1. Introduction
Heterozygous familial hypercholesterolemia (HeFH) is a common genetic disease characterised by lifelong elevation of low-density lipoprotein cholesterol (LDL-C), which can lead to early-onset atherosclerosis and increased risk of cardiovascular disease (CVD) [
1,
2]. In the last decades, growing data emphasizes the importance of early detection and intervention in HeFH subjects, as research findings clearly demonstrate that the cumulative risk of exposure to elevated LDL-C levels from birth accelerates the progression of atherosclerosis. Thus, lowering cholesterol levels at a young age reduces the risk of CVD in adulthood [
1,
2,
3].
Unfortunately, childhood HeFH is mostly underdiagnosed or undertreated [
4,
5]. Recent data shows that the majority of hypercholesterolemic children, even those under hypolipidemic drug therapy, do not reach the LDL-C lowering goals [
1,
2,
3]. Although therapeutic lifestyle interventions, including dietary modifications and intense physical activity, constitute the fundamental strategy for managing dyslipidemia in children, they rarely achieve to reduce LDL-C levels sufficiently in children affected by genetic dyslipidemias. Evidence shows that an improved diet and exercise regimen
lowers LDL-C by 10%–15% [
1,
2].
Recently, many nutraceuticals (NCs), alone or in combination, are gaining a growing interest, as they seem to be an effective alternative approach for the management of adult patients with mild or modest hypercholesterolemia or with intolerance to conventional drug therapy. However, there are few studies evaluating the efficacy and safety of the NCs combinations compared to that of each component they contain [
6,
7,
8,
9]. Notably, the International Lipid Expert Panel as well as the European Society of Cardiology and the European Atherosclerosis Society (EAS) guidelines, recommend the use of dietary supplements and a balanced diet to improve lipid profile [
10,
11].
Red yeast rice (RYR), a Chinese rice cultivated with the mold
Μonascus purpureus, is currently the most well studied, after soluble fibers and phytosterols, as an effective lipid lowering nutraceutical [
12]. Its main bioactive compound is a natural statin, the monacolin K, a 3-hydroxy-3-methyl-glutaryl-coenzyme A (HMG-CoA) reductase inhibitor, structurally and functionally similar to lovastatin. RYR efficacy as well as its safety profile is considered to be comparable to those of low dose statins [
8,
12]. Data show that daily consumption of monacolin K leads to a reduction of low density lipoprotein—cholesterol (LDL-C) levels, up to 15-25% within 6 to 8 weeks. Furthermore, growing evidence support that RYR use improves the structure and function of vessels as well as inflammatory biomarkers [
10,
12]. Adverse effects are similar to those of statins but are rare when low doses of monacolin K are used. Moreover, many adverse effects have been attributed to citrinin, a substance produced during rice fermentation by
Μonascus purpureus, in experimental models. Thus, the recommendation is to use citrinin-free RYR products [
7,
8,
10].
As NCs have various mechanisms of lipid lowering action, their combined use could have additive or synergistic effects. Recent data support the potential use of nutraceutical combinations, with the majority of them containing RYR combined with other substances such as policosanols, berberine, astaxanthin, coenzyme Q10 and folic acid [
7,
8]. Policosanols are long-chain aliphatic alcohols, which inhibit the action of HMG-CoA reductase and the bile acids absorption, with the results of studies about its hypolipidemic action being conflicting. Berberine is a plant derived alkaloid drug which improves LDL-C excretion by different mechanisms and has a beneficial effect on glucose metabolism and blood pressure [
7]. Coenzyme Q10, an antioxidant and anti-inflammatory factor, inhibits LDL-C oxidation, but a significant reduction in LDL-C levels has not been reported. Astaxanthin has an antioxidant and anti-inflammatory action, and an effect on lipid metabolism, although its supplementation had no effect on total cholesterol levels in adult patients [
7,
8,
10]. Finally, folic acid administration resulted in a significant reduction in total cholesterol (TC) and LDL-C levels in adults with atherosclerotic risk factors [
13].
The majority of clinical evidence evaluating efficacy and safety of NCs involve adult dyslipidemic patients [
7]. In the latest EAS guidelines NCs’ use has also been indicated for children, older than 6 years, with familial hypercholesterolemia [
14]. There are few studies concerning the administration of NCs in children and adolescents with dyslipidemia. Most of them refer to the administration of soluble fibers and phytosterols to a limited number of subjects, while only one study refers to the early effect of supplementation with RYR combined with policosanols on lipid profile in childhood [
15,
16].
As existing data for RYR use in childhood are extremely limited, the aim of the present study was to evaluate the efficacy and safety of the long-term consumption of a dietary supplement containing RYR, combined with other natural compounds, in children and adolescents with primary hypercholesterolemia.
2. Material and Methods
2.1. Study Design
A prospective single center cohort study was conducted at Outpatient Lipid Unit of 2nd Department of Pediatrics of the National and Kapodistrian University of Athens at the “Panagiotis & Aglaia Kyriakou” Children’s Hospital in Greece. The study complies with the Declaration of Helsinki and the protocol was reviewed and approved by the “Panagiotis & Aglaia Kyriakou” Children’s Hospital ethical committee (approval number: 1474/10 Jan 2020). The study has been registered in ClinicalTrials.gov (NCT06045377). Parental informed written consent was obtained prior to enrollment.
2.2. Study Population
Ninety children and adolescents, aged 7 to 16 years, with primary dyslipidemia were enrolled in the study. The cohort enrolment criteria were age ≥ 7 years and LDL-C >150 mg/dl in more than two measurements, after therapeutic lifestyle changes for at least 6 months. The exclusion criteria were: secondary hypercholesterolemia, presence of any chronic disease or growth and developmental disorders, abnormal liver, kidney or thyroid function and prior use of hypolipidemic or other medication, at least 6 months before participation in the study.
All participants had a positive family history for hypercholesterolemia (LDL
> 95th percentile) and most of them had a positive family history for premature CVD (<55 years for males and <60 years for females) in first or/and second-degree relatives. Eleven of them had a genetically confirmed diagnosis of HeFH, while the rest had a probable or positive HeFH according to the Dutch Lipid Clinic Network criteria [
17]. None of them had moderate or significant hypertriglyceridemia, even those with increased body mass index (BMI).
All children followed a low saturated fat and low cholesterol diet by a trained dietician and had moderate or intense physical activity, for at least 6 months before the participation in the study. In addition, during the last 6 months, 19 of them consumed 1.5–2.5g of plant sterols daily in the form of a yogurt drink or spread. Lifestyle and eating habits were maintained throughout the study.
A nutraceutical containing five natural substances formulated as a tablet with the commercial name Armolipid (Rottapharm S.p.A., Monza, Italia), was recommended in all participants. Every tablet contained 200 mg red yeast rice (RYR) extract equivalent to 3 mg of monacolin K, 10 mg policosanols, 0.2 mg folic acid, 2.0 mg coenzyme Q10 and 0.5 mg astaxanthin, and was citrinin-free. It was administered once-daily with lunch.
All participants as well as their parents were interviewed using a data collection form, created specifically for this research. Details concerning compliance to supplement intake, as well as possible adverse effects were recorded and analysed. Six out of 90 children were excluded from the study because they did not comply with the recommendation for taking the supplement (compliance rate 93.3%). All the rest 84 children had one and 64 of them had two evaluations under Armolipid treatment.
2.3. Clinical and Labolatory Evaluation
A clinical and laboratory evaluation of all participants took place right before (Time 0, T0) and once (n=84, Time 1, T1) or twice (n=64, T1 and Time 2, T2) after the start of nutraceutical supplementation.
The body weight (BW) in kg, the height (H) in cm were measured to the nearest 0.1 kg and 0.5 cm, respectively (TANITA, Corporation Tokyo), with children barefoot and lightly dressed. BMI was calculated as BW in kg per H in m
2. The standard deviation scores (z-scores) of BW, H and BMI were also calculated according to a standardized age- and sex- specific calculator. Waist circumference (WC) in cm was measured and the ratio of WC/Height was calculated. Systolic (SBP) and diastolic blood pressure (DBP) in mmHg were measured three consecutive times using an automated oscillometric device (Dinamap V100, GE Medical Systems Information Technologies) and the average value of the three measurements was used in the statistical analysis. The stage of puberty was recorded according to Tanner stages (I-V) for boys and girls [
18].
A full lipid profile including TC, LDL-C, high density lipoprotein—cholesterol (HDL-C), non-high density lipoprotein—cholesterol (non-HDL-C), triglycerides (TGs), apolipoprotein A1 (Apo-A1), apolipoprotein B (Apo-B), and lipoprotein (a) [Lp(a)] levels was evaluated in serum, after an overnight fast. Serum creatinine, glucose, aspartate and alanine aminotransferases (AST and ALT), creatine kinase (CK), and thyroid-stimulating hormone (TSH) were also assessed. A full blood count was performed in all participants.
TC, HDL-C, LDL-C, TGs, glucose, ALT, AST, CK and creatinine were measured using an enzymatic method (Roche Diagnostics) on an automatic analyzer (Cobass Integra 800), and Apo-A1, Apo-B, and Lp(a) by an immunonephelometric assay (Siemens BNII Nephelometer Analyzer), with an intra-assay and inter-assay variation <5% for all tests. Non-HDL-C was measured as TC minus HDL-C. All lipid values are expressed in mg/dL. Haematological parameters (full blood count) were analyzed using the SysmexXE-2100 automated haematology analyzer (Roche Diagnostics). Manufacturers’ instructions of instrument were strictly followed.
At the time of the examination, all children were healthy and none of them had a febrile or afebrile infection in the two weeks preceding the check-up.
The primary outcome was the reduction of LDL-C serum levels. Secondary outcomes included the improvement of the other parameters of lipid profile, as well as the evaluation of possible adverse effects such as gastrointestinal symptoms or hepatic and muscle enzymes elevation.
2.4. Statistical Analysis
Qualitative data is presented with absolute and relative frequencies (%). Depending on the distribution (normal or not), quantitative data is presented with Mean ± standard deviation or Median (Q25, Q75). Regularity was checked using the Shapiro-Wilk criterion, and graphically with the use of histograms, normal Q-Q plots and boxplots. To evaluate changes in lipid profile before and after Armolipid consumption in total study population, a Friedman test was used (because of non-normality of the data). An individual Wilcoxon Signed Rank Test was conducted (using a Bonferroni adjusted alpha value) to control for Type 1 error. A one-way repeated measures ANOVA was conducted to evaluate changes in lipid profile after Armolipid supplementation in participants with no prior consumption of phytosterols (because of normality of the data in this group of children). Mixed between-within subjects’ analysis of variance was used for the comparison of Armolipid effect on lipid profile of population subgroups. The impact of Tanner alteration in lipid profile improvement was examined by using a Mann-Whitney U Test, while a paired-samples t-test was conducted to evaluate differences in lipid levels before and after phytosterols consumption. Statistical analysis was performed with the statistical package SPSS v25 and a probability value of p<0.05 was considered statistically significant.
3. Results
3.1. Descriptive Characteristics of the Study Population
Six out of the 90 children and adolescents who were recommended Armolipid supplementation daily, were excluded from the study because of bad compliance. Finally, 84 Caucasian children and adolescents, 41 (48.8%) males and 43 (51.2%) females, aged 7-16 years old (mean age: 9.9 years, + 2.1) were included in statistical analyses. Nineteen of the participants (22.6%) were consuming phytosterols, 1.5-2.5g daily, at least 3 months before the enrollment in the study. The consumption was discontinued from the day of their inclusion to the study.
All 84 participants were evaluated, clinically and biochemically, once (T1) and 64 (76.2%) twice (T1 and T2), under Armolipid treatment. The median intervals between the baseline (T0) and 1st (T1) and 2nd (T2) evaluation were 6 (Q25-Q75: 5-8) and 16 (Q25-Q75: 11-19.7) months respectively. The mean age of participants in T0, T1 and T2 was 9.9 (SD:2.0), 10.5 (SD:2.0) and 11.2 (SD:2.0) years, respectively. At baseline (T0), 47 (56%) of subjects were in pre-pubertal (Tanner stage 1) and 37 (44%) in pubertal stage (Tanner stages 2-5). At the T1 evaluation, 47.6% were in pre-puberty and 52.4% in puberty, while at the T2 evaluation the respective percentages were 39% and 61%.
The clinical and biochemical variables of the 64 participants who were evaluated twice under Armolipid treatment are shown in
Table 1.
3.2. Changes in Lipid Profile After Armolipid Supplementation in Total Study Population
A significant reduction in TC, LDL-C, non-HDL-C and Apo-B levels was observed after Armolipid treatment (p<0.001), while HDL-C, Apo-A1 and TGs levels didn’t change significantly (
Table 1). The absolute (mg/dl) and the percentage changes of lipids, lipoproteins and apolipoproteins levels after Armolipid consumption are presented in
Table 2 (the new variables conform to normal distribution, so they are expressed as Mean (SD)).
At 1st evaluation after Armolipid administration (T1), a decrease of LDL-C, non-HDL-C and Apo-B ≥10% was observed in 60.7%, 63.1% and 66.7% of 84 participants. At the 2nd evaluation (T2, N=64), the respective percentages were 78.1%, 70.3% and 59.4%. Moreover, a decrease of LDL-C, non-HDL-C and Apo-B from 5% to <10% from baseline levels was found in 14.3%, 11.9% and 7.1% of participants in T1 and in 6.3%, 14.1% and 12.5% in T2 evaluation.
None of the participants presented any gastrointestinal symptom or any liver, muscle or renal enzymes elevations, during the study period (
Table 1).
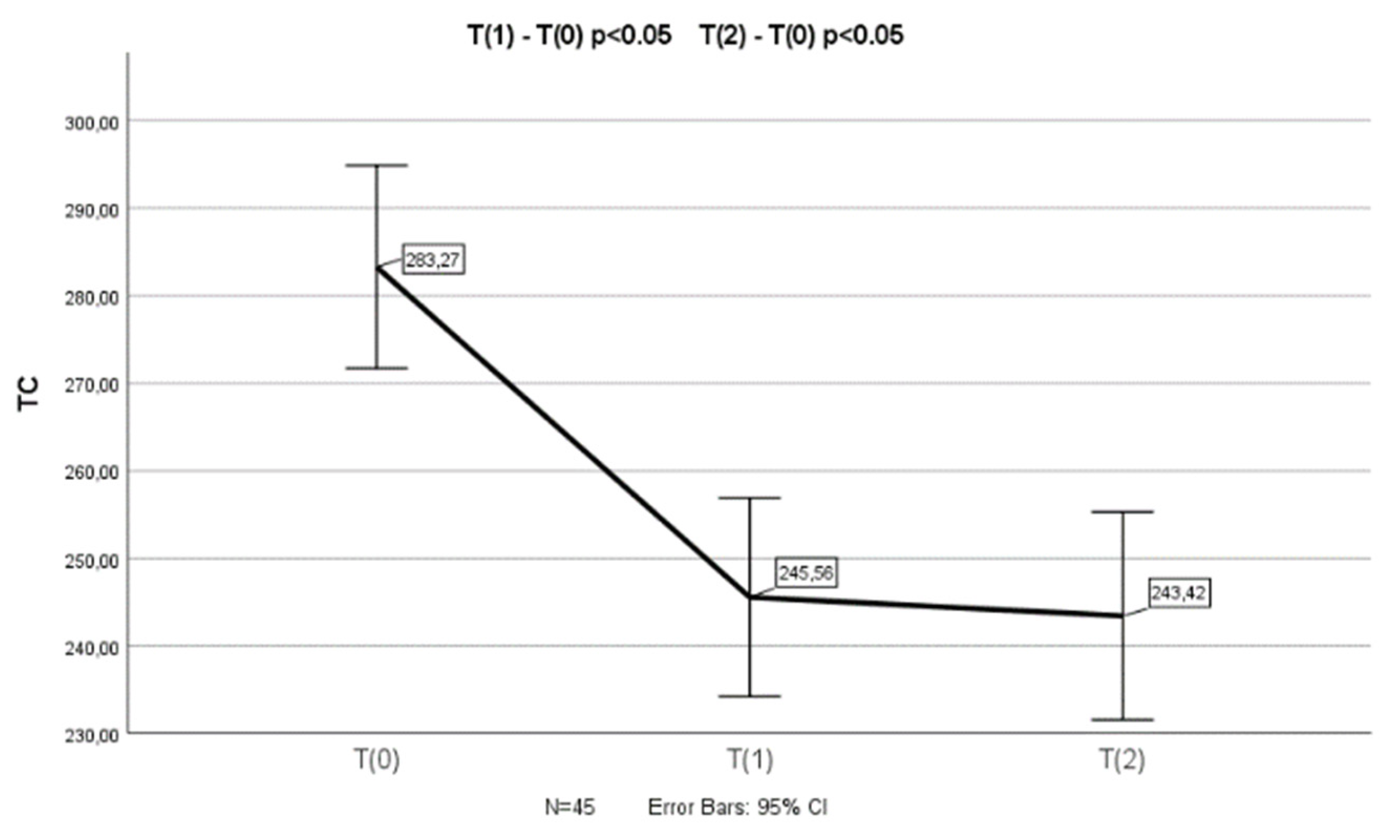
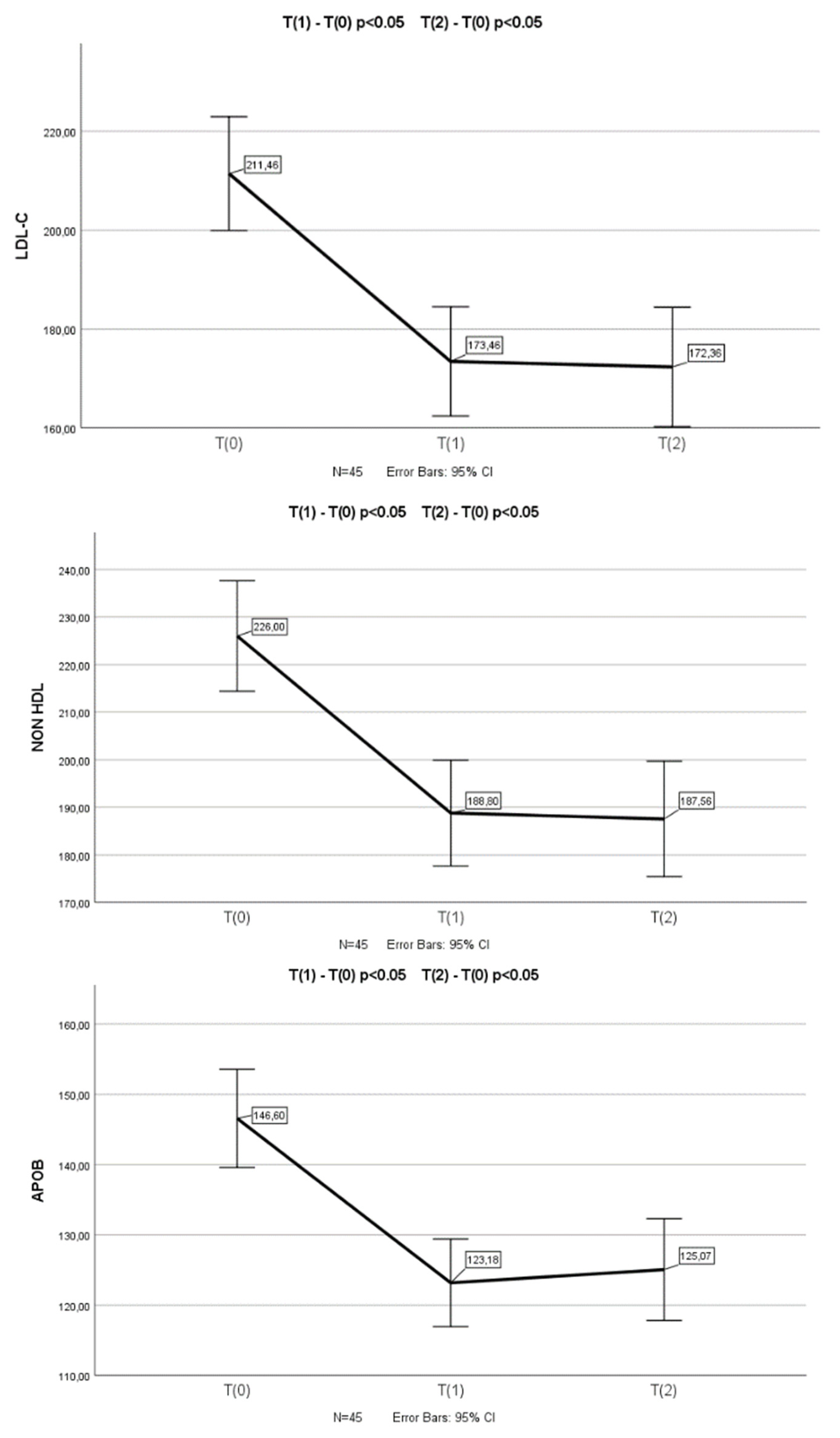
3.3. Changes in Lipid Profile After Armolipid Supplementation in Participants with no Prior Consumption of Phytosterols
In the group of children who had not previously consumed plant sterols (n=64), a statistically significant reduction in TC (p<0.001), LDL-C (p<0.001), non-HDL-C (p<0.001) and Apo-B (p<0.001) levels was found in T
1 and T
2 (n=45) measurements compared to the baseline (T
0) (a one-way repeated measures ANOVA was conducted (because of normality of the data in this group of children)). Appropriate post-hoc tests (pairwise comparisons) revealed a significant decrease in TC (p<0.001 and p<0.001), LDL-C (p<0.001 and p<0.001), non-HDL-C (p<0.001 and p<0.001) and Apo-B (p<0.001 and p<0.001) levels between T
0 and T
1, as well as between T
0 and T
2 measurements (
Figure 1). There was not any significant difference in TC, LDL-C, non-HDL-C and Apo-B levels between T
1 and T
2 measurements.
A one-way repeated measures ANOVA was conducted to compare scores on TC, LDL-C, non-HDL-C and Apo-B in 3 time period. There was a significant effect for time (p < .001). Post-hoc comparisons test, for all 4 parameters, indicated that the mean score for T(0) was significantly different from T(1) and from T(2). Also, the mean score for T(1) did not differ significantly from T(2).
No significant changes in HDL-C, TGs, Apo-A1 and Lp(a) levels were observed after the supplementation with Armolipid (T0 vs. T1 & T2).
No significant differences were found between BMI z-score, WC/H ratio, other biochemical parameters and TSH levels in times 0, 1 and 2. In contrary, SBP, DBP and creatinine levels were significantly higher at T1 and T2 evaluation when compared with T0 (p< 0.001, age statistically significant, with a beta value for SBP, DBP and creatinine =4.13, p<0.001, =2.95, p<0.001 and =0.03, p=0.001, respectively). The alterations in Tanner stage during the study had no significant effect on the reduction of LDL-C, non–HDL-C and Apo-B levels.
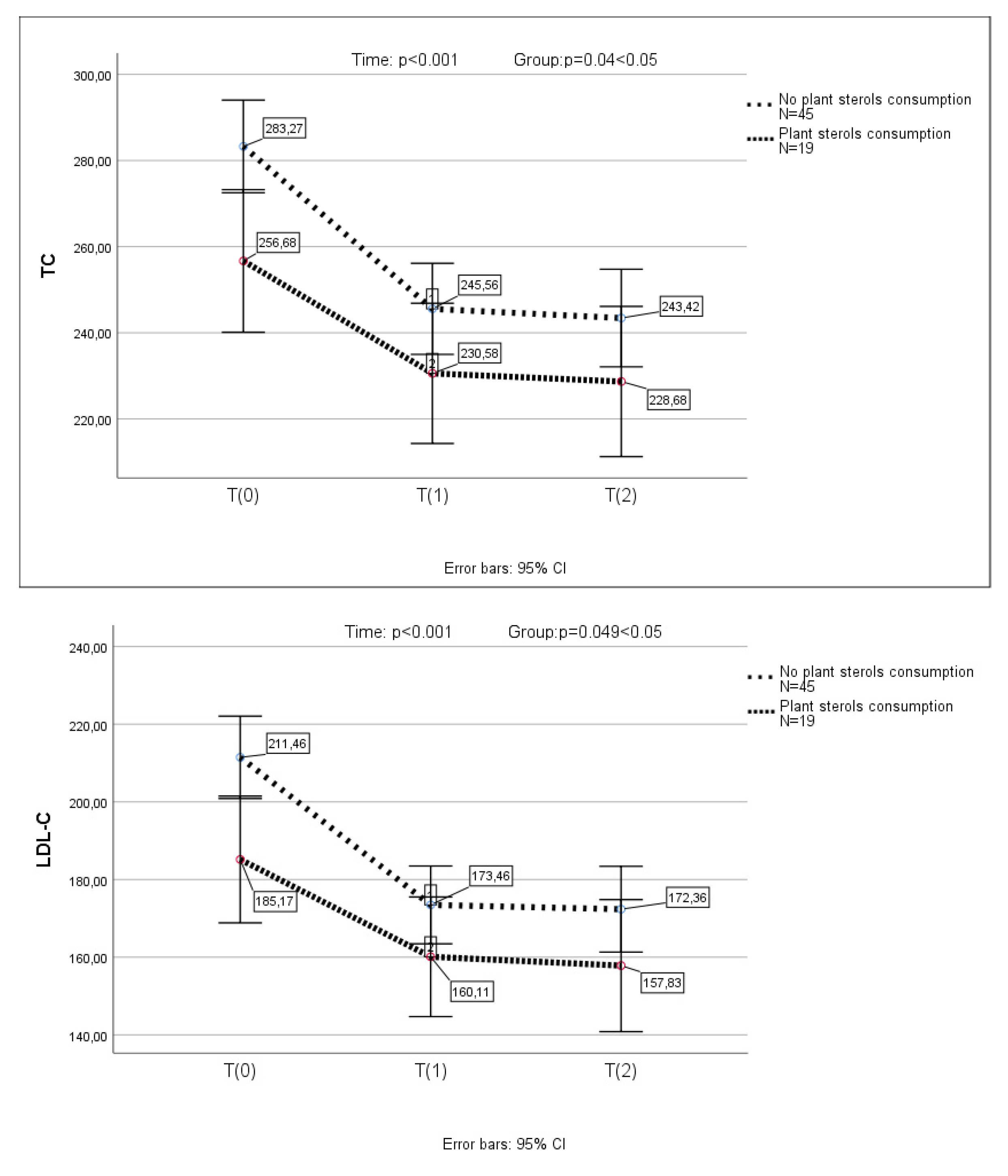
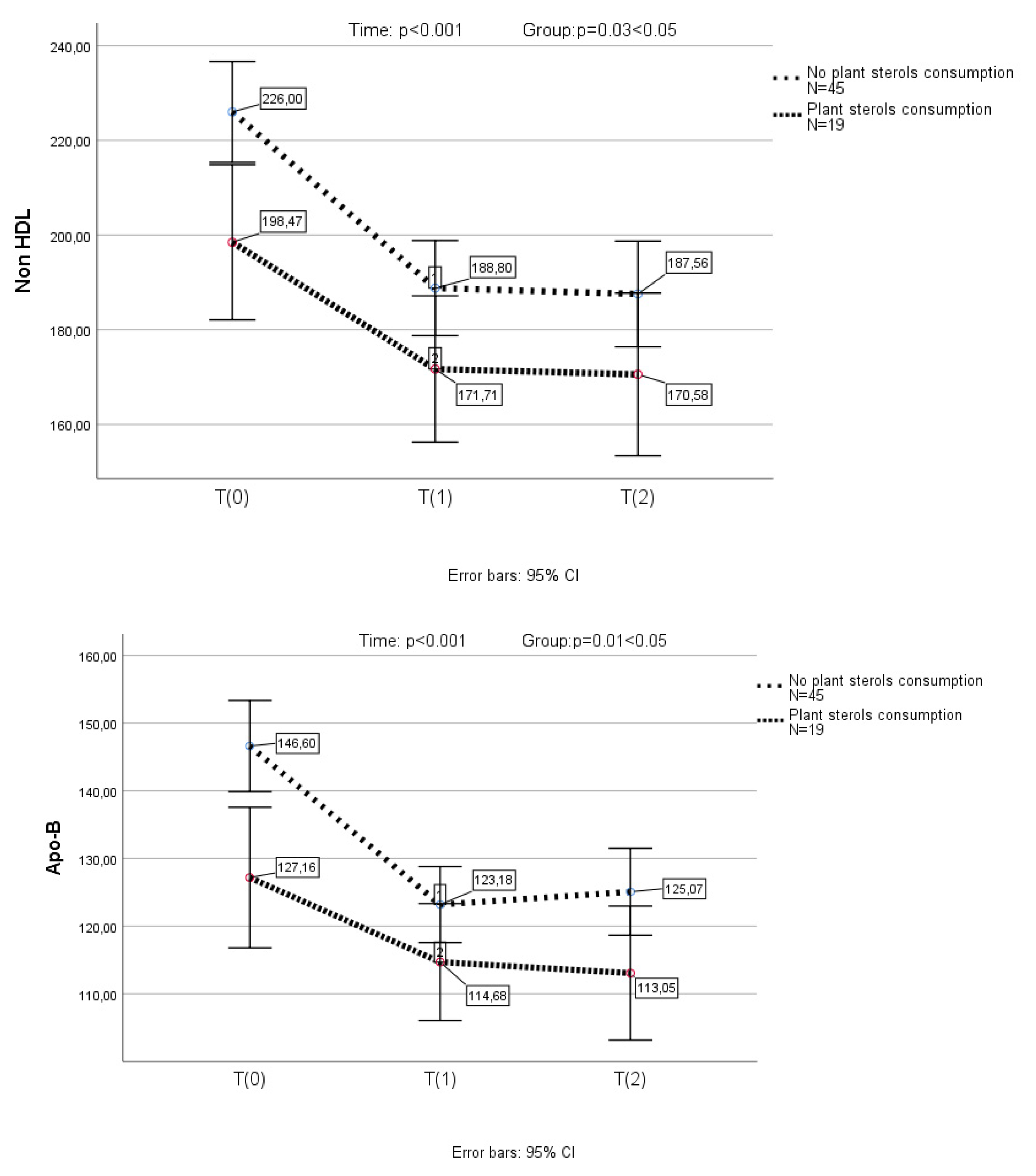
3.4. Changes in Lipid Profile After Armolipid Supplementation in Participants with Prior Consumption of Phytosterols
In the subgroup of children (n=19), who had previously received plant sterols, previous supplementation with sterols resulted in a significant reduction in LDL-C levels (p<0.001) (a one-way repeated measures ANOVA was conducted (because of normality of the data in this group of children)). In this group of children, TC, LDL-C, non-HDL-C and Apo-B further decreased significantly after the substitution of plant sterols by Armolipid (
Figure 2). None of the above parameters presented any further reduction in the 2nd measurement.
A one-way repeated measures ANOVA was conducted to compare scores on TC, LDL-C, non-HDL-C and Apo-B in 3 time period. There was a significant effect for time (p < .001). Post-hoc comparisons test, for all 4 parameters, indicated that the mean score for T(0) was significantly different from T(1) and from T(2). Also, the mean score for T(1) did not differ significantly from T(2).
A mixed between-within subjects analysis of variance was conducted to assess the impact of plant sterols consumption (No plant sterols consumption group, plant sterols consumption group) on participants’ scores on the TC, LDL-C, non-HDL-C and Apo-B across three time periods. Comparing the two groups, with and without prior plant sterols consumption, a significant difference in TC, LDL-C, non-HDL-C and Apo-B reduction, was observed (p=0.04, p=0.049, p=0.03 and p=0.01, respectively) (
Figure 2).
4. Discussion
In the present study, the long-term daily administration of 200 mg RYR extract equivalent to 3 mg of monacolin K, 10 mg policosanols, 0.2 mg folic acid, 2.0 mg coenzyme Q10 and 0.5 mg astaxanthin (commercial name: Armolipid) resulted in a significant long-lasting decrease of TC, LDL-C, non-HDL-C and Apo-B levels, in children with moderate and severe primary hypercholesterolemia. Almost 75% of participants had a reduction in atherogenic lipoproteins equal or higher than 5%, with more than 60% presenting a decrease of equal or above 10%. In contrast, Armolipid consumption did not have any significant effect on the levels of HDL-C, TGs, Apo-A1 and Lp(a). Moreover, in children who had previously consumed phytosterols, its substitution by Armolipid resulted in a further significant decrease. In total population, the reduction of atherogenic lipoproteins was independent of treatment duration or baseline lipid levels. Finally, no adverse effects of Armolipid were observed.
To our knowledge, there is only one study evaluating the early effect of Armolipid on lipid profile in children with dyslipidemia. In that study, similarly to our results, daily consumption of Armolipid for 4 weeks significantly reduced TC by 18.5%, LDL-C by 25.1% and Apo-B by 25.3%, compared to placebo. No significant differences were observed in HDL-C and Apo-A1 levels. Furthermore, opposite to our results, a significant reduction in TGs levels was also reported [
16].
In adults, there is sufficient evidence showing the beneficial effect of RYR supplementation in the improvement of lipid profile in patients with hypercholesterolemia. [
12]. A recent meta-analysis of 15 high—quality RCTs, including 1,012 participants, showed that daily consumption of RYR in a dose of 200–4,800 mg could be an effective and safe alternative for the treatment of dyslipidemic patients. RYR was effective in reducing TC, TG, LDL-C, apo-B and increasing HDL-C, and showed a synergistic action with other NCs [
19].
Furthermore, growing evidence supports the association of RYR consumption, alone or in combination with other natural compounds, with the improvement of inflammatory biomarkers, endothelial function and arterial stiffness in adult dyslipidemic patients, as well as with the reduction of CVD risk in adults with previous myocardial infraction [
8,
10]. According to a recent meta-analysis, RYR consumption significantly reduced the risk of fatal and nonfatal cardiovascular events in patients with borderline hypercholesterolemia, already affected by coronary artery disease [
20].
The most well studied combination of lipid-lowering NCs in adults is the one of RYR, polycosanols and berberine (commercial name: Armolipid Plus). According to a recent meta-analysis of 12 studies, including 1,050 subjects, the supplementation of the above combination had a significant effect on the improvement of BMI, lipid profile and inflammatory markers, which is consistent with improved cardiometabolic health [
21]. The study of Galletti et al., in 158 adult patients with metabolic syndrome, showed a reduction in atherogenic lipoproteins, including small-dense LDL-C (sdLDL-C), after Armolipid Plus supplementation when compared with placebo [
22]. Administration of Armolipid Plus in patients with familial combined hyperlipidemia had similar effect on sdLDL-C levels, which suggests a potential role in atherosclerotic risk reduction [
23]. However, there are no studies on the effect of RYR alone on sdLDL-C levels [
24].
Other natural compounds, such as coenzyme Q10 and folic acid, in combination with RYR, had also a beneficial effect on lipid profile [
13,
25]. Moreover, an improvement in endothelial function and arterial stiffness, after the consumption of RYR combined with coenzyme Q10 for six months, has been reported in adults with moderate hypercholesterolemia [
26].
In the present study, in children in whom prior consumption of plant sterols was replaced by Armolipid, a further significant reduction in LDL-C and other atherogenic lipoproteins levels, similar to that of children with no prior intake of phytosterols, was observed. In most of these children, the plant sterols supplementation had already resulted in a significant decrease in LDL-C, non-HDL-C and Apo-B levels. These results indicate a stronger hypolipidemic effect of Armolipid compared to plant sterols. Given the fact that plant sterols and RYR have different mechanisms of action, their combined use could have synergistic effect in the improvement of lipid profile [
7,
8,
10]. In the study of Cicero et al., including 90 adults with hypercholesterolemia, the daily administration of 800mg phytosterols for 2 months had not any effect on lipid profile. In contrast, the use of RYR extract, equivalent with 5 mg monacolin K, for 2 months had a beneficial effect on lipids, while an additive hypolipidemic effect with the simultaneous consumption of phytosterols and RYR for 2 months was observed [
27].
Results of studies evaluating a possible anti-hypertensive effect of RYR are controversial [
28,
29]. In our study, SBP and DBP were significantly higher at T
1 and T
2 evaluation when compared with T
0, possibly due to older age of the participants.
Regarding the safety of RYR, a substance that mirrors statin effects, it is shown that it is similar to that of low-dose statins [
10]. In a recent study, a nutrivigilance-derived data analysis showed that adverse effects from the consumption of products containing RYR are very rare (0.037% of consumers), with only 0.0003% of consumers having serious adverse effects [
30]. In the only short-term study in children, daily consumption of Armolipid had no serious adverse events [
16]. Our findings are consistent with these data, as the long-term consumption of Armolipid was well tolerated and had not any clinical or biochemical adverse effect.
This study has some limitations. First, there is no control group. Secondly, the number of participants who were under plant sterols consumption prior the initiation of Armolipid is small. In addition, the evaluation of participants’ compliance both with dietary instructions or Armolipid intake was based on information given by the participants as well as their parents. Finally, we did not include a group of children taking simultaneously phytosterols and Armolipid.
The advantages of the present report are the prospective design, the long-term administration of the nutraceutical, the homogeneity of the study group and the inclusion in the study of children not only with moderate but also with severe dyslipidemia (LDL-C >190 mg/dl).