Submitted:
12 December 2025
Posted:
15 December 2025
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Materials and Methods
2.1. Clinical Samples, Ethical Aspects, and Eligibility
2.2. Assay Design and Optimization
2.2.1. Oligo Design
2.2.2. In Silico Specificity Evaluation
2.2.3. Synthetic controls: Ultramer™ Duplex
2.2.4. Ultramer™ Duplex Control Preparation: Stock Solution
2.2.5. Assay Optimization
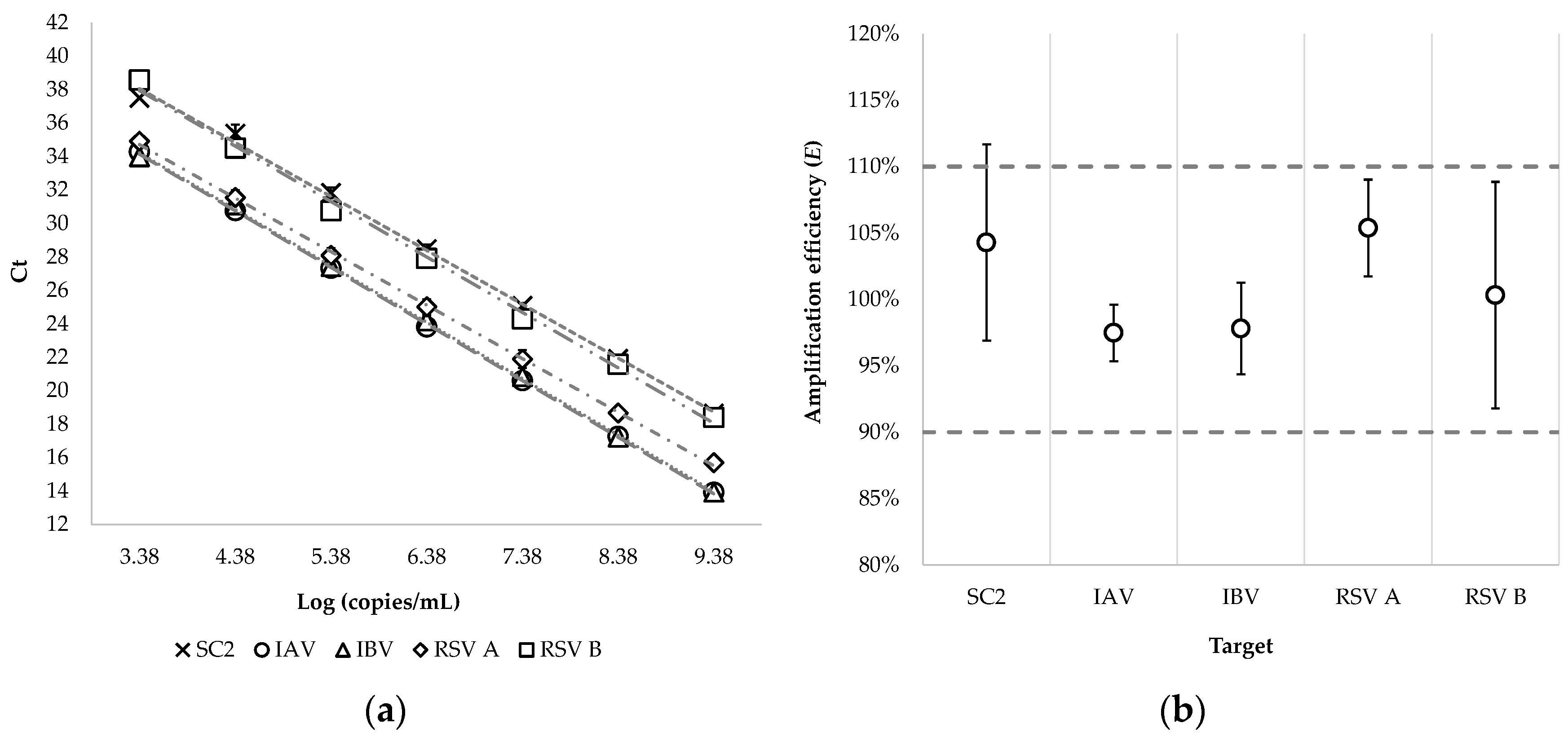
2.2.6. Amplification Efficiency and Multiplex Compatibility
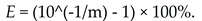
2.2.7. Panther Fusion® Open Access™ RNA/DNA Enzyme Cartridge: Off-Label Protocol
2.3. Analytical Performance
2.3.1. Analytical Sensitivity (Limit of Detection)
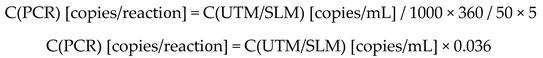

2.3.2. Analytical Specificity: Cross-Reactivity (In Vitro Exclusivity) and In Vitro Inclusivity
2.3.3. Precision
2.3.4. Method Comparison: Agreement Assessment
Allplex™ SARS-CoV-2/FluA/FluB/RSV Assay (APRA)
LDRA and Panther Fusion® SARS-CoV-2/Flu A/B/RSV Assay (PFRA)
2.4. Diagnostic Performance
2.5. Mixed Infection Detection Capability
2.6. Statistical Analysis
3. Results
3.1. Design Evaluation and Assay Optimization
3.1.1. In Silico Specificity Evaluation
3.1.2. Amplification Efficiency and Multiplex Compatibility
3.2. Analytical Performance
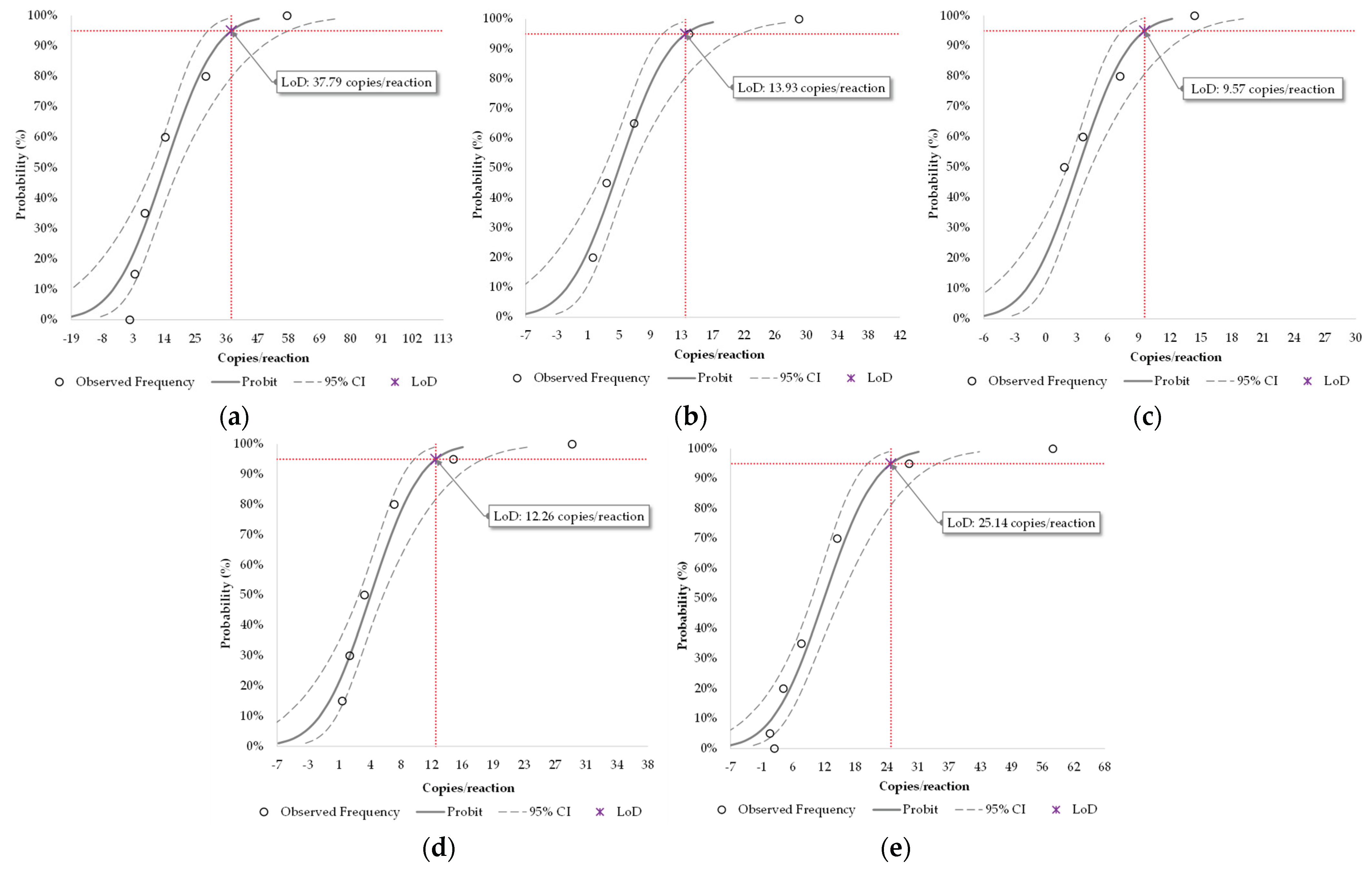
3.2.1. Analytical Sensitivity (Limit of Detection)
3.2.2. Analytical Specificity
In Vitro Inclusivity
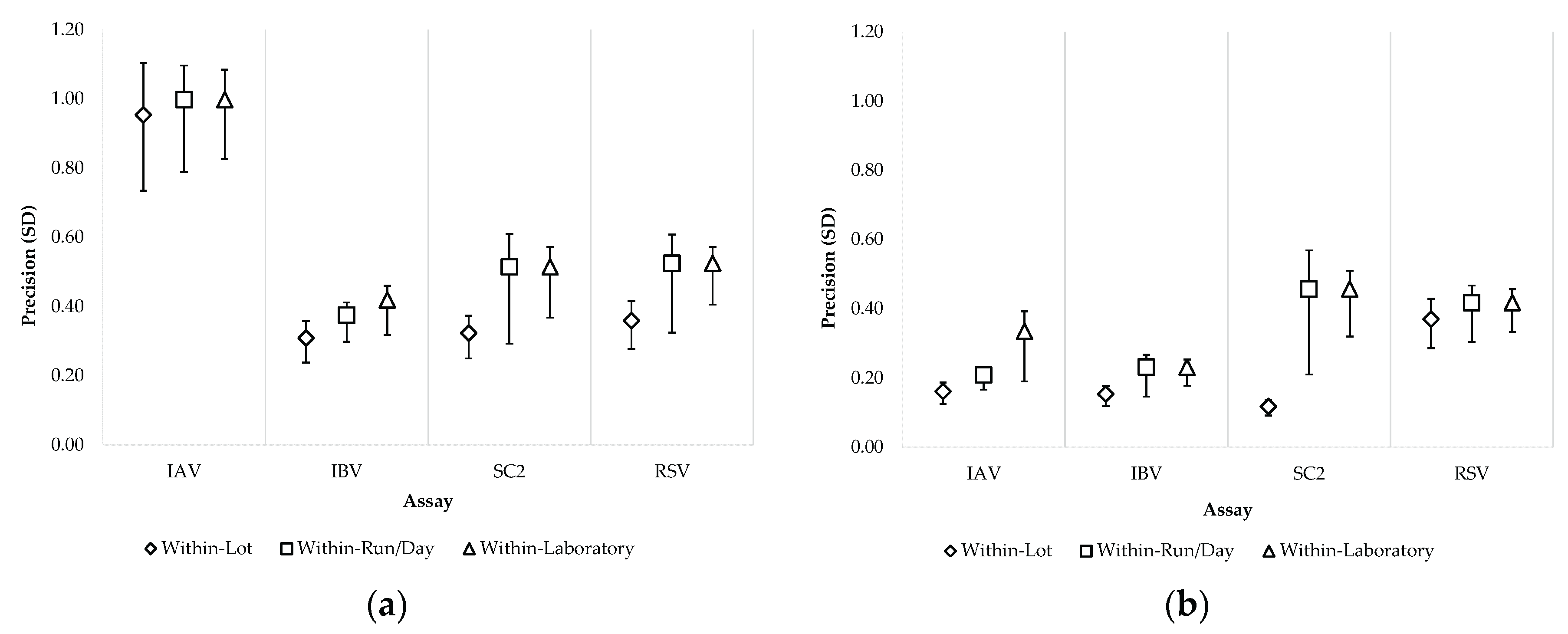
3.2.3. Precision
3.2.4. Method Comparison: Agreement Assessment
3.3. Diagnostic Performance
3.4. Mixed Infection Detection Capability
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| 95% CI | 95% confidence interval |
| ANOVA | Analysis of variance |
| APRA | Allplex™ SARS-CoV-2/FluA/FluB/RSV assay (Allplex respiratory assay) |
| ASRC | Amplirun® SARS-CoV-2 RNA Controls |
| CAP | College of American Pathologist |
| CDC | Centers for Disease Control and Prevention |
| CV | Coefficient of variation |
| Cy5 | Cyanine 5 dye |
| Cy5.5 | Cyanine 5.5 dye |
| DNA | Deoxyribonucleic acid |
| dSens | Diagnostic sensitivity |
| dSpec | Diagnostic specificity |
| E | Amplification efficiency |
| EQA | External quality assessment |
| E-value | Expectation value |
| FAM | 6-carboxifluoresceína dye |
| FN | False negative |
| FP | False positive |
| fwd | Forward primer |
| GISAID | Global Initiative on Sharing Influenza Data |
| H1N1pdm09 | H1N1 subtype derived from the 2009 pandemic |
| H1N1sea | Seasonal H1N1 subtype |
| IAV | Influenza A virus |
| IBV | Influenza B virus |
| IC | Internal control |
| ID3 | CAP Nucleic Acid Amp, Respiratory Ltd EQA Programme |
| IDR | CAP Infectious Disease, Respiratory EQA Programme |
| INFTP24 | QCMD 2024 Influenza Typing EQA Programme |
| k | Kappa index |
| LDRA | Laboratory-developed respiratory assay |
| LDT | Laboratory-developed test |
| LDT RSV | RSV amplification and detection assay |
| LoD | Limit of detection |
| MAFFT | Multiple alignment using fast Fourier transform |
| MixPA | Mixed infection percent agreement |
| MMX | Master mix |
| n | Total number of samples/replicates |
| NAAT | Nucleic acid amplification test |
| NATRVP 2.1 | NATtrol Respiratory Verification Panel 2.1 |
| NCBI | National Center for Biotechnology Information |
| Neg | Negative result |
| NPA | Negative percent agreement |
| NPV | Negative predictive value |
| nt | Nucleotide |
| OPA | Overall percent agreement |
| PFRA | Panther Fusion® SARS-CoV-2/Flu A/B/RSV assay (Panther Fusion respiratory assay) |
| PPA | Positive percent agreement |
| PPR | Primers and probes reconstitution solution |
| PPV | Positive predictive value |
| PR | Positive rate |
| prb | Probe |
| QCMD | Quality Control for Molecular Diagnostics |
| qPCR | Real-time PCR or Quantitative PCR |
| RdRp | RNA-dependent RNA polymerase |
| rev | Reverse primer |
| RFU | Relative fluorescence units |
| RNA | Ribonucleic acid |
| RNase P | RNase P humana |
| RSV | Respiratory syncytial virus |
| RT | Reverse transcription |
| RT-qPCR | Real-time PCR with reverse transcription |
| RTX#QC | Respiratory Multiplex (1 to 5) Q Control |
| SC2 | SARS-CoV-2 |
| SD | Standard deviation |
| SinPA | Single infection percent agreement |
| SLM | Medium of the Panther Fusion® Specimen Lysis Tube (specimen lysis medium) |
| SOP | Standard operating procedure |
| TAT | Turnaround time |
| TexRd-XN | Texas Red-XN dye |
| Ta | Annealing temperature |
| Tm | Melting temperature |
| TN | True negative |
| TP | True positive |
| UTM | Universal transport medium |
| UTM/SLM | Disolución de UTM de hisopados nasofaríngeos negativos en SLM a la proporción de 0.5:0.71 mL. |
| WHO | World Helath Organization |
| YakYel | Yakima Yellow dye |
| 1 | Amplirun® SARS-CoV-2 B.1.1.7 (Alpha) RNA Control, Amplirun® SARS-CoV-2 B.1.351 (Beta) RNA Control, Amplirun® SARS-CoV-2 B.1.617.2 (Delta) RNA Control, Amplirun® SARS-CoV-2 P.1 (Gamma) RNA Control, Amplirun® SARS-CoV-2 BA.1 (Omicron) RNA Control. |
References
- Olsen, S.J.; Azziz-Baumgartner, E.; Budd, A.P.; Brammer, L.; Sullivan, S.; Pineda, R.F.; Cohen, C.; Fry, A.M. Decreased Influenza Activity During the COVID-19 Pandemic - United States, Australia, Chile, and South Africa, 2020. MMWR Morb Mortal Wkly Rep 2020, 69, 1305–1309. [Google Scholar] [CrossRef] [PubMed]
- Park, M.; Choi, W.S.; Cowling, B.J. Shifts in Influenza and Respiratory Syncytial Virus Infection Patterns in Korea After the COVID-19 Pandemic Resulting From Immunity Debt: Retrospective Observational Study. JMIR Public Health Surveill 2025, 11, e68058. [Google Scholar] [CrossRef] [PubMed]
- Boukli, N.; Flamand, C.; Chea, K.L.; Heng, L.; Keo, S.; Sour, K.; In, S.; Chhim, P.; Chhor, B.; Kruy, L.; et al. One assay to test them all: Multiplex assays for expansion of respiratory virus surveillance. Frontiers in Medicine 2023, 10. [Google Scholar] [CrossRef] [PubMed]
- Komu, J.G.; Jamsransuren, D.; Matsuda, S.; Ogawa, H.; Takeda, Y. Performance Evaluation of a Fully Automated Molecular Diagnostic System for Multiplex Detection of SARS-CoV-2, Influenza A/B Viruses, and Respiratory Syncytial Virus. Diagnostics 2025, 15, 1791. [Google Scholar] [CrossRef]
- Yun, J.; Park, J.H.; Kim, N.; Roh, E.Y.; Shin, S.; Yoon, J.H.; Kim, T.S.; Park, H. Evaluation of Three Multiplex Real-time Reverse Transcription PCR Assays for Simultaneous Detection of SARS-CoV-2, Influenza A/B, and Respiratory Syncytial Virus in Nasopharyngeal Swabs. J Korean Med Sci 2021, 36, e328. [Google Scholar] [CrossRef]
- Upadhyay, P.; Surur, F.; Singh, V. Performance Assessment of a Multiplex Real-Time PCR Assay for Detection of Viruses Causing Respiratory Tract Infections. Diagnostics (Basel) 2024, 14. [Google Scholar] [CrossRef]
- World Health Organization. Influenza (seasonal). Available online: https://www.who.int/news-room/fact-sheets/detail/influenza-%28seasonal%29 (accessed on 16 September 2025).
- World Health Organization. COVID-19 epidemiological update –. Available online: https://www.who.int/publications/m/item/covid-19-epidemiological-update---24-december-2024 (accessed on 16 September 2025).
- World Health Organization. Respiratory syncytial virus (RSV). Available online: https://www.who.int/news-room/fact-sheets/detail/respiratory-syncytial-virus-(rsv) (accessed on 16 September 2025).
- Center for Disease Control and Prevention. RSV in Adults. Available online: https://www.cdc.gov/rsv/adults/index.html (accessed on 16 September 2025).
- Tai, C.-S.; Jian, M., Jr.; Lin, T.-H.; Chung, H.-Y.; Chang, C.-K.; Perng, C.-L.; Hsieh, P.-S.; Shang, H.-S. Analytical performance evaluation of a multiplex real-time RT-PCR kit for simultaneous detection of SARS-CoV-2, influenza A/B, and RSV. PeerJ 2025, 13, e19693. [Google Scholar] [CrossRef]
- Lee, J.S.; Ahn, J.J.; Kim, S.J.; Yu, S.Y.; Koh, E.J.; Kim, S.H.; Sung, H.S.; Huh, J.W.; Hwang, S.Y. POCT Detection of 14 Respiratory Viruses Using Multiplex RT-PCR. BioChip Journal 2021, 15, 371–380. [Google Scholar] [CrossRef]
- Pabbaraju, K.; Wong, A.A.; Ma, R.; Zelyas, N.; Tipples, G.A. Development and validation of a multiplex reverse transcriptase-PCR assay for simultaneous testing of influenza A, influenza B and SARS-CoV-2. Journal of virological methods 2021, 293, 114151. [Google Scholar] [CrossRef]
- Kim, T.Y.; Bae, G.E.; Kim, J.Y.; Kang, M.; Jang, J.H.; Huh, H.J.; Chung, D.R.; Lee, N.Y. Evaluation of the Kaira COVID-19/Flu/RSV Detection Kit for detection of SARS-CoV-2, influenza A/B, and respiratory syncytial virus: A comparative study with the PowerChek SARS-CoV-2, influenza A&B, RSV Multiplex Real-time PCR Kit. PloS one 2022, 17, e0278530. [Google Scholar] [CrossRef]
- Chan, W.S.; Wong, K.P.; Yau, S.K.; Wong, C.Y.; Chan, T.C.; Hung, J.; Lai, K.T.; Leung, C.P.; Wang, C.L.; Au, C.H.; et al. Clinical Evaluation of Xpert Xpress CoV-2/Flu/RSV plus and Alinity m Resp-4-Plex Assay. Diagnostics (Basel) 2024, 14. [Google Scholar] [CrossRef] [PubMed]
- Centers for Disease Control and Prevention (CDC). Clinical Guidance for Patients with Acute Respiratory Illness Being Hospitalized When SARS-CoV-2 and Influenza Viruses are Co-Circulating. Available online: https://www.cdc.gov/flu/hcp/clinical-guidance/testing-guidance-for-clinicians-hospitalized.html (accessed on 17 September 2025).
- Sinha, R. The role and impact of new technologies on healthcare systems. Discover Health Systems 2024, 3. [Google Scholar] [CrossRef]
- Abdullah, A.; Sam, I.C.; Ong, Y.J.; Theo, C.H.; Pukhari, M.H.; Chan, Y.F. Comparative Evaluation of a Standard M10 Assay with Xpert Xpress for the Rapid Molecular Diagnosis of SARS-CoV-2, Influenza A/B Virus, and Respiratory Syncytial Virus. Diagnostics 2023, 13, 3507. [Google Scholar] [CrossRef]
- McElvania, E.; Rao, D.; Greninger, A.L.; Harnett, G.; Larcena, A.; Patel, A.; Webster, B.; Ulen, C.; Green, D.F.; King, D.; et al. Evaluation of Cepheid Xpert Xpress CoV-2/Flu/RSV plus for nasal and nasopharyngeal specimens tested in CLIA-accredited and CLIA-waived settings. Journal of clinical virology: the official publication of the Pan American Society for Clinical Virology 2025, 180, 105851. [Google Scholar] [CrossRef]
- Matic, N.; Lawson, T.; Ritchie, G.; Lowe, C.F.; Romney, M.G. Testing the limits of multiplex respiratory virus assays for SARS-CoV-2 at high cycle threshold values: Comparative performance of cobas 6800/8800 SARS-CoV-2 & Influenza A/B, Xpert Xpress SARS-CoV-2/Flu/RSV, and cobas Liat SARS-CoV-2 & Influenza A. Journal of the Association of Medical Microbiology and Infectious Disease Canada 2024, 8, 328–335. [Google Scholar] [CrossRef] [PubMed]
- Sasidharan, A.; Selvarangan, R.; Konrad, K.; Faron, M.L.; Shakir, S.M.; Hillyard, D.; McCall, R.K.; McHardy, I.H.; Goldberg, D.C.; Dunn, J.J.; et al. Multi-center clinical evaluation of the Panther Fusion SARS-CoV-2/Flu A/B/RSV assay in nasopharyngeal swab specimens from symptomatic individuals. Journal of clinical microbiology 2023, 61, e0082723. [Google Scholar] [CrossRef]
- Zhen, W.; Manji, R.; Smith, E.; Wuitschick, J.; Lucic, D.; Berry, G.J. Evaluation of the Alinity m Resp-4-Plex Assay for the Detection of Severe Acute Respiratory Syndrome Coronavirus 2, Influenza A Virus, Influenza B Virus, and Respiratory Syncytial Virus. Microbiol Spectr 2022, 10, e0109021. [Google Scholar] [CrossRef]
- Chung, H.Y.; Jian, M.J.; Chang, C.K.; Lin, J.C.; Yeh, K.M.; Yang, Y.S.; Chen, C.W.; Hsieh, S.S.; Tang, S.H.; Perng, C.L.; et al. Multicenter study evaluating one multiplex RT-PCR assay to detect SARS-CoV-2, influenza A/B, and respiratory syncytia virus using the LabTurbo AIO open platform: epidemiological features, automated sample-to-result, and high-throughput testing. Aging (Albany NY) 2021, 13, 24931–24942. [Google Scholar] [CrossRef]
- Shu, B.; Kirby, M.K.; Davis, W.G.; Warnes, C.; Liddell, J.; Liu, J.; Wu, K.H.; Hassell, N.; Benitez, A.J.; Wilson, M.M.; et al. Multiplex Real-Time Reverse Transcription PCR for Influenza A Virus, Influenza B Virus, and Severe Acute Respiratory Syndrome Coronavirus 2. Emerg Infect Dis 2021, 27, 1821–1830. [Google Scholar] [CrossRef]
- Caballero Méndez, A.; Reynoso de la Rosa, R.A.; Abreu Bencosme, M.E.; Sosa Ortiz, M.N.; Pichardo Beltre, E.; de la Cruz Garcia, D.M.; Pinero Santana, N.J.; Bacalhau de Leon, J.C. Development and performance evaluation of a qPCR-based assay for the fully automated detection of group B Streptococcus (GBS) on the Panther Fusion Open Access system. Microbiol Spectr 2024, 12, e0005724. [Google Scholar] [CrossRef]
- Caballero Méndez, A.; Reynoso de La Rosa, R.A.; Abreu Bencosme, M.E.; Sosa Ortiz, M.N.; Pichardo Beltré, E.; de La Cruz García, D.M.; Piñero Santana, N.J.; Bacalhau de León, J.C. Screening for Streptococcus agalactiae: Development of an Automated qPCR-Based Laboratory-Developed Test Using Panther Fusion Open AccessTM. Bio-protocol 2025, 15, e5255. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez, A.; Rodriguez, M.; Cordoba, J.J.; Andrade, M.J. Design of primers and probes for quantitative real-time PCR methods. Methods Mol Biol 2015, 1275, 31–56. [Google Scholar] [CrossRef] [PubMed]
- Wang, K.; Li, H.; Xu, Y.; Shao, Q.; Yi, J.; Wang, R.; Cai, W.; Hang, X.; Zhang, C.; Cai, H.; et al. MFEprimer-3.0: quality control for PCR primers. Nucleic acids research 2019, 47, W610–W613. [Google Scholar] [CrossRef] [PubMed]
- Galaxy, C. The Galaxy platform for accessible, reproducible, and collaborative data analyses: 2024 update. Nucleic acids research 2024, 52, W83–W94. [Google Scholar] [CrossRef]
- Hologic Inc. Panther Fusion® Specimen Lysis. 2022, 001, AW-26255-001. [Google Scholar]
- Roche Inc. MagNA Pure 96 DNA andViral NA Large Volume Kit. 2021, 10. [Google Scholar]
- Seegene Inc. 12/2023 V2.01_(EN); Allplex™ SARS-CoV-2/FluA/FluB/RSV Assay. 2023.
- Hologic Inc. Panther Fusion® SARS-CoV-2/Flu A/B/RSV Assay. 2025, 002, 32326–001001. [Google Scholar]
- Silva, D.T.; Starke-Buzetti, W.A.; Alves-Martin, M.F.; Paixao Mdos, S.; Tenorio Mda, S.; Lopes, M.L. Comparative evaluation of several methods for Canine Visceral Leishmaniasis diagnosis. Rev Bras Parasitol Vet 2014, 23, 179–186. [Google Scholar] [CrossRef]
- Tahir, B.; Weldegebreal, F.; Ayele, F.; Ayana, D.A. Comparative evaluation of saliva and nasopharyngeal swab for SARS-CoV-2 detection using RT-qPCR among COVID-19 suspected patients at Jigjiga, Eastern Ethiopia. PloS one 2023, 18, e0282976. [Google Scholar] [CrossRef]
- Erdoğan, S.; Gülhan, O.T. Alternative Confidence Interval Methods Used in the Diagnostic Accuracy Studies. Computational and Mathematical Methods in Medicine 2016, 2016, 1–7. [Google Scholar] [CrossRef]
- Centers for Disease Control and Prevention. Multiplex Assays Authorized for Simultaneous Detection of Influenza Viruses and SARS-CoV-2 by FDA. Available online: https://www.cdc.gov/flu/hcp/testing-methods/flu-covid19-detection.html?utm (accessed on 10 October 2025).
- Koçer, İ.; Demirbakan, H.; Aktaş, A. Temporal dynamics and forecasting of respiratory viral infections during and after the SARS-CoV-2 pandemic (2020–2027): a multiplex PCR and ARIMA-based study. Frontiers in Microbiology 2025, 16. [Google Scholar] [CrossRef] [PubMed]
- Leung, E.C.; Chow, V.C.; Lee, M.K.; Tang, K.P.; Li, D.K.; Lai, R.W. Evaluation of the Xpert Xpress SARS-CoV-2/Flu/RSV Assay for Simultaneous Detection of SARS-CoV-2, Influenza A and B Viruses, and Respiratory Syncytial Virus in Nasopharyngeal Specimens. Journal of clinical microbiology 2021, 59. [Google Scholar] [CrossRef] [PubMed]
- Spitzenberger, F.; Patel, J.; Gebuhr, I.; Kruttwig, K.; Safi, A.; Meisel, C. Laboratory-Developed Tests: Design of a Regulatory Strategy in Compliance with the International State-of-the-Art and the Regulation (EU) 2017/746 (EU IVDR [In Vitro Diagnostic Medical Device Regulation]). Therapeutic Innovation & Regulatory Science 2022, 56, 47–64. [Google Scholar] [CrossRef]
- Gao, J.; Jennings, L.J. Quality Assurance and Quality Control in Molecular Diagnostic Laboratories. In Practical Oncologic Molecular Pathology: Frequently Asked Questions; Ding, Y., Zhang, L., Eds.; Springer International Publishing: Cham, 2021; pp. 77–85. [Google Scholar]
- Teirlinck, A.C.; Broberg, E.K.; Stuwitz Berg, A.; Campbell, H.; Reeves, R.M.; Carnahan, A.; Lina, B.; Pakarna, G.; Bøås, H.; Nohynek, H.; et al. Recommendations for respiratory syncytial virus surveillance at the national level. European Respiratory Journal 2021, 58, 2003766. [Google Scholar] [CrossRef]
- Fan, G.; Qian, Q.; Tang, Y.; Liu, J.; Yang, L.; Peng, Y.; Lin, Y.; Ou, G.; Luo, Y.; Shen, C.; et al. The dynamic etiology and epidemiological patterns of acute respiratory tract infections during and post non-pharmacological interventions of SARS-CoV-2 in Shenzhen, China: a two years’ prospective cohort study from June 2022. Frontiers in Cellular and Infection Microbiology 2025, 15. [Google Scholar] [CrossRef]
- Tang, H.T.; Norz, D.; Grunwald, M.; Giersch, K.; Pfefferle, S.; Fischer, N.; Aepfelbacher, M.; Rohde, H.; Lutgehetmann, M. Analytical and clinical validation of a novel, laboratory-developed, modular multiplex-PCR panel for fully automated high-throughput detection of 16 respiratory viruses. Journal of clinical virology: the official publication of the Pan American Society for Clinical Virology 2024, 173, 105693. [Google Scholar] [CrossRef]
- Wang, L.; Piedra, P.A.; Avadhanula, V.; Durigon, E.L.; Machablishvili, A.; López, M.-R.; Thornburg, N.J.; Peret, T.C.T. Duplex real-time RT-PCR assay for detection and subgroup-specific identification of human respiratory syncytial virus. J. Virol. Methods 2019, 271, 113676. [Google Scholar] [CrossRef]
- Williams, T.; Jackson, S.; Barr, I.; Bi, S.; Bhiman, J.; Ellis, J.; Von Gottberg, A.; Lindstrom, S.; Peret, T.; Rughooputh, S.; et al. Results from the second WHO external quality assessment for the molecular detection of respiratory syncytial virus, 2019–2020. Influenza and Other Respiratory Viruses 2023, 17. [Google Scholar] [CrossRef]
- Vogels, C.B.F.; Brito, A.F.; Wyllie, A.L.; Fauver, J.R.; Ott, I.M.; Kalinich, C.C.; Petrone, M.E.; Casanovas-Massana, A.; Muenker, Catherine; Moore, M.A.J.; et al. Analytical sensitivity and efficiency comparisons of SARS-CoV-2 RT–qPCR primer–probe sets. Nature Microbiology 2020, 5, 1299–1305. [Google Scholar] [CrossRef]
- Kim, T.Y.; Kim, J.Y.; Shim, H.J.; Yun, S.A.; Jang, J.H.; Huh, H.J.; Kim, J.W.; Lee, N.Y. Performance Evaluation of the PowerChek SARS-CoV-2, Influenza A & B Multiplex Real-Time PCR Kit in Comparison with the BioFire Respiratory Panel. Ann Lab Med 2022, 42, 473–477. [Google Scholar] [CrossRef]
- Havasi, A.; Visan, S.; Cainap, C.; Cainap, S.S.; Mihaila, A.A.; Pop, L.A. Influenza A, Influenza B, and SARS-CoV-2 Similarities and Differences - A Focus on Diagnosis. Front Microbiol 2022, 13, 908525. [Google Scholar] [CrossRef] [PubMed]
- Huang, Z.; Song, J.; Shi, C.; Fan, X.; Xiao, Y.; Wang, X. Development and validation of an automated and high-throughput quadruplex RT–ddPCR assay for the detection of influenza A, influenza B, respiratory syncytial virus, and SARS-CoV-2. Frontiers in Cellular and Infection Microbiology 2025, 15. [Google Scholar] [CrossRef] [PubMed]
- Hays, A.; Islam, R.; Matys, K.; Williams, D. Best Practices in qPCR and dPCR Validation in Regulated Bioanalytical Laboratories: Best Practices in qPCR and dPCR Validation in Regulated Bioanalytical Laboratories. The AAPS journal 2022, 24, 36. [Google Scholar] [CrossRef] [PubMed]
- Kim, T.Y.; Kim, J.Y.; Shim, H.J.; Yun, S.A.; Jang, J.H.; Huh, H.J.; Kim, J.W.; Lee, N.Y. Comparison of the PowerChek SARS-CoV-2, Influenza A&B, RSV Multiplex Real-time PCR Kit and BioFire Respiratory Panel 2.1 for simultaneous detection of SARS-CoV-2, influenza A and B, and respiratory syncytial virus. Journal of virological methods 2021, 298, 114304. [Google Scholar] [CrossRef]
- U.S Food; Drug Administration. MAUDE Adverse Event Report: HOLOGIC, INC. FUSION SARS COV-2/FLU/RSV; PANTHER FUSION SARS COV-2/FLU A/B/RSV ASSAY. Available online: https://www.accessdata.fda.gov/scripts/cdrh/cfdocs/cfmaude/detail.cfm?mdrfoi__id=20263844 (accessed on 30 September 2025).
- U.S Food; Drug Administration. 510(k) SUBSTANTIAL EQUIVALENCE DETERMINATION. Available online: https://www.accessdata.fda.gov/cdrh_docs/reviews/K241240.pdf (accessed on 12 September 2025).
- Wolters, F.; Grunberg, M.; Huber, M.; Kessler, H.H.; Pruller, F.; Saleh, L.; Febreau, C.; Rahamat-Langendoen, J.; Thibault, V.; Melchers, W.J.G. European multicenter evaluation of Xpert(R) Xpress SARS-CoV-2/Flu/RSV test. Journal of medical virology 2021, 93, 5798–5804. [Google Scholar] [CrossRef]
- Tang, M.L.; Li, Y.Q.; Chen, X.; Lin, H.; Jiang, Z.C.; Gu, D.L.; Chen, X.; Tang, C.X.; Xie, Z.Q. Co-Infection with Common Respiratory Pathogens and SARS-CoV-2 in Patients with COVID-19 Pneumonia and Laboratory Biochemistry Findings: A Retrospective Cross-Sectional Study of 78 Patients from a Single Center in China. Med Sci Monit 2021, 27, e929783. [Google Scholar] [CrossRef]
- Alvares, P.A. SARS-CoV-2 and Respiratory Syncytial Virus Coinfection in Hospitalized Pediatric Patients. Pediatr Infect Dis J 2021, 40, e164–e166. [Google Scholar] [CrossRef]
- Zandi, M.; Soltani, S.; Fani, M.; Abbasi, S.; Ebrahimi, S.; Ramezani, A. Severe acute respiratory syndrome coronavirus 2 and respiratory syncytial virus coinfection in children. Osong Public Health Res Perspect 2021, 12, 286–292. [Google Scholar] [CrossRef]
- Cong, B.; Deng, S.; Wang, X.; Li, Y. The role of respiratory co-infection with influenza or respiratory syncytial virus in the clinical severity of COVID-19 patients: A systematic review and meta-analysis. Journal of Global Health 2022, 12. [Google Scholar] [CrossRef]
- Krumbein, H.; Kümmel, L.S.; Fragkou, P.C.; Thölken, C.; Hünerbein, B.L.; Reiter, R.; Papathanasiou, K.A.; Renz, H.; Skevaki, C. Respiratory viral co-infections in patients with COVID-19 and associated outcomes: A systematic review and meta-analysis. Rev. Med. Virol. 2023, 33. [Google Scholar] [CrossRef]
- Padilha, D.A.; Barazzetti, F.H.; Schörner, M.A.; Grisard, H.B.D.S.; Filho, V.B.; Kawagoe, E.K.; Souza, D.S.M.; Bazzo, M.L.; Wagner, G.; Fongaro, G. Respiratory Viruses Coinfections During the COVID-19 Pandemic in Southern Brazil. COVID 2025, 5, 133. [Google Scholar] [CrossRef]
- Trifonova, I.; Korsun, N.; Madzharova, I.; Alexiev, I.; Ivanov, I.; Levterova, V.; Grigorova, L.; Stoikov, I.; Donchev, D.; Christova, I. Epidemiological and Genetic Characteristics of Respiratory Viral Coinfections with Different Variants of Severe Acute Respiratory Syndrome Coronavirus 2 (SARS-CoV-2). Viruses 2024, 16, 958. [Google Scholar] [CrossRef]
- Pratt, G.W.; Wong, C.L.; Rao, L.V. Prevalence and Co-Detection Rates of SARS-CoV-2, Influenza, and Respiratory Syncytial Virus: A Retrospective Analysis. APMIS: acta pathologica, microbiologica, et immunologica Scandinavica 2025, 133, e70010. [Google Scholar] [CrossRef]
- An, S.H.; Kim, N.Y.; Heo, G.B.; Kang, Y.M.; Lee, Y.J.; Lee, K.N. Development and evaluation of a multiplex real-time RT-PCR assay for simultaneous detection of H5, H7, and H9 subtype avian influenza viruses. Journal of virological methods 2024, 327, 114942. [Google Scholar] [CrossRef]
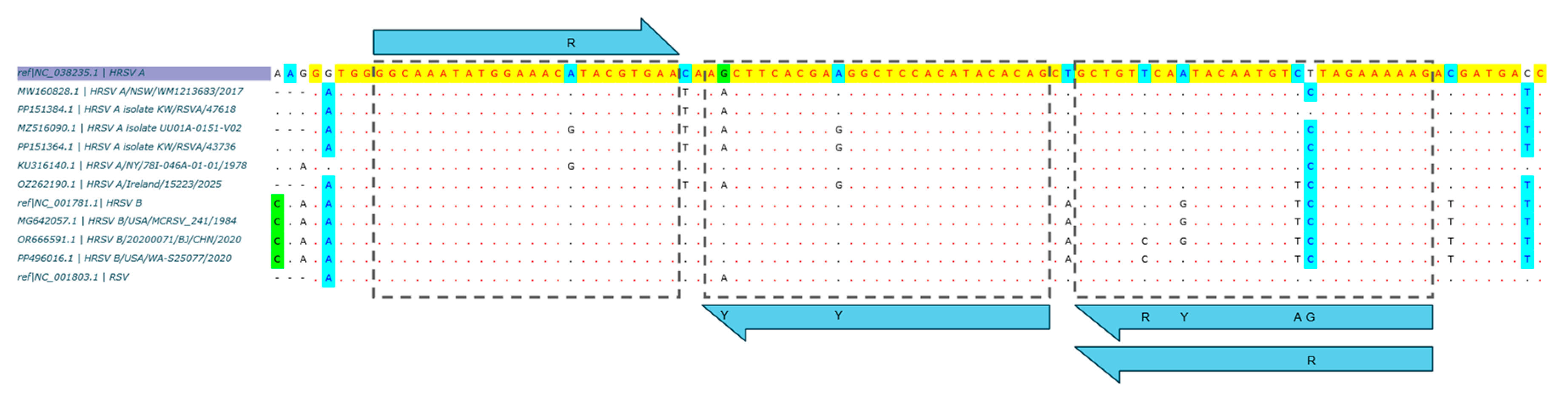
| Assay_Oligo type | Target gene * | Nucleotide position * | Oligo sequence (5’→3’) | Tm ** (°C) | Amplicon size (nt) | Ref |
|---|---|---|---|---|---|---|
| IAV_fwd1 | M | 143–167 | CAA GAC CAA TCY TGT CAC CTC TGA C | 65.4 # | 106 | [24] |
| IAV_fwd2 | 143–167 | CAA GAC CAA TYC TGT CAC CTY TGA C | 65 # | |||
| IAV_rev1 | 248–226 | GCA TTY TGG ACA AAV CGT CTA CG | 64.3 # | |||
| IAV_rev2 | 248–226 | GCA TTT TGG ATA AAG CGT CTA CG | 62.2 | |||
| IAV_prb 1 | 224–201 | /FAM/TGC AGT CCT /ZEN/ CGC TCA CTG GGC ACG/IABkFQ/ | 73.1 | |||
| IBV_fwd | NS2 | 758–779 | TCC TCA AYT CAC TCT TCG AGC G | 64.7 # | 103 | [24] |
| IBV_rev | 860–840 | CGG TGC TCT TGA CCA AAT TGG | 63.8 | |||
| IBV_prb 2 | 802–828 | /YakYel/CCA ATT CGA /ZEN/ GCA GCT GAA ACT GCG GTG/IABkFQ/ | 70.7 | |||
| SC2_fwd | N | 29,463–29,485 | CTG CAG ATT TGG ATG ATT TCT CC | 61.7 | 92 | [24] |
| SC2_rev | 29,554–29,530 | CCT TGT GTG GTC TGC ATG AGT TTA G | 65.2 | |||
| SC2_prb 3 | 29,491–29,520 | /TexRd-XN/ATT GCA ACA /TAO/ ATC CAT GAG CMG TGC TGA CTC/IAbRQSp/ | 70.9 # | |||
| RSV A/B_fwd | M | 3,226–3,249 | GGC AAA TAT GGA AAC RTA CGT GAA | 62.9 # | 80 | This study |
| RSV A_rev | 3,308–3,281 | CTT TTT CTA RGA CAT TGT ATT GAA CAG C | 62.5 # | |||
| RSV B_rev | 3,308–3,281 | CTT TTT CTA GAA CAT TGT AYT GRA CAG C | 63 # | |||
| RSV A/B_prb 4 | 3,278–3,252 | /CY5/CTG TGT ATG /TAO/ TGG AGC CYT CGT GAA GYT/IAbRQSp/ | 69.2 # | |||
| RNaseP_fwd | Human RNase P | 28–46 | AGA TTT GGA CCT GCG AGC G | 64.4 | 65 | [24] |
| RNaseP_rev | 92–73 | GAG CGG CTG TCT CCA CAA GT | 66.2 | |||
| RNaseP_prb 5 | 49–71 | /CY5.5/TTC TGA CCT GAA GGC TCT GCG CG/IAbRQSp/ | 70 |
| Reagent | Concentration | Volume | |||
|---|---|---|---|---|---|
| Stock/Working | Reaction (PCR) | PPR (1.25x) |
Unitarian 1 | PPR (40 tests) 2 |
|
| Water, PCR grade | n/a | n/a | n/a | 13.04 µL | 782.25 µL |
| K+ | 1 M | 75 mM | 93.75 mM | 1.88 µL | 112.5 µL |
| Mg++ | 1 M | 4 mM | 5 mM | 0.1 µL | 6 µL |
| TRIS | 1 M | 8 mM | 10 mM | 0.2 µL | 12 µL |
| IAV_fwd1 | 100 µM | 0.4 µM | 0.5 µM | 0.1 µL | 6 µL |
| IAV_fwd2 | 100 µM | 0.4 µM | 0.5 µM | 0.1 µL | 6 µL |
| IAV_rev1 | 100 µM | 0.6 µM | 0.75 µM | 0.15 µL | 9 µL |
| IAV_rev2 | 100 µM | 0.2 µM | 0.25 µM | 0.05 µL | 3 µL |
| IAV_prb | 100 µM | 0.2 µM | 0.25 µM | 0.05 µL | 3 µL |
| IBV_fwd | 100 µM | 0.8 µM | 1 µM | 0.2 µL | 12 µL |
| IBV_rev | 100 µM | 0.8 µM | 1 µM | 0.2 µL | 12 µL |
| IBV_prb | 100 µM | 0.4 µM | 0.5 µM | 0.1 µL | 6 µL |
| SC2_fwd | 100 µM | 0.8 µM | 1 µM | 0.2 µL | 12 µL |
| SC2_rev | 100 µM | 0.8 µM | 1 µM | 0.2 µL | 12 µL |
| SC2_prb | 100 µM | 0.2 µM | 0.25 µM | 0.05 µL | 3 µL |
| RSV A/B_fwd | 100 µM | 0.8 µM | 1 µM | 0.2 µL | 12 µL |
| RSV A_rev | 100 µM | 0.8 µM | 1 µM | 0.2 µL | 12 µL |
| RSV B_rev | 100 µM | 0.8 µM | 1 µM | 0.2 µL | 12 µL |
| RSV A/B_prb | 100 µM | 0.4 µM | 0.5 µM | 0.1 µL | 6 µL |
| RNaseP_fwd | 100 µM | 0.3 µM | 0.38 µM | 0.08 µL | 4.5 µL |
| RNaseP_rev | 100 µM | 0.3 µM | 0.38 µM | 0.08 µL | 4.5 µL |
| RNaseP_prb | 100 µM | 0.15 µM | 0.19 µM | 0.04 µL | 2.25 µL |
| Betaine | 5 M | 0.5 M | 0.63 M | 2.5 µL | 150 µL |
| Total volume | n/a | n/a | n/a | 20 µL | 1,200 µL |
| Target | R2 * | Slope (95% CI) | E** (95% CI) | ||
|---|---|---|---|---|---|
| IAV | 0.9998 | -3.38 | (-3.44–-3.33) | 97% | (95.3–99.7%) |
| IBV | 0.9995 | -3.38 | (-3.47–-3.29) | 98% | (94.3–101.5%) |
| SC2 | 0.9978 | -3.22 | (-3.4–-3.05) | 104% | (96.9–112.8%) |
| RSV A | 0.9995 | -3.20 | (-3.28–-3.12) | 105% | (101.7–109.3%) |
| RSV B | 0.9966 | -3.31 | (-3.54–-3.09) | 100% | (91.8–110.6%) |
| Level | Concentration | Observed Frequency (Hit Rate) | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| copies/mL 1 | copies/reaction | IAV | IBV | SC2 | RSV A | RSV B | ||||||
| 1 | 15,488 | 230.4 | 20/20 | (100%) | 20/20 | (100%) | 20/20 | (100%) | 20/20 | (100%) | 20/20 | (100%) |
| 2 | 7,744 | 115.2 | 20/20 | (100%) | 20/20 | (100%) | 20/20 | (100%) | 20/20 | (100%) | 20/20 | (100%) |
| 3 | 3,872 | 57.6 | 20/20 | (100%) | 20/20 | (100%) | 20/20 | (100%) | 20/20 | (100%) | 20/20 | (100%) |
| 4 | 1,936 | 28.8 | 16/20 | (80%) | 20/20 | (100%) | 20/20 | (100%) | 20/20 | (100%) | 19/20 | (95%) |
| 5 | 968 | 14.4 | 12/20 | (60%) | 19/20 | (95%) | 20/20 | (100%) | 19/20 | (95%) | 14/20 | (70%) |
| 6 | 484 | 7.2 | 7/20 | (35%) | 13/20 | (65%) | 16/20 | (80%) | 16/20 | (80%) | 7/20 | (35%) |
| 7 | 242 | 3.6 | 3/20 | (15%) | 9/20 | (45%) | 12/20 | (60%) | 10/20 | (50%) | 4/20 | (20%) |
| 8 | 121 | 1.8 | 0/20 | (0%) | 4/20 | (20%) | 10/20 | (50%) | 6/20 | (30%) | 0/20 | (0%) |
| Pathogen | LoD (95% CI) | |||
|---|---|---|---|---|
| copies/mL 1 | copies/reaction | |||
| IAV | 2,541 | (1,981–3,899) | 37.8 | (29.5–58.0) |
| IBV | 936 | (736–1,420) | 13.9 | (11.0–21.1) |
| SC2 | 643 | (496–982) | 9.6 | (7.4–14.6) |
| RSV A | 824 | (648–1,208) | 12.3 | (9.6–18.0) |
| RSV B | 1,690 | (1,374–2,313) | 25.1 | (20.4–34.4) |
| Pathogen | Variant, Subtype, Lineage or Strain |
Source | Result | |||
|---|---|---|---|---|---|---|
| IAV | IBV | SC2 | RSV | |||
| Adenovirus 1 | n/a | NATRVP 2.1 | – | – | – | – |
| Adenovirus 3 | n/a | – | – | – | – | |
| Adenovirus 31 | n/a | – | – | – | – | |
| Bordetella parapertussis | A747 | – | – | – | – | |
| Bordetella pertussis | A639 | – | – | – | – | |
| Chlamydia pneumoniae | CWL-029 | – | – | – | – | |
| Coronavirus 229E | n/a | – | – | – | – | |
| Coronavirus HKU-1 | n/a | – | – | – | – | |
| Coronavirus NL63 | n/a | – | – | – | – | |
| Coronavirus OC43 | n/a | – | – | – | – | |
| Influenza A | A/NY/02/09 (H1N1pdm09) | + | – | – | – | |
| Influenza B | B/Florida/02/06 (Victoria) | – | + | – | – | |
| Mycoplasma pneumoniae | M129 | – | – | – | – | |
| Metapneumovirus 8 | Peru6-2003 | – | – | – | – | |
| Parainfluenza 1 | n/a | – | – | – | – | |
| Parainfluenza 2 | n/a | – | – | – | – | |
| Parainfluenza 3 | n/a | – | – | – | – | |
| Parainfluenza 4 | n/a | – | – | – | – | |
| Rhinovirus 1A | n/a | – | – | – | – | |
| RSV A | n/a | – | – | – | + | |
| SARS-CoV-2 | USA-WA1/2020 | – | – | + | – | |
| Adenovirus 14 | n/a | RTX1-5QC | – | – | – | – |
| Enterovirus A16 | n/a | – | – | – | – | |
| Enterovirus 68 | n/a | – | – | – | – | |
| Metapneumovirus A2 | n/a | – | – | – | – | |
| Rhinovirus 16 | n/a | – | – | – | – | |
| Adenovirus A | n/a | IDR-C 2024 | – | – | – | – |
| Enterovirus A71 | n/a | IDR-A 2023 | – | – | – | – |
| Legionella pneumophila | Philadelphia Group 1 | – | – | – | – | |
| Metapneumovirus B2 | n/a | – | – | – | – | |
| Pathogen | Variant, Subtype, Lineage or Strain |
Source | Result | |||
|---|---|---|---|---|---|---|
| IAV | IBV | SC2 | RSV | |||
| Influenza A | A/NY/02/09 (H1N1pdm09) | NATRVP 2.1 | + | – | – | – |
| A/NewCaledonia/20/99 (H1N1sea) | + | – | – | – | ||
| A/Brisbane/10/07 (H3N2) | + | – | – | – | ||
| A/Brisbane/02/2018 (H1N1pdm09) | IDR-A 2023 | + | – | – | – | |
| A/HongKong/2671/2019 (H3N2) | IDR-B 2023 | + | – | – | – | |
| A/Victoria/2570/2019 (H1N1pdm09) | ID3-C 2024 | + | – | – | – | |
| A/Cambodia/e0826360/2020 (H3N2) | ID3-A 2024 | + | – | – | – | |
| A/Kansas/14/2017 (H3N2) | ID3-B 2023 | + | – | – | – | |
| A/Netherlands/1250/2016 (H1N1pdm09) | INFTP24 | + | – | – | – | |
| A/Hong Kong/213/2003 (H5N1) | + | – | – | – | ||
| A/Netherlands/398/2014 (H3N2) | + | – | – | – | ||
| A/Netherlands/2393/2015 (H3N2) | + | – | – | – | ||
| A/Mallard/Netherlands/2/2009 (H7N7) | + | – | – | – | ||
| A/NIBRG-14 (H5N1) * | ASRC | + | – | – | – | |
| A/Brisbane/59/2007 (H1N1sea) | + | – | – | – | ||
| A/Perth/16/2009 (H3N2) | + | – | – | – | ||
| A/cattle/Texas/56283/2024 (H5N1) | APLH5N1 | + | – | – | – | |
| Influenza B | B/Florida/02/06 (Victoria) | NATRVP 2.1 | – | + | – | – |
| B/Washington/02/2019 (Victoria) | ID3-A 2024 | – | + | – | – | |
| B/Brisbane/60/2008 (Yamagata) | ATSFR | – | + | – | – | |
| B/Phuket/3073/2013 (Yamagata) | ID3-B 2024 | – | + | – | – | |
| SARS-CoV-2 | B.1.1.7 (Alpha) | ASRC | – | – | + | – |
| B.1.351 (Beta) | – | – | + | – | ||
| P.1 (Gamma) | – | – | + | – | ||
| B.1.617.2 (Delta) | – | – | + | – | ||
| BA.1 (Omicron) | – | – | + | – | ||
| A (USA-WA1/2020) | ID3-A 2024 | – | – | + | – | |
| B (Italy-INMI1/2020) | ID3-B 2024 | – | – | + | – | |
| RSV | n/a (Type A) | NATRVP 2.1 | – | – | – | + |
| RTX1QC | – | – | – | + | ||
| ID3-A 2024 | – | – | – | + | ||
| n/a (Type B) | RTX5QC | – | – | – | + | |
| IDR-B 2024 | – | – | – | + | ||
| 4/2015 (Type B) | IDR-B 2023 | – | – | – | + | |
| 9320 (Type B) | ATSFR | – | – | – | + | |
| Member panel | Content | Pathogen | Level | Agreed/n | Agreement | Mean Ct | Within Lot |
Between Lot |
Between Instrument |
Between Run/Day |
Within Run/Day |
Within Laboratory (Total) |
||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| SD (95% CI) |
CV | SD (95% CI) |
CV | SD (95% CI) |
CV | SD (95% CI) |
CV | SD (95% CI) |
CV | SD (95% CI) |
CV | |||||||
| 1 | Neg | n/a | n/a | 112/112 | 100% | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
| 2 | IAV/SC2 | IAV | Mod | 112/112 | 100% | 27.93 | 0.16 | 0.6% | 0.13 | 0.5% | 0 | 0% | 0.26 | 0.9% | 0.21 | 0.7% | 0.33 | 1.2% |
| (0.14–0.2) | (0.07–0.21) | (0–0.12) | (0.18–0.43) | (0.19–0.25) | (0.28–0.48) | |||||||||||||
| SC2 | Low | 112/112 | 100% | 34-40 | 0.32 | 0.9% | 0 | 0% | 0.4 | 1.2% | 0 | 0% | 0.51 | 1.15% | 0.51 | 1.5% | ||
| (0.27–0.4) | (0–0.21) | (0.27–0.66) | (0–0.33) | (0.42–0.74) | (0.46–0.66) | |||||||||||||
| 3 | SC2/IAV | IAV | Low | 111/112 1 | 99.1% | 34.84 | 0.95 | 2.7% | 00 | 0% | 0.3 | 0.8% | 0 | 0% | 1 | 2.9% | 1 | 2.9% |
| (0.8–1.17) | (0–0.55) | (0–0.68) | (0–0.43) | (0.9–1.21) | (0.91–1.17) | |||||||||||||
| SC2 | Mod | 112/112 | 100% | 25.49 | 0.12 | 0.5% | 0.1 | 0.4% | 0.43 | 1.7% | 0 | 0% | 0.46 | 1.8% | 0.46 | 1.8% | ||
| (0.1–0.14) | (0.06–0.16) | (0.31–0.69) | (0–0.22) | (0.35–0.7) | (0.4–0.59) | |||||||||||||
| 4 | IBV/RSV | IBV | Mod | 112/112 | 100% | 25.30 | 0.15 | 0.6% | 0.1 | 0.4% | 0.14 | 0.5% | 0 | 0% | 0.23 | 0.9% | 0.23 | 0.9% |
| (0.13–0.19) | (0.03–0.17) | (0.05–0.25) | (0–0.12) | (0.2–0.32) | (0.21–0.29) | |||||||||||||
| RSV | Low | 111/112 1 | 99.1% | 34.72 | 0.36 | 1% | 0.17 | 0.5% | 0.34 | 1% | 0 | 0% | 0.52 | 1.5% | 0.52 | 1.5% | ||
| (0.3–0.44) | (0–0.33) | (0.18–0.6) | (0–0.24) | (0.44–0.72) | (0.48–0.64) | |||||||||||||
| 5 | RSV/IBV | IBV | Low | 111/112 2 | 99.1% | 32.21 | 0.31 | 1% | 0.21 | 0.7% | 0 | 0% | 0.19 | 0.6% | 0.38 | 1.2% | 0.42 | 1.3% |
| (0.26–0.38) | (0.05–0.35) | (0–0.22) | (0.06–0.34) | (0.34–0.45) | (0.38–0.52) | |||||||||||||
| RSV | Mod | 111/112 2 | 99.1% | 26.07 | 0.37 | 1.4% | 0.12 | 0.5% | 0.15 | 0.6% | 0 | 0% | 0.42 | 1.6% | 0.42 | 1.6% | ||
| (031–0.46) | (0–0.29) | (0–0.34) | (0–0.2) | (0.37–0.53) | (0.38–0.5) | |||||||||||||
| Comparator RT-qPCR Assay | Target | Candidate RT-qPCR Assay: LDRA | n | NPA (95% CI) |
PPA (95% CI) |
OPA (95% CI) |
k (95% CI) |
||
|---|---|---|---|---|---|---|---|---|---|
| + | – | ||||||||
| PFRA | IAV | + | 65 | 0 | 405 | 100% | 100% | 100% | 1.000 |
| – | 0 | 340 | (98.9–100%) | (94.4–100%) | (99.1–100%) | (1.000–1.000) | |||
| IBV | + | 79 | 0 | 405 | 100% | 100% | 100% | 1.000 | |
| – | 0 | 326 | (98.8–100%) | (95.4–100%) | (99.1–100%) | (1.000–1.000) | |||
| SC2 | + | 88 * | 4 | 405 | 100% | 95.7% | 99.0% | 0.971 | |
| – | 0 | 313 | (98.8–100%) | (89.3–98.3%) | (97.5–99.6%) | (0.944–0.999) | |||
| RSV | + | 111 | 1 | 405 | 100% | 99.1% | 99.8% | 0.994 | |
| – | 0 | 293 | (98.7–100%) | (95.1–99.8%) | (98.6–100%) | (0.982–1.000) | |||
| APRA | IAV | + | 64 | 0 | 405 | 99.7% | 100% | 99.8% | 0.991 |
| – | 1 | 340 | (98.4–99.9%) | (94.3–100%) | (98.6–100%) | (0.973–1.000) | |||
| IBV | + | 78 | 0 | 405 | 99.7% | 100% | 99.8% | 0.992 | |
| – | 1 | 326 | (98.3–99.9%) | (95.3–100%) | (98.6–100%) | (0.977–1.000) | |||
| SC2 | + | 88 * | 0 | 405 | 100% | 100% | 100% | 1.000 | |
| – | 0 | 317 | (98.8–100%) | (95.8–100%) | (99.1–100%) | (1.000–1.000) | |||
| RSV | + | 110 | 0 | 405 | 99.7% | 100% | 99.8% | 0.994 | |
| – | 1 | 294 | (98.1–99.9%) | (96.6–100%) | (98.6–100%) | (0.982–1.000) | |||
| RT-qPCR Assay |
Target | TP | FP | FN | TN | n | PR (95% CI) |
dSens (95% CI) |
dSpec (95% CI) |
PPV (95% CI) |
NPV (95% CI) |
|---|---|---|---|---|---|---|---|---|---|---|---|
| LDRA | IAV | 65 | 0 | 0 | 340 | 405 | 16.0% | 100% | 100% | 100% | 100% |
| (12.8–19.9%) | (94.4–100%) | (98.9–100%) | (94.5–100%) | (98.9–100%) | |||||||
| IBV | 79 | 0 | 0 | 326 | 405 | 19.5% | 100% | 100% | 100% | 100% | |
| (15.9–23.6%) | (95.4–100%) | (98.8–100%) | (95.4–100%) | (98.9–100%) | |||||||
| SC2 | 88 | 0 | 0 | 317 | 405 | 21.7% | 100% | 100% | 100% | 100% | |
| (18.0–26.0%) | (95.8–100%) | (98.8–100%) | (95.9–100%) | (98.8–100%) | |||||||
| RSV | 111 | 0 | 0 | 294 | 405 | 27.4% | 100% | 100% | 100% | 100% | |
| (23.3–32.0%) | (96.7–100%) | (98.7–100%) | (96.7–100%) | (98.8–100%) | |||||||
| PFRA | IAV | 65 | 0 | 0 | 340 | 405 | 16.0% | 100% | 100% | 100% | 100% |
| (12.8–19.9%) | (94.4–100%) | (98.9–100%) | (94.5–100%) | (98.9–100%) | |||||||
| IBV | 79 | 0 | 0 | 326 | 405 | 19.5% | 100% | 100% | 100% | 100% | |
| (15.9–23.6%) | (95.4–100%) | (98.8–100%) | (95.4–100%) | (98.9–100%) | |||||||
| SC2 | 88 | 4 | 0 | 313 | 405 | 22.7% | 100% | 98.7% | 95.7% | 100% | |
| (18.9–27.0%) | (95.8–100%) | (96.8–99.5%) | (89.3–98.3%) | (98.8–100%) | |||||||
| RSV | 111 | 1 | 0 | 293 | 405 | 27.7% | 100% | 99.7% | 99.1% | 100% | |
| (23.5–32.2%) | (96.7–100%) | (98.1–99.9%) | (94.0–99.9%) | (98.8–99.9%) | |||||||
| APRA | IAV | 64 | 0 | 1 | 340 | 405 | 15.8% | 98.5% | 100% | 100% | 99.7% |
| (12.6–19.7%) | (91.8–99.7%) | (98.9–100%) | (94.4–100%) | (98.0–100%) | |||||||
| IBV | 78 | 0 | 1 | 326 | 405 | 19.3% | 98.7% | 100% | 100% | 99.7% | |
| (15.7–23.4%) | (93.2–99.8%) | (98.8–100%) | (95.4–100%) | (97.9–100%) | |||||||
| SC2 | 88 | 0 | 0 | 317 | 405 | 21.7% | 100% | 100% | 100% | 100% | |
| (18.0–26.0%) | (95.8–100%) | (98.8–100%) | (95.9–100%) | (98.8–100%) | |||||||
| RSV | 110 | 0 | 1 | 294 | 405 | 27.2% | 99.1% | 100% | 100% | 99.7% | |
| (23.1–31.7%) | (95.1–99.8%) | (98.7–100%) | (96.7–100%) | (97.7–100%) |
| RT-qPCR Assay |
Consensus | n | SinPA (95% CI) |
MixPA (95% CI) |
OPA (95% CI) |
k (95% CI) |
||
|---|---|---|---|---|---|---|---|---|
| Mix | Sin | |||||||
| LDRA | Mix | 7 | 0 | 335 | 100% | 100% | 100% | 1.000 |
| Sin | 0 | 328 | (98.8–100%) | (64.6–100%) | (98.9–100%) | (1.000–1.000) | ||
| PFRA | Mix | 7 | 3 | 335 | 99.1% | 100% | 99.1% | 0.819 |
| Sin | 0 | 325 | (97.3–99.7%) | (64.6–100%) | (97.4–99.7%) | (0.619–1.000) | ||
| APRA | Mix | 5 | 0 | 335 | 100% | 71.4% | 99.4% | 0.830 |
| Sin | 2 | 328 | (98.8–100%) | (35.9–91.8%) | (97.8–99.8%) | (0.599–1.000) | ||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





