1. Introduction
Greater Trochanteric Pain Syndrome (GTPS) is a frequent and complex clinical condition characterized by acute, chronic, and more or less relapsing pain in the lateral region of the hip around the greater trochanter [
1,
2].
In the past, lateral hip pain was attributed exclusively to an inflammation state of the peri-trochanteric bursae, described as “trochanteric bursitis” [
3].
GTPS, instead, can result from different mechanical and inflammatory conditions involving peri-trochanteric structures and can be traced back to three primary entities. External snapping hip syndrome, trochanteric bursitis, and, more frequently, degenerative gluteus medius and minimus muscle tendinopathies [
4,
5,
6,
7].
Traditionally, the treatment of GTPS is initially conservative and includes rest, ice, Non-steroidal anti-inflammatory drugs (NSAIDs), and physical therapy focusing on postural exercises and stretching of the iliotibial band [
8,
9,
10]. The use of steroids is highly controversial and does not appear to be effective in the long term [
8,
11]. In recent years, few studies have been conducted with Platelet Rich Plasma (PRP); however, only one study has shown valid results at 12 weeks [
12,
13,
14,
15,
16]. In two in vitro studies [
17,
18], the effects of swine-derived Type I Collagen, MD-Tissue (GUNA S.p.a. Milan, Italy), on a culture of human tenocytes were described, focusing on collagen turnover pathways to understand how this medical device could improve tendon biology. MD-Tissue is an injectable medical device, non-FDA approved, based on type I porcine collagen; the collagen content is 100µg/2mL per vial. Porcine collagen is similar to human collagen and highly compatible; it presents very low risks of inducing adverse effects, so it is used in various clinical settings [
19,
20]. The Authors used MD-Tissue as a coating for cell cultures of human tenocytes harvested from the gluteus minimus tendon. The results suggested that MD-Tissue can induce an anabolic phenotype in tenocytes by stimulating their proliferation and migration; furthermore, it promotes synthesis, maturation, and secretion of COL-I, thus positively regulating homeostasis.
For this reason, MD-Tissue could be a valid biological treatment for GTPS. It is less invasive and less expensive than PRP and autologous tenocytes.
The primary endpoint of this study was to evaluate, through NRS, pain reduction in patients affected by GTPS.
Secondary endpoints were the pre- and post-procedural modified Harris Hip Score, hip abductor strength, and the peritrochanteric space MRI images. Furthermore, the amount of painkiller consumption and dropouts, as well as all adverse events, were recorded.
2. Materials and Methods
The study is a single-center pilot clinical investigation based on a one-sample design and was approved by the Institutional Review Board (protocol code OSMAMI-14/05/2021-0021380-U in date 14/5/2021); National Clinical Trial number - NCT number: 05486078.
The subjects were selected among patients who met the inclusion criteria, did not meet the exclusion criteria, and were willing to sign the informed consent.
Male and female subjects aged between 18 and 70 who met the following criteria were considered eligible: palpatory lateral pain lasting at least one month; positive Faber (flexion-abduction-external rotation) test; pain elicited by resisted abduction and resisted external derotation; pain level ≥ 5 assessed according to the NRS; capability to collaborate, understand, and sign in the informed consent.
Pre-procedural (T0) diagnostic exams included a Pelvis X-ray and Dunn 45° axial views of the hip and a high field MRI (1,5 Tesla) with different sequences: coronal T1 and STIR (FAT suppression), axial T1, and STIR.
The exclusion criteria were the following: actual hip joint pain (with positive flexion-adduction-internal rotation test); ESHS (External Snapping Hip Syndrome); total hip replacement in the affected hip; radiological and clinical evidence of gluteus minimus and/or medius tendons tears with an indication for surgical repair; evidence of tendon calcifications documented radiographically; hip osteoarthritis (Tönnis classification >1); lumbar spine pathology and sacroiliac joint pathology; fibromyalgia; fluoroquinolones treatment within 30 days before enrollment; hip injection with hyaluronic acids or steroids within four weeks before the enrollment; local or systemic infection; chronic treatment with steroids or immunosuppressants; drug and/or alcohol addiction, psychiatric disorders or clinical conditions that may compromise the correct interpretation of Patient-Reported Outcome Measures (PROMs) or follow-up; coagulopathies, platelet aggregation disorders, or treatment with oral anticoagulants or antiplatelets that cannot be suspended during the study period; pregnancy and breastfeeding; allergy to porcine collagen.
The all-study group was treated with an ultrasound-guided injection of 2 ml of MD-Tissue—one injection once a week for three consecutive weeks.
The injections were guided by an ultrasound device (MyLab™ XPRO 80, Esaote S.p.a., Genova, Italy). The procedures were performed with the patient in a lateral decubitus. After preparing a sterile field, a linear (15-18 MegaHertz) probe was placed longitudinally along the gluteus tendons, precisely at the most painful trigger point and where the most significant area of microstructural inhomogeneity was visible. At that point, a 20-22 Gauge spinal needle was inserted under constant ultrasound guidance. MD-Tissue was injected into the trochanteric bursa and at the level of the tendons, particularly at the most degenerated insertional areas.
The subjects were then evaluated at six different times: at baseline (time T0), after one week (T1s), after two weeks (T2s), after six weeks (T6s/FU), after ten weeks (T10s/FU), and after 24 weeks (T24s/FU) when a post-procedural Pelvis MRI (High-field MRI 1,5 T) was performed for each patient for further radiological evaluation. Two highly qualified musculoskeletal radiologists performed MRI evaluations focusing on perilesional edema on axial and coronal STIR-weighted images.
The strength is assessed with the subject supine through the mean of 3 repeated measurements of the abduction force of the hip affected by GTPS, using a dynamometer, with 30-second interval between each measurement to avoid muscle fatigue and ensure consistency.
Since this is a one-sample study, it is assumed that the proportion of successes (patients who have a reduction of at least 3 points in the NRS scale) in the absence of treatment cannot exceed 25% (H0), while it is assumed a success rate equal to at least 50% (HA) in treated subjects.
With these premises, a one-tailed exact binomial test applied to a sample of 49 subjects reaches a power of 95.7% in discriminating the difference equal to 25% between the proportion predicted by HA and that predicted by H0 at a significance level equal to 0.025.
Possible dropout subjects cannot determine sample size inflation, as each subject of this type will automatically be considered a failure.
Incidentally, it should also be noted that upon reaching the 19th success, the null hypothesis can already be rejected, as 19 successes out of 49 would represent a proportion of 38.776%, which has an exact binomial confidence interval at 95%, which varies between 25.197 % and 53.761%, so the maximum proportion of 25% predicted by H0 would be below the lower margin of the confidence interval. All the variables were subjected to the appropriate descriptive analysis after validation. According to distribution, continuous variables were presented as mean and standard deviation or median and range. Categorical variables were expressed in terms of case numbers or frequencies. Statistical analyses were conducted with Stata/SE 17.0 (StataCorp LLC, College Station, USA).
The primary endpoint was assessed with a one-tailed exact binomial test. It was evaluated at T10s/FU, and the NRS score at T10s/FU was compared to T0. Reducing at least 3 points in the NRS scale in at least 50% of the treated subjects was considered clinically significant.
The within-subject repeated measures ANOVA or Friedman test was used to evaluate the NRS score, mHHS score, and abductor strength at T6s/FU and T24s/FU compared to time T0.
The Student’s t-test for paired data or Wilcoxon test was used to assess the evidence of resolution or decrease in inflammatory signs in the peri-trochanteric region of the hip affected according to MRI images at T24s/FU compared to T0.
The repeated measures ANOVA or Friedman test was used to evaluate painkiller consumption based on the clinical diary at T0, T1s, T2s, T6s/FU, T10s/FU, and T24s/FU.
The absolute and relative frequency and 95% confidence interval of the fraction of subjects who abandoned the study concerning Adverse Events were also reported.
3. Results
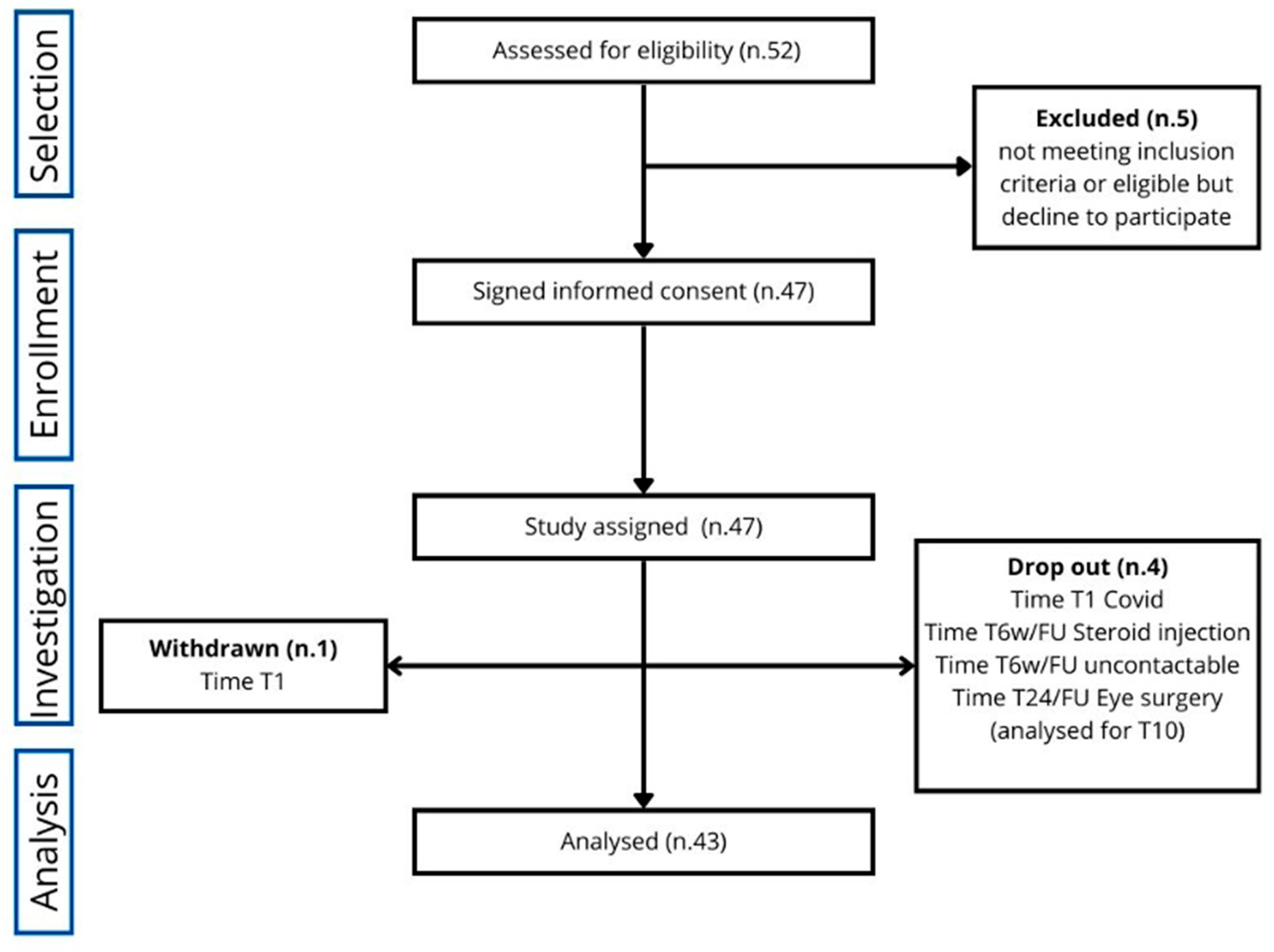
Of the initially eligible 52 subjects, 47 were enrolled in the study for 13 months, from October 2021 to November 2022. Of these 47 patients, two withdrew at time T1 (one independently and one due to SarsCov2 infection) and two at T6/FU (one due to steroid injection, one untraceable). One was considered a dropout only from MRI analysis at T24/FU for incompatibility because of an artificial lens inserted in the meantime (cataract surgery).
Therefore, the subjects who completed the study for the primary endpoint were 43, while those participating in the entire study [primary and secondary endpoints (T0-T24/FU)] were 42, as illustrated in
Figure 1.
3.1. Primary Endpoint
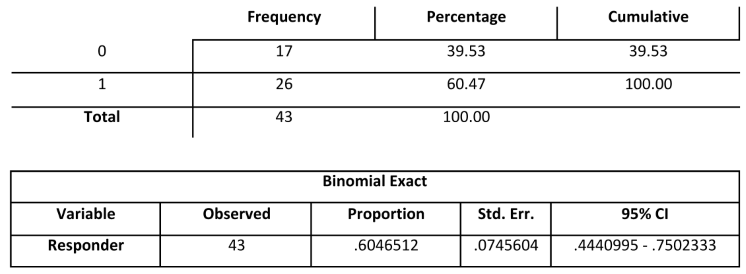
Twenty-six patients (60.5%) showed a significant pain reduction (more than 3 NRS points) at T10/FU (primary endpoint); 17 patients (39.5%) did not reach the primary endpoint (
Table 1). The primary endpoint was achieved since 60.5% of patients declared a reduction of more than 3 NRS points.
3.2. Secondary endpoints
3.2.1. NRS Pre- and Post-Procedural
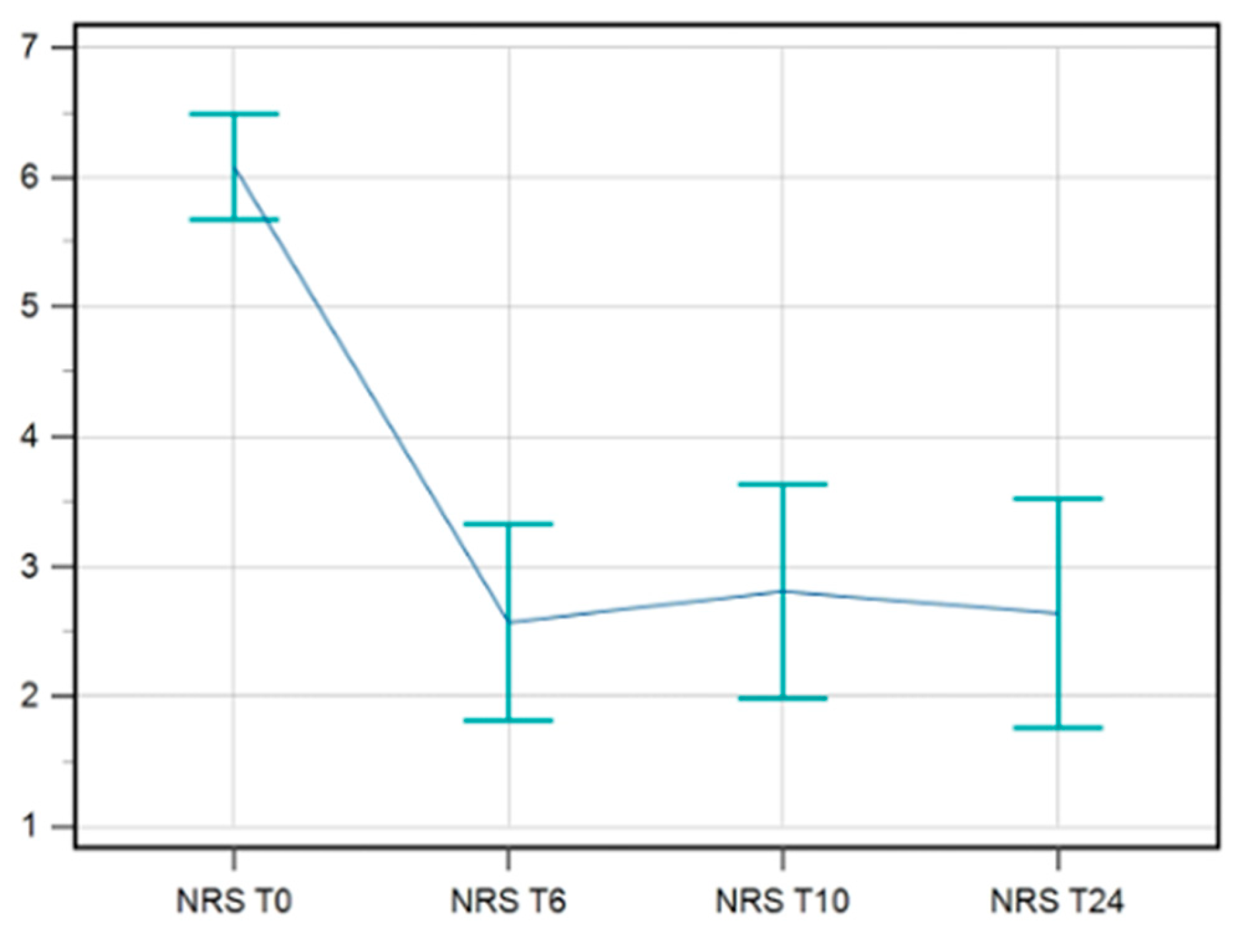
Data available for NRS till T24/FU were complete for 42 patients. A statistical improvement in pain was maintained till T24/FU. The F-test for within-subject variation was significant (p < 0.001) under any assumption regarding sphericity. Pairwise tests corrected with the Bonferroni method show that the values at T0 significantly differed from those at T6s/FU, T10s/FU, and T24s/FU (p < 0.0001 in all cases) (
Figure 2).
3.2.2. mHHS
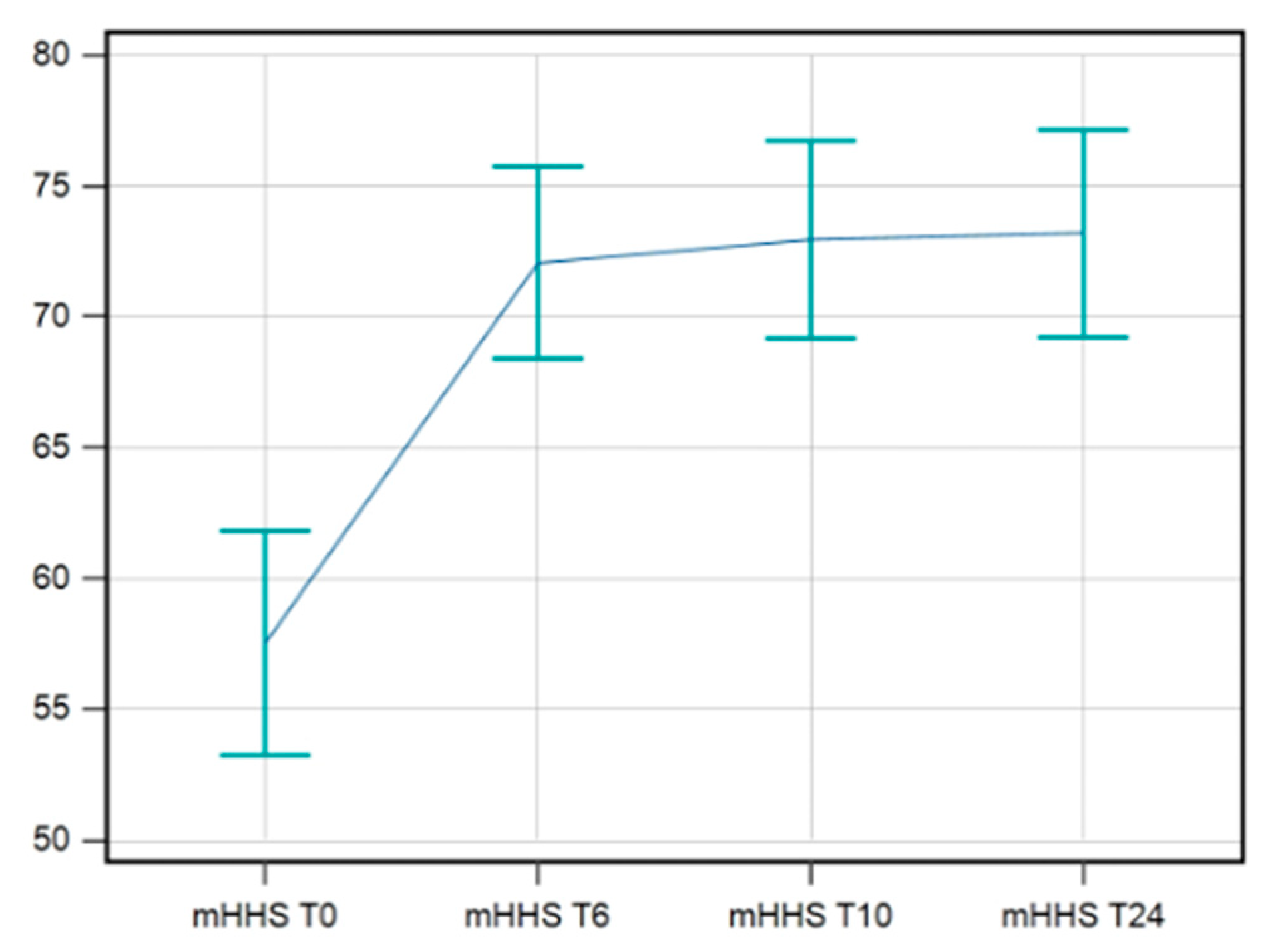
The data available for mHHS were complete for 42 patients. A statistical improvement in mHHS was maintained till T24/FU. The F-test for within-subject variation was significant (p < 0.001) under any assumption regarding sphericity. Pairwise tests corrected with the Bonferroni method show that the values at T0 significantly differed from those at T6s/FU, T10s/FU, and T24s/FU (p < 0.0001 in all cases) (
Figure 3).
3.2.3. Abduction Strength
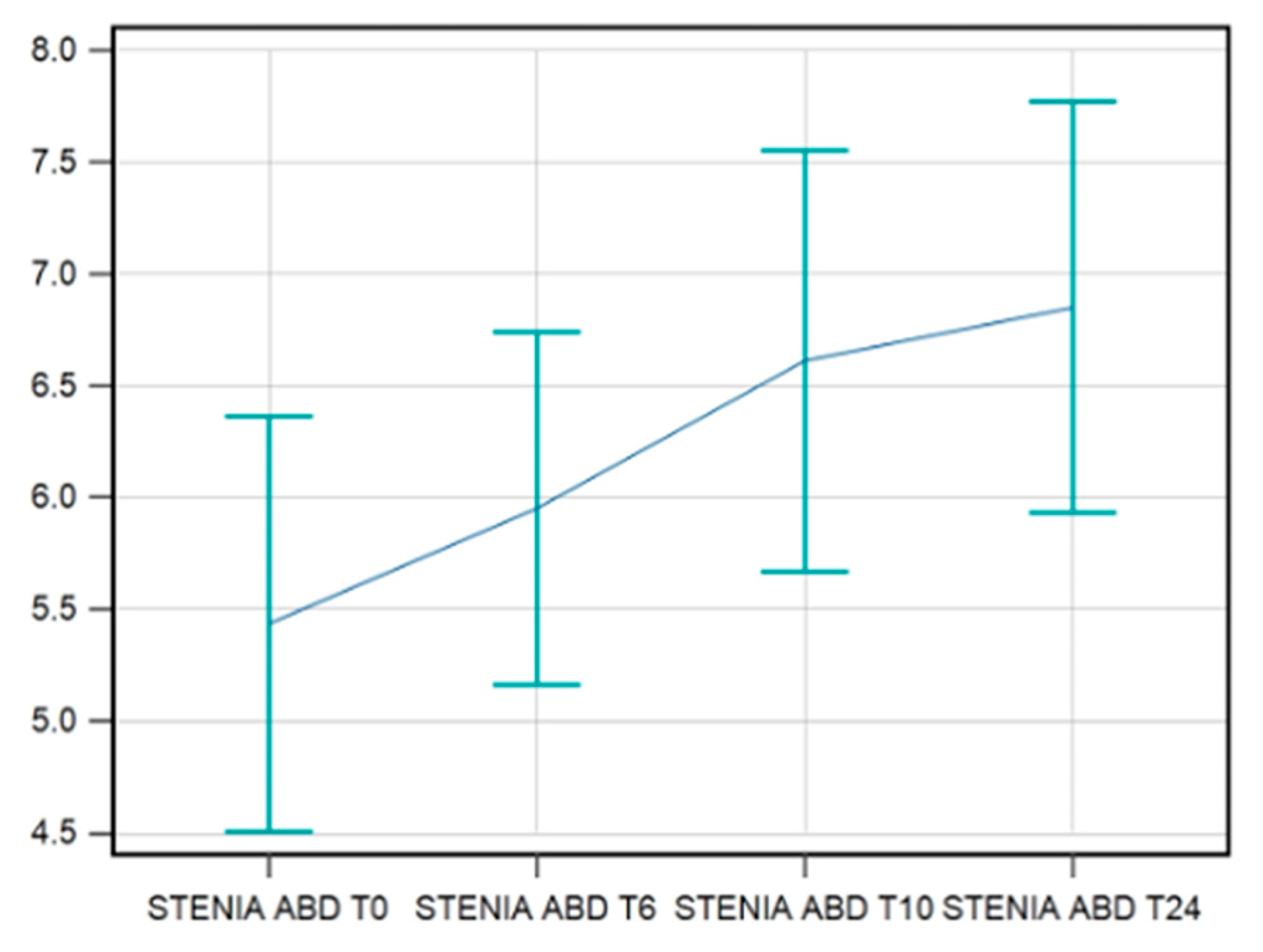
The data available for the strength in abduction were complete for 42 patients. A statistical improvement in strength in hip abduction was recorded till T24/FU but with a different behavior. The F-test for within-subject variation was significant (p < 0.001) for any sphericity assumption. Pairwise tests corrected with the Bonferroni method show that the values at T0 were significantly different compared to those at T10s/FU (p = 0.0003) and T24s/FU (p = 0.0001) (
Figure 4).
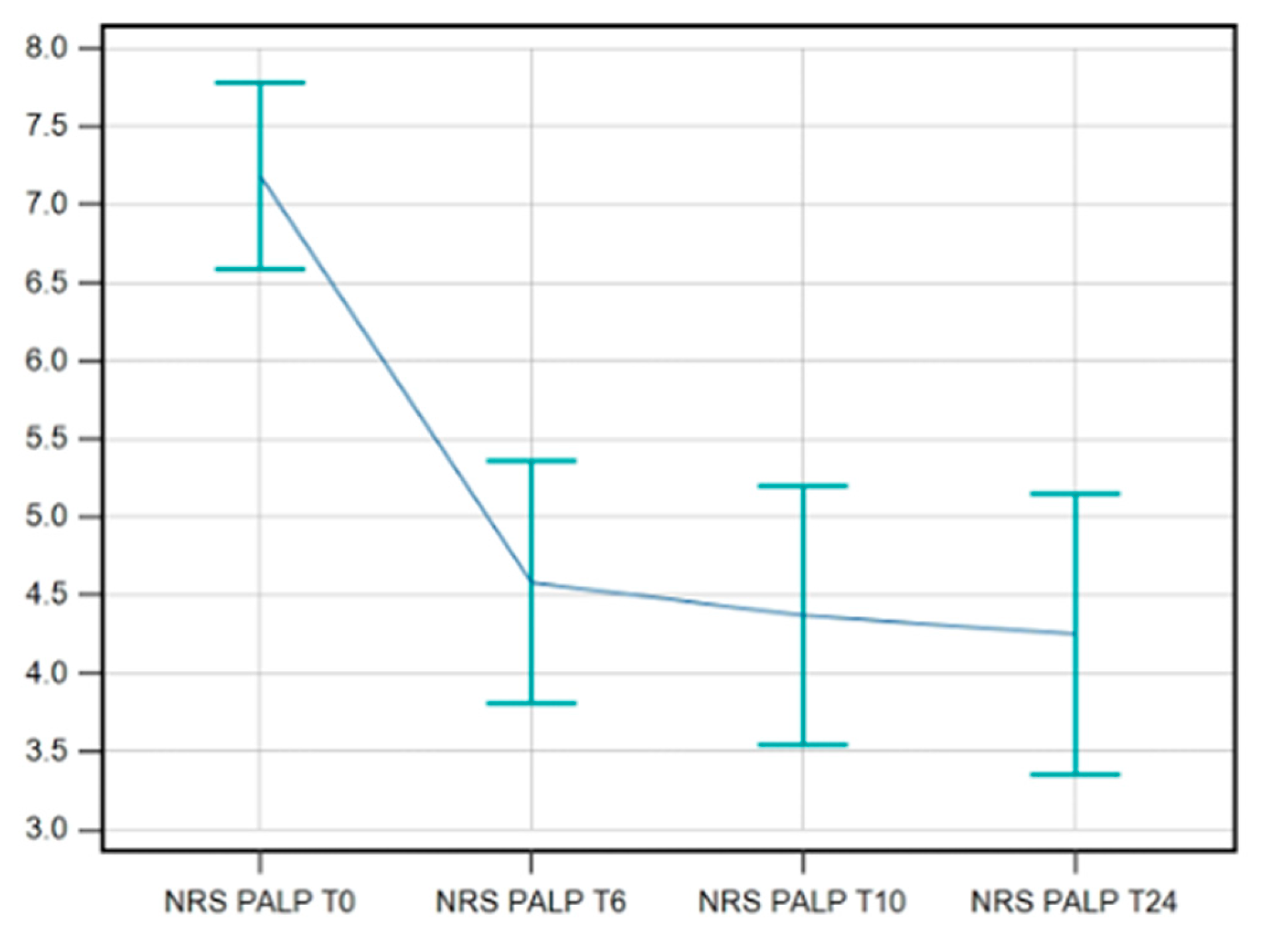
3.2.4. NRS on Palpation
The data available for NRS on palpation showed an improvement maintained at T24/FU. Again, the F-test for within-subject variation was significant (p < 0.001) for any sphericity assumption. The Pairwise tests corrected with the Bonferroni method show that the values at T0 significantly differ from those at T6s/FU, T10s/FU, and T24s/FU (p < 0.0001 in all cases) (
Figure 5).
3.2.5. Painkiller Consumption
Data on painkiller consumption were available for 43 patients and did not increase significantly during the study (Friedman test: p = 0.992). However, in two patients, between T10s/FU and T24s/FU, an abnormal increase in painkiller consumption was observed, going from 1 to 12 and from 1 to 13, respectively.
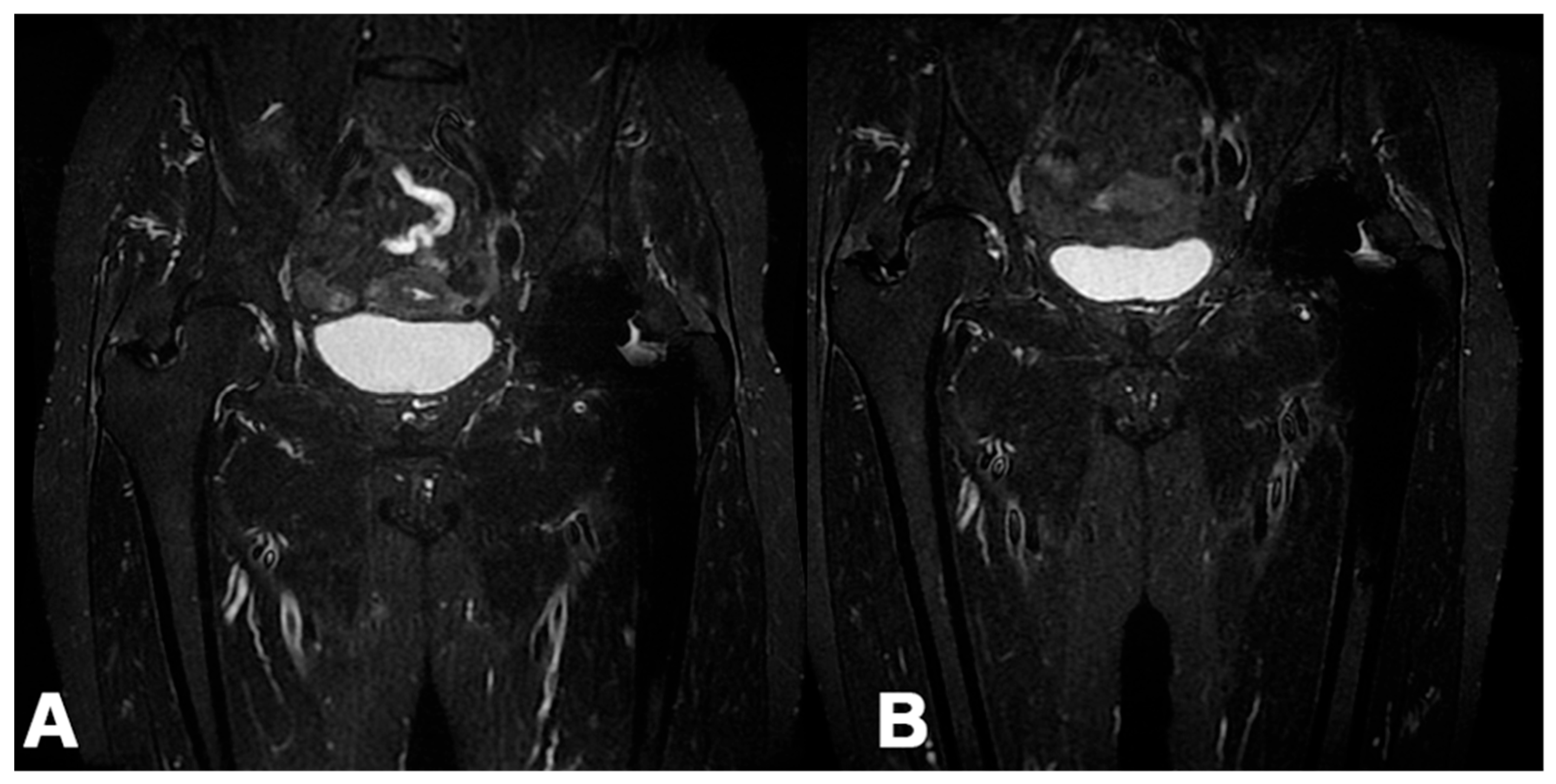
3.2.6. MRI
MRI images of the peritrochanteric area after six months (T24/FU) were available for 40 patients. In 25 patients (62.5%), a reduction in peri-tendinous edema was visible between the MRI performed before treatment and the one obtained at T24s/FU (
Figure 6), while in 15 (37.5%), no changes were found. The paired samples t-test was statistically significant at p < 0.001. Interestingly, a linear correlation between pain reduction and improved MRI images was not always found, and vice versa.
4. Discussion
The main finding of this study was patients’ significant improvement, still at six months follow-up, after three ultrasound-guided peritrochanteric injections of MD-Tissue for GTPS, with no reported adverse effects.
No other study has previously evaluated the effect of a collagen-based medical device administered with ultrasound-guided injections as a therapy for GTPS.
The main strengths of the present study are the prospectively collected data, the quantitative evaluation of the abductor strength, and the qualitative investigation through MRI.
Lateral peri trochanteric pain, defined as Greater Trochanter Pain Syndrome (GTPS) since the pioneering research of Karpinski in 1985 [
21], is due to a minimus and medius glutei tendinopathy and represents one of the most frequent causes of pain around the hip worldwide. Various treatments have been proposed, but no one has shown a clear superiority. The orthopedic community has recently moved from classic treatments, such as physiotherapy, focal shock wave therapy, and steroid injections, to a new era focused on restoring biological homeostasis. Ultrasound-guided injections of platelet-rich plasma, bone marrow aspirate [
22], autologous tenocytes [
23], and percutaneous fenestrations [
24] are examples of this trend. Among these biological treatments, Porcine collagen is one of the most recent, at least regarding its use in the specific field of GTPS.
Swine-origin type I collagen has an excellent scientific basis in vitro studies [
17,
18] and some evidence of its effectiveness in the clinical practice for different musculoskeletal disorders [
19,
20,
25,
26]. It has been used for epicondylitis [
27], plantar fasciitis [
28], knee osteoarthritis [
29], supraspinatus tendinopathy [
19], and myofascial pain treatment [
30].
A 2023 Bayesian analysis [
31] of 596 GTPS patients treated with ultrasound-guided infiltrations of PRP, corticosteroids, or focal shock waves showed that PRP was the most efficacious pain reduction agent, followed by shock waves. The study demonstrated that both therapies were safe and effective, but neither the abduction strength nor MRI imaging were analyzed.
Heaver C. [
32], in a single-blinded, double-arm randomized controlled trial, compared the results of focal shock wave therapy with corticosteroids ultrasound-guided infiltrations. The primary endpoint was pain reduction. At one year follow-up, the group treated with focal shock waves showed a statistically significant improvement compared to those treated with corticosteroids. This study also demonstrated an improvement in the Trendelenburg sign. The improvement was maintained over time in the group treated with focal shock waves but not in the group treated with corticosteroid injections.
A 2021 systematic review [
33] of randomized controlled trials, with 1034 patients analyzed, demonstrated that PRP and focal shock waves significantly improve pain reduction. Unfortunately, the authors also showed that none of the two therapies maintained these clinical improvements over time.
In this study, hip strength in abduction remained constant and progressively increased up to 6 months after treatment. This is an understandable trend since myotendinous junction and tendon fibers take time to regain physiological functionality.
MD-Tissue treatment also showed favorable MRI imaging and reduced peri-tendinous edema in more than 60% of treated patients. However, imaging improvements did not always correlate with clinical ones. The inadequacy of the follow-up could partly explain this difference. A visible biological response at MRI imaging, as an end-up result of cellular metabolic changes, may take longer to be observable. On the other hand, some patients without significant pain improvement showed a subjective reduction in peri-tendinous edema. This finding could be explained by the intrinsic difficulties in evaluating the peri-trochanteric space, the subjectivity of the methodology, and a need for complete knowledge about pain generators of the area.
The present study has some limitations. First, the small sample size risks type II errors, even though 90% of the patients enrolled have completed the study. Second, the need for a control group limits this study.
Another issue is that the Modified Harris Hip Score is less suited for use in patients with GTPS, as it was developed primarily for elderly patients undergoing total hip arthroplasty [
34]. Finally, the opportunity to compare these results to previous ones is limited due to this study’s unicity and the literature’s heterogeneity.
The mean age of the cohort patients could also be a source of bias since younger patients could respond faster and better to biologically active treatment.
5. Conclusions
Ultrasound-guided peritrochanteric injections of MD-Tissue are a safe and effective treatment for the majority of the Patients affected by Greater Trochanteric Pain Syndrome.
Author Contributions
Conceptualization, F.R.; methodology, M.G.M., A.M; software, A.R; validation, A.F, and A.R.; formal analysis, A.F.; investigation, A.F., A.R., L.R., L.V.; data curation, A.F., A.M.; writing—original draft preparation, A.F., M.G.M.; writing—review and editing, F.R., A.R., L.R., L.V., A.M. All authors have read and agreed to the published version of the manuscript.
Funding
The institution has received funding from Guna S.p.a., Via Palmanova, 71, 20132 Milano, Italia.
Institutional Review Board Statement
The study has been performed according to the Declaration of Helsinki (Fortaleza 64th 2013), with Good Clinical Practices (GCP), and has been approved by the Institutional Review Board of Milano Area 2 (protocol code OSMAMI-14/05/2021-0021380-U in date 14/5/2021)—National Clinical Trial number—NCT number: 05486078. Informed consent was obtained from all subjects involved in the study.
Informed Consent Statement
Informed consent was obtained from all subjects involved in the study
Data Availability Statement
All the data are available upon reasonable request. The corresponding author will share the article’s data upon reasonable request.
Acknowledgments
We thank GUNA S.p.a., Milan, Italy, for their unconditional support in this study. In particular, Dr. Vincenzo Miranda and Dr. Kamilia Laarej of the GUNA Clinical Research Unit for the ethical and scientific rigor with which they followed the study.
Conflicts of Interest
The Authors declare that there is no conflict of interest
References
- Strauss, E.J.; Nho, S.J.; Kelly, B.T. Greater Trochanteric Pain Syndrome. Sports Med Arthrosc Rev 2010, 18, 113–119. [Google Scholar] [CrossRef] [PubMed]
- Sunil Kumar, K.H.; Rawal, J.; Nakano, N.; Sarmento, A.; Khanduja, V. Pathogenesis and Contemporary Diagnoses for Lateral Hip Pain: A Scoping Review. Knee Surg Sports Traumatol Arthrosc 2021, 29, 2408–2416. [Google Scholar] [CrossRef] [PubMed]
- Williams, B.S.; Cohen, S.P. Greater Trochanteric Pain Syndrome: A Review of Anatomy, Diagnosis and Treatment. Anesth Analg 2009, 108, 1662–1670. [Google Scholar] [CrossRef]
- Aprato, A.; Jayasekera, N.; Bajwa, A.; Villar, R.N. Peri-Articular Diseases of the Hip: Emerging Frontiers in Arthroscopic and Endoscopic Treatments. J Orthopaed Traumatol 2014, 15, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Fearon, A.M.; Scarvell, J.M.; Cook, J.L.; Smith, P.N. Does Ultrasound Correlate with Surgical or Histologic Findings in Greater Trochanteric Pain Syndrome? A Pilot Study. Clinical Orthopaedics and Related Research® 2010, 468, 1838. [Google Scholar] [CrossRef]
- Fearon, A.M.; Scarvell, J.M.; Neeman, T.; Cook, J.L.; Cormick, W.; Smith, P.N. Greater Trochanteric Pain Syndrome: Defining the Clinical Syndrome. Br J Sports Med 2013, 47, 649–653. [Google Scholar] [CrossRef]
- Klauser, A.S.; Martinoli, C.; Tagliafico, A.; Bellmann-Weiler, R.; Feuchtner, G.M.; Wick, M.; Jaschke, W.R. Greater Trochanteric Pain Syndrome. Semin Musculoskelet Radiol 2013, 17, 43–48. [Google Scholar] [CrossRef]
- Frizziero, A.; Vittadini, F.; Pignataro, A.; Gasparre, G.; Biz, C.; Ruggieri, P.; Masiero, S. Conservative Management of Tendinopathies around Hip. Muscles Ligaments Tendons J 2016, 6, 281–292. [Google Scholar] [CrossRef]
- Grimaldi, A.; Mellor, R.; Hodges, P.; Bennell, K.; Wajswelner, H.; Vicenzino, B. Gluteal Tendinopathy: A Review of Mechanisms, Assessment and Management. Sports Med 2015, 45, 1107–1119. [Google Scholar] [CrossRef]
- Marín-Pena, O.; Papavasiliou, A.V.; Olivero, M.; Galanis, N.; Tey-Pons, M.; Khanduja, V. Non-Surgical Treatment as the First Step to Manage Peritrochanteric Space Disorders. Knee Surg Sports Traumatol Arthrosc 2021, 29, 2417–2423. [Google Scholar] [CrossRef]
- Walker, P.; Kannangara, S.; Bruce, W.J.; Michael, D.; Van der Wall, H. Lateral Hip Pain: Does Imaging Predict Response to Localized Injection? Clinical Orthopaedics and Related Research® 2007, 457, 144. [Google Scholar] [CrossRef] [PubMed]
- Ali, M.; Oderuth, E.; Atchia, I.; Malviya, A. The Use of Platelet-Rich Plasma in the Treatment of Greater Trochanteric Pain Syndrome: A Systematic Literature Review. J Hip Preserv Surg 2018, 5, 209–219. [Google Scholar] [CrossRef]
- Dettoni, F.; Pellegrino, P.; La Russa, M.R.; Bonasia, D.E.; Blonna, D.; Bruzzone, M.; Castoldi, F.; Rossi, R. Validation and Cross Cultural Adaptation of the Italian Version of the Harris Hip Score. Hip Int 2015, 25, 91–97. [Google Scholar] [CrossRef] [PubMed]
- Fitzpatrick, J.; Bulsara, M.K.; O’Donnell, J.; McCrory, P.R.; Zheng, M.H. The Effectiveness of Platelet-Rich Plasma Injections in Gluteal Tendinopathy: A Randomized, Double-Blind Controlled Trial Comparing a Single Platelet-Rich Plasma Injection With a Single Corticosteroid Injection. Am J Sports Med 2018, 46, 933–939. [Google Scholar] [CrossRef] [PubMed]
- Galea, V.P.; Florissi, I.; Rojanasopondist, P.; Connelly, J.W.; Ingelsrud, L.H.; Bragdon, C.; Malchau, H.; Troelsen, A. The Patient Acceptable Symptom State for the Harris Hip Score Following Total Hip Arthroplasty: Validated Thresholds at 3-Month, 1-, 3-, 5-, and 7-Year Follow-Up. J Arthroplasty 2020, 35, 145–152e2. [Google Scholar] [CrossRef]
- Singh, J.A.; Schleck, C.; Harmsen, S.; Lewallen, D. Clinically Important Improvement Thresholds for Harris Hip Score and Its Ability to Predict Revision Risk after Primary Total Hip Arthroplasty. BMC Musculoskelet Disord 2016, 17, 256. [Google Scholar] [CrossRef]
- Randelli, F.; Menon, A.; Giai Via, A.; Mazzoleni, M.G.; Sciancalepore, F.; Brioschi, M.; Gagliano, N. Effect of a Collagen-Based Compound on Morpho-Functional Properties of Cultured Human Tenocytes. Cells 2018, 7, 246. [Google Scholar] [CrossRef]
- Randelli, F.; Sartori, P.; Carlomagno, C.; Bedoni, M.; Menon, A.; Vezzoli, E.; Sommariva, M.; Gagliano, N. The Collagen-Based Medical Device MD-Tissue Acts as a Mechanical Scaffold Influencing Morpho-Functional Properties of Cultured Human Tenocytes. Cells 2020, 9, 2641. [Google Scholar] [CrossRef]
- Corrado, B.; Bonini, I.; Chirico, V.A.; Filippini, E.; Liguori, L.; Magliulo, G.; Mazzuoccolo, G.; Rosano, N.; Gisonni, P. Ultrasound-Guided Collagen Injections in the Treatment of Supraspinatus Tendinopathy: A Case Series Pilot Study. J Biol Regul Homeost Agents 2020, 34, 33–39, ADVANCES IN MUSCULOSKELETAL DISEASES AND INFECTIONS-SOTIMI 2019. [Google Scholar]
- Martin Martin, L.S.; Massafra, U.; Bizzi, E.; Migliore, A. A Double Blind Randomized Active-Controlled Clinical Trial on the Intra-Articular Use of Md-Knee versus Sodium Hyaluronate in Patients with Knee Osteoarthritis (“Joint”). BMC Musculoskelet Disord 2016, 17, 94. [Google Scholar] [CrossRef]
- Karpinski, M.R.; Piggott, H. Greater Trochanteric Pain Syndrome. A Report of 15 Cases. J Bone Joint Surg Br 1985, 67, 762–763. [Google Scholar] [CrossRef] [PubMed]
- Rosário, D.A.V.; Faleiro, T.B.; Franco, B.A.F.M.; Daltro, G.D.C.; Marchetto, R. COMPARISON BETWEEN CONCENTRATED BONE MARROW ASPIRATE AND CORTICOID IN GLUTEAL TENDINOPATHY. Acta Ortop Bras 2021, 29, 26–29. [Google Scholar] [CrossRef] [PubMed]
- Bucher, T.A.; Ebert, J.R.; Smith, A.; Breidahl, W.; Fallon, M.; Wang, T.; Zheng, M.-H.; Janes, G.C. Autologous Tenocyte Injection for the Treatment of Chronic Recalcitrant Gluteal Tendinopathy: A Prospective Pilot Study. Orthop J Sports Med 2017, 5, 2325967116688866. [Google Scholar] [CrossRef] [PubMed]
- Jacobson, J.A.; Yablon, C.M.; Henning, P.T.; Kazmers, I.S.; Urquhart, A.; Hallstrom, B.; Bedi, A.; Parameswaran, A. Greater Trochanteric Pain Syndrome: Percutaneous Tendon Fenestration Versus Platelet-Rich Plasma Injection for Treatment of Gluteal Tendinosis. J Ultrasound Med 2016, 35, 2413–2420. [Google Scholar] [CrossRef] [PubMed]
- Corrado, B.; Bonini, I.; Alessio Chirico, V.; Rosano, N.; Gisonni, P. Use of Injectable Collagen in Partial-Thickness Tears of the Supraspinatus Tendon: A Case Report. Oxf Med Case Reports 2020, 2020, omaa103. [Google Scholar] [CrossRef]
- Damjanov, N.; Micu, M.C. Ultrasound Guided Injection with Collagen-Based Medical Device: Real-Life Evaluation of Efficacy and Safety in Hip Osteoarthritis. Med Ultrason 2023. [CrossRef]
- Farkash, U.; Avisar, E.; Volk, I.; Slevin, O.; Shohat, N.; El Haj, M.; Dolev, E.; Ashraf, E.; Luria, S. First Clinical Experience with a New Injectable Recombinant Human Collagen Scaffold Combined with Autologous Platelet-Rich Plasma for the Treatment of Lateral Epicondylar Tendinopathy (Tennis Elbow). J Shoulder Elbow Surg 2019, 28, 503–509. [Google Scholar] [CrossRef]
- Kim, M.; Choi, Y.S.; You, M.-W.; Kim, J.S.; Young, K.W. Sonoelastography in the Evaluation of Plantar Fasciitis Treatment: 3-Month Follow-Up After Collagen Injection. Ultrasound Q 2016, 32, 327–332. [Google Scholar] [CrossRef]
- De Luca, P.; Colombini, A.; Carimati, G.; Beggio, M.; de Girolamo, L.; Volpi, P. Intra-Articular Injection of Hydrolyzed Collagen to Treat Symptoms of Knee Osteoarthritis. A Functional In Vitro Investigation and a Pilot Retrospective Clinical Study. J Clin Med 2019, 8, 975. [Google Scholar] [CrossRef]
- Nitecka-Buchta, A.; Walczynska-Dragon, K.; Batko-Kapustecka, J.; Wieckiewicz, M. Comparison between Collagen and Lidocaine Intramuscular Injections in Terms of Their Efficiency in Decreasing Myofascial Pain within Masseter Muscles: A Randomized, Single-Blind Controlled Trial. Pain Res Manag 2018, 2018, 8261090. [Google Scholar] [CrossRef]
- He, Y.; Lin, Y.; He, X.; Li, C.; Lu, Q.; He, J. The Conservative Management for Improving Visual Analog Scale (VAS) Pain Scoring in Greater Trochanteric Pain Syndrome: A Bayesian Analysis. BMC Musculoskelet Disord 2023, 24, 423. [Google Scholar] [CrossRef] [PubMed]
- Heaver, C.; Pinches, M.; Kuiper, J.H.; Thomas, G.; Lewthwaite, S.; Burston, B.J.; Banerjee, R.D. Greater Trochanteric Pain Syndrome: Focused Shockwave Therapy versus an Ultrasound Guided Injection: A Randomised Control Trial. Hip Int 2023, 33, 490–499. [Google Scholar] [CrossRef] [PubMed]
- Gazendam, A.; Ekhtiari, S.; Axelrod, D.; Gouveia, K.; Gyemi, L.; Ayeni, O.; Bhandari, M. Comparative Efficacy of Nonoperative Treatments for Greater Trochanteric Pain Syndrome: A Systematic Review and Network Meta-Analysis of Randomized Controlled Trials. Clin J Sport Med 2022, 32, 427–432. [Google Scholar] [CrossRef] [PubMed]
- Kemp, J.L.; Collins, N.J.; Roos, E.M.; Crossley, K.M. Psychometric Properties of Patient-Reported Outcome Measures for Hip Arthroscopic Surgery. Am J Sports Med 2013, 41, 2065–2073. [Google Scholar] [CrossRef] [PubMed]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).