Submitted:
01 November 2024
Posted:
01 November 2024
You are already at the latest version
Abstract
Carob pulp is a valuable source of cellulose-rich fraction (CRF) for many food applications. This study aimed to obtain and characterize a CRF derived from carob pulp waste, after sugar removal, and to evaluate its potential use in the 3D printing of cellulose-rich foods. Thus, extraction of the CRF present in carob pulp (by obtaining the alcohol-insoluble residue) was carried out, accounting for nearly 45 % dm (dry matter) of this by-product. The CRF contained about 24 % dm of cellulose. The functional properties (swelling capacity, water retention and fat adsorption) related to this fraction were determined, which showed a value of 5.9 mL/g of CRF, 4.0 and 6.5 g/g of CRF, respectively. Different gels were formulated with a total solids content of 15 % wm (wet matter), using potato peel flour as a base and partially substituting with CRF (0-8 % wm). The cellulose-based gels were characterized in terms of viscosity, water distribution (low-field NMR), and printability, while the 3D-printed samples were assessed for their textural properties. As the percentage of added CRF increased, the viscosity decreased while the water retention increased. Printability improved when small proportions of CRF (2-4%) were used, while deteriorated for higher percentages (6-8 %). The textural properties (hardness, adhesiveness, cohesiveness, and gumminess) showed significant changes caused by the addition of CRF, with gels containing 3-4 % CRF exhibiting the most suitable printing values. In summary, CRF extracted from carob pulp waste can be used as an ingredient in the 3D printing of novel cellulose-rich foods, reducing food waste within the framework of the circular economy.
Keywords:
1. Introduction
2. Results and Discussion
2.1. Characterization of Carob Cellulose-Rich Fraction (CRF)
2.1.1. Analysis of Carbohydrate Composition
2.1.2. Functional Properties
2.2. Characterization of Cellulose-Based Gels
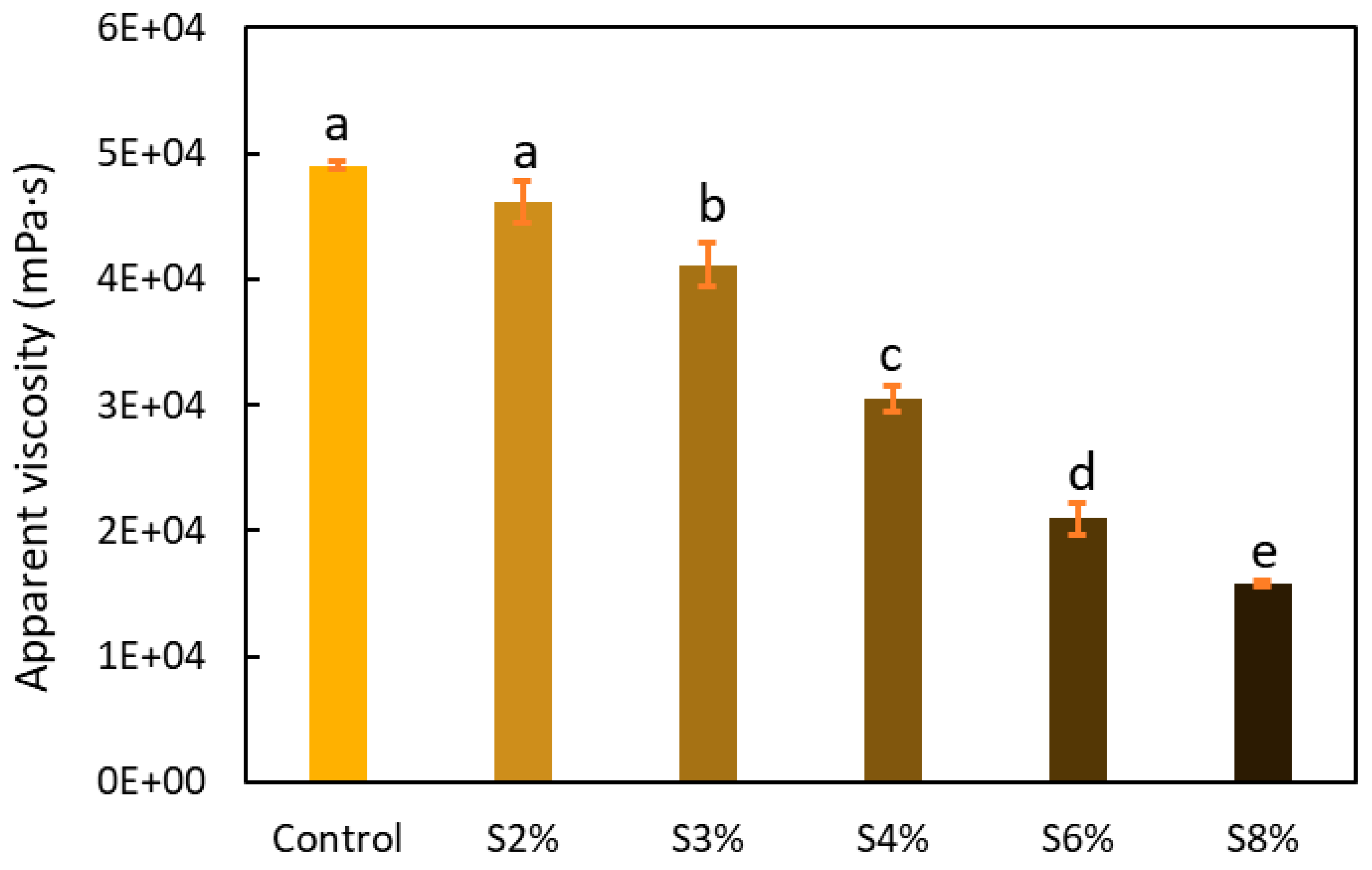
2.2.1. Apparent Viscosity
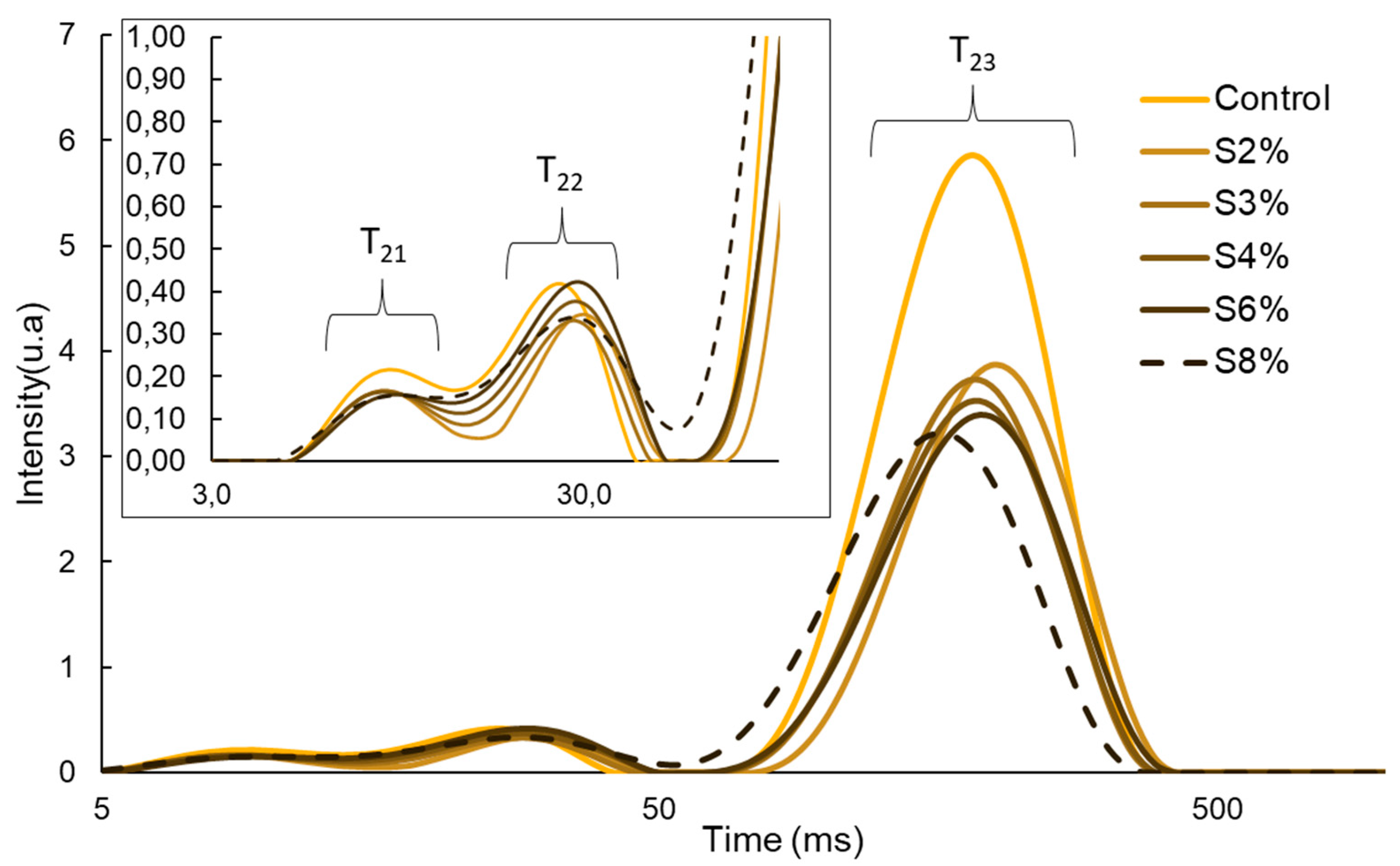
2.2.2. Water Distribution
2.2.3. Printability
2.3. Characterization of 3D Printed Samples
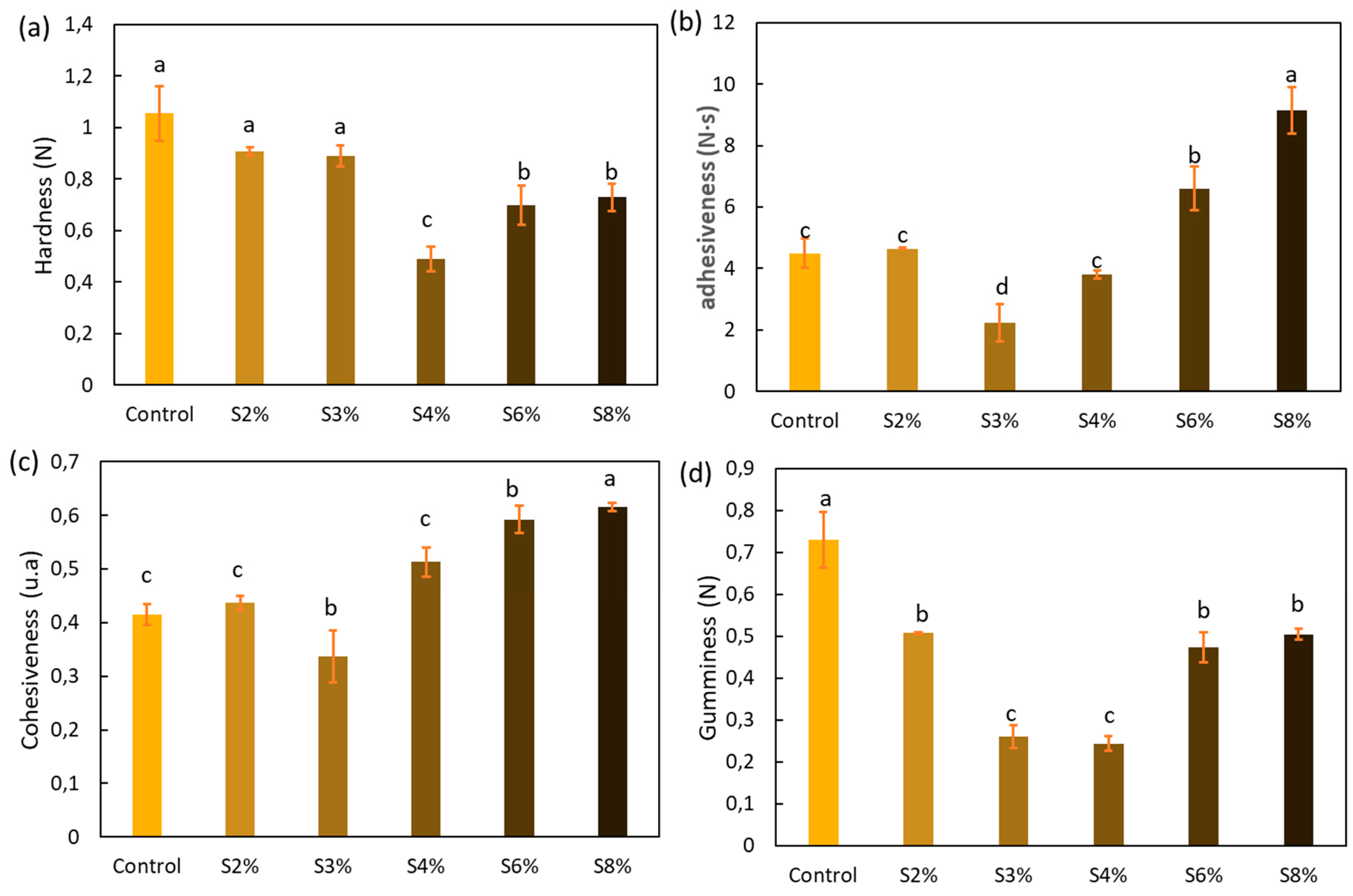
2.3.1. Textural Properties
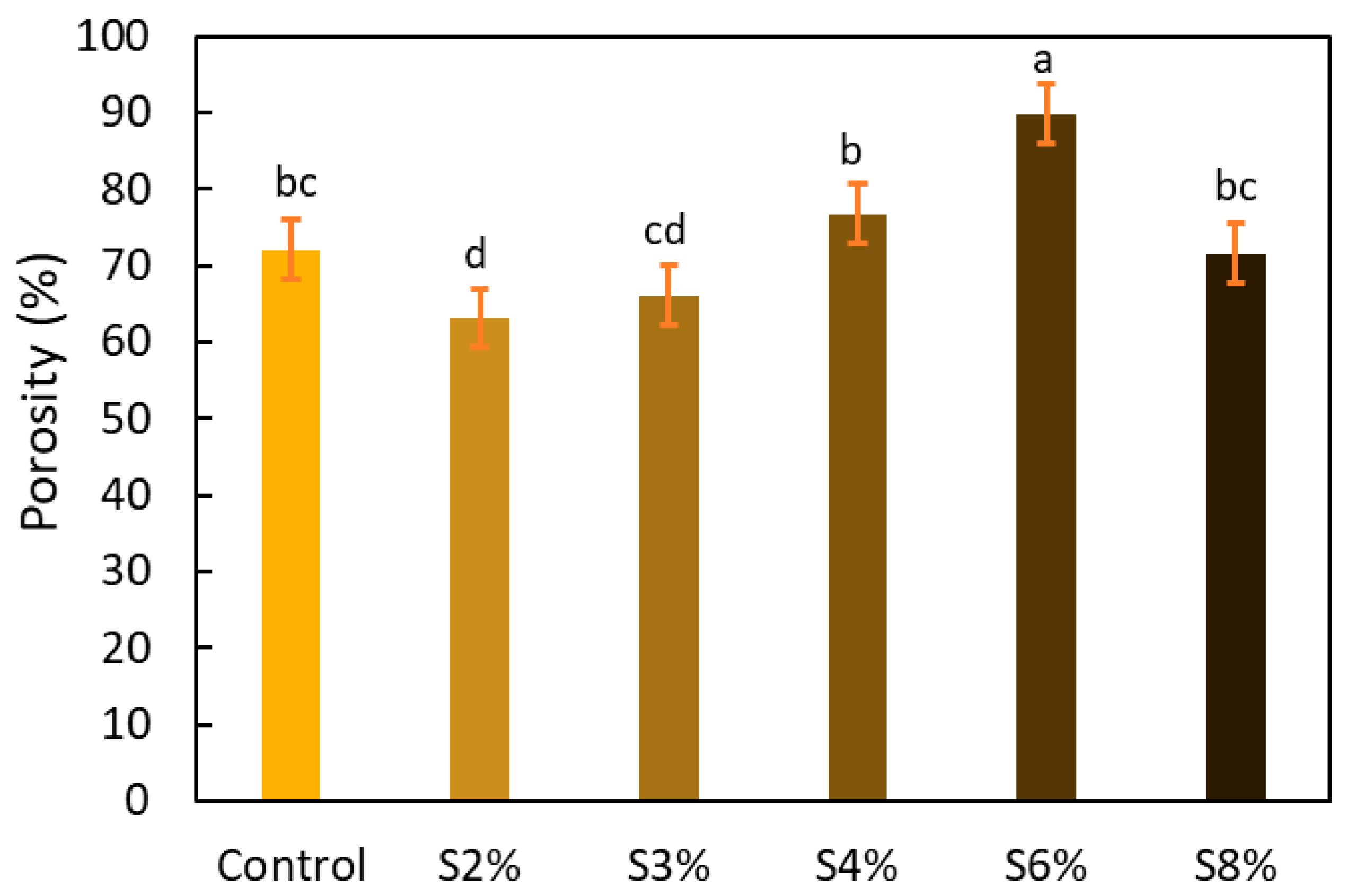
2.3.2. Microstructure
3. Conclusions
4. Materials and Methods
4.1. Raw Matter
4.2. Obtainment of the Cellulose Rich-Fraction (CRF)
4.3. Composition of the CRF
4.4. CRF Functional Properties
4.5. Preparation of the Cellulose-Based Gel for 3D Printing
4.6. Characterization of the Cellulose-Based Gel
4.6.1. Apparent Viscosity
4.6.2. Water Distribution
4.6.3. Printability
4.7. Characterization of 3D Printed Samples
4.7.1. Textural Properties
4.7.2. Porosity
4.8. Statistical Analysis
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Singh, L.; Kaur, S.; Aggarwal, P. Techno and Bio Functional Characterization of Industrial Potato Waste for Formulation of Phytonutrients Rich Snack Product. Food Biosci 2022, 49, 101824. [Google Scholar] [CrossRef]
- Phanthong, P.; Reubroycharoen, P.; Hao, X.; Xu, G.; Abudula, A.; Guan, G. Nanocellulose: Extraction and Application. 2018. [Google Scholar] [CrossRef]
- Katahira, R.; Elder, T.J.; Beckham, G.T. Chapter 1. A Brief Introduction to Lignin Structure. In; 2018; pp. 1–20.
- Brassesco, M.E.; Brandão, T.R.S.; Silva, C.L.M.; Pintado, M. Carob Bean (Ceratonia Siliqua L.): A New Perspective for Functional Food. Trends Food Sci Technol 2021, 114, 310–322. [Google Scholar] [CrossRef]
- Caliskan, A.; Abdullah, N.; Ishak, N.; Caliskan, I.T. Physicochemical, Microbial and Sensory Properties of Wild Carob Bar: A Shelf-Life Study. Int J Gastron Food Sci 2023, 31. [Google Scholar] [CrossRef]
- Ikram, A.; Khalid, W.; Wajeeha Zafar, K. ul; Ali, A.; Afzal, M.F.; Aziz, A.; Faiz ul Rasool, I.; Al-Farga, A.; Aqlan, F.; Koraqi, H. Nutritional, Biochemical, and Clinical Applications of Carob: A Review. Food Sci Nutr 2023, 11, 3641–3654. [Google Scholar] [CrossRef]
- Loullis, A.; Pinakoulaki, E. Carob as Cocoa Substitute: A Review on Composition, Health Benefits and Food Applications. European Food Research and Technology 2017 244:6 2017, 244, 959–977. [Google Scholar] [CrossRef]
- Correa, M.J.; Salinas, M. V.; Carbas, B.; Ferrero, C.; Brites, C.; Puppo, M.C. Technological Quality of Dough and Breads from Commercial Algarroba–Wheat Flour Blends. J Food Sci Technol 2017, 54, 2104–2114. [Google Scholar] [CrossRef]
- Restuccia, D.; Esposito, L.; Spizzirri, U.G.; Martuscelli, M.; Caputo, P.; Rossi, C.O.; Clodoveo, M.L.; Pujia, R.; Mazza, E.; Pujia, A.; et al. Formulation of a Gluten-Free Carob-Based Bakery Product: Evaluation of Glycemic Index, Antioxidant Activity, Rheological Properties, and Sensory Features. Fermentation 2023, 9. [Google Scholar] [CrossRef]
- Hopson, C.; Rigual, V.; Domínguez, J.C.; Alonso, M.V.; Oliet, M.; Rodríguez, F. A New Approach for the Use of Cellulose-Rich Solids from Biorefinery in the Formulation of Gel-like Materials. Ind Crops Prod 2022, 186. [Google Scholar] [CrossRef]
- Dai, L.; Cheng, T.; Duan, C.; Zhao, W.; Zhang, W.; Zou, X.; Aspler, J.; Ni, Y. 3D Printing Using Plant-Derived Cellulose and Its Derivatives: A Review. Carbohydr Polym 2019, 203, 71–86. [Google Scholar] [CrossRef]
- Lille, M.; Nurmela, A.; Nordlund, E.; Metsä-Kortelainen, S.; Sozer, N. Applicability of Protein and Fiber-Rich Food Materials in Extrusion-Based 3D Printing. J Food Eng 2018, 220, 20–27. [Google Scholar] [CrossRef]
- Guo, C.; Zhang, M.; Devahastin, S. 3D Extrusion-Based Printability Evaluation of Selected Cereal Grains by Computational Fluid Dynamic Simulation. J Food Eng 2020, 286, 110113. [Google Scholar] [CrossRef]
- Liu, Z.; Chen, H.; Zheng, B.; Xie, F.; Chen, L. Understanding the Structure and Rheological Properties of Potato Starch Induced by Hot-Extrusion 3D Printing. Food Hydrocoll 2020, 105, 105812. [Google Scholar] [CrossRef]
- Godoi, F.C.; Prakash, S.; Bhandari, B.R. 3d Printing Technologies Applied for Food Design: Status and Prospects. J Food Eng 2016, 179, 44–54. [Google Scholar] [CrossRef]
- Zheng, L.; Yu, Y.; Tong, Z.; Zou, Q.; Han, S.; Jiang, H. The Characteristics of Starch Gels Molded by 3D Printing. J Food Process Preserv 2019, 43. [Google Scholar] [CrossRef]
- Jagadiswaran, B.; Alagarasan, V.; Palanivelu, P.; Theagarajan, R.; Moses, J.A.; Anandharamakrishnan, C. Valorization of Food Industry Waste and By-Products Using 3D Printing: A Study on the Development of Value-Added Functional Cookies. Future Foods 2021, 4, 100036. [Google Scholar] [CrossRef]
- Reche, C.; Umaña, M.; Dalmau, E.; Carcel, J.A.; Eim, V. Improving 3D Printed Food Characteristics by Using Mushroom By-Products and Olive Oil in the Formulation. LWT 2024, 202. [Google Scholar] [CrossRef]
- Muthurajan, M.; Veeramani, A.; Rahul, T.; Gupta, R.K.; Anukiruthika, T.; Moses, J.A.; Anandharamakrishnan, C. Valorization of Food Industry Waste Streams Using 3D Food Printing: A Study on Noodles Prepared from Potato Peel Waste. Food Bioproc Tech 2021, 14, 1817–1834. [Google Scholar] [CrossRef]
- Cikrikci Erunsal, S.; Basturk, Z.S.; Canturkoglu, I.; Ozturk, H.I. Development of 3D Printed Dark Chocolate Sweetened with Carob Extract. Int J Gastron Food Sci 2023, 34, 100794. [Google Scholar] [CrossRef]
- Pavičić, T.V.; Grgić, T.; Ivanov, M.; Novotni, D.; Herceg, Z. Influence of Flour and Fat Type on Dough Rheology and Technological Characteristics of 3d-Printed Cookies. Foods 2021, 10. [Google Scholar] [CrossRef]
- Nasar-Abbas, S.M.; e-Huma, Z.; Vu, T.H.; Khan, M.K.; Esbenshade, H.; Jayasena, V. Carob Kibble: A Bioactive-Rich Food Ingredient. Compr Rev Food Sci Food Saf 2016, 15, 63–72. [Google Scholar] [CrossRef] [PubMed]
- Femenia, A.; Sánchez, E.S.; Simal, S.; Rosselló, C. Compositional Features of Polysaccharides from Aloe Vera (Aloe Barbadensis Miller) Plant Tissues. Carbohydr Polym 1999, 39, 109–117. [Google Scholar] [CrossRef]
- Zhu, B.J.; Zayed, M.Z.; Zhu, H.X.; Zhao, J.; Li, S.P. Functional Polysaccharides of Carob Fruit: A Review. Chinese Medicine (United Kingdom) 2019, 14, 1–10. [Google Scholar] [CrossRef]
- Petkova, N.; Petrova, I.; Ivanov, I.; Mihov, R.; Hadjikinova, R.; Ognyanov, M.; Nikolova, V. Nutritional and Antioxidant Potential of Carob (Ceratonia Siliqua) Flour and Evaluation of Functional Properties of Its Polysaccharide Fraction.
- Fidan, H.; Stankov, S.; Petkova, N.; Petkova, Z.; Iliev, A.; Stoyanova, M.; Ivanova, T.; Zhelyazkov, N.; Ibrahim, S.; Stoyanova, A.; et al. Evaluation of Chemical Composition, Antioxidant Potential and Functional Properties of Carob (Ceratonia Siliqua L.) Seeds. J Food Sci Technol 2020, 57, 2404. [Google Scholar] [CrossRef]
- Boulos, N.N.; Greenfield, H.; Wills, R.B.H. Water Holding Capacity of Selected Soluble and Insoluble Dietary Fibre. Int J Food Prop 2000, 3, 217–231. [Google Scholar] [CrossRef]
- Liu, C.; Ho, C.; Wang, J. The Development of 3D Food Printer for Printing Fibrous Meat Materials. [CrossRef]
- Reche, C.; Umaña, M.; Dalmau, E.; Carcel, J.A.; Eim, V. Improving 3D Printed Food Characteristics by Using Mushroom By-Products and Olive Oil in the Formulation. LWT 2024, 202, 116238. [Google Scholar] [CrossRef]
- Chen, Y.; Zhang, M.; Phuhongsung, P. 3D Printing of Protein-Based Composite Fruit and Vegetable Gel System. LWT 2021, 141, 110978. [Google Scholar] [CrossRef]
- Phuhongsung, P.; Zhang, M.; Devahastin, S. Investigation on 3D Printing Ability of Soybean Protein Isolate Gels and Correlations with Their Rheological and Textural Properties via LF-NMR Spectroscopic Characteristics. LWT 2020, 122, 109019. [Google Scholar] [CrossRef]
- Liu, Z.; Chen, H.; Zheng, B.; Xie, F.; Chen, L. Understanding the Structure and Rheological Properties of Potato Starch Induced by Hot-Extrusion 3D Printing. Food Hydrocoll 2020, 105, 105812. [Google Scholar] [CrossRef]
- Lille, M.; Nurmela, A.; Nordlund, E.; Metsä-Kortelainen, S.; Sozer, N. Applicability of Protein and Fiber-Rich Food Materials in Extrusion-Based 3D Printing. J Food Eng 2018, 220, 20–27. [Google Scholar] [CrossRef]
- Mirazimi, F.; Saldo, J.; Sepulcre, F.; Gràcia, A.; Pujola, M. Enriched Puree Potato with Soy Protein for Dysphagia Patients by Using 3D Printing. Food Front 2022, 3, 706–715. [Google Scholar] [CrossRef]
- Zhang, C.; Wang, C.S.; Therriault, D.; Heuzey, M.C. Development of Aqueous Protein/Polysaccharide Mixture-Based Inks for 3D Printing towards Food Applications. Food Hydrocoll 2022, 131, 107742. [Google Scholar] [CrossRef]
- Siamand, R.; Deeth, H.C.; Al-Saadi, J.M.S. Textural and Sensory Properties of a Calcium-Induced Milk Gel. J Food Eng 2014, 139, 10–12. [Google Scholar] [CrossRef]
- Rosenthal, A.J.; Thompson, P. What Is Cohesiveness?—A Linguistic Exploration of the Food Texture Testing Literature. J Texture Stud 2021, 52, 294–302. [Google Scholar] [CrossRef]
- Shaikh, M.; Ali, T.M.; Hasnain, A. Utilization of Chemically Modified Pearl Millet Starches in Preparation of Custards with Improved Cold Storage Stability. Int J Biol Macromol 2017, 104, 360–366. [Google Scholar] [CrossRef]
- Femenia, A.; Sánchez, E.S.; Simal, S.; Rosselló, C. Compositional Features of Polysaccharides from Aloe Vera (Aloe Barbadensis Miller) Plant Tissues. Carbohydr Polym 1999, 39, 109–117. [Google Scholar] [CrossRef]
- Dalmau, M.E.; Bornhorst, G.M.; Eim, V.; Rosselló, C.; Simal, S. Effects of Freezing, Freeze Drying and Convective Drying on in Vitro Gastric Digestion of Apples. Food Chem 2017, 215, 7–16. [Google Scholar] [CrossRef]
- Coimbra, M. a; Delgadillo, I.; Waldron, K.W.; Selvendran, R.R. Isolation and Analysis of Cell Wall Polymers from Olive Pulp. Modern Methods of Plant Analysis 1996, 17, 19–44. [Google Scholar] [CrossRef]
- Khatun, M.M.; Li, Y.H.; Liu, C.G.; Zhao, X.Q.; Bai, F.W. Fed-Batch Saccharification and Ethanol Fermentation of Jerusalem Artichoke Stalks by an Inulinase Producing Saccharomyces Cerevisiae MK01. RSC Adv 2015, 5, 107112–107118. [Google Scholar] [CrossRef]
- Kuniak, L.; Marchessault, R.H. Study of the Crosslinking Reaction between Epichlorohydrin and Starch. Starch - Stärke 1972, 24, 110–116. [Google Scholar] [CrossRef]
- González-Centeno, *!!! REPLACE !!!*; Rosselló, C.; Simal, S.; Garau, M.C.; López, F.; Femenia, A. Physico-Chemical Properties of Cell Wall Materials Obtained from Ten Grape Varieties and Their Byproducts: Grape Pomaces and Stems. LWT - Food Science and Technology 2010, 43, 1580–1586. [Google Scholar] [CrossRef]
- Liu, Z.; Zhang, M.; Bhandari, B.; Yang, C. Impact of Rheological Properties of Mashed Potatoes on 3D Printing. J Food Eng 2018, 220, 76–82. [Google Scholar] [CrossRef]
- Liu, Z.; Zhang, M.; Yang, C. hui Dual Extrusion 3D Printing of Mashed Potatoes/Strawberry Juice Gel. LWT 2018, 96, 589–596. [Google Scholar] [CrossRef]
- Rodríguez, Ó.; Llabrés, P.J.; Simal, S.; Femenia, A.; Rosselló, C. Intensification of Predrying Treatments by Means of Ultrasonic Assistance: Effects on Water Mobility, PPO Activity, Microstructure, and Drying Kinetics of Apple. Food Bioproc Tech 2015, 8, 503–515. [Google Scholar] [CrossRef]
- Yang, G.; Tao, Y.; Wang, P.; Xu, X.; Zhu, X. Optimizing 3D Printing of Chicken Meat by Response Surface Methodology and Genetic Algorithm: Feasibility Study of 3D Printed Chicken Product. LWT 2022, 154, 112693. [Google Scholar] [CrossRef]
- Sánchez-Alonso, I.; Martinez, I.; Sánchez-Valencia, J.; Careche, M. Estimation of Freezing Storage Time and Quality Changes in Hake (Merluccius Merluccius, L.) by Low Field NMR. Food Chem 2012, 135, 1626–1634. [Google Scholar] [CrossRef]
- Bourne, M.C. Food Texture and Viscosity: 4 Principles of Objective Texture Measurements; 2002; ISBN 9780121190620.
- Reche, C.; Rosselló, C.; Dalmau, E.; Eim, V.; Simal, S. Quantification of Microstructural Changes in Artichoke By-Products by Image Analysis after High-Power Ultrasound-Assisted Extraction of Bioactive Compounds. LWT 2022, 171, 114127. [Google Scholar] [CrossRef]
- Baniasadi, H.; Ajdary, R.; Trifol, J.; Rojas, O.J.; Seppälä, J. Direct Ink Writing of Aloe Vera/Cellulose Nanofibrils Bio-Hydrogels. Carbohydr Polym 2021, 266, 118114. [Google Scholar] [CrossRef]
- R Core Team R: A Language and Environment for Statistical Computing 2017.
- RStudio Team RStudio: Integrated Development for, R. RStudio 2022.
| Monosaccharid | mg/g CRF |
|---|---|
| Rhamnose | 10.7 ± 1.6 |
| Fucose | 7.5 ± 1.1 |
| Arabinose | 78.4 ± 6.6 |
| Xylose | 158.2 ± 13.8 |
| Mannose | 24.8 ± 3.1 |
| Galactose | 47.4 ± 4.3 |
| Glucose | 179.0 ± 18.6 |
| Uronic Acids | 153.2 ± 9.0 |
| Total | 659.5 ± 42.2 |
| Sw (ml/g) | FAC (g/g) | WRC (g/g) |
|---|---|---|
| 5,9 ± 0,5 | 6,5 ± 0,5 | 4,0 ± 0,1 |
| Top View | Front View | Scale | |
| Control |  |
 |
4 |
| S2% |  |
 |
5 |
| S3% |  |
 |
5 |
| S4% |  |
 |
4 |
| S6% |  |
 |
3 |
| S8% |  |
 |
2 |
| Control |  |
S2% |  |
| S3% |  |
S4% | 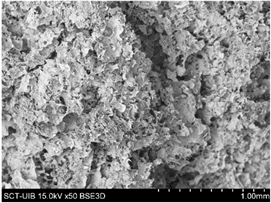 |
| S6% |  |
S8% | 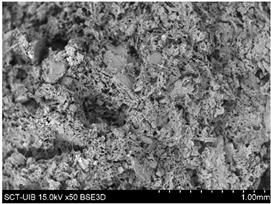 |
| Inks | CRF content (%) | Potato peel poder (%) |
|---|---|---|
| Control | 0 | 15 |
| S 2 | 2 | 13 |
| S 3 | 3 | 12 |
| S 4 | 4 | 11 |
| S 6 | 6 | 9 |
| S 8 | 8 | 7 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).





