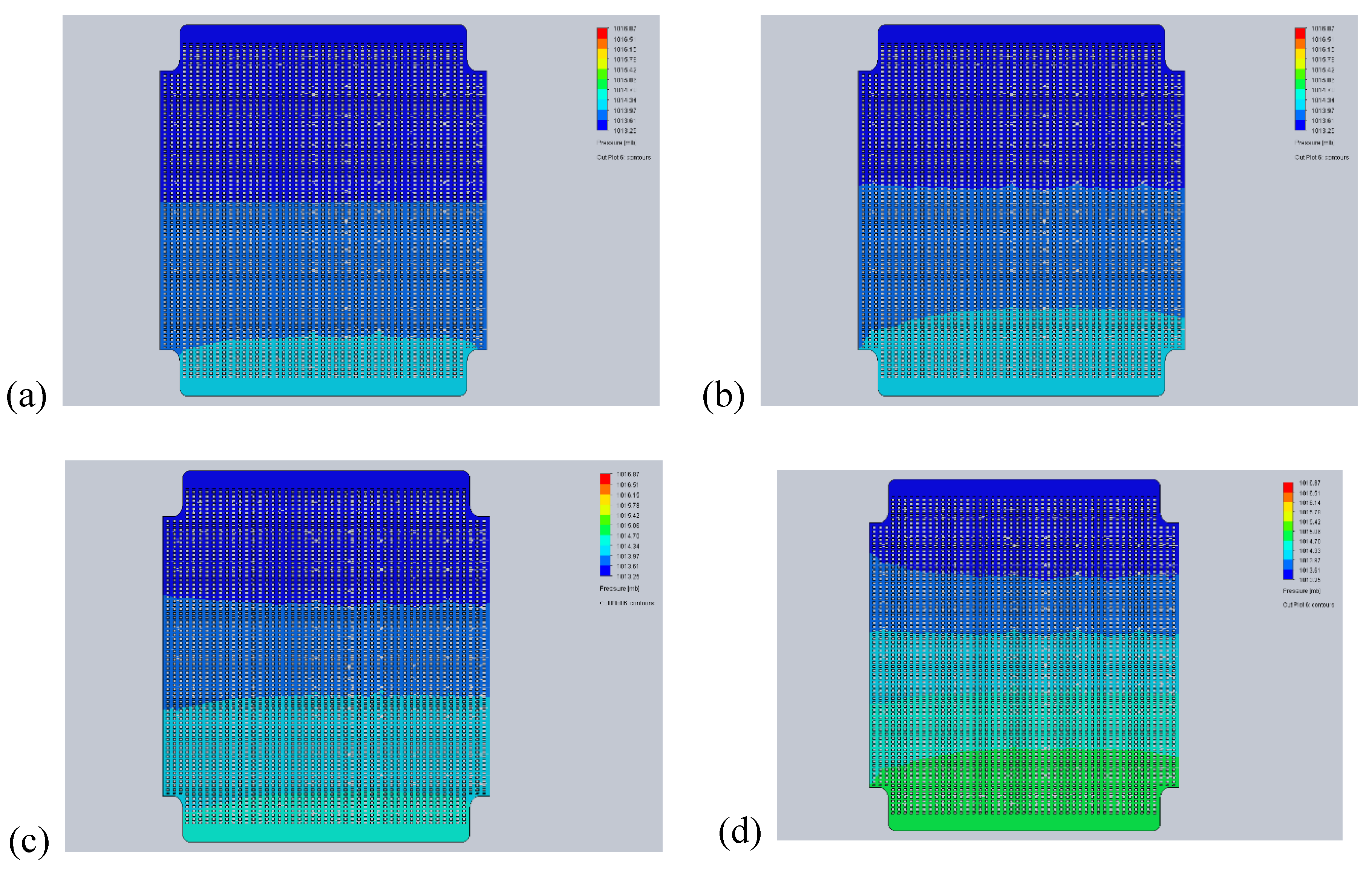
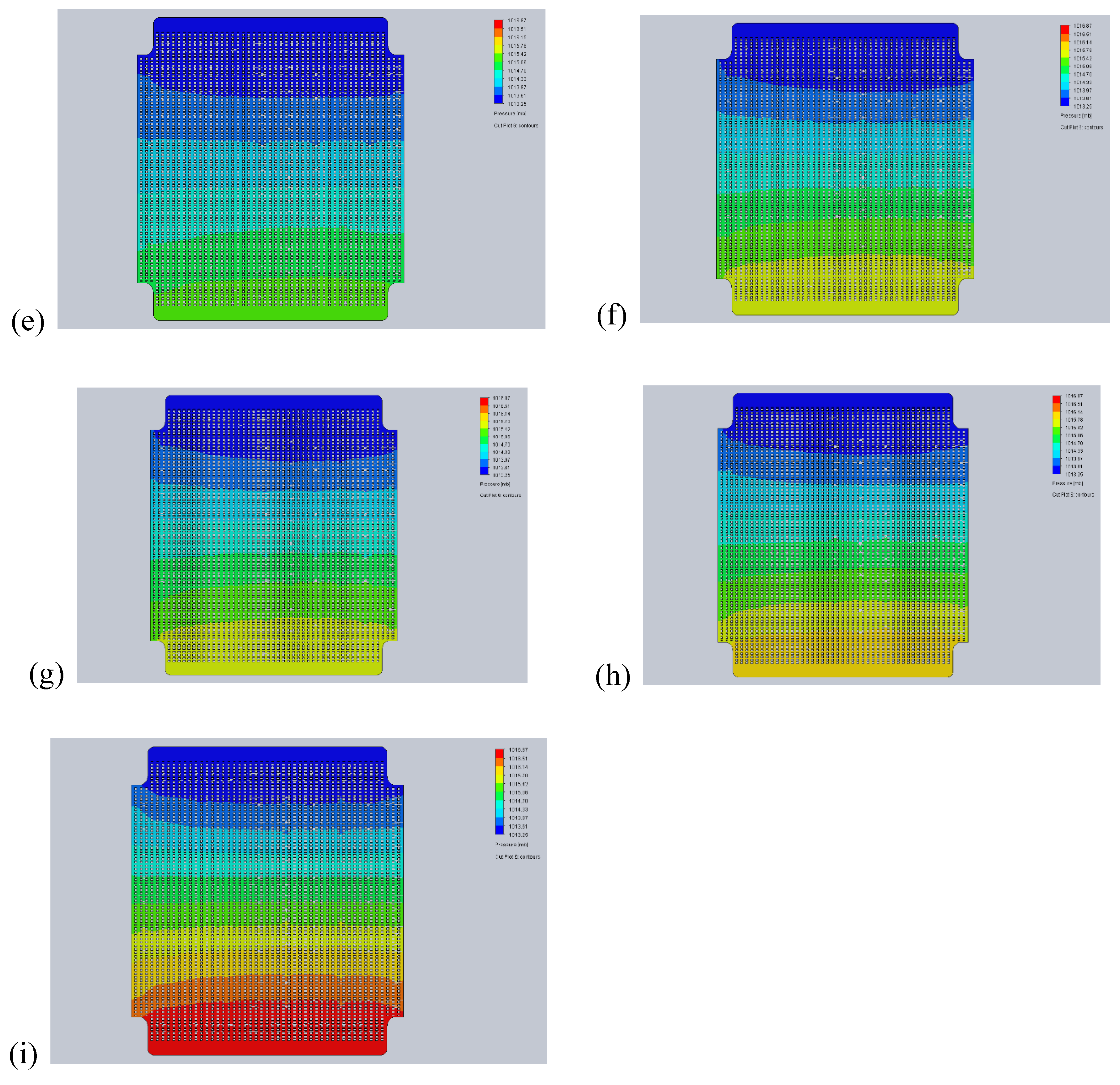
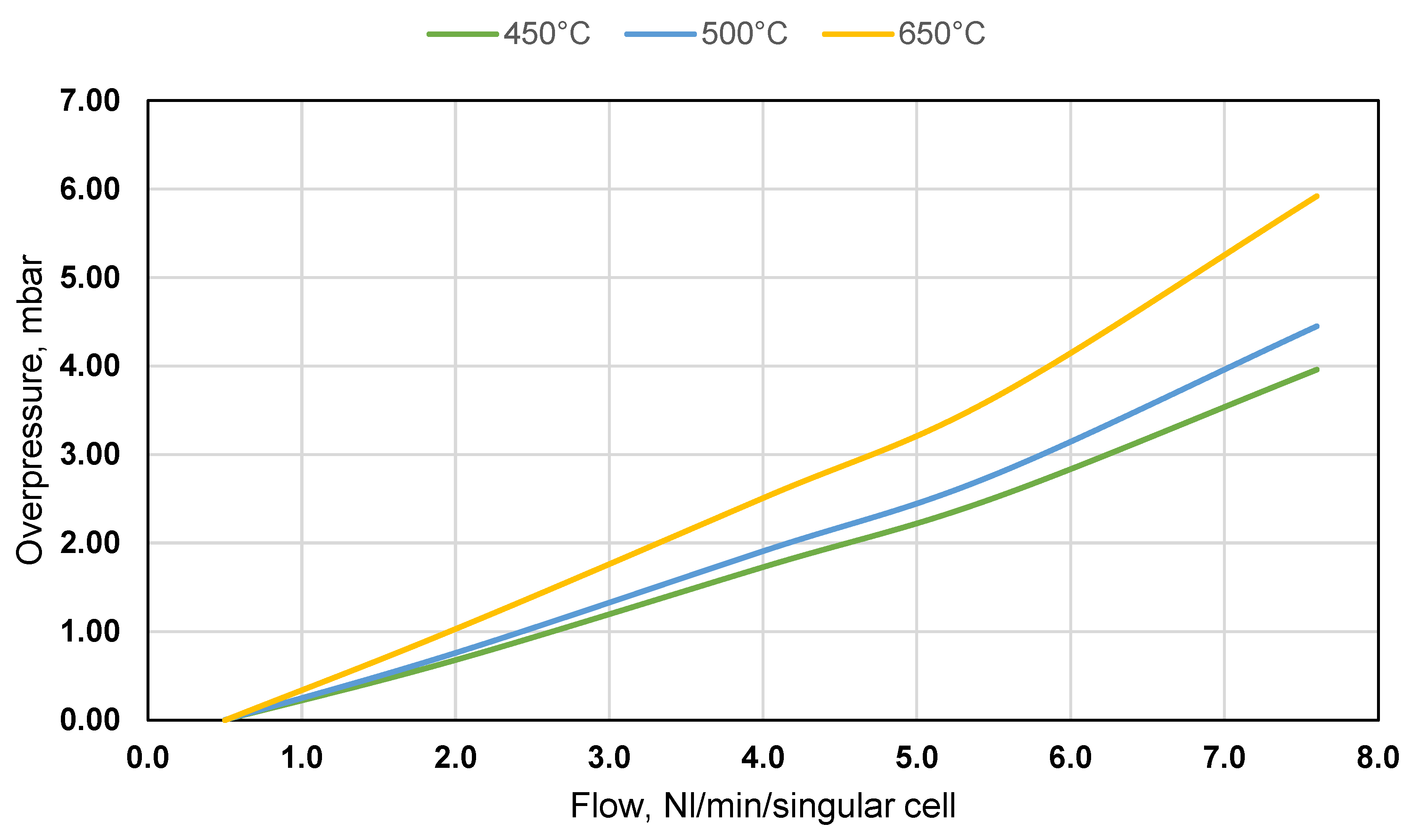
- 450°C – melting temperature of electrolyte. Below 450°C, electrolyte is in powder form, which could be easily pushed out by a large overpressure.
- 500°C – the minimum operating temperature of MCFC. The flow distribution is examined to deliver data for the MCFC control strategy.
- 650°C – the nominal operating temperature of MCFC. At this operating point, flow distribution is the primary focus of interest.
Conclusions
4. Acknowledgements
References
- Comission, E. Energy Union Package, A Framework Strategy for a Resilient Energy Union with a Forward-Looking Climate Change Policy. 2015, COM(2015).
- Nations, U. “Paris Agreement.” Report of the Conference of the Parties to the United Nations Framework Convention on Climate Change. (21st Sess.
- Liu, H.; Khan, M.Y.A.; Yuan, X. Hybrid Maximum Power Extraction Methods for Photovoltaic Systems: A Comprehensive Review. Energies 2023, 16. [Google Scholar] [CrossRef]
- Ishikawa, T.; Yasue, H. Start-up, Testing and Operation of 1000 KW Class MCFC Power Plant. J. Power Sources 2000, 86, 145–150. [Google Scholar] [CrossRef]
- Bischoff, M.; Huppmann, G. Operating Experience with a 250 KWel Molten Carbonate Fuel Cell (MCFC) Power Plant. J. Power Sources 2002, 105, 216–221. [Google Scholar] [CrossRef]
- Milewski, J.; Zdeb, J.; Szcz\keśniak, A.; Martsinchyk, A.; Kupecki, J.; Dybiński, O. Concept of a Solid Oxide Electrolysis-Molten Carbonate Fuel Cell Hybrid System to Support a Power-to-Gas Installation. Energy Convers. Manag. 2023, 276, 116582. [Google Scholar] [CrossRef]
- Dybiński, O.; Milewski, J.; Szabłowski, Ł.; Szczęśniak, A.; Martinchyk, A. Methanol, Ethanol, Propanol, Butanol and Glycerol as Hydrogen Carriers for Direct Utilization in Molten Carbonate Fuel Cells. Int. J. Hydrogen Energy 2023. [Google Scholar] [CrossRef]
- Dybiński, O.; Milewski, J.; Szcz\keśniak, A.; Martsinchyk, A.; Szabłowski, Ł. Experimental Investigation of Porous Anode Degradation of a Molten Carbonate Fuel Cell Fed with Direct Fermentation Product Composed of Bioethanol. Int. J. Hydrogen Energy 2023. [Google Scholar] [CrossRef]
- Szczęśniak, A.; Milewski, J.; Szabłowski, Ł.; Dybiński, O.; Futyma, K. Numerical Analysis of a Molten Carbonate Fuel Cell Stack in Emergency Scenarios. J. Energy Resour. Technol. 2020, 142. [Google Scholar] [CrossRef]
- Szczęśniak, A.; Milewski, J.; Szabłowski, Ł.; Bujalski, W.; Dybiński, O. Dynamic Model of a Molten Carbonate Fuel Cell 1 KW Stack. Energy 2020, 200, 117442. [Google Scholar] [CrossRef]
- Milewski, J.; Wołowicz, M.; Miller, A.; Bernat, R. A Reduced Order Model of Molten Carbonate Fuel Cell: A Proposal. Int. J. Hydrogen Energy 2013, 38. [Google Scholar] [CrossRef]
- Shuhayeu, P.; Martsinchyk, A.; Martsinchyk, K.; Szcz\keśniak, A.; Szabłowski, Ł.; Dybiński, O.; Milewski, J. Model-Based Quantitative Characterization of Anode Microstructure and Its Effect on the Performance of Molten Carbonate Fuel Cell. Int. J. Hydrogen Energy 2023. [Google Scholar] [CrossRef]
- Kim, Y.; Lim, K.; Salihi, H.; Heo, S.; Ju, H. The Effects of Stack Configurations on the Thermal Management Capabilities of Solid Oxide Electrolysis Cells. Energies 2023, 17, 125. [Google Scholar] [CrossRef]
- Verda, V.; Sciacovelli, A. Design Improvement of Circular Molten Carbonate Fuel Cell Stack through CFD Analysis. Appl. Therm. Eng. 2011, 31, 2740–2748. [Google Scholar] [CrossRef]
- Roh, G.; Na, Y.; Park, J.-Y.; Kim, H. Analysis of Internal Gas Leaks in an MCFC System Package for an LNG-Fueled Ship. Appl. Sci. 2019, 9, 2330. [Google Scholar] [CrossRef]
- Kim, S.-G.; Choi, Y.-J.; Jun, J.; Lim, H.-C.; Lee, J.-E. Verification of CFD Modeling for 5kW Class MCFC Stack Composed of 7 Unit Cells with 7,500cm 2 in Effective Electrode Area. ECS Trans. 2008, 12, 467–474. [Google Scholar] [CrossRef]
- Marra, D.; Bosio, B.; Arato, E. Fluid-Dynamic Characterisation of MCFC Gas Distributors. Chem. Eng. Process. Process Intensif. 2009, 48, 797–807. [Google Scholar] [CrossRef]
- Kim, S.-G.; Kuk, S.T.; Choi, Y.-J.; Jun, J.; Kim, D.-H.; Kim, B.; Lim, H.-C. Operation Results of the External Reforming Type 75kW Class MCFC Stack. ECS Trans. 2010, 26, 399–406. [Google Scholar] [CrossRef]
- Roh, G.; Na, Y.; Park, J.-Y.; Kim, H.W.H.; Kim, S.-G.; Choi, Y.-J.; Jun, J.; Lim, H.-C.C.; Lee, J.-E.; Seo, H.K.; et al. Computational Fluid Dynamic Analyses of Catalytic Combustors for 100 KW-Class Molten Carbonate Fuel Cell. Int. J. Hydrogen Energy 2009, 26, 72–78. [Google Scholar] [CrossRef]
- Cho 2008 Numerical Analysis of the Gas Flow Distribution Characteristics in the Anode Flow Channel of the Molten Carbonate Fuel Cell (MCFC).Pdf.
- Liu, X.; Chen, B.; Zhao, Y.; Jung, K.-S.; Zhang, K.; Lee, C.-W. Simulation of Internal Manifold-Type Molten Carbonate Fuel Cells (MCFCs) with Different Operating Conditions. Energies 2023, Vol. 16, Page 2700 2023, 16, 2700. [Google Scholar] [CrossRef]
- Yu, J.-H.; Lee, C.-W. Effect of Cell Size on the Performance and Temperature Distribution of Molten Carbonate Fuel Cells. Energies 2020, 13, 1361. [Google Scholar] [CrossRef]
- Matsson, J.E. An Introduction to SolidWorks ® Flow Simulation 2011.
- Wallace, J.S. INVESTIGATION OF SOLIDWORKS FLOW SIMULATION AS A VALID TOOL FOR ANALYZING AIRFOIL PERFORMANCE CHARACTERISTICS IN LOW REYNOLDS NUMBER FLOWS. 2019.
- Omo-Oghogho, E.; Sadjere, E.G. Analysis of a Simulated Flat Plate Solar Collector System Using Solidworks Flow Simulator Interface. Int. J. Eng. Innov. Res. 2020, 2, 121–128. [Google Scholar]
- Khoshim, B.S.; Akhmatov, A.A. DETERMINATION OF OPTIMAL QUANTITIES AND SIZES OF TANGENTIAL SWIRLERS OF VORTEX DEVICES IN SOLIDWORKS FLOW SIMULATION. J. Chem. Technol. 2021, 29, 442–448. [Google Scholar] [CrossRef]
- Ramlan, Imadduddin and Darlis, N. Comparison between Solidworks and Ansys Flow Simulation on Aerodynamic Studies. J. Des. Sustain. Environ. 2020, 2. [Google Scholar]

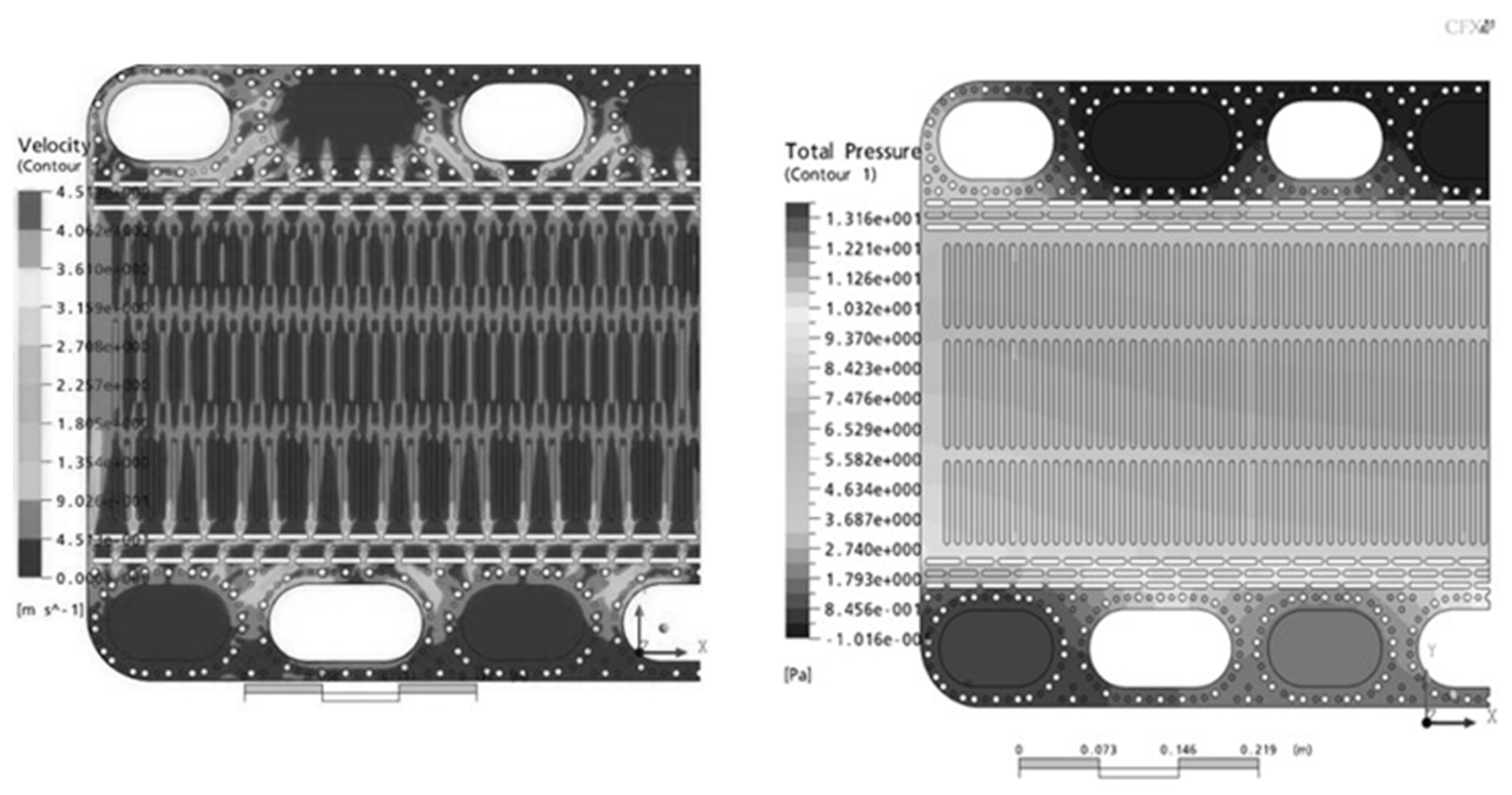

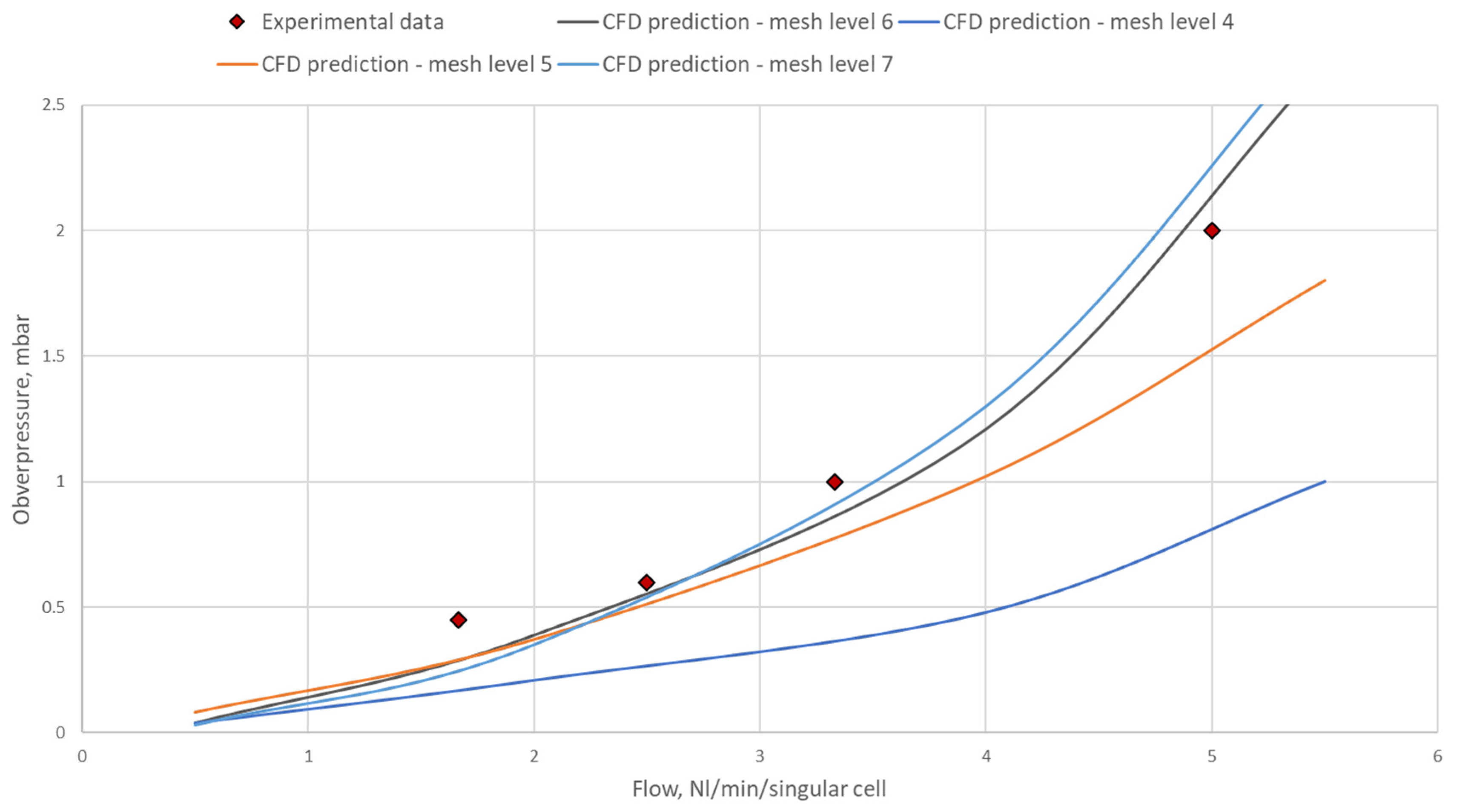
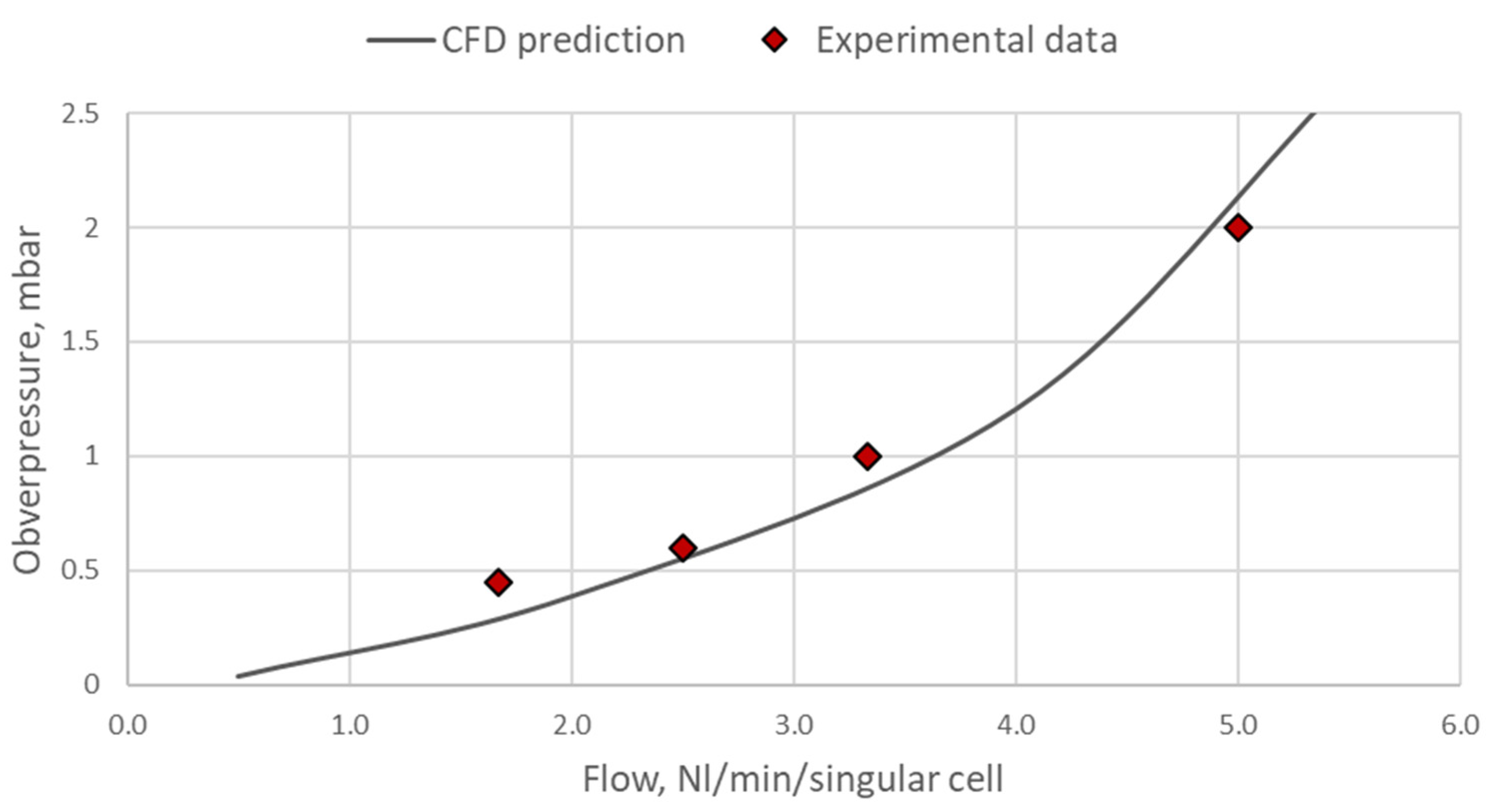
| Inlet opening | Outlet opening | |
|---|---|---|
| Type | Flow | Environmental pressure |
| Fluid mixture | pure N2 | - |
| Flow | 50 .. 300 Nl/min | - |
| Ambient temperature | 20°C | - |
| Parameter | Value |
|---|---|
| Anodic gas flow rate | H2: 1.75 Nl/min |
| Cathodic gas flow rate | CO2: 1.8 Nl/min |
| Air: 3.2 Nl/min | |
| Anodic gas temperature inlet | 650°C |
| Cathodic gas temperature inlet | 650°C |
| Current drawn from a cell | 0 A |
| Active cell area | 650 cm2 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





