Submitted:
30 November 2024
Posted:
02 December 2024
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Calculation Details
3. Results and Discussion
3.1. Structure Optimization
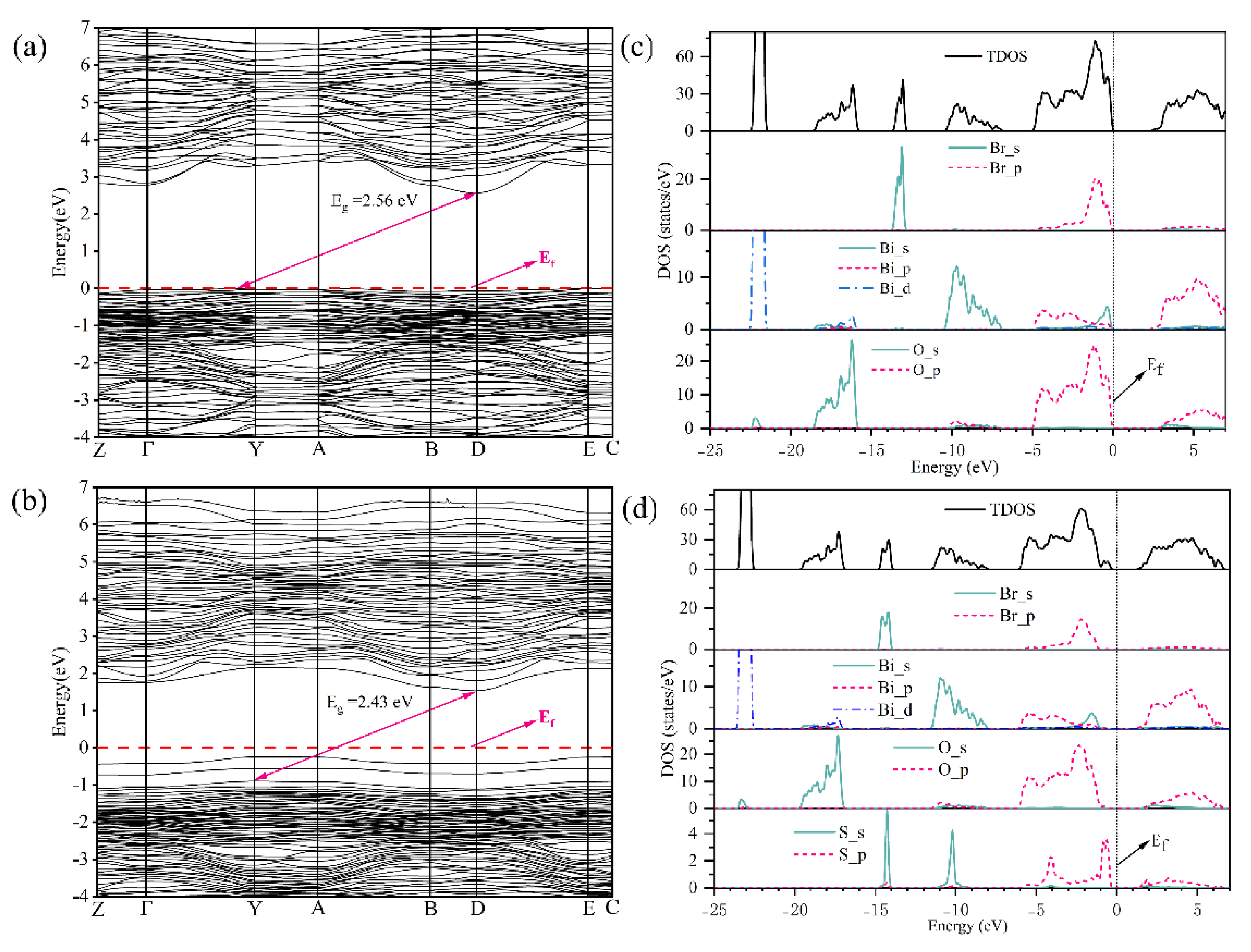
3.2. Energy Band Structure and Density of States
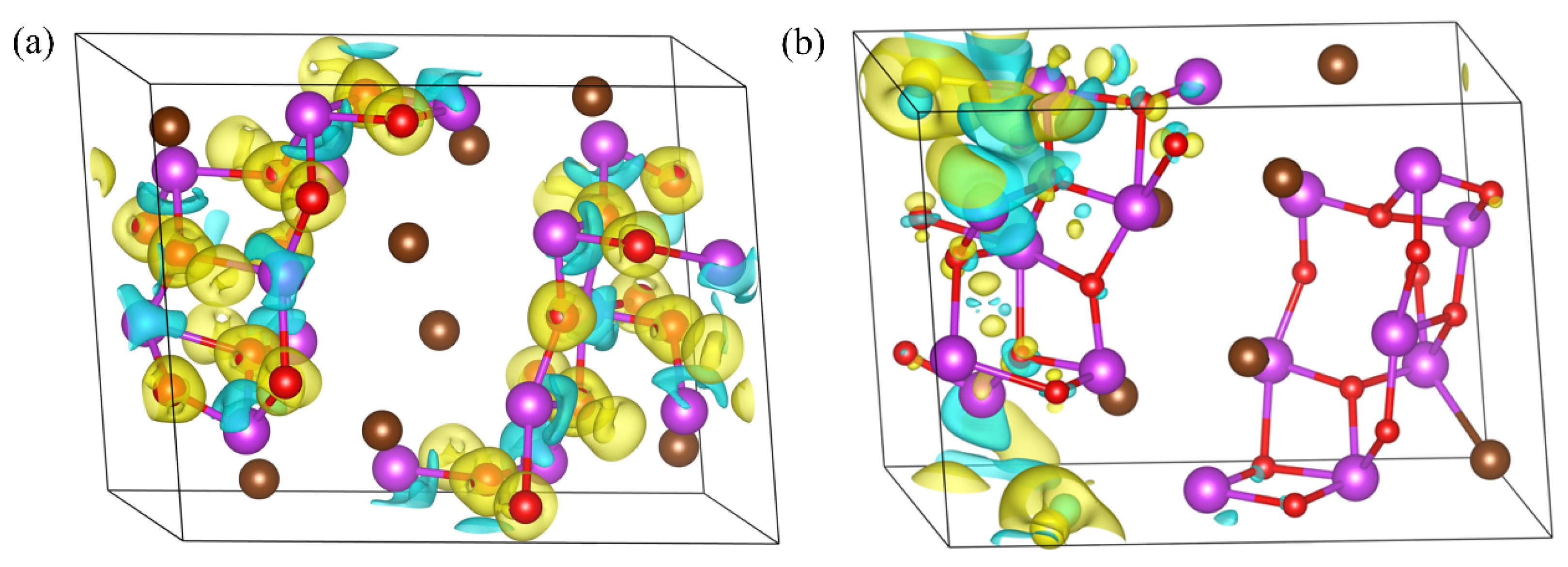
3.3. Differential Charge Density
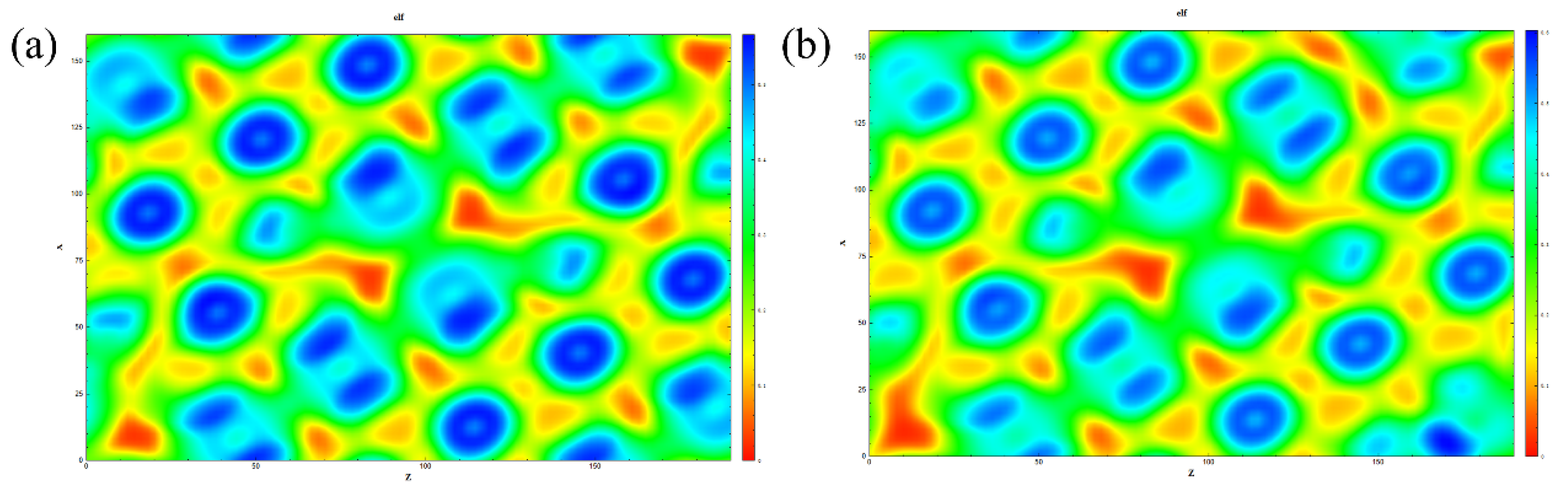
3.4. Electron Localization Function
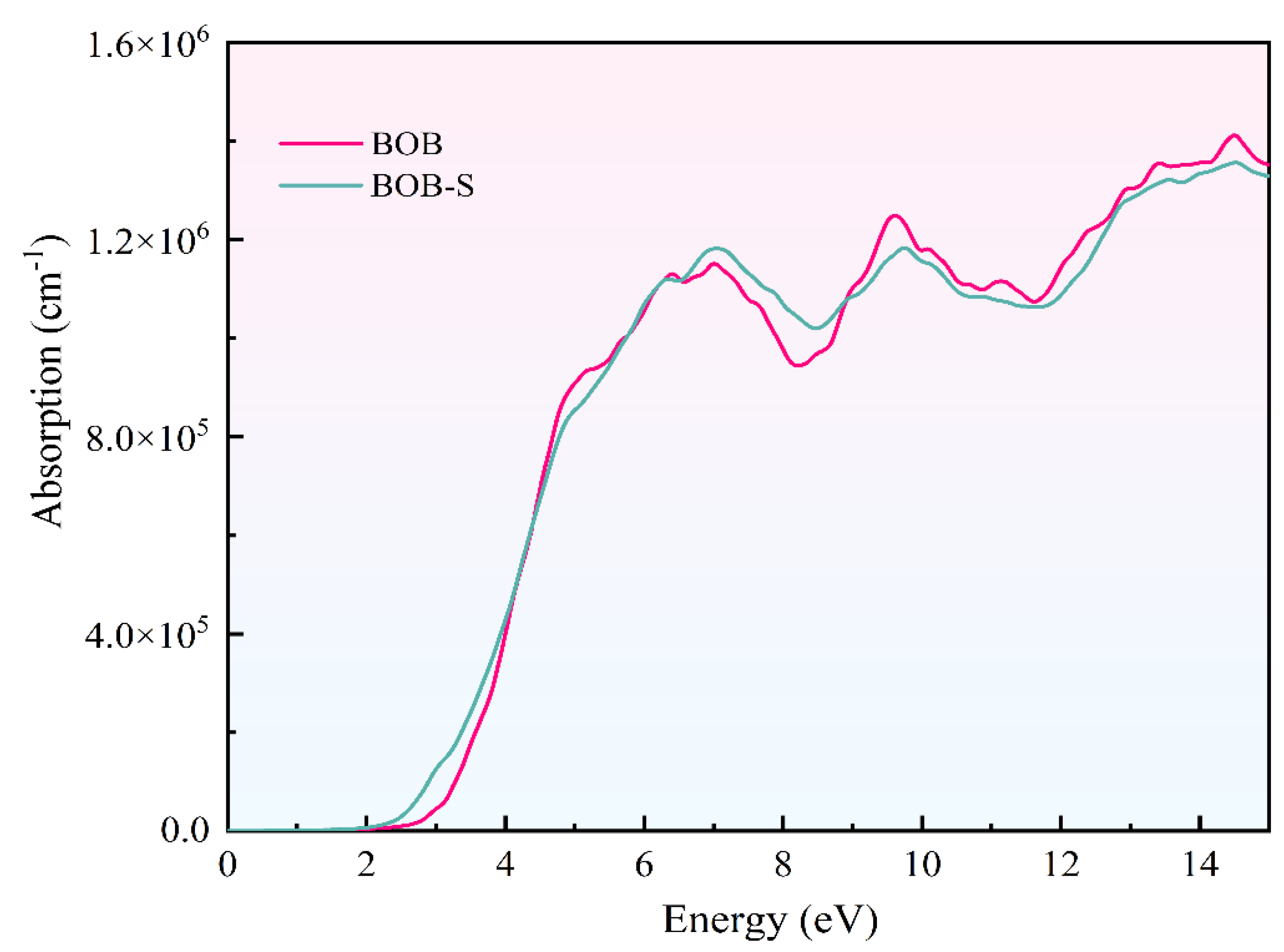
3.5. Absorption Spectrum
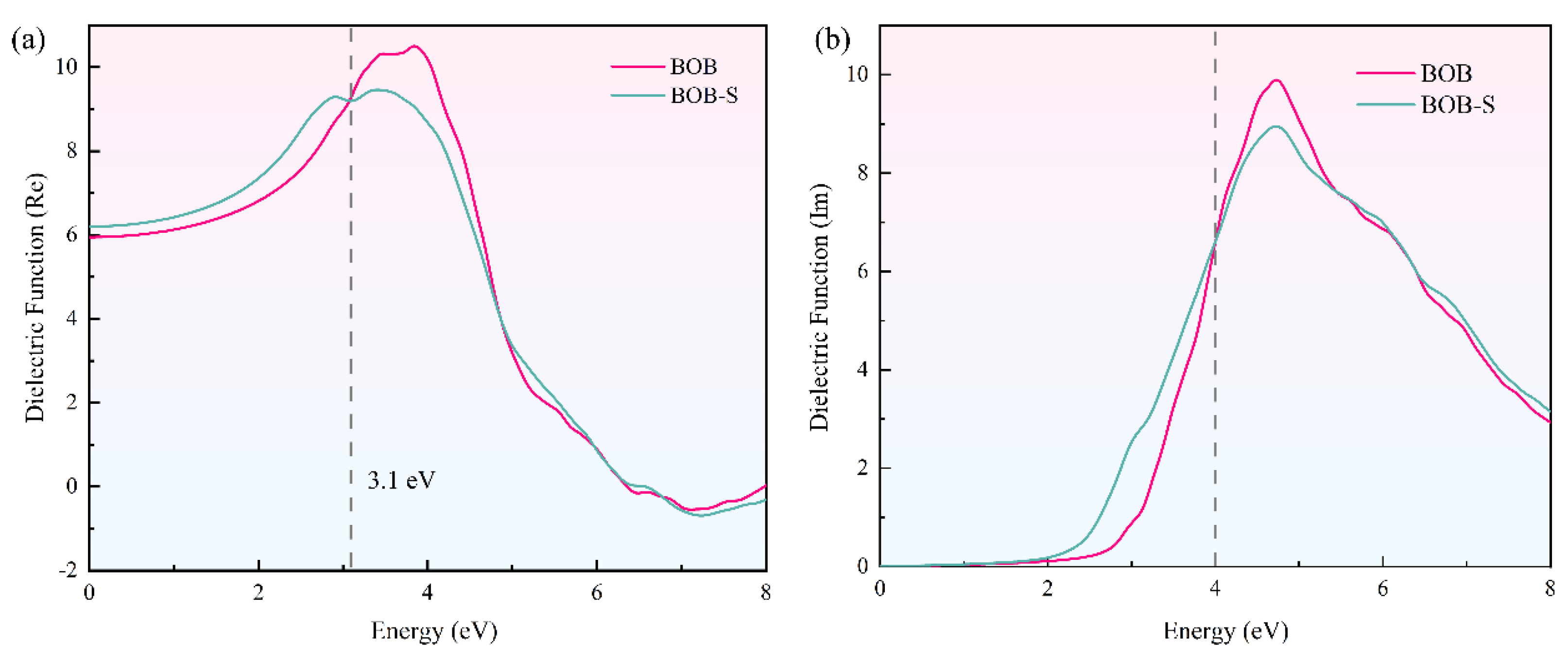
3.6. Dielectric Function
4. Conclusion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Rashid, J.; Abbas, A.; Chang, L.C.; Iqbal, A.; Haq, I.U.; Rehman, A.; Awan, S.U.; Arshad, M.; Rafique, M.; Barakat, M.A. Butterfly cluster like lamellar BiOBr/TiO2 nanocomposite for enhanced sunlight photocatalytic mineralization of aqueous ciprofloxacin. Sci. Total Environ. 2019, 665, 668–677. [Google Scholar] [CrossRef] [PubMed]
- Ning, J.; Zhang, B.; Siqin, L.; Liu, G.; Wu, Q.; Xue, S.; Shao, T.; Zhang, F.; Zhang, W.; Liu, X. Designing advanced S-scheme CdS QDs/La-Bi2WO6 photocatalysts for efficient degradation of RhB. Exploration 2023, 3, 20230050. [Google Scholar] [CrossRef]
- Zhang, B.; Fang, C.; Ning, J.; Dai, R.; Liu, Y.; Wu, Q.; Zhang, F.; Zhang, W.; Dou, S.; Liu, X. Unusual aliovalent Cd doped γ-Bi2MoO6 nanomaterial for efficient photocatalytic degradation of sulfamethoxazole and rhodamine B under visible light irradiation. Carbon Neutralization 2023, 2, 646–660. [Google Scholar] [CrossRef]
- Ye, L.; Su, Y.; Jin, X.; Xie, H.; Zhang, C. Recent advances in BiOX (X = Cl, Br and I) photocatalysts: Synthesis, modification, facet effects and mechanisms. Environ. Sci-Nano 2014, 1, 90. [Google Scholar] [CrossRef]
- Li, T.; Li, Y.; Guo, C.; Hu, Y. Dual-defect semiconductor photocatalysts for solar-to-chemical conversion: Advances and challenges. Chem. Commun. 2024, 60, 2320–2348. [Google Scholar] [CrossRef]
- Luo, S.; Zeng, Z.; Zeng, G.; Liu, Z.; Xiao, R.; Xu, P.; Wang, H.; Huang, D.; Liu, Y.; Shao, B.; Liang, Q.; Wang, D.; He, Q.; Qin, L.; Fu, Y. Recent advances in conjugated microporous polymers for photocatalysis: Designs, applications, and prospects. J. Mater. Chem. A 2020, 8, 6434–6470. [Google Scholar] [CrossRef]
- Wei, X.; Akbar, M.U.; Raza, A.; Li, G. A review on bismuth oxyhalide based materials for photocatalysis. Nanoscale Adv. 2021, 3, 3353–3372. [Google Scholar] [CrossRef]
- Mao, D.; Lü, X.; Jiang, Z.; Xie, J.; Lu, X.; Wei, W.; Showkot Hossain, A.M. Ionic liquid-assisted hydrothermal synthesis of square BiOBr nanoplates with highly efficient photocatalytic activity. Mater. Lett. 2014, 118, 154–157. [Google Scholar] [CrossRef]
- Li, Y.; Jiang, H.; Wang, X.; Hong, X.; Liang, B. Recent advances in bismuth oxyhalide photocatalysts for degradation of organic pollutants in wastewater. RSC Adv. 2021, 11, 26855–26875. [Google Scholar] [CrossRef]
- Zhu, G.; Hojamberdiev, M.; Zhang, W.; Taj Ud Din, S.; Joong Kim, Y.; Lee, J.; Yang, W. Enhanced photocatalytic activity of Fe-doped Bi4O5Br2 nanosheets decorated with Au nanoparticles for pollutants removal. Appl. Surf. Sci. 2020, 526, 146760. [Google Scholar] [CrossRef]
- Sheng, H.; Zhang, X.; Xin, S.; Shi, H.; Liu, G.; Wu, Q.; Xue, S.; Wang, X.; Shao, T.; Liu, Y.; Zhang, F.; Liu, X. First-Principles Study of Electronic Structure and Optical Properties of Ni-Doped Bi4O5Br2. Coatings 2024, 14, 67. [Google Scholar] [CrossRef]
- Lu, S.; Li, J.; Duan, F.; Duan, L.; Du, M.; Chen, M. One-step preparation of Bi4O5BrxI2−x solid solution with superior photocatalytic performance for organic pollutants degradation under visible light. Appl. Surf. Sci. 2019, 475, 577–586. [Google Scholar] [CrossRef]
- Di, J.; Ji, M.; Xia, J.; Li, X.; Fan, W.; Zhang, Q.; Li, H. Bi4O5Br2 ultrasmall nanosheets in situ strong coupling to MWCNT and improved photocatalytic activity for tetracycline hydrochloride degradation. J. Mol. Catal. A-Chem. 2016, 424, 331–341. [Google Scholar] [CrossRef]
- Qian, X.; Ma, Y.; Xia, X.; Xia, J.; Ye, J.; He, G.; Chen, H. Recent progress on Bi4O5Br2-based photocatalysts for environmental remediation and energy conversion. Catal. Sci. Technol. 2024, 14, 1085–1104. [Google Scholar] [CrossRef]
- Li, Y.; Zhao, X.; Yang, J.; Liu, Q.; Huang, X.; Liu, Y.; Kuang, X. Solvothermal synthesis of Cu-Bi4O5Br2 photocatalyst enhanced visible light degradation of ciprofloxacin. Catal. Lett. 2024, 154, 5022–5034. [Google Scholar] [CrossRef]
- Chen, Y.; Zhao, C.; Ma, S.; Xing, P.; Hu, X.; Wu, Y.; He, Y. Fabrication of a Z-scheme AgBr/Bi4O5Br2 nanocomposite and its high efficiency in photocatalytic N2 fixation and dye degradation. Inorg. Chem. Fron. 2019, 6, 3083–3092. [Google Scholar] [CrossRef]
- Zhao, C.; Li, X.; Yue, L.; Ren, X.; Yuan, S.; Zeng, Z.; Hu, X.; Wu, Y.; He, Y. Bi4O5Br2 nanoflower and CdWO4 nanorod heterojunctions for photocatalytic synthesis of ammonia. ACS Appl. Nano Mater. 2023, 6, 15709–15720. [Google Scholar] [CrossRef]
- Yang, W.; Sun, K.; Wan, J.; Ma, Y.-A.; Liu, J.; Zhu, B.; Liu, L.; Fu, F. Boosting holes generation and O2 activation by bifunctional NiCoP modified Bi4O5Br2 for efficient photocatalytic aerobic oxidation. Appl. Catal. B:-Environ. Energy 2023, 320, 121978. [Google Scholar] [CrossRef]
- Dong, X. a.; Cui, Z.; Shi, X.; Yan, P.; Wang, Z.; Co, A.C.; Dong, F. Insights into dynamic surface bromide sites in Bi4O5Br2 for sustainable N2 photofixation. Angew. Chem. Int. Edit. 2022, 61, e202200937. [Google Scholar] [CrossRef] [PubMed]
- Li, N.; Zhu, G.; Hojamberdiev, M.; Zhu, R.; Chang, J.; Gao, J.; Guo, Q.; Liu, P. Pd nanoparticle-decorated Bi4O5Br2 nanosheets with enhanced visible-light photocatalytic activity for degradation of Bisphenol A. J. Photoch. and Photobio. A. 2018, 356, 440–450. [Google Scholar] [CrossRef]
- Cai, Z.; Huang, Y.; Ji, H.; Liu, W.; Fu, J.; Sun, X. Type-II surface heterojunction of bismuth-rich Bi4O5Br2 on nitrogen-rich g-C3N5 nanosheets for efficient photocatalytic degradation of antibiotics. Sep. Purif. Technol. 2022, 280, 119772. [Google Scholar] [CrossRef]
- Wang, C.; Sun, X.; Zhang, M.; Wang, Y.; Tan, Z.; Li, J.; Xi, B. Ultrasound-assisted room-temperature in situ precipitation synthesis of BC doped Bi4O5Br2 for enhanced photocatalytic activity in pollutants degradation under visible light. J. Alloy. Compd. 2021, 889, 161609. [Google Scholar] [CrossRef]
- Zhang, W.-B.; Xiao, X.; Wu, Q.-F.; Fan, Q.; Chen, S.; Yang, W.-X.; Zhang, F.-C. Facile synthesis of novel Mn-doped Bi4O5Br2 for enhanced photocatalytic NO removal activity. J. Alloy. Compd. 2020, 826, 154204. [Google Scholar] [CrossRef]
- Cao, W.; Jiang, C.; Chen, C.; Zhou, H.; Wang, Y. A novel Z-scheme CdS/Bi4O5Br2 heterostructure with mechanism analysis: Enhanced photocatalytic performance. J. Alloy. Compd. 2021, 861, 158554. [Google Scholar] [CrossRef]
- Li, X. Preparation and photocatalytic properties of Bi-based composite photocataltsts doped by non-metals. Professional Master ‘s Thesis, Zhejiang Sci-Tech University, 2014. (in Chinese).
- Zhang, Y.; Liu, S.; Guo, X.; Mikulčić, H.; Xiao, R.; Wang, X. F-doped Bi2MoO6 nanosheets for photoreduction of CO2 with H2O. J. Photoch. Photobio. A. 2024, 447, 115278. [Google Scholar] [CrossRef]
- Weng, P.; Cai, Q.; Wu, H.; Zhang, L.; Wu, K.; Guo, J. Facile synthesis of flower-like CQDs/S-Bi4O5Br2 composites as a highly efficient visible-light response photocatalyst for ciprofloxacin degradation. J. Mater. Sci. 2022, 57, 1977–1993. [Google Scholar] [CrossRef]
- Zhou, X.; Liu, C.; Zhang, Z.; Chen, P.; Yang, Y.; Yang, S. Construction of bismuth-deposited Bi4O5Br2 complex and exploration of its photocatalytic properties. J. Mate. Sci-Mater. El. 2023, 34, 558. [Google Scholar] [CrossRef]
- Lu, M.; Xiao, X.; Zhang, L.; Liu, F.; Nan, J. Photocatalytic degradation of methyl 2,4-dihydroxybenzoate with I-doped Bi4O5Br2 under visible light irradiation. Journal of South China Normal University (Natural Science Edition) 2018, 51, 22–27. (in Chinese). [Google Scholar]
- Zhang, W.; Zhu, G.; Yang, W.; Sun, Q.; Wu, Q.; Tian, Y.; Zhang, Z.; Zhang, S.; Cheng, S.; Zhang, C.; Chen, S.; Zhang, F. Fe-doped Bi4O5Br2 visible light photocatalyst: A first principles investigation. J. Theor. Compu. Chem. 2018, 17, 1850031. [Google Scholar] [CrossRef]
- Blochl, P.E. Projector augmented-wave method. Phys. Rev. B. 1994, 50, 17953–17979. [Google Scholar] [CrossRef]
- Grimme, S. Semiempirical GGA-type density functional constructed with a long-range dispersion correction. J. Comput. Chem. 2006, 27, 1787–1799. [Google Scholar] [CrossRef]
- Liu, G.; Dai, R.; Shi, H.; Dong, N.; Zhang, B.; Li, S.; Wang, W.; Liu, Y.; Shao, T.; Zhang, M.; Subramaniam, V.; Ramachandran, K.; Zhang, F.; Liu, X. Using Er/Cd-Co doped Bi4O5Br2 microspheres to enhance antibiotic degradation under visible illumination: A combined experimental and DFT investigation. J. Phys. Chem. B 2024, 128, 9373–9384. [Google Scholar] [CrossRef]
- Wang, M.; Han, J.; Guo, P.; Sun, M.; Zhang, Y.; Tong, Z.; You, M.; Lv, C. Hydrothermal synthesis of B-doped Bi2MoO6 and its high photocatalytic performance for the degradation of Rhodamine B. J. Phys. Chem. Solids 2018, 113, 86–93. [Google Scholar] [CrossRef]
- Yang, H. Study on the preparation and photocatalytic performance of modified Bi4O5Br2. Professional Master ‘s Thesis, Northwest University 2021. (in Chinese).
- Liu, Z.; Wu, B.; Zhao, Y.; Niu, J.; Zhu, Y. Solvothermal synthesis and photocatalytic activity of Al-doped BiOBr microspheres. Ceram. Int. 2014, 40, 5597–5603. [Google Scholar] [CrossRef]
- Huang, M.; Li, J.; Su, W.; Huang, X.; Li, B.; Fan, M.; Dong, L.; He, H. Oriented construction of S-doped, exposed {001} facet BiOBr nanosheets with abundant oxygen vacancies and promoted visible-light-driven photocatalytic performance. CrystEngComm 2020, 22, 7684–7692. [Google Scholar] [CrossRef]
- Wang, Y.; Yin, L.; Wu, J.; Li, N.; He, N.; Zhao, H.; Li, X.; Lai, X.; Wu, Q. Enhanced charge separation efficiency of sulfur-doped TiO2 nanorod arrays for an improved photoelectrochemical glucose sensing performance. J. Mater. Sci. 2022, 57, 1362–1372. [Google Scholar] [CrossRef]
- Sun, M., Li jieyuan, Dong, Fan. The research progress on the structural regulation methods of Bi-based photocatalytic materials and their applications in the field of environmental and energy. Journal of Huazhong Agricultural University 2020, 39, 17–25. (in Chinese). [Google Scholar]
- Jin, Y.; Li, F.; Li, T.; Xing, X.; Fan, W.; Zhang, L.; Hu, C. Enhanced internal electric field in S-doped BiOBr for intercalation, adsorption and degradation of ciprofloxacin by photoinitiation. App. Cata. B-Environ.Energy 2022, 302, 120824. [Google Scholar] [CrossRef]
- Wang, R.; Li, D.; Wang, H.; Liu, C.; Xu, L. Preparation, Characterization, and Performance Analysis of S-Doped Bi2MoO6 Nanosheets. Nanomaterials 2019, 9, 1341. [Google Scholar] [CrossRef] [PubMed]
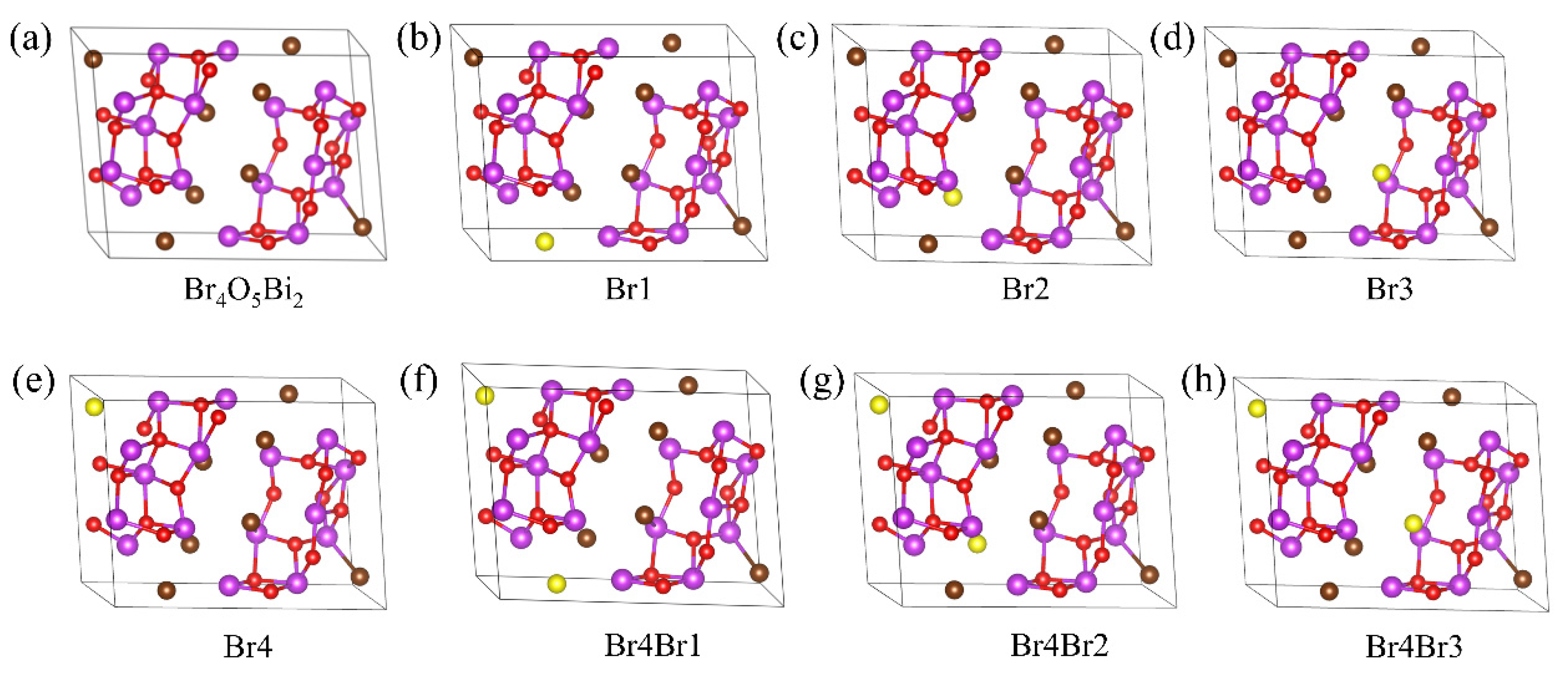
| Configuration | Br1 | Br2 | Br3 | Br4 | Br4Br1 | Br4Br2 | Br4Br3 |
| Energy (eV) | 2.72 | 2.73 | 2.74 | 2.62 | 2.53 | 2.98 | 3.23 |
| Species | a (Å) | b (Å) | c (Å) | α (°) | β (°) | γ (°) |
| Bi4O5Br2 | 10.89 | 5.67 | 14.60 | 90 | 97.72 | 90 |
| S-BOB | 10.92 | 5.67 | 14.58 | 90.64 | 97.97 | 91.44 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





