3.1. Reduction of the in Contact Area Real Pressure and the Physiochemical Implications
One of the possibilities for reducing wear is the reduction of the in contact area real pressure of the friction pairs in selective transfer conditions. It is known that the real contact surface is about 10 - 100 times smaller than the nominal friction surface [
3,
17,
18], it changes very little during the working process if it is properly run, because it is related to the self-adjustment mechanism and obtaining optimal roughness during operation [
17,
19]. Along with the conditions that the lubricants must meet in real contact, the stress, the materials of the friction pairs and many other factors have an influence. In addition, during the operation of friction pairs, a large part of the friction surface is not used, which means that there is a reserve.
In the contact areas, high real pressures arise, which act on the surface even at low nominal loads, which can lead to elastic and plastic deformations of certain areas. For this reason, to decrease the value of the real contact pressure, the nominal contact surface must be increased in several cases [
3,
17,
18,
19], for example in the case of a bearing. This is in contradiction with the requirement and effort of the designers to reduce the mass of a construction, simultaneously increasing its resistance.
The friction process under the conditions of a transfer takes place through the electrochemical processes activation, with the dissolution of the anodic alloying elements at high tensions in the areas of the contact surfaces. By dissolving the anodic components of the metal, surfactants are formed, which are adsorbed by the areas that play the role of cathodes. As a result, the resistance is reduced, and the formation of colloidal particles is favored because surfactants and colloids are very good lubricants.
It is expected that when the real contact surface increases and when the stresses from the plastic deformation range pass to lower values, the process of increasing the surface will slow down. However, the joint influence of the selective solution of the structure components and the reduction of resistance by adsorption, together with the rest of the solution of the cathode alloying components, lead to the formation of a dense layer of these components. From the point of view of density, the layer is similar to a liquid, something proven by Rybaboka and Sevenko in ref. [
4] and confirmed by Ilia in ref. [
20]. The fact that this layer is in a special structural state also conditions its lubrication capacity. This makes it possible for the friction to take place under conditions of a much higher pressure than with the friction of mixed or adhesion layers.
Increasing the real contact surface and the corresponding reduction of the real contact stress are possibilities for reducing friction and wear and increasing the load-carrying capacity. This is necessary for the protection of the layer against breakage, a change in its deformation during the friction process.
Therefore, research has shown that a contact actual pressure reduction increases the safety of friction pairs, as well as their bearing capacity.
3.2 Reduction of Shear Resistance and Deformation of Superficial Layers
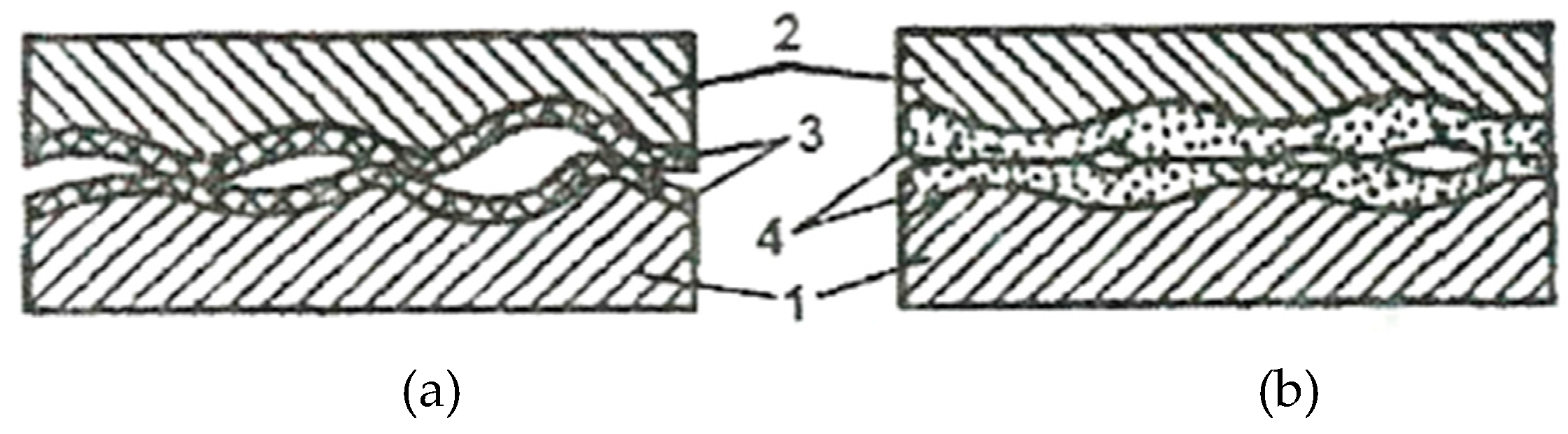
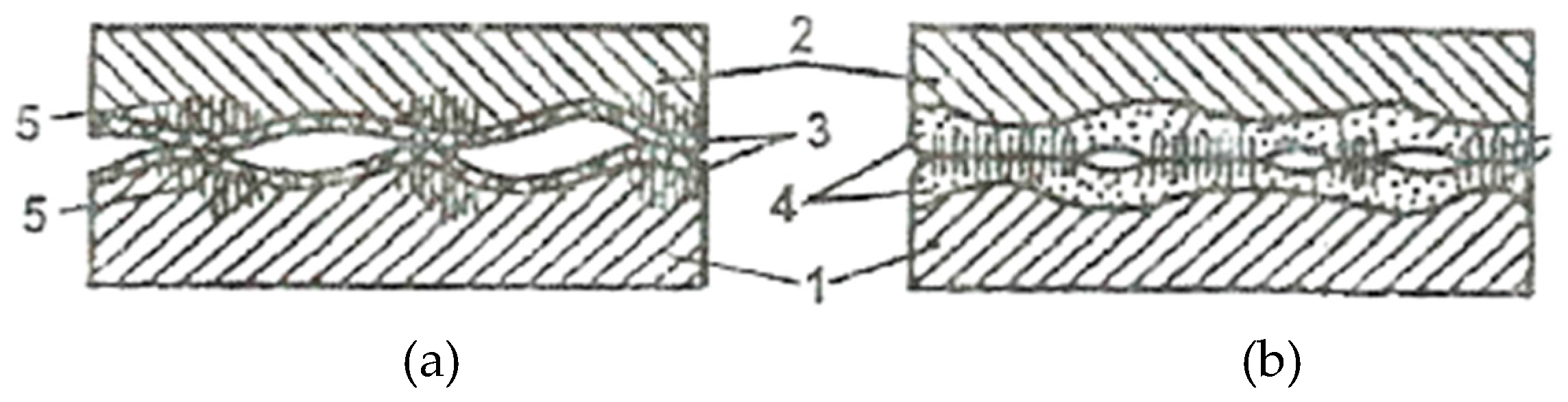
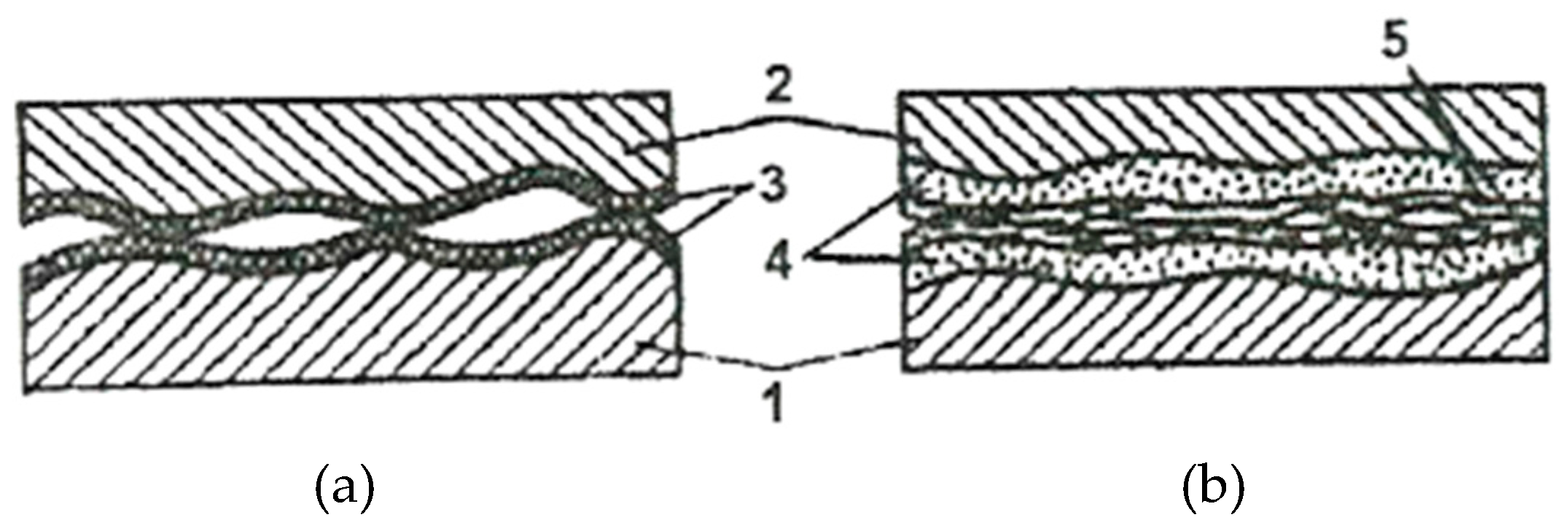
The reduction of the real contact pressure is the result of the formation of the servowitte film on the friction surfaces as a result of the selective dissolution of a thin superficial layer, in the friction process. This film (layer) ensures during deformation processes a dislocations agglomeration, similar to the malleable materials, and, by this, protects it against destruction. In the presence of organic compounds and surfactants, this layer allows obtaining a coefficient of friction (COF) comparable to fluid friction.
From the experimental research presented in the papers [
4,
13], it turned out that this layer has a high density of point defects (vacancies) of 1021 atoms/cm3, which exceeds the number of vacancies (1018 - 1019 atoms/cm3) that are determined in normal heating or deformation conditions.
The selective dissolution of the alloying components of a copper alloy causes a surplus of defects both in chemical compounds and in the crystalline network of this solid solution. Apart from this, defects arise also at the deformation of the superficial areas and, at the same time, the exit of dislocations on the surface. The layer thickness is about 1 - 3 μm (on average) and it is extremely porous, thereby reducing its thickness even more, and its dimensions are compared with the stresses field of dislocations.
The surfactant substances that are in the pores of this layer reduce the resistance of the pore walls, and the high mobility of dislocations in the layer is caused by the following factors: the high density of defects, the Rebinder effect, and the reduced thickness of the pore walls.
Increasing the real contact area to approximately the nominal contact area size and reducing the coefficient of friction led to the assumption that friction does not unfold between the solid areas of the surface but between limited areas with an interaction very reduced inside these areas. Researching this state of the layers is difficult because it exists only in the friction process and only under conditions of very high pressure, at a well-established temperature, and only during the development of special tribotechnical processes. When the rubbing process is finished, this layer ceases to exist.
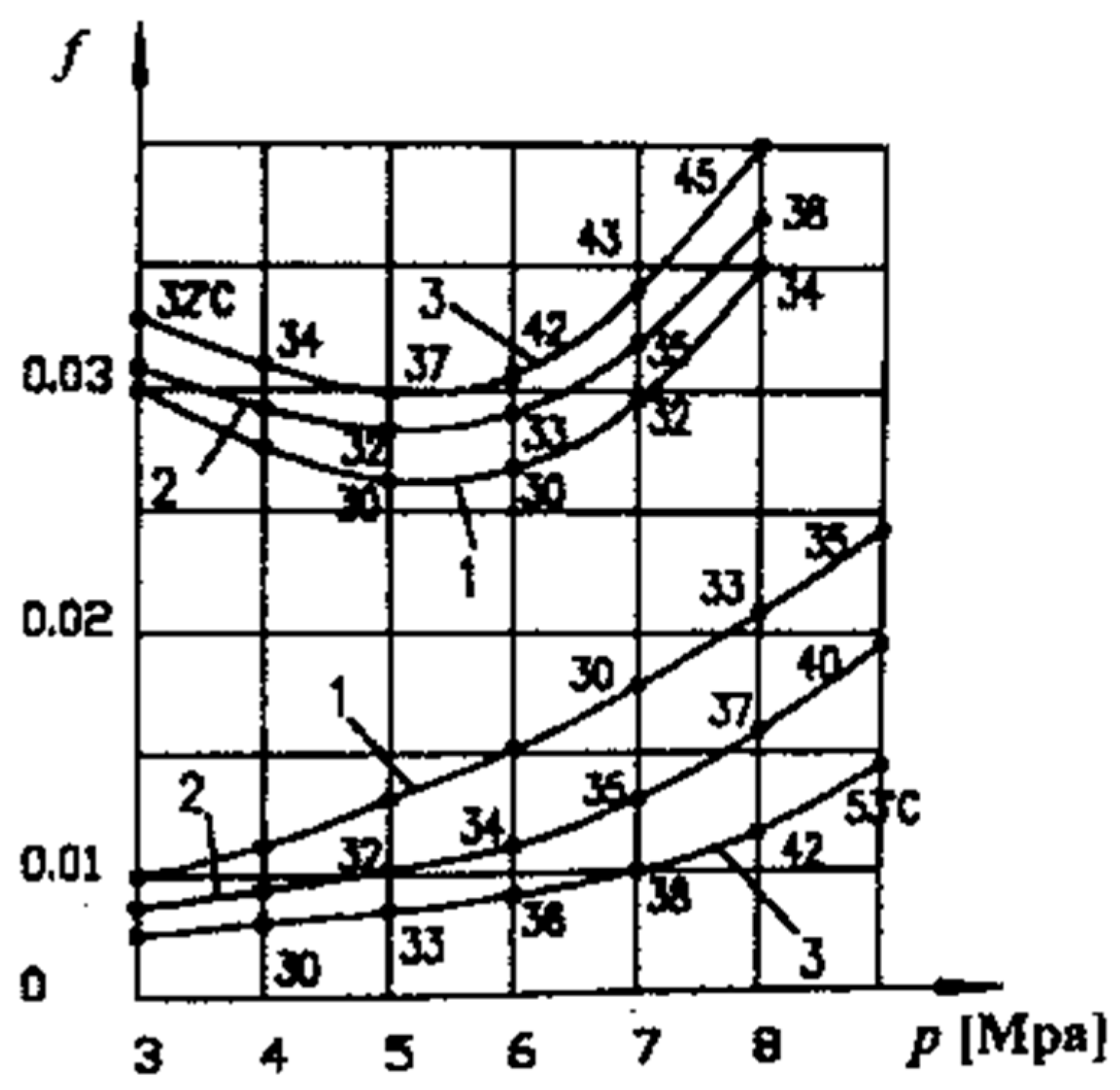
Figure 6 shows the variation of COF with pressure, p, at different sliding speeds under the friction conditions between the adherent layers (upper curves) and selective transfer (lower curves). Average temperature values are shown on the curves, which resulted in the COF values corresponding to the given pressures (see
Figure 6) and were determined experimentally on copper-based alloy specimens (CuSn12T equivalent to UNS-C90800) in contact with steel specimens (OLC 45 equivalent to AISI/SAE 1045) [
2,
21] of roller - shoe type, tested on the Amsler installation.
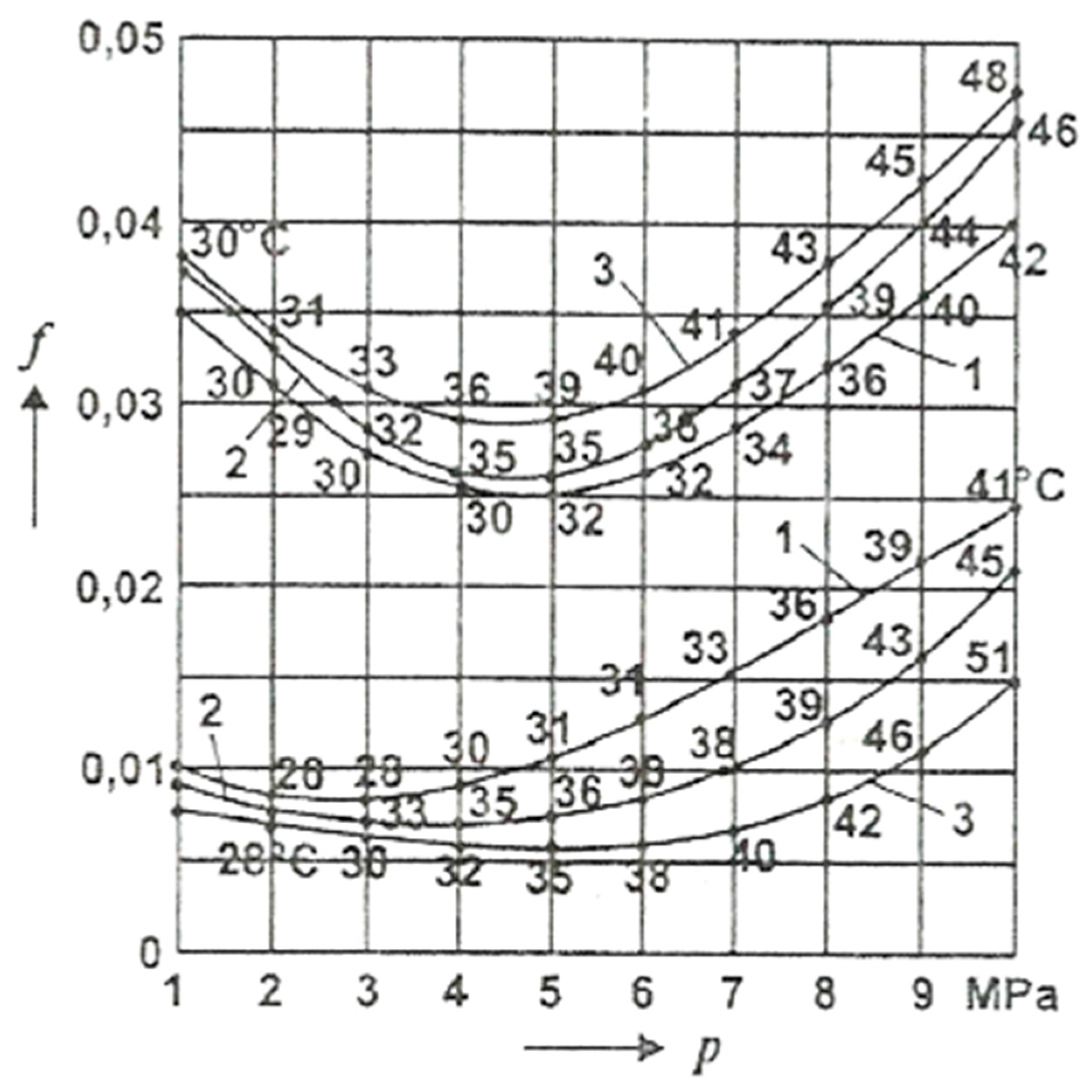
For comparison,
Figure 7 presents the COF variation with the average pressure, p under conditions of selective transfer at different sliding speeds in the spindle-bearing installation (spindle made of OLC 45 (AISI/SAE 1045), bronze bearing CuSn12T (UNS-C90800) ), lubricated with glycerin, coefficient obtained experimentally.
It can be observed that the values of these coefficients are close, having approximately the same shape, with slightly higher values, at the same pressures, than in the case presented in
Figure 6. At the same time, it is noted (in both situations) that the temperature rise due to the friction of the adherent layers causes the increase of the coefficient of friction with the increase of speed, while in the conditions of selective transfer, there is a reduction of the coefficient of friction with the increase of temperature.
The reactions that take place during the selective transfer determine an improvement in lubrication and a reduction in the viscosity of this layer, during the chemisorption processes. This is not possible with the usual friction between the adherent layers. Similar reactions take place in conditions where high demands suddenly appear during the selective transfer.
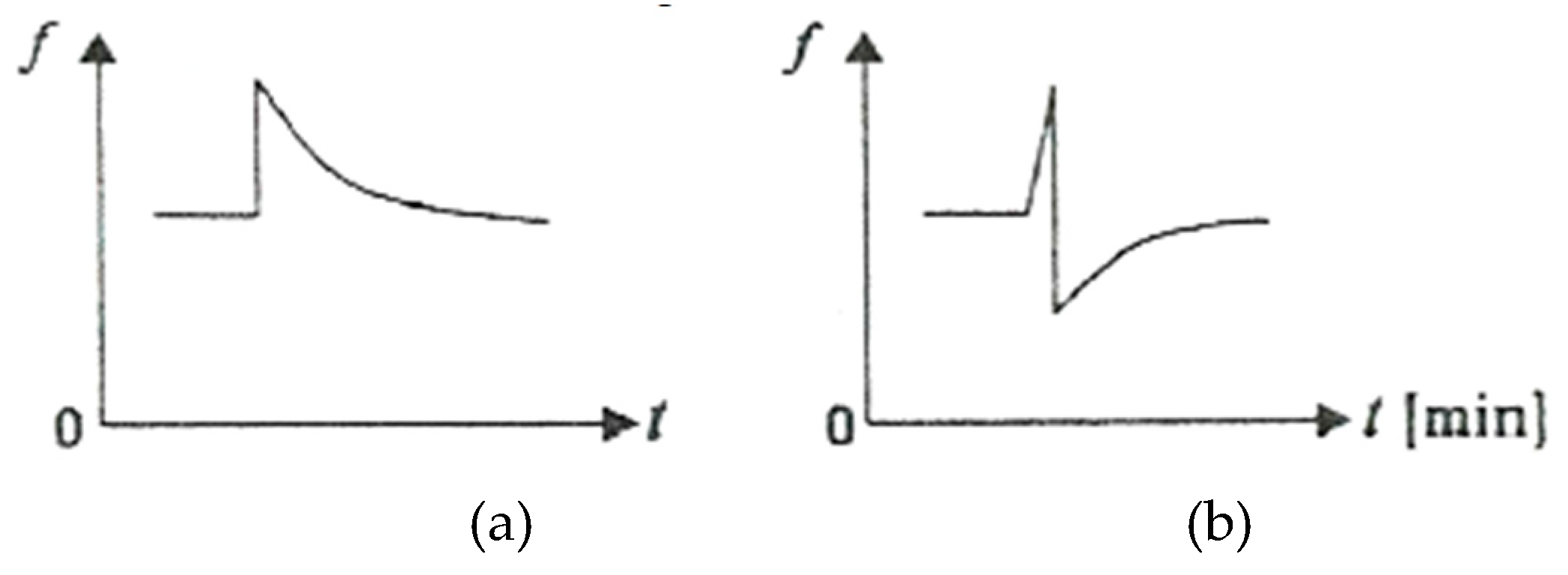
Figure 8 shows schematically the variation of COF as a function of time t, upon the sudden appearance of an additional stress under conditions of friction between adherent layers (
Figure 8, a) and under conditions of selective transfer (
Figure 8, b) [
1,
2,
22].
After removing the additional load, the COF value gradually returns to the initial value under the friction conditions between the adherent layers and selective transfer conditions, the COF decreases greatly, to gradually return to the initial value.
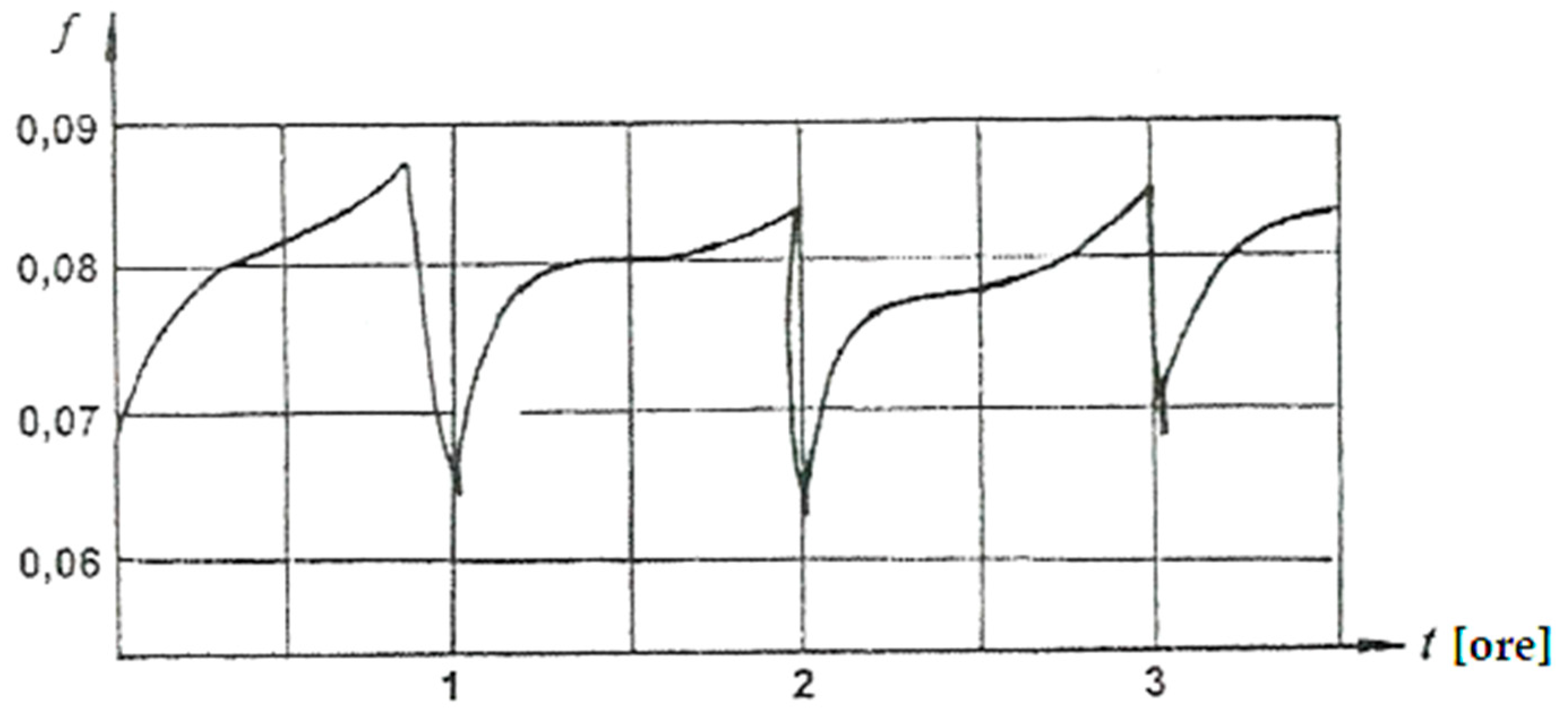
The way COF varies over time for a pressure of 4.5 MPa and speed of 0.2 m/s is shown in
Figure 9.
It is observed that the COF varies around the value of 0.08, after an almost repeatable cycle, the duration of the cycle being approximately 1 hour. The form of the typical relaxation of the oscillations is due to the unloading of dislocation clusters and the accumulation of vacancies [69]. The surface reactions are the same as when the temperature increases, namely the transition from adsorption to chemisorption, with additional dissolution and formation of surface-active lubricants.
The sevowitte layer depends (in terms of the protective effect against abrasive, corrosive wear, as well as in terms of the effect of reducing the friction force) on the particularities of the copper alloy, such as the properties and qualities of the alloying elements, the limit of solubility and the properties of surfactants that arise during friction.
If friction takes place only in a single medium composed of dissolved mineral salts and metal particles, then the layer that is formed provides protection against wear, but the protective effect is considerably reduced, and the COF value is, therefore, higher than with the servowitte layer which contains surface-active lubricants, called “dividal” (layer formed by the combination of the surface-active substances from the base lubricant and the metal ions that arise through electrochemical processes - ionic lubrication - in the friction zone) [
1,
2].
In the absence of organic hydrocarbon molecules in the lubricant, which carry out the selective transfer, there are no more processes of compensating the deformation of the surface layer or polarization. In addition, the absence of obstacles in the superficial layer prevents the penetration of oxygen, due to the surfactants, and thus an oxidation on the surface, where this layer is not in a state of contact. Wear is continuously reduced, as the friction surfaces are separated from this layer in the friction process.
3.3. Electrical Phenomena Under Conditions of Selective Transfer
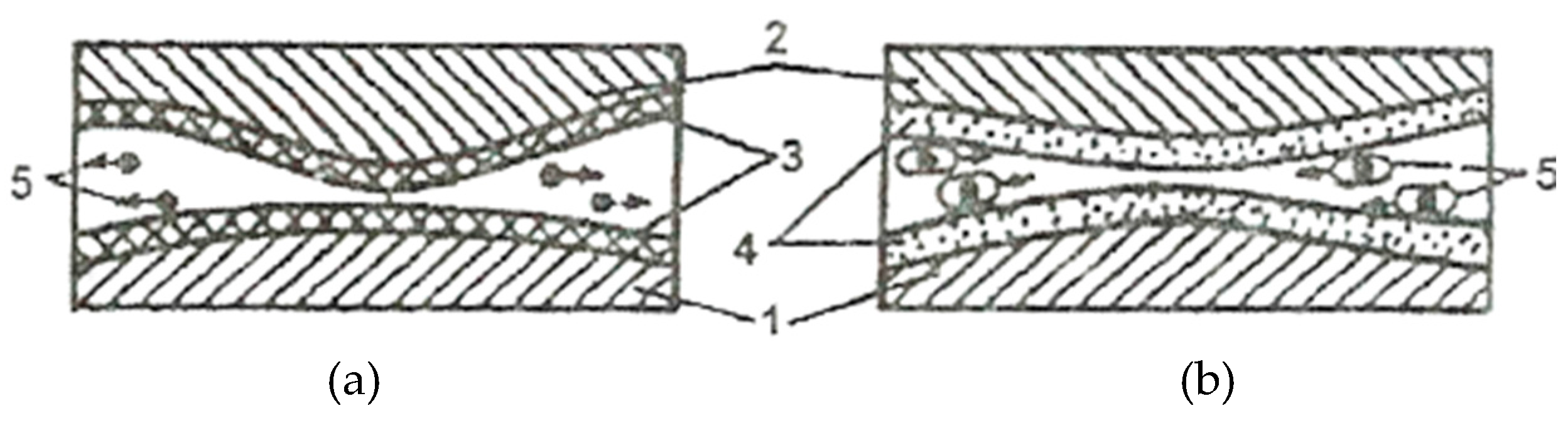
Under conditions of selective transfer, electrical phenomena also occur in the contact area, which lead to the formation of a double layer with electrical charges. But, as a result of tribochemical processes and a metal dispersion in the friction process, inorganic layers, complex and metal-organic compounds, colloids, and simple electrically charged particles are formed, which are the cause of electro-kinetic phenomena.
The considerable potentials that are formed during frictional stress in the dispersion medium cause an electrophoretic movement and the precipitation of particles in the area of the frictional contacts (electrophoresis is the directed movement of particles in a solution, which arises from the effect of an electric field). The processes of electrophoresis were also confirmed experimentally [
7,
23] and realized practically, in the different forms of selective transfer.
Thus, the copper particles transfer to steel surface, but also the gradual reduction of the alloying components in the surface layer led to a decrease in the potential from the friction initial stage to a value approaching zero, because there is a difference of potential between the contact area and the one without contact. In the situation where one of the friction surfaces, or both, of a friction pair are dielectric, the electrically charged double layer can be a result of the so-called triboelectrification.
For this reason, in the lubrication slot, between the friction surfaces, both larger and smaller forces act as a shoulder of the electric field, which also causes electro-kinetic phenomena, and through the deposition of colloid particles or particles of the cathode metal, a reduction of frictional force and erosion. Such particles are in the lubricant and, therefore, also in the field of the electrically charged double layer. The exception is the lubricant, where its tribodestruction is lower and the friction occurs between the same metals, a case found in the friction of adherent layers.
Under operating conditions, the process in a friction pair also has a depolarizing effect on the polarized surface layers and thereby imposes a kind of "cleaning" of the surfaces, which leads to a transition of the servowitte layer into the metal. On this occasion, they are entrained together with the particles and molecules of the surfactant lubricant, which are then adsorbed on the surfaces of these particles. These molecules cause certain porosity of the layer, an additional lubrication effect, and, in particular, an adsorbing effect on the layer, creating the possibility that they prolong the existence of adsorption defects. A typical example of electro-kinetic deposition and precipitation of copper particles in the contact zone by friction could be observed in refrigeration units. This is where copper is removed, through the Freon solution with a slightly corrosive effect, from the copper pipes in the friction areas of the compressor.
This example is very interesting because there is no copper in the friction pairs contact area. Here, the frictional pair are steel/steel and, for this reason, the transfer of copper particles and the formation of a servowitte layer from these particles are of particular interest [
28].
In practice, pairs of friction surfaces of different materials and different lubricants are used and therefore different conditions for a selective transfer.
Table 1 shows some examples of the use of different materials, under appropriate conditions, where electro-kinetic phenomena have been proven, and the operation taking place with selective transfer [
1].
It should be noted that the precipitation of particles in the lubrication slot is not a sufficient condition for the formation of a servowitte layer. The layer takes deformations, without being destroyed, because the lubricants contain organic compounds and surfactants. Otherwise, a dividing layer must be created because it also favors the electro-kinetic capture of particles.
3.4. Protection Against Metal Oxidation
In the friction pairs contact area, due to the friction, the temperature increases; this causes the acceleration of metal oxidation reactions. A separate share of the wear of metal surfaces through the friction of adherent layers and friction without lubricant is formed by the wear of oxide layers.
In the friction pairs that work at high pressures, an increase in wear is observed, as a result of the oxide layer destruction and the formation of some local welding bridges. When the temperature of the friction pair elements increases as a result of friction (through external heating or improper heat removal), an increase of the oxide layer thickness is observed, and implicitly, an increase in wear, an inevitable phenomenon in the friction mixed or of adhesion of layers.
Under conditions of selective transfer, the adsorbed layer, which also contains surface-active substances, assumes the function of protection against oxidation of the micro-weld [
1,
2,
3]. Surfactant substances are formed in the initial stage of friction, during selective dissolution, through the destruction of the anodic components of an alloy [
1,
3]. Because the layer passes on the cathode surfaces, it blocks them against the deposition of oxygen molecules. At the same time, the resistance decreases, as a result of the adsorption effect, and facilitates the dispersion of the metal.
By dispersion, colloidal particles are formed, which, through the electrically charged double layer, are attracted to the contact area, where they discharge and combine with the metal, forming a layer. In this situation, the areas of friction surfaces have reducing properties; during the tribodestruction and oxidation of the lubricant, a series of substances with reducing action can be formed [
1].
If the lubricant lacks organic compounds, the dividing layer in formation is in the friction process under reducing conditions and does not oxidize, and in the absence of a friction function, the oxidation takes place in the usual way.
3.5. Formation of the Polymer Protective Layer
It is known that when lubricating with mineral oils, the mixed or adhesion layer does not provide sufficient protection against wear; their properties are improved by adding anti-wear, anti-oxidation, and other special additives. This is economical in terms of lubricant consumption and increases the durability of the friction pairs. Under these conditions, certain analogies with selective transfer result. Such a layer has a resistance to deformation against the moving backlash, as usually occurs with a layer of liquid on ordinary friction surfaces.
These processes take place at high pressure on the copper layer, taking place adsorption, as well as a catalytic effect of the metal when the oxide layer is worn. It is assumed that, following the heating of the contact areas at higher stresses, welding of the forming layer with the basic metal material (polymer layer) takes place. Through the wear of the polymer layer, a new layer is formed, due to the magnitude of the frictional force and the increase in temperature.
In the specialized literature [
32,
33] it is indicated as a special additive soluble in lubricants, such as mixtures of metallic compounds of polybasic acids and polyamines, to form the polymer layer.
If we compare the friction of the adherent layers with the friction during the selective transfer, then in the initial stage those processes are mainly carried out, which create favorable conditions for the formation of a solid bond between the polymerization products and the metal. For this, low pressures of the surfaces, comparable to the resistance of the layer, are needed, as well as free chemical combinations, which are formed in the initial stage of friction, when the alloying elements of an alloy are selectively detached. These combinations are important for the interaction between the friction surfaces and the polymers that form on them; also the absence of oxide layers on the friction surfaces favors the interaction.
The chemical analysis and that by mass spectroscopy showed [
8] that, following the mechanic-chemical processes on the friction surfaces between brass or bronze and steel lubricated with glycerin, a series of products derived from glycerin (aldehyde, glycerin acid, acrolein, formic acid aldehyde, etc.), but through a triboactivation they can also directly polymerize hydrocarbons.
The polymers that form help the other processes with friction and wear, creating new areas of sliding surfaces. Apart from this, the polymerized products have a so-called polyliquid consistency, as was also observed in practice [
3].
The characteristic of adhesion-friction deformation is one of the bases for increasing the reliability of friction pairs. In the present case, the forces are taken over by the polymer layer and we speak of its so-called servo properties.
Under practice conditions, for achieving a selective transfer in the friction pairs, there are different possibilities. For example, a selective transfer can also be achieved in the friction pairs of the steel/steel type, cast iron/teflon with copper insert, steel/glass, etc. Among the possibilities, we can mention brass coating, and the use of bronze additions [
2,
3]. The advantage of using such methods and processes to reduce friction and wear is very great.