Submitted:
20 November 2023
Posted:
21 November 2023
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Results and Discussion
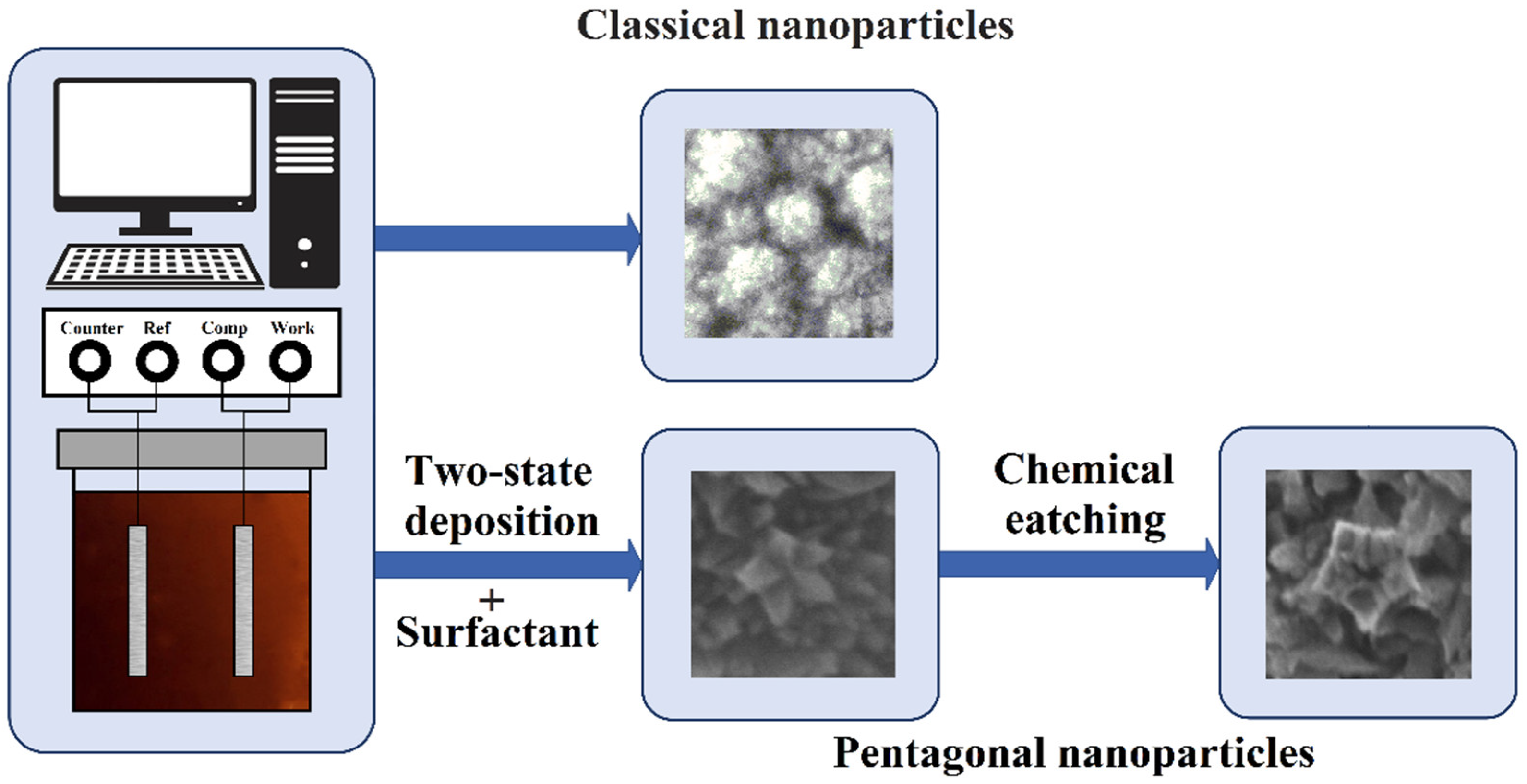
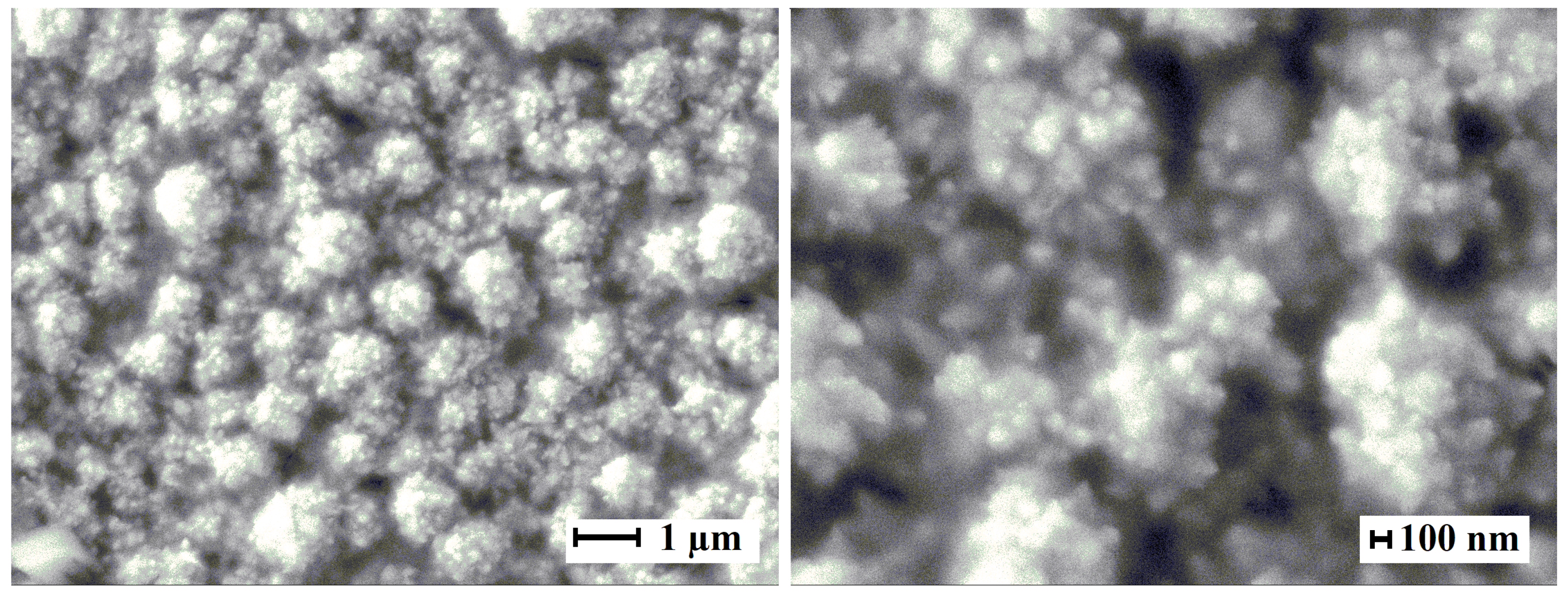
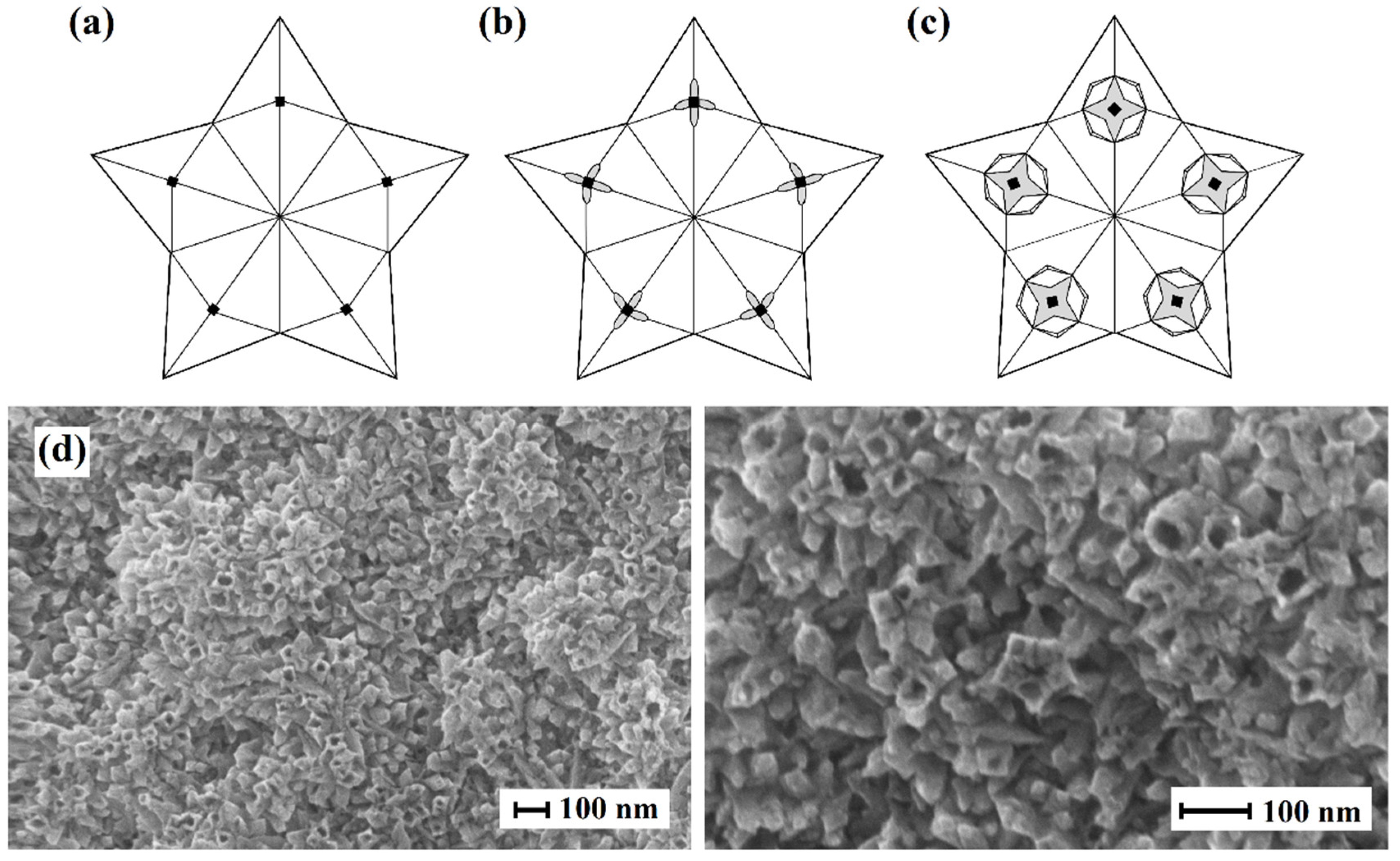
2.1. Morphology, structure features and preparation of nanoparticles on Pd basis
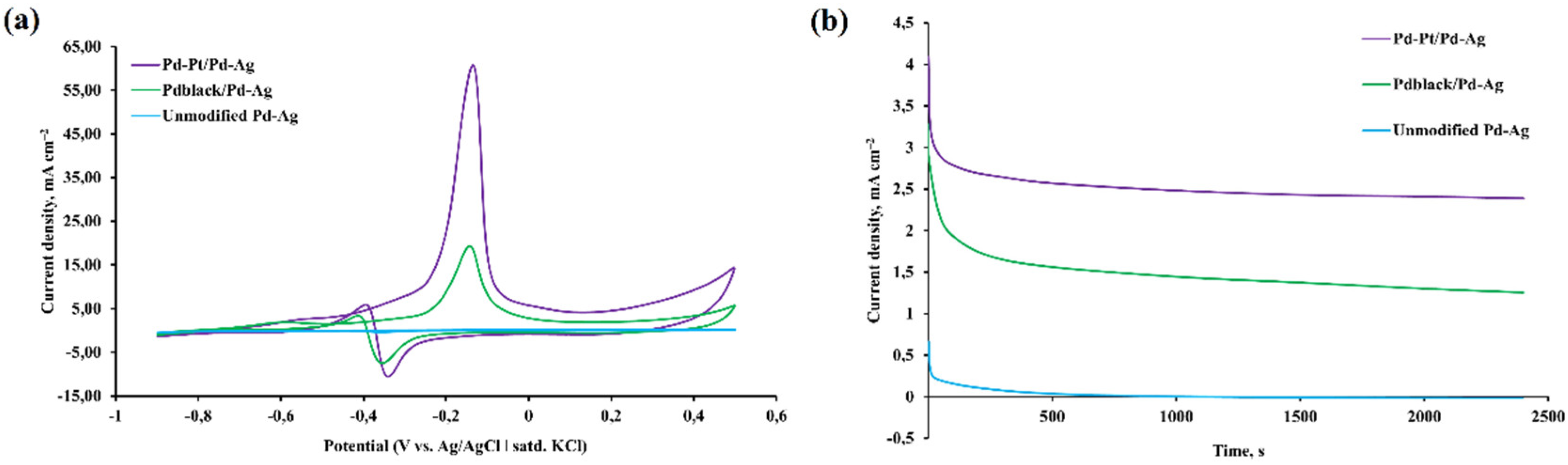
2.2. Catalytic characteristics of the bimetallic Pd-Pt modifier on pentatwinned nanoparticles basis
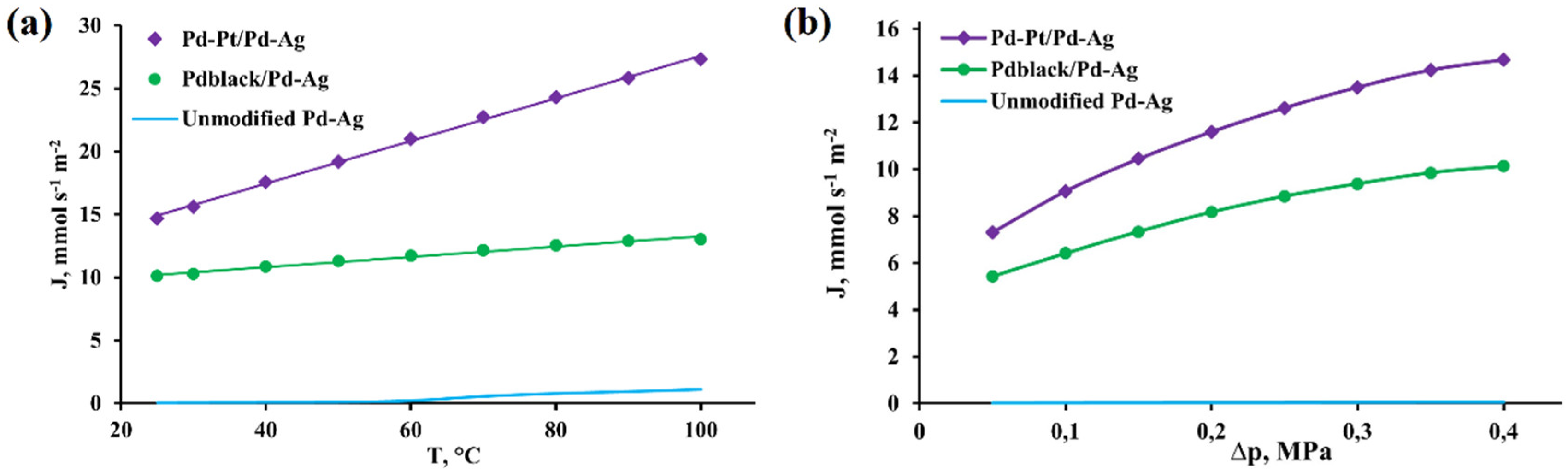
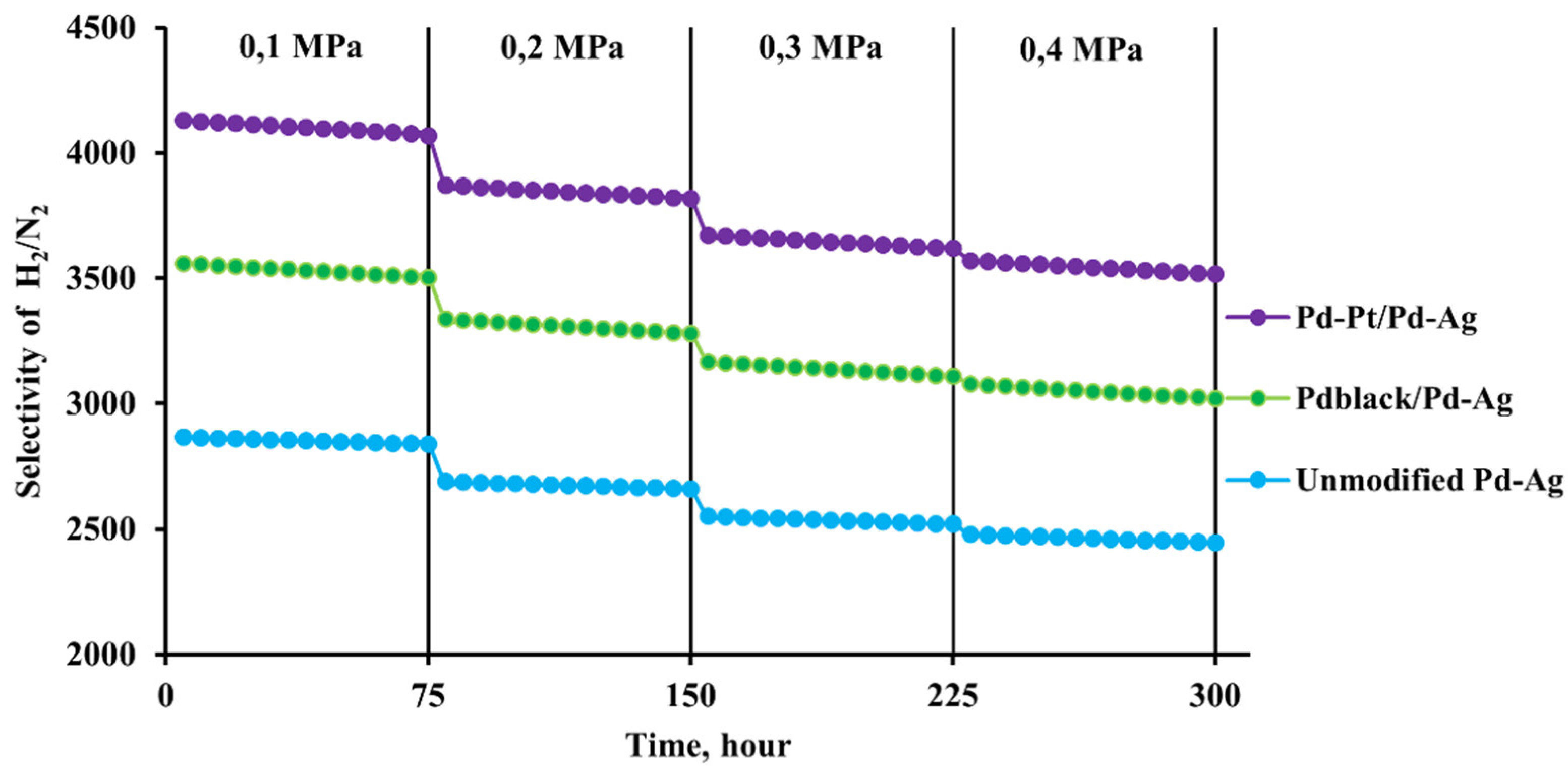
2.3. Diffusion and selective characteristics of bimetallic Pd-Pt modifier on pentatwinned nanoparticles basis
3. Materials and Methods
Creation of a membrane basis, methods of its modification and study of its surface morphology
Membrane research in catalytic and gas transportation processes
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Filippova, S.P.; Yaroslavtsev, A.B. Hydrogen energy: development prospects and materials. Russ. Chem. Rev. 2021, 90, 627–643. [Google Scholar] [CrossRef]
- Mamun, A.; Kiari, M.; Sabantina, L. A Recent Review of Electrospun Porous Carbon Nanofiber Mats for Energy Storage and Generation Applications. Membranes 2023, 13, 830. [Google Scholar] [CrossRef] [PubMed]
- Perović, K.; Morović, S.; Jukić, A.; Košutić, K. Alternative to Conventional Solutions in the Development of Membranes and Hydrogen Evolution Electrocatalysts for Application in Proton Exchange Membrane Water Electrolysis: A Review. Materials 2023, 16, 6319. [Google Scholar] [CrossRef] [PubMed]
- Migliari, L.; Micheletto, D.; Cocco, D. Performance Analysis of a Diabatic Compressed Air Energy Storage System Fueled with Green Hydrogen. Energies 2023, 16, 7023. [Google Scholar] [CrossRef]
- Binazadeh, M.; Mamivand, S.; Sohrabi, R.; Taghvaei, H.; Iulianelli, A. Membrane reactors for hydrogen generation: From single stage to integrated systems. Int. J. Hydrogen Energy 2023, In Press. [CrossRef]
- Nemitallah, M.A. Characteristics of hydrogen separation and methane steam reforming in a Pd-based membrane reactor of shell and tube design. Case Stud. Therm. Eng. 2023, 45, 102939. [Google Scholar] [CrossRef]
- Aditiya, H.B.; Aziz, M. Prospect of hydrogen energy in Asia-Pacific: A perspective review on techno-socio-economy nexus. Int. J. Hydrogen Energy 2021, 46, 35027–35056. [Google Scholar] [CrossRef]
- Solomin, E.; Salah, Z.; Osintsev, K.; Aliukov, S.; Kuskarbekova, S.; Konchakov, V.; Olinichenko, A.; Karelin, A.; Tarasova, T. Ecological Hydrogen Production and Water Sterilization: An Innovative Approach to the Trigeneration of Renewable Energy Sources for Water Desalination: A Review. Energies 2023, 16, 6118. [Google Scholar] [CrossRef]
- Zhang, L.; Xie, G.; Liu, F.; Ji, H. High hydrogen selectivity Pd-Ni alloy film hydrogen sensor with hybrid organosilica membranes. J. Alloys Compd. 2023, 941, 168898. [Google Scholar] [CrossRef]
- Xu, F.; Ma, J.; Li, C.; Ma, C.; Li, J.; Guan, B.-O.; Chen, K. Fabry–Pérot Cavities with Suspended Palladium Membranes on Optical Fibers for Highly Sensitive Hydrogen Sensing. Molecules 2023, 28, 6984. [Google Scholar] [CrossRef]
- Jung, W.; Chang, D. Deep Reinforcement Learning-Based Energy Management for Liquid Hydrogen-Fueled Hybrid Electric Ship Propulsion System. J. Mar. Sci. Eng. 2023, 11, 2007. [Google Scholar] [CrossRef]
- Parra, D.; Valverde, L.; Pino, F.J.; Patel, M.K. A review on the role, cost and value of hydrogen energy systems for deep decarbonisation. Renew. Sust. Energ. Rev. 2019, 101, 279–294. [Google Scholar] [CrossRef]
- Yin, Z.; Yang, Z.; Tong, Y.; Du, M.; Mi, J.; Yu, Q.; Li, S. Improved sulfur tolerance of Pd–Ru membranes: Influence of H2S concentration and exposure time on the hydrogen flux. Int. J. Hydrogen Energy 2023, In Press. [CrossRef]
- Buonomenna, M.G. Proton-Conducting Ceramic Membranes for the Production of Hydrogen via Decarbonized Heat: Overview and Prospects. Hydrogen 2023, 4, 807–830. [Google Scholar] [CrossRef]
- Bernardo, G.; Araújo, T.; Lopes, T.S.; Sousa, J.; Mendes, A. Recent advances in membrane technologies for hydrogen purification. Int. J. Hydrogen Energy 2020, 45, 7313–7338. [Google Scholar] [CrossRef]
- Skolotneva, E.; Tsygurina, K.; Mareev, S.; Melnikova, E.; Pismenskaya, N.; Nikonenko, V. High Diffusion Permeability of Anion-Exchange Membranes for Ammonium Chloride: Experiment and Modeling. Int. J. Mol. Sci. 2022, 23, 5782. [Google Scholar] [CrossRef] [PubMed]
- Mareev, S.; Gorobchenko, A.; Ivanov, D.; Anokhin, D.; Nikonenko, V. Ion and Water Transport in Ion-Exchange Membranes for Power Generation Systems: Guidelines for Modeling. Int. J. Mol. Sci. 2023, 24, 34. [Google Scholar] [CrossRef] [PubMed]
- Zhang, K.; Way, J.D. Palladium-copper membranes for hydrogen separation. Sep. Purif. Technol. 2017, 186, 39–44. [Google Scholar] [CrossRef]
- Mamivand, S.; Binazadeh, M.; Sohrabi, R. Applicability of membrane reactor technology in industrial hydrogen producing reactions: Current effort and future directions. J. Ind. Eng. Chem. 2021, 104, 212–230. [Google Scholar] [CrossRef]
- Petriev, I.S.; Lutsenko, I.S.; Pushankina, P.D.; Frolov, V.Yu.; Glazkova, Yu.S.; Malkov, T.I.; Gladkikh, A.M.; Otkidach, M.A.; Sypalo, E.B.; Baryshev, P.M.; Shostak, N.A.; Kopytov, G.F. Hydrogen Transport through Palladium-Coated Niobium Membranes. Russ. Phys. J. 2022, 65, 312–316. [Google Scholar] [CrossRef]
- Lytkina, A.A.; Orekhova, N.V.; Ermilova, M.M.; Petriev, I.S.; Baryshev, M.G.; Yaroslavtsev, A.B. Ru–Rh based catalysts for hydrogen production via methanol steam reforming in conventional and membrane reactors. Int. J. Hydrogen Energy 2019, 44, 13310–13322. [Google Scholar] [CrossRef]
- Chen, W.-H.; Kuo, P.-C.; Lin, Y.-L. Evolutionary computation for maximizing CO2 and H2 separation in multiple-tube palladium-membrane systems. Appl. Energy 2019, 235, 299–310. [Google Scholar] [CrossRef]
- Flanagan, T.B.; Oates, W.A. The Palladium-Hydrogen System. Annu. Rev. Mater. Sci. 1991, 21, 269–304. [Google Scholar] [CrossRef]
- Bosko, M.L.; Fontana, A.D.; Tarditi, A.; Cornaglia, L. Advances in hydrogen selective membranes based on palladium ternary alloys. Int. J. Hydrogen Energy 2021, 46, 15572–15594. [Google Scholar] [CrossRef]
- Zhu, K.; Li, X.; Zhang, Y.; Zhao, X.; Liu, Z.; Guo, J. Tailoring the hydrogen transport properties of highly permeable Nb51W5Ti23Ni21 alloy membrane by Pd substitution. Int. J. Hydrogen Energy 2022, 47, 6734–6744. [Google Scholar] [CrossRef]
- Alrashed, F.S.; Paglieri, S.N.; Alismail, Z.S.; Khalaf, H.; Harale, A.; Overbeek, J.P.; van Veen, H.M.; Hakeem, A.S. Steam reforming of simulated pre-reformed naphtha in a PdAu membrane reactor. Int. J. Hydrogen Energy 2021, 46, 21939–21952. [Google Scholar] [CrossRef]
- Suzuki, A.; Yukawa, H. Analysis for Reverse Temperature Dependence of Hydrogen Permeability through Pd-X (X = Y, Ho, Ni) Alloy Membranes Based on Hydrogen Chemical Potential. Membranes 2020, 10, 123. [Google Scholar] [CrossRef]
- Zhou, Q.; Luo, S.; Zhang, M.; Liao, N. Selective and efficient hydrogen separation of Pd–Au–Ag ternary alloy membrane. Int. J. Hydrogen Energy 2022, 47, 13054–13061. [Google Scholar] [CrossRef]
- Petriev, I.S.; Pushankina, P.D.; Lutsenko, I.S.; Baryshev, M.G. Anomalous Kinetic Characteristics of Hydrogen Transport through Pd–Cu Membranes Modified by Pentatwinned Flower-Shaped Palladium Nanocrystallites with High-Index Facets. Tech. Phys. Lett. 2021, 47, 803–806. [Google Scholar] [CrossRef]
- Petriev, I.S.; Lutsenko, I.S.; Voronin, K.A.; Pushankina, P.D.; Baryshev, M.G. Hydrogen permeability of surface-modified Pd-Ag membranes at low temperatures. IOP Conf. Ser. Mater. Sci. Eng. 2020, 791, 012058. [Google Scholar] [CrossRef]
- Petriev, I.; Pushankina, P.; Bolotin, S.; Lutsenko, I.; Kukueva, E.; Baryshev, M. The influence of modifying nanoflower and nanostar type Pd coatings on low temperature hydrogen permeability through Pd-containing membranes. J. Membr. Sci. 2021, 620, 118894. [Google Scholar] [CrossRef]
- Vicinanza, N.; Svenum, I.-H.; Peters, T.; Bredesen, R.; Venvik, H. New Insight to the Effects of Heat Treatment in Air on the Permeation Properties of Thin Pd77%Ag23% Membranes. Membranes 2018, 8, 92. [Google Scholar] [CrossRef]
- Petriev, I.; Pushankina, P.; Shostak, N.; Baryshev, M. Gas-Transport Characteristics of PdCu–Nb–PdCu Membranes Modified with Nanostructured Palladium Coating. Int. J. Mol. Sci. 2022, 23, 228. [Google Scholar] [CrossRef]
- Kozmai, A.; Pismenskaya, N.; Nikonenko, V. Mathematical Description of the Increase in Selectivity of an Anion-Exchange Membrane Due to Its Modification with a Perfluorosulfonated Ionomer. Int. J. Mol. Sci. 2022, 23, 2238. [Google Scholar] [CrossRef]
- Petriev, I.; Pushankina, P.; Glazkova, Y.; Andreev, G.; Baryshev, M. Investigation of the Dependence of Electrocatalytic Activity of Copper and Palladium Nanoparticles on Morphology and Shape Formation. Coatings 2023, 13, 621. [Google Scholar] [CrossRef]
- Shan, H.; Wang, W.; Wang, Z.; Ge, J.; Liu, Q.; Zhang, W.; Fu, Q. General synthesis of flexible CuO nanoparticles-anchored ZrO2 nanofibrous membranes for catalytic oxidation of tetracycline. J. Chem. Eng. 2023, 466, 143063. [Google Scholar] [CrossRef]
- Shkirskaya, S.A.; Kononenko, N.A.; Timofeev, S.V. Structural and Electrotransport Properties of Perfluorinated Sulfocationic Membranes Modified by Silica and Zirconium Hydrophosphate. Membranes 2022, 12, 979. [Google Scholar] [CrossRef]
- Basov, A.; Dzhimak, S.; Sokolov, M.; Malyshko, V.; Moiseev, A.; Butina, E.; Elkina, A.; Baryshev, M. Changes in Number and Antibacterial Activity of Silver Nanoparticles on the Surface of Suture Materials during Cyclic Freezing. Nanomaterials 2022, 12, 1164. [Google Scholar] [CrossRef] [PubMed]
- Mutalik, C.; Saukani, M.; Khafid, M.; Krisnawati, D.I.; Widodo; Darmayanti, R.; Puspitasari, B.; Cheng, T.-M.; Kuo, T.-R. Gold-Based Nanostructures for Antibacterial Application. Int. J. Mol. Sci. 2023, 24, 10006. [CrossRef]
- Stenina, I.; Yurova, P.; Achoh, A.; Zabolotsky, V.; Wu, L.; Yaroslavtsev, A. Improvement of Selectivity of RALEX-CM Membranes via Modification by Ceria with a Functionalized Surface. Polymers 2023, 15, 647. [Google Scholar] [CrossRef] [PubMed]
- Ramos-Zúñiga, J.; Bruna, N.; Pérez-Donoso, J.M. Toxicity Mechanisms of Copper Nanoparticles and Copper Surfaces on Bacterial Cells and Viruses. Int. J. Mol. Sci. 2023, 24, 10503. [Google Scholar] [CrossRef]
- Sogorb, M.A.; Candela, H.; Estévez, J.; Vilanova, E. Investigation of the Effects of Metallic Nanoparticles on Fertility Outcomes and Endocrine Modification of the Hypothalamic-Pituitary-Gonadal Axis. Int. J. Mol. Sci. 2023, 24, 11687. [Google Scholar] [CrossRef]
- Petriev, I.S.; Pushankina, P.D.; Lutsenko, I.S.; Baryshev, M.G. The influence of a crystallographically atypical pentagonal nanostructured coating on the limiting stage of low-temperature hydrogen transport through Pd–Cu membranes. Dokl. Phys. 2021, 66, 209–213. [Google Scholar] [CrossRef]
- Theerthagiri, J.; Lee, S.J.; Murthy, A.P.; Madhavan, J.; Choi, M.Y. Fundamental aspects and recent advances in transition metal nitrides as electrocatalysts for hydrogen evolution reaction: A review. Curr. Opin. Solid State Mater. Sci. 2020, 24, 100805. [Google Scholar] [CrossRef]
- Bianchini, C.; Shen, P.K. Palladium-based electrocatalysts for alcohol oxidation in half cells and in direct alcohol fuel cells. Chem. Rev. 2009, 109, 4183–4206. [Google Scholar] [CrossRef] [PubMed]
- Alaqarbeh, M.; Adil, S.F.; Ghrear, T.; Khan, M.; Bouachrine, M.; Al-Warthan, A. Recent Progress in the Application of Palladium Nanoparticles: A Review. Catalysts 2023, 13, 1343. [Google Scholar] [CrossRef]
- Sanap, K.K.; Mali, S.S.; Tyagi, D.; Shirsat, A.N.; Phapale, S.B.; Waghmode, S.B.; Varma, S. Development of a Simple Electroless Method for Depositing Metallic Pt-Pd Nanoparticles over Wire Gauge Support for Removal of Hydrogen in a Nuclear Reactor. Materials 2023, 16, 6541. [Google Scholar] [CrossRef] [PubMed]
- Pushankina, P.; Baryshev, M.; Petriev, I. Synthesis and Study of Palladium Mono- and Bimetallic (with Ag and Pt) Nanoparticles in Catalytic and Membrane Hydrogen Processes. Nanomaterials 2022, 12, 4178. [Google Scholar] [CrossRef] [PubMed]
- Ruditskiy, A.; Choi, S.I.; Peng, H.C.; Xia, Y. Shape-controlled metal nanocrystals for catalytic applications. MRS Bulletin 2014, 39, 727–737. [Google Scholar] [CrossRef]
- Liu, Q.; Rzepka, P.; Frey, H.; Tripp, J.; Beck, A.; Artiglia, L.; Ranocchiari, M.; van Bokhoven, J.A. Sintering behavior of carbon-supported Pt nanoparticles and the effect of surface overcoating. Mater. Today Nano 2022, 20, 100273. [Google Scholar] [CrossRef]
- Dong, K.; Dai, H.; Pu, H.; Zhang, T.; Wang, Y.; Deng, Y. Enhanced electrocatalytic activity and stability of Pd-based bimetallic icosahedral nanoparticles towards alcohol oxidation reactions. Int. J. Hydrogen Energy 2023, 48, 12288–12298. [Google Scholar] [CrossRef]
- Chen, Y.; Dai, Q.; Zhang, Q.; Huang, Y. Precisely deposited Pd on ZnO (002) facets derived from complex reduction strategy for methanol steam reforming. Int. J. Hydrogen Energy 2022, 47, 14869–14883. [Google Scholar] [CrossRef]
- Karaman, C. Engineering of N,P,S-Triple doped 3-dimensional graphene architecture: Catalyst-support for “surface-clean” Pd nanoparticles to boost the electrocatalysis of ethanol oxidation reaction. Int. J. Hydrogen Energy 2023, 48, 6691–6701. [Google Scholar] [CrossRef]
- Pushankina, P.; Andreev, G.; Petriev, I. Hydrogen Permeability of Composite Pd–Au/Pd–Cu Membranes and Methods for Their Preparation. Membranes 2023, 13, 649. [Google Scholar] [CrossRef]
- Paz-Borbón, L.O.; Johnston, R.L.; Barcaro, G.; Fortunelli, A. Structural motifs, mixing, and segregation effects in 38-atom binary clusters. J. Chem. Phys. 2008, 128, 134517. [Google Scholar] [CrossRef]
- Jung, H.; King, M.E.; Personick, M.L. Strategic synergy: advances in the shape control of bimetallic nanoparticles with dilute alloyed surfaces. Curr. Opin. Colloid Interface Sci. 2019, 40, 104–117. [Google Scholar] [CrossRef]
- Gutkin, M.Yu.; Kolesnikova, A.L.; Yasnikov, I.S.; Vikarchuk, A.A.; Aifantis, E.C.; Romanov, A.E. Fracture of hollow multiply-twinned particles under chemical etching. Eur. J. Mech. A/Solids 2018, 68, 133–139. [Google Scholar] [CrossRef]
- Yousaf, A.B.; Khan, R.; Imran, M.; Fernandez, C.; Yuan, C.-Z.; Song, L. Synergistic Electronic Pull of Graphene Oxide Supported Pd Nanoparticles on Enhancing Catalytic Activity of Electro Deposited Pt Nanoparticles for Methanol Oxidation Reaction. Int. J. Electrochem. Sci. 2016, 11, 6735–6746. [Google Scholar] [CrossRef]
- Kim, S.-M.; Lee, Y.-J.; Kim, J.-W.; Lee, S.-Y. Facile synthesis of Pt–Pd bimetallic nanoparticles by plasma discharge in liquid and their electrocatalytic activity toward methanol oxidation in alkaline media. Thin Solid Films 2014, 572, 260–265. [Google Scholar] [CrossRef]
- Zhan, F.; Bian, T.; Zhao, W.; Zhang, H.; Jin, M.; Yang, D. Facile synthesis of Pd–Pt alloy concave nanocubes with high-index facets as electrocatalysts for methanol oxidation. CrystEngComm, 2014, 16, 2411–2416. [Google Scholar] [CrossRef]
- Hanifah, M.F.R.; Jaafar, J.; Othman, M.H.D.; Ismail, A.F.; Rahman, M.A.; Yusof, N.; Aziz, F.; Rahman, N.A. One-pot synthesis of efficient reduced graphene oxide supported binary Pt-Pd alloy nanoparticles as superior electro-catalyst and its electro-catalytic performance toward methanol electro-oxidation reaction in direct methanol fuel cell. J. Alloys Compd. 2019, 793, 232–246. [Google Scholar] [CrossRef]
- Woo, S.; Lee, J.; Park, S.-K.; Kim, H.; Chung, T.D.; Piao, Y. Electrochemical codeposition of Pt/graphene catalyst for improved methanol oxidation. Curr. Appl. Phys. 2015, 15, 219–225. [Google Scholar] [CrossRef]
- Chen, D.; Zhao, Y.; Fan, Y.; Wang, W.; Li, X.; Peng, X.; Wang, X.; Tian, J. Preparation and characterization of core–shell-like PbPt nanoparticles electro-catalyst supported on graphene for methanol oxidation. Int. J. Hydrogen Energy 2014, 39, 16053–16060. [Google Scholar] [CrossRef]
- Zhang, Y.; Chang, G.; Shu, H.; Oyama, M.; Liu, X.; He, Y. Synthesis of Pt–Pd bimetallic nanoparticles anchored on graphene for highly active methanol electro-oxidation. J. Power Sources 2014, 262, 279–285. [Google Scholar] [CrossRef]
- Kim, T.-W.; Lee, E.-H.; Byun, S.; Seo, D.-W.; Hwang, H.-J.; Yoon, H.-C.; Kim, H.; Ryi, S.-K. Highly selective Pd composite membrane on porous metal support for high-purity hydrogen production through effective ammonia decomposition. Energy 2022, 260, 125209. [Google Scholar] [CrossRef]
- Iulianelli, A.; Ghasemzadeh, K.; Marelli, M.; Evangelisti, C. A supported Pd-Cu/Al2O3 membrane from solvated metal atoms for hydrogen separation/purification. Fuel Process. Technol. 2019, 195, 106141. [Google Scholar] [CrossRef]
- Yin, Z.; Yang, Z.; Du, M.; Mi b, J.; Hao, L.; Tong, Y.; Feng, Y.; Li, S. Effect of annealing process on the hydrogen permeation through Pd–Ru membrane. J. Membr. Sci. 2022, 654, 120572. [Google Scholar] [CrossRef]
- Petriev, I.S.; Pushankina, P.D.; Andreev, G.A. Investigation of Low-Temperature Hydrogen Permeability of Surface Modified Pd–Cu Membranes. Membr. Membr. Technol. 2023, 5, 360–369. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).




